Abstract
Background
Current guidelines do not recommend performing aeroallergen skin prick testing (SPT) in chronic spontaneous urticaria (CSU).
Objective
The objective of this review was to investigate the presence of aeroallergen sensitization and markers of T2 inflammation in subjects with CSU.
Methods
Systematic literature reviews to identify all studies that evaluated the presence of T2 markers of allergic inflammation in CSU subjects were performed.
Results
In 16 studies that assessed the prevalence of positive SPT to multiple aeroallergens in CSU, 38.5% of CSU subjects had positive SPT. In three controlled studies, 34.2% of CSU subjects had positive SPT to multiple aeroallergens, compared to 13.6% of controls (p = 0.047). In 18 studies that assessed the prevalence of house dust mite (HDM) positive SPT in CSU, 27.5% of CSU subjects had positive SPT. In three controlled studies, 27.5% of CSU subjects had positive SPT to HDM, compared to 2.1% of controls (p = 0.047). Overall, CSU subjects were 3.1 times more likely to be aeroallergen-sensitized (95% CI 1.7–5.8, p = 0.0002) and 6.1 times more likely to be HDM-sensitized (95% CI 3.7–9.9, p < 0.00001) than controls. Mean total serum IgE (tIgE) levels were 238 kU/L and median tIgE levels were 164 kU/L, which was greater than the upper 90th percentile of normal (< 137 kU/L). Compared to healthy controls, CSU subjects were 6.5 times more likely to have IgG autoantibody against FcεR1α (p = 0.001), 2.4 times more likely to have IgG anti-IgE antibody (p = 0.03) and 5 times more likely to have anti-thyroid peroxidase (anti-TPO) antibody (p = 0.02). When corticosteroids were withheld for ≥ 28 days, mean blood eosinophil percentage was elevated at 5.9% (normal < 4%), but other studies reporting absolute count found the mean was in the normal range, 239 L (normal < 400 L).
Conclusion
Increased aeroallergen sensitization, tIgE, autoantibodies and blood eosinophil percentage in the CSU subjects indicates the possible importance of T2 inflammation in the pathogenesis of CSU. Further studies may be warranted to determine if specific allergen avoidance, desensitization or improvement in the mucosal allergic inflammation present in asthma and/or rhinitis has any benefit in the management of CSU.
Keywords: Chronic spontaneous urticaria, Aeroallergen, IgE, Eosinophils, FcεR1α, Autoantibody, Anti-IgE, House dust mite, Rhinitis, T2 inflammation
Background
Urticaria is a vascular reaction in the superficial skin characterized by dark-red and slightly raised wheals, that are often associated with pruritus [1]. Typically, most cases are self-limiting, benign and of short duration, with episodes rarely persisting for more than several days; however, hives and swelling can persist for weeks to years and can significantly decrease one’s quality of life. Conventionally, chronic spontaneous urticaria (CSU) is defined as continuous or recurring intermittent episodes of 6 weeks or longer [1]. The prevalence of CSU in the general population has been estimated to range from 0.5 to 5% [2]. While specific mechanisms have been implicated in the pathogenesis of CSU, autoimmunity has been suggested to be a frequent cause of the condition [3]. However, in approximately 50% of patients, its etiology remains largely unknown [4].
Skin prick testing (SPT) is a highly reliable clinical procedure used to detect the presence of IgE antibodies or sensitization to an allergen of interest and can provide clinical evidence for a diagnosis of a suspected Type 1 Immunoglobulin E (IgE)-dependent allergic disease. While the potential role of immediate hypersensitivity in the pathogenesis of CSU has been considered in only a few reports, current guidelines state that “rarely, IgE-mediated reactions from foods, drugs, or other allergens might result in CSU” and that “Immediate hypersensitivity skin or serologic testing for food or other allergens is rarely useful and not recommended on a routine basis.” [2] SPT is not felt to be relevant, as international guidelines state “Type I allergy is an extremely rare cause of CSU” [5].
However, omalizumab (anti-IgE) has been shown to be an effective therapy for CSU, suggesting the importance of IgE in this condition. The presence of immunoglobulin G (IgG) autoantibody to the high-affinity Fc region of IgE (FcεR1α) in some subjects has been proposed as a possible cause of CSU, however autoantibodies may be more commonly found when atopy is present [6–9]. Other autoantibodies including IgG anti-IgE and anti-thyroid peroxidase (anti TPO) have also been described [10, 11]. A group in New York, USA found that 67% of CSU patients with allergic rhinitis in their clinic were sensitized to mugwort compared to 31% positive SPT in subjects with allergic rhinitis alone (p = 0.004) [12]. They speculated that cross reactivity to foods and spices may play a role in CSU, but didn’t consider the effects of inhaled mugwort or other aeroallergens as a possible cause. Recently, it was reported that following house dust mite (HDM) immunotherapy treatment in two patients with persistent HDM allergic rhinitis (AR) and CSU, both their nasal and CSU symptoms significantly improved [13]. In addition, there are case reports of three different anti-IL5 monoclonal antibodies [14–16] as well as a case series of the anti-IL4 and anti-IL13 monoclonal antibody, dupilumab [17] improving symptoms of CSU in subjects with severe eosinophilic asthma. Recently, a CSU cohort was found to have IgE against staphylococcal enterotoxin B (SEB), commonly found in subjects with chronic rhinosinusitis with nasal polyposis (CRSwNP) [18]. SEB IgE level was positively correlated with CSU disease activity, elevated total serum IgE (tIgE) and increased basophil histamine-release.
Here, we systematically evaluated the literature for the presence of T2 markers of allergic inflammation in CSU subjects, specifically the presence of sensitization to aeroallergens in CSU, the presence of IgG autoantibody to FcεR1α and other markers of T2 inflammation including tIgE, blood eosinophil count and concomitant AR and asthma.
Methods
Methods 1.1
A search for all original studies referring to the presence of positive SPT to inhalant allergens in subjects > 18 years old with CSU was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for reporting. We performed electronic searches within the PubMed, Ovid Medline, EMBASE and Cochrane Database of Systematic Reviews and Cochrane Controlled Register of Trials (CENTRAL) databases for English language articles published in peer-reviewed journals up to May 2020. The key words and search terms were as follows: (chronic urticaria OR chronic hives OR chronic spontaneous urticaria OR chronic idiopathic urticaria) AND (inhalant allergen* OR aeroallergen* OR allergen* OR mite* OR pollen OR skin prick test*). Manual searches of the Annals of Allergy journals, which were not available in electronic databases were also executed. Reference lists of identified articles were scanned to collect additional records.
Methods 1.2
A search for all original studies referring to the levels of tIgE in adolescent and adult subjects with CSU treated with omalizumab was conducted following the PRISMA guidelines for reporting. We performed electronic searches within the PubMed, Ovid Medline and CENTRAL databases for English language articles published in peer-reviewed journals up to May 2020. The key words and search terms were as follows: (chronic urticaria OR chronic hives OR chronic spontaneous urticaria OR chronic idiopathic urticaria) AND (omalizumab). Reference lists of identified articles were scanned to collect additional records.
Methods 1.3
A search for all original studies assessing the presence of IgG autoantibody to FcεR1α, IgG anti-IgE antibody and anti-TPO antibody in patients with CSU was also conducted following the PRISMA guidelines for reporting. We performed electronic searches within the PubMed, Ovid Medline and CENTRAL databases for English language articles published in peer-reviewed journals up to May 2020. The key words and search terms were as follows: (chronic urticaria OR chronic hives OR chronic spontaneous urticaria OR chronic idiopathic urticaria) AND (autoantibod* OR FcepsilonRI OR FcεR1α OR high-affinity IgE receptor).
Methods 1.4
A search for all original studies assessing the total eosinophil percentage and/or absolute eosinophil counts in adolescent and adult subjects with CSU was conducted following the PRISMA guidelines for reporting. We performed electronic searches within the PubMed, Ovid Medline and CENTRAL databases for English language articles published in peer-reviewed journals up to May 2020. The key words and search terms were as follows: (chronic urticaria OR chronic hives OR chronic spontaneous urticaria OR chronic idiopathic urticaria) AND (eosino*). Reference lists of identified articles were scanned to collect additional records.
Eligibility criteria
The search was limited to studies that met the following pre-defined inclusion criteria:
Methods 1.1
Participants: Male or female subjects with CSU who underwent testing for aeroallergen sensitization (subjects > 18 years of age).
Intervention: Male or female subjects diagnosed with CSU by explicit diagnostic criteria.
Control: Healthy male or female subjects with no history of atopy.
Outcomes: A measure of SPT results.
Types of studies: Any observational study including case control trials, retrospective chart reviews, cross-sectional studies, and case series.
Methods 1.2
Participants: Male or female subjects with CSU who underwent testing for tIgE levels (subjects > 18 years of age).
Intervention: Male or female subjects diagnosed with CSU by explicit diagnostic criteria.
Control: Healthy male or female subjects with no history of atopy.
Outcomes: A measure of tIgE levels.
Types of studies: Any observational study including case control trials, cross-sectional studies, and case series.
Methods 1.3
Participants: Male or female subjects with CSU who underwent testing for IgG autoantibody to FcεR1α (subjects > 18 years of age).
Intervention: Male or female subjects diagnosed with CSU by explicit diagnostic criteria.
Control: Healthy male or female subjects with no history of atopy.
Outcomes: A measure of IgG autoantibody to FcεR1α IgG anti-IgE antibody and anti- TPO antibody.
Types of studies: Any observational study including case control trials, cross-sectional studies, and case series.
Methods 1.4
Participants: Male or female subjects with CSU who underwent testing for total eosinophil percentage and/or absolute eosinophil counts (pediatric and adult subjects).
Intervention: Male or female subjects diagnosed with CSU by explicit diagnostic criteria.
Control: Healthy male or female subjects with no history of atopy.
Outcomes: A measure of total eosinophil percentage and/or absolute eosinophil counts.
Types of studies: Any observational study including case control trials, cross-sectional studies, retrospective chart reviews and case series.
Articles describing acute urticaria or inducible urticaria were excluded. Non-full text publications, letters to the editor, case reports, as well as articles that were not published in the English language were also excluded. Finally, abstract-only publications were not considered due to lack of detailed raw data.
Data collection and quality assessment
Two reviewers independently assessed the titles and abstracts of all retrieved publications to identify eligible and relevant studies. Full texts of selected publications were then retrieved if necessary and eligibility criteria was considered. Any discrepancies that were raised during data collection were resolved through consensus. Should the publication have met all eligibility criteria, information regarding the first author, year of publication, country that the study was conducted in, definition of CSU, indication of treatment provided, and the:
1.1: CSU population who underwent skin prick testing, number of inhalant allergens tested, SPT results.
1.2: CSU population who underwent IgE testing and the resulting mean tIgE levels.
1.3. CSU population who underwent testing for IgG autoantibody to FcεR1α, IgG anti-IgE antibody and anti-TPO antibody.
1.4: Mean and median total eosinophil levels as well as mean and median absolute eosinophil counts, was extracted.
Effect measures
Forest plots were used to assess the association of CSU with positive SPT to aeroallergens including HDM, as well as with the presence of IgG autoantibody to FcεR1α, IgG anti-IgE antibody and anti-TPO antibody. We used Review Manager version 5.3.4 to calculate mean differences and risk ratios with associated 95% CIs for dichotomous outcomes. The Mantel–Haenszel approach was chosen by using a random-effects model according to methods suggested in the Cochrane Handbook for Systematic Reviews of Interventions.
Results
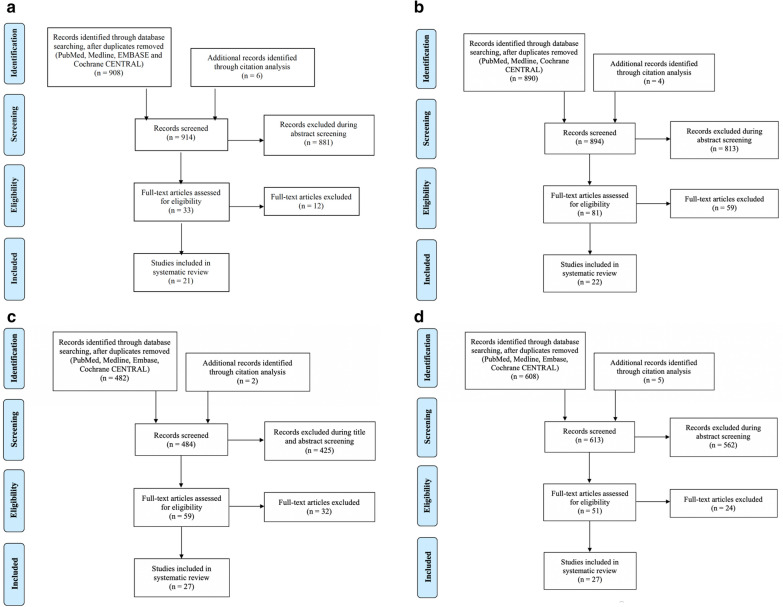
To identify the presence of positive SPT to aeroallergens in CSU subjects, 908 publications were retrieved from database searches following duplicate removal, as well as an additional 6 publications identified through citation analysis. 33 were screened for full-text review. Of these, 12 did not meet the outlined eligibility criteria, yielding 21 full texts from 11 countries involving a total of 2982 CSU subjects that were included for analysis (Fig. 1a).
Fig. 1.
a PRISMA flow diagram of literature review process for the presence of aeroallergen positive SPT in CSU subjects. b PRISMA flow diagram of literature review process for total IgE levels in CSU subjects. c PRISMA flow diagram of literature review process for autoantibodies in CSU subjects. d PRISMA flow diagram of literature review process for total eosinophil percentage and/or absolute eosinophil counts in CSU subjects
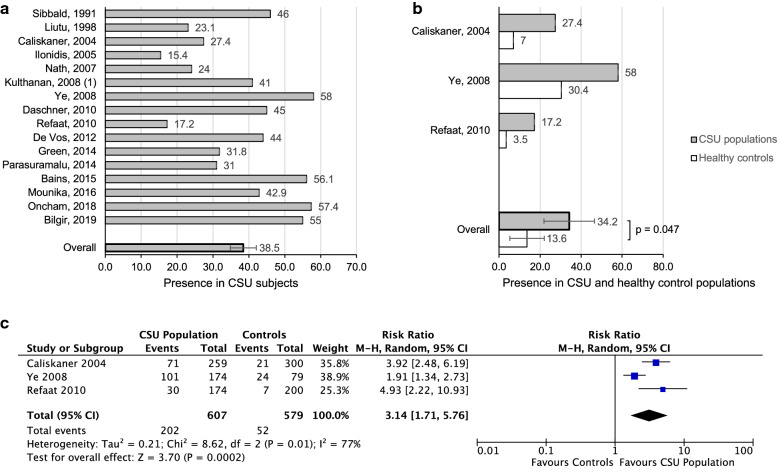
16 studies focused on multiple allergen extracts, namely pollens, animal dander and moulds (Table 1). In addition, some studies [12, 19–21] did not report mean numbers of positive SPTs; however, they indicated what percentage of participants were allergic to at least one antigen. As such, we included these values at the bottom of Table 1. As shown in Fig. 2a, the presence of multiple aeroallergen positive SPT in all study groups was 38.5% (standard error of the mean [SEM] 3.6).
Table 1.
Summary of all studies measuring multiple aeroallergen SPT in subjects with CSU
| Reference (author, year) | Country | Definition of CSU in weeks | Number of inhalant allergens tested | Number of CSU subjects undergoing SPT | % of positive SPT for aeroallergens in CSU patients | Number of healthy controls undergoing SPT | % of positive SPT for aeroallergens in controls | Number of antihistamine (AH) and/or systemic corticosteroid (SC)-free days | % of CSU subjects with atopic diseases: allergic rhinitis (AR) and/or asthma (A) | Total IgE levels in CSU subjects |
|---|---|---|---|---|---|---|---|---|---|---|
| Sibbald, 1991 [53] | Canada | – | 12 | 75 | 46 | – | – | – | 10 (A) | 23% > 200 IU/L |
| Liutu, 1998 [54] | Finland | > 8 | ≥ 4 | 91 | 23.1 | – | – | – | – | 49.3% > 111 IU/L |
| Caliskaner, 2004 [51] | Turkey | > 6 | 56 | 259 | 27.4 | 300 | 7 | 7 (AH) | Excluded AR/A | – |
| Ilonidis, 2005 [55] | Greece | – | 8 | 130 | 15.4 | – | – | – | – | – |
| Kulthanan, 2008 [56] | Thailand | > 6 | 12 | 88 | 41 | – | – |
3 (AH) 28 (SC) |
20.5 (AR) 6.8 (A) |
– |
| Ye, 2008 [57] | Korea | > 6 | 50 | 174 | 58.0 | 79 | 30.4 |
3 (AH) 3 (SC) |
36.1 (AR) 7.7 (A) |
– |
| Daschner, 2010 [58] | Spain | – | 10 | 80 | 45 | 522 (with AR/A) | – | – | ||
| Refaat, 2010 [59] | Egypt | – | 9 | 174 | 17.2 | 200 | 3.5 | 5 (AH) | Excluded AR/A | – |
| Green, 2014 [60] | Canada | – | 30 | 44 | 31.8 | – | – | – | – | – |
| Bains, 2015 [52] | India | – | 66 | 41 | 56.1 | – | – |
3 (AH) 14 (SC) |
Excluded AR/A | – |
| Oncham, 2018 [61] | Thailand | – | 17 | 140 | 57.4 | – | – | – | – | – |
| Bilgir, 2019 [62] | Turkey | > 6 | – | 302 | 55 | – | – | – | – | – |
| Nath, 2007 [19] | India | > 6 | 11 | 50 | ≥ 24 | – | – | – | – | – |
| De Vos, 2012 [12] | United States | > 6 | 22 | 19 | ≥ 44 | 19 | ≥26 | – | Excluded AR | – |
| Parasuramalu, 2014 [20] | India | > 6 | 26 | 300 | 1 | – | – | 15 (AH) |
11.3 (AR) 2.3 (A) |
– |
| Mounika, 2017 [21, 63] | India | > 6 | 40 | 56 | ≥ 42.9 | – | – |
2 (AH) 14 (SC) |
– | – |
AH antihistamine, SC systemic corticosteroid, AR allergic rhinitis, A asthma
Fig. 2.
a Presence of positive SPT to multiple or single aeroallergens in CSU populations in 16 studies. b Presence of positive SPT to multiple or single antigens in CSU populations in 3 studies, compared to healthy control groups with no history of atopy (p = 0.047). c Forest plot graphic assessing the relative risk of positive SPT to multiple or single aeroallergens. M–H, Mantel–Haenszel approach
Only three studies (607 CSU participants) that investigated the association of CSU with positive SPT for multiple aeroallergens included a healthy control population with no history of atopy. In each of the three publications, the CSU group had a higher percentage of positive SPT to aeroallergens compared to the control group, as shown in Fig. 2b (p = 0.047). On average, 34.2% of the total study population had positive skin tests to aeroallergens (SEM 12.3) while a review of control groups revealed a presence of 13.6% (SEM 8.4). Due to the lack of a control group in the remaining studies, it becomes more difficult to determine if the increased percentage of positive SPT results are due to a diagnosis of CSU or a result of other factors. Combined relative risk (RR) was 3.1 (95% CI 1.7–5.8) with significant heterogeneity (p = 0.0002; I2 = 77%) (Fig. 2c). Overall, the final estimate from the forest plots revealed an increased risk of positive SPT to aeroallergens in subjects with CSU.
The most frequently assessed allergen was HDM, which was highlighted in 18 studies (Table 2). The presence of HDM positive SPT in all 18 study groups was 27.5% (SEM 4.3) (Fig. 3a). Only three studies (452 CSU participants) investigated the association of HDM positive SPT in both CSU and healthy control populations with no history of atopy. On average, 27.5% of the study population (SEM 8.8) were sensitive to HDM, while only 2.1% of the control group (SEM 1.4) tested positive to HDM (p = 0.047) (Fig. 3b). Results reveal a combined RR of 6.1 (95% CI 3.7–9.9) with no heterogeneity (p < 0.00001; I2 = 0%), as shown in Fig. 3c. Overall, the final estimates revealed an increased risk of positive SPT to HDM in subjects with CSU. Mahesh et al. found a high number of positive HDM results in control subjects, 23%, but did not read skin tests in a standard fashion, as the authors interpreted a positive skin test as 50% or more compared to the histamine test. As such, the data from this study was not included in Fig. 3b or c. In the Nath et al. study, only 12% patients had positive SPT to HDM, but they only tested one HDM, Dermatophagoides farinae.
Table 2.
Summary of studies performing house dust mite allergen SPT in subjects with CSU
| Reference (author, year) | Region | Definition of CSU in weeks | Number of CSU subjects undergoing SPT | % of positive SPT for House Dust Mite in Study Group | Number of healthy controls undergoing SPT | % of positive SPT for House Dust Mite in Control Group | Number of antihistamine (AH) and/or systemic corticosteroid (SC)-free days | % of subjects with atopic diseases: allergic rhinitis (AR) and/or asthma (A) | Total IgE levels in CSU subjects |
|---|---|---|---|---|---|---|---|---|---|
| Sibbald, 1991 [53] | Canada | – | 75 | 27 | – | – | – | – | 23% > 200 IU/L |
| Liutu, 1998 [54] | Finland | > 8 | 91 | 9.8 | – | – | – | – | 49.3% > 111 IU/L |
| Caliskaner, 2004 [51] | Turkey | > 6 | 259 | 24.7 | 300 | 4.7 | 7 (AH) | Excluded AR/A | – |
| Ilonidis, 2005 [55] | Greece | – | 130 | > 2.3 | – | – | – | – | – |
| Mahesh, 2005 [64] | India | > 6 | 122 | 64 | 25 | 28 | – |
28.7 (AR) 23.8 (A) |
– |
| Nath, 2007 [19] | India | > 6 | 50 | 12 (D. farinae only) | – | – | – | – | – |
| Kulthanan, 2008 [56] | Thailand | > 6 | 88 | 38.6 | – | – |
3 (AH) 28 (SC) |
20.5 (AR) 6.8 (A) |
– |
| Kulthanan, 2008 [65] | Thailand | > 6 | 172 | 34.9 | – | – |
3 (AH) 28 (SC) |
20.4 (AR) 4.7 (A) |
– |
| Daschner, 2010 [58] | Spain | – | 80 | 26.3 | 522 (with AR/A) | – | – | – | – |
| Refaat, 2010 [59] | Egypt | – | 174 | 13.8 | 200 | 1.5 | 5 (AH) | Excluded AR/A | – |
| Geçer, 2012 [66] | Turkey | – | 50 | 16 | – | – | – | – | – |
| De Vos, 2012 [12] | United States | > 6 | 19 | 44 | 19 | 0 | – | Excluded AR | – |
| Song, 2013 [67] | China | > 6 | 862 | 17.7 | – | – | – |
4.5 (AR) 1.7 (A) |
– |
| Green, 2014 [60] | Canada | – | 44 | 15.9 | – | – | – | – | |
| Parasuramalu, 2014 [20] | India | > 6 | 300 | 20 | – | – | 15 (AH) |
11.3 (AR) 2.3 (A) |
– |
| Mounika, 2017 [63] | India | > 6 | 56 | 14.2 | – | – | – | – | – |
| Oncham, 2018 [61] | Thailand | – | 140 | 67.9 | – | – | – | – | – |
| Bilgir, 2019 [62] | Turkey | > 6 | 302 | 43.7 | – | – | – | – | – |
Fig. 3.
a Presence of positive SPT to house dust mite antigen in CSU subjects in 18 studies. b Presence of positive SPT to house dust mite antigen in CSU populations in 3 studies, compared to healthy control groups with no history of atopy (p = 0.047). c Forest plot graphic assessing the relative risk of positive SPT to house dust mite antigen in CSU populations compared to healthy controls, M-H, Mantel–Haenszel approach
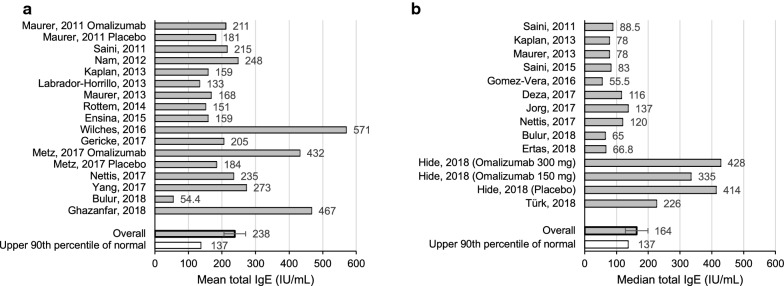
In identifying studies that evaluated mean tIgE levels in CSU subjects undergoing treatment with omalizumab, from 890 publications retrieved from database searches following duplicate removal, as well as an additional 4 publications identified through citation analysis, 81 were screened for full-text review. Of these, 59 did not meet the outlined eligibility criteria, yielding 22 full texts that were included for analysis (Fig. 1b). Of the 22 studies, 15 provided the mean tIgE levels in the CSU population undergoing treatment with omalizumab, with absolute values ranging from 0 to 5600 kU/L (Table 3). A recent study by Omenaas et al. reveals the 90th percentile of the distribution of total IgE in a general population was 137 kU/L [22]. While a number of studies have attempted to quantitate tIgE in a general population, these studies may have used different methodologies in their analyses and thus, comparisons should be made with caution. An average of these results revealed an overall mean total of 238 kU/L (SEM 32.1), compared to 137 kU/L, the upper 90th percentile of a general population, kU/L (Fig. 4a) and an overall median total of 164 kU/L (SEM 35.4) (Fig. 4b).
Table 3.
Summary of studies showing mean and median total IgE levels in subjects with CSU undergoing treatment with omalizumab or placebo
| Reference (author, year) | Region | Definition of CSU in weeks | Treatment | Number of CSU subjects undergoing IgE testing | Mean total IgE (kU/L) (SD) | Median Total IgE (kU/L) (IQR) | % of subjects previously on systemic corticosteroids (SC) | Number of antihistamine (AH) or systemic corticosteroid-free days | Range (kU/L) |
|---|---|---|---|---|---|---|---|---|---|
| Maurer, 2011 [68] | Germany | > 6 | Omalizumab | 27 | 211 (158) | – | – | – | – |
| Placebo | 22 | 181 (136) | – | – | – | – | |||
| Saini, 2011 [69] | USA and Germany | > 6 | Omalizumab or Placebo | 90 | 215 (432) | 88.5 | – | – | 2–3510 |
| Nam, 2012 [70] | Korea | > 6 | Omalizumab | 26 | 248 (275) | – | 42.3 | – | – |
| Kaplan, 2013 [71] | Various countries | > 6 | Omalizumab or Placebo | 335 | 159 (288) | 78 | 57.9 | 16 | 1–3050 |
| Labrador-Horrillo, 2013 [72] | Spain | > 6 | Omalizumab | 107 | 133 | – | 77.3 | – | 2–1042 |
| Maurer, 2013 [73] | Various countries | > 6 | Omalizumab or Placebo | 322 | 168 (232) | 78 | – | 30 (SC) | – |
| Rottem 2014 [74] | Israel | > 6 | Omalizumab | 43 | 151 (255) | – | 88 | – | – |
| Saini, 2015 [35] | Various countries | > 6 | Omalizumab or Placebo | 318 | – | 83 | – | 16 (SC) | 1–5000 |
| Ensina, 2016 [75] | Brazil | – | Omalizumab | 27 | 159 | – | – | – | 0.2–774 |
| Gomez-Vera, 2016 [25] | Mexico | > 6 | Omalizumab | 26 | – | 55.5 | – | – | 31.5–186 |
| Wilches, 2016 [76] | Ecuador | > 6 | Omalizumab | 26 | 571 | – | – | – | 55–2500 |
| Deza 2017 [77] | Spain | > 6 | Omalizumab | 47 | – | 116 | – | – | 42–277 |
| Gericke, 2017 [78] | Germany | > 6 | Omalizumab | 56 | 205 (230) | – | – | – | – |
| Jorg, 2017 [79] | Switzerland | > 6 | Omalizumab or Placebo | 30 | – | 137 (37.8–311) | – | AH, SC allowed | – |
| Metz, 2017 [80] | Germany | > 6 | Omalizumab | 20 | 432 (1031) | – | – | 3 (AH), 30 (SC) | – |
| Placebo | 10 | 184 (242) | – | – | 3 (AH), 30 (SC) | – | |||
| Nettis, 2017 [81] | Italy | > 6 | Omalizumab | 290 | 235 (526) | 120 | 73.5 | Allowed (AH, SC) | 0–5237 |
| Yang, 2017 [82] | Taiwan | > 6 | Omalizumab | 17 | 272.6 | – | 23.5 | – | 8.9–1510 |
| Bulur, 2018 [83] | Turkey | > 6 | Omalizumab | 132 | 54.4 (150) | 65 | 62.1 | Allowed (AH, SC) | 7–978.9 |
| Ertas, 2018 [84] | Turkey | > 6 | Omalizumab | 96 | – | 66.8 | – | – | 20.4–127 |
| Ghazanfar, 2018 [85] | Denmark | > 6 | Omalizumab | 117 | 467 (1076) | – | – | Allowed (AH) | – |
| Hide, 2018 [86] | Japan | > 6 | Omalizumab 150 mg | 71 | – | 335 | 26.5 | 30 | 15–2360 |
| Omalizumab 300 mg | 73 | – | 428 | 37.1 | 20–4950 | ||||
| Placebo | 73 | – | 414 | 36.1 | 0–5600 | ||||
| Türk, 2018 [87] | Turkey | > 6 | Omalizumab | 25 | – | 226 | – | – | 56–340 |
AH antihistamine, SC systemic corticosteroids
Fig. 4.
a Mean total IgE in CSU populations undergoing treatment with omalizumab from 15 studies, compared to the upper limit (90th percentile) of normal total IgE. b Median total IgE in CSU populations undergoing treatment with omalizumab or placebo from 12 studies
To identify studies evaluating the association between the presence of IgG autoantibody to FcεR1α (Table 4), IgG anti-IgE antibody (Table 5) and anti-TPO antibody (Table 6) in CSU subjects and healthy controls, 482 papers were retrieved from database searches following duplicate removal. 57 full texts were reviewed including an additional two papers from citation analysis, and 27 publications were accepted (Fig. 1C).
Table 4.
Summary of studies detecting IgG autoantibody to FcεR1α in subjects with CSU
| Reference (author, year) | Region | Definition of CSU in weeks | Number of CSU patients undergoing testing | Number of healthy controls undergoing testing | % prevalence of IgG autoantibody to FcεR1α in study group | Functional assay (F) or non-functional assay (N) | % prevalence of IgG autoantibody to FcεR1α in control group | Number of antihistamine (AH) and/or systemic corticosteroid (SC)-free days |
|---|---|---|---|---|---|---|---|---|
| Hide, 1993 [88] | England | > 8 | 26 + ASST | 10 | 46.1 | F | 0 | 2 (AH) |
| Fiebiger, 1995 [11] | Austria | > 8 | 32 | 15 | 37 | N | 0 | Not stated |
| Zweiman, 1996 [89] | United States | – | 70 | 20 | 30 | F | 15 | Not stated |
| Ferrer 1998 [10] | United States | > 12 | 53 | 24 | 64 | N | 0 | 2 (AH) |
| Fiebiger, 1998 [90] | Austria | > 8 | 281 | 41 | 38 | N | – | Not stated |
| Zuberbier, 2000 [91] | Austria | > 6 | 48 | 5 | 35 | N | 0 | Not stated |
| Sabroe, 2002 [92] | England | > 6 | 78 | 39 |
26 15 |
F N |
0 | 3 (AH), 28 (SC) |
| Hidvegi, 2003 [93] | Hungary | > 6 | 50 | 9 | 34 | N | 0 | 3 (AH), 28 (SC) |
| Eckman, 2008 [94] | United States | > 6 | 73 | 23 | 59 | N | 57 | Not stated |
| Lee, 2014 [95] | Taiwan | > 6 | 40 (20 + ASST) (20 − ASST) | 20 | 47.5 (70) (25) | N | 5 | Not stated |
| Ulambayar, 2017 [96] | Taiwan | > 6 | 125 (64 + ASST) (61 − ASST) | 64 | 24.8 (32.8) (16.4) | N | 3.1 | 5 (AH) |
| Baioumy, 2018 [97] | Egypt | > 8 | 40 (18 + ASST) (22 − ASST) | 40 | 52.5 (83.3) (27.3) | N | 2.5 | 5 (AH) |
| Schoepke, 2019 [98] | Multinational | > 6 | 182 (107 + ASST) | 0 | 58 | N | – | 3 (AH), 28 (SC) |
| Asero, 2020 [99] | Italy | > 6 | 20 | 20 | 50 | N | – | Not stated |
AH antihistamine, SC systemic corticosteroids
Table 5.
Summary of studies detecting IgG anti-IgE antibodies in subjects with CSU and healthy controls
| Reference (author, year) | Region | Definition of CSU in weeks | Number of CSU patients undergoing testing | Number of healthy controls undergoing testing | % prevalence of IgG anti-IgE autoantibody in study group | Functional assay (F) or non-functional assay (N) | % prevalence of IgG anti-IgE autoantibody in control group | Number of antihistamine (AH) and/or systemic corticosteroid (SC)-free days |
|---|---|---|---|---|---|---|---|---|
| Gruber, 1988 [100] | United States | – | 6 | 32 | 50 | N | 0 | Not stated |
| Fiebiger, 1995 [11] | Austria | > 8 | 32 | 15 | 69 | N | 26 | Not stated |
| Tong, 1997 [101] | United States | > 12 | 50 | 20 | 12 | F | 5 | 2 (AH) |
| Eckman, 2008 [94] | United States | > 6 | 73 | 23 | 45 | N | 30 | Not stated |
| Rojanapremsuk, 2015 [102] | United States | > 6 | 438 | – | 33 | N | – | Not stated |
AH antihistamine, SC systemic corticosteroids
Table 6.
Summary of studies detecting antithyroid peroxidase (anti-TPO) antibodies in subjects with CSU and healthy controls
| Reference (author, year) | Region | Definition of CSU in weeks | Number of CSU patients undergoing testing | Number of healthy controls undergoing testing | % prevalence of anti-TPO antibody in study group | % prevalence of anti-TPO antibody in control group | Number of antihistamine (AH) and/or systemic corticosteroid (SC)-free days |
|---|---|---|---|---|---|---|---|
| Verneuil, 2004 [103] | France | – | 45 | 30 | 17.8 | 3.3 | Not stated |
| Gangemi, 2009 [104] | Italy | > 6 | 95 | 0 | 26.3 | – | Not stated |
| Nuzzo, 2011 [105] | Italy | > 6 | 54 | 108 | 22.2 | 1.9 | Not allowed |
| Alpay, 2013 [106] | Turkey | > 6 | 50 | 50 | 12 | 4 | 3 (AH) |
| Cho, 2013 [107] | United States | > 6 | 27 | 20 | 11.1 | 20.0 | Not stated |
| Wan, 2013 [108] | Taiwan | > 6 | 60 | 40 | 8.3 | 0 | Allowed (AH) |
| Yadav, 2013 [109] | India | > 6 | 80 | 0 | 17.5 | – | 3 (AH), 21 (SC) |
| Kim, 2016 [110] | Korea | > 6 | 184 | 0 | 13.6 | – | Not stated |
| Czarnecka-Operacz, 2017 [111] | Poland | > 6 | 145 | 35 | 15.9 | – | Not stated |
| Kasumagic-Halilovic, 2017 [112] | Bosnia and Herzegovina | > 6 | 70 | 70 | 30 | 1.4 | Not stated |
| Schoepke, 2019 [98] | Multinational | > 6 | 182 | 0 | 20.9 | – | 3 (AH), 28 (SC) |
Anti-TPO antithyroid peroxidase, AH antihistamine, SC systemic corticosteroids
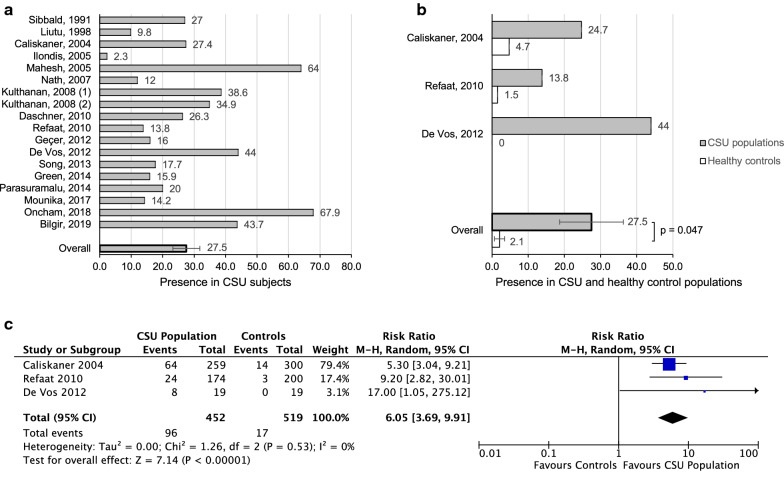
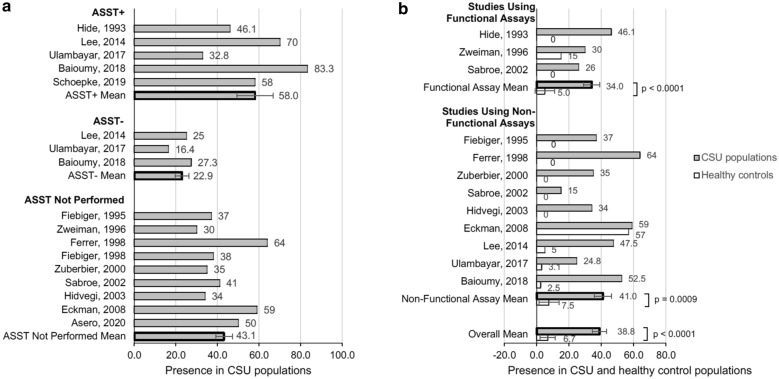
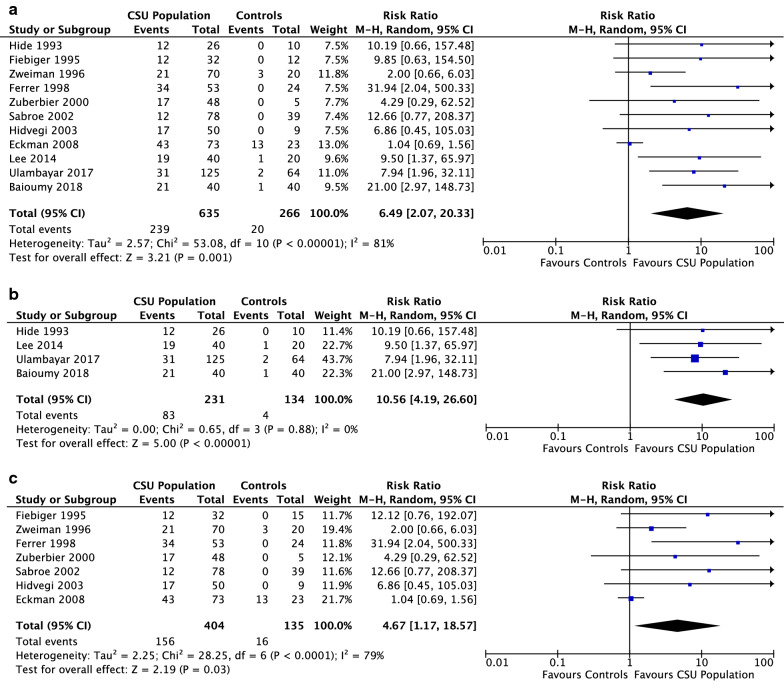
14 studies assessed the presence of IgG autoantibody directed to FcεR1α in CSU subjects. In nine studies that did not perform autologous serum skin tests (ASST), IgG anti-FcεR1α autoantibody was detected in 43.1% (SEM 3.4) of CSU subjects (Fig. 5a). In five papers that studied positive ASST CSU subjects, 58% of the subjects (SEM 8.8) had IgG anti-FcεR1α autoantibody, while in 3 papers that studied negative ASST CSU subjects, the autoantibody was detected in 22.9% of subjects. 11 of the 14 total studies that investigated the association of CSU with IgG anti-FcεR1α reactivity included a healthy control population with no history of atopy. On average, 38.8% of the CSU population (SEM 4.3) had IgG autoantibody directed to FcεR1α while only 6.7% of the healthy control group (SEM 4.7) had IgG anti-FcεR1α present (p < 0.0001) (Fig. 5b). In three controlled studies that used a functional assay to detect IgG anti-FcεR1α in CSU subjects, 34% of the CSU subjects had the autoantibody (SEM 5.4) compared to a 5% presence within control populations. The Hide 1993 study was the only study to include only ASST + CSU subjects. In nine controlled studies that used a non-functional assay, IgG anti-FcεR1α was detected in 41% of CSU subjects, while only 7.5% of the control group had the autoantibody present. In the Lee 2014, Ulambayar 2017 and Baioumy 2018 studies, the values from all CSU subjects (ASST positive and negative), were compared to the controls, in whom no ASST was performed. Combined RR was 6.5 (95% CI 2.1–20.3) with significant heterogeneity (p = 0.001; I2 = 81%), as shown in Fig. 6a. In four studies that performed ASSTs on CSU subjects, the combined RR was 10.6 (95% CI 4.2–26.6) with no heterogeneity (p < 0.00001; I2 = 0%) (Fig. 6b). In comparison, in 7 studies that did not perform ASSTs on CSU subjects, the combined RR was 4.7 (95% CI 1.2–18.6) with significant heterogeneity (p = 0.03; I2 = 79%) (Fig. 6c). Overall, the final estimate from the forest plots revealed an increased risk of presence of IgG autoantibody to FcεR1α in subjects with CSU.
Fig. 5.
a Presence of IgG autoantibody to FcεR1α in CSU populations with and without ASST+. b Presence of IgG autoantibody to FcεR1α in CSU populations in 11 studies, compared to healthy control groups (p < 0.0001)
Fig. 6.
a Forest plot graphic assessing the relative risk of the presence of IgG Autoantibody to FcεR1α in all studies. M-H, Mantel–Haenszel approach. b Forest plot graphic assessing the relative risk of the presence of IgG Autoantibody to FcεR1α in studies that performed the ASST. M–H, Mantel–Haenszel approach. c Forest plot graphic assessing the relative risk of the presence of IgG Autoantibody to FcεR1α in studies that did not perform the ASST. M–H, Mantel–Haenszel approach
The presence of IgG anti-IgE autoantibody in five CSU populations was 41.8% (SEM 9.4) (A). Four out of these five studies included a healthy control population with no history of atopy. 44% of the CSU groups (SEM 11.9) had IgG anti-IgE antibody, compared to only 15.3% in the healthy control groups (SEM 7.5) (p = 0.3) (Fig. 7b). Combined RR was 2.4 (95% CI 1.1–5.1), (p = 0.03, I2 = 39%), as shown in Fig. 7c.
Fig. 7.
a Presence of with IgG anti-IgE antibody in CSU populations. b Presence of IgG anti-IgE antibody in CSU populations in 5 studies, compared to healthy control groups. c Forest plot graphic assessing the relative risk of the presence of IgG anti-IgE antibody in CSU populations compared to healthy controls. M–H, Mantel–Haenszel approach
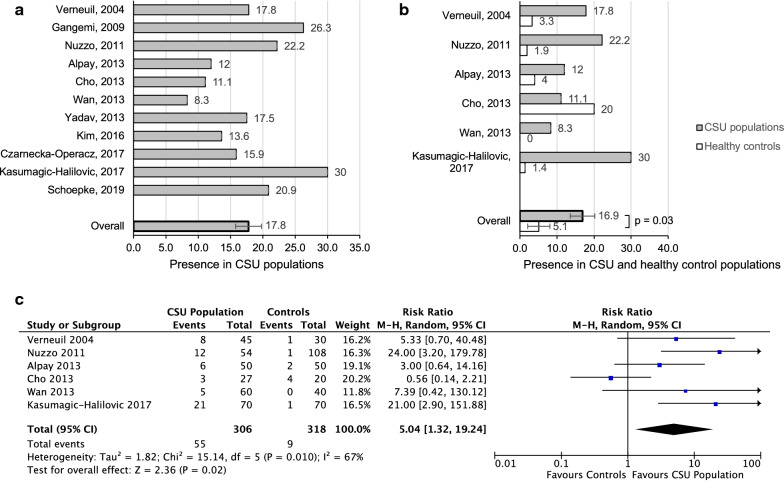
The presence of anti-TPO antibody in 11 CSU groups was 17.8 (SEM 2.0) (Fig. 8a). Six out of these 11 studies included a healthy control population with no history of atopy. Anti-TPO antibody was detected in 16.9% of the CSU populations (SEM 3.3), compared to 5.1% in healthy control groups (SEM 3) (p = 0.03) (Fig. 8b). Combined RR was 5.0 (95% CI 1.3–19.2), (p = 0.02, I2 = 67%), as shown in Fig. 8c.
Fig. 8.
a Presence of anti-thyroid peroxidase antibody in CSU populations. b Presence of anti-thyroid peroxidase antibody in CSU populations in 11 studies, compared to healthy control groups. c Forest plot graphic assessing the relative risk of the presence of anti-thyroid peroxidase antibody in CSU populations compared to healthy controls, M–H, Mantel–Haenszel approach
Assessing the total blood eosinophil percentage and/or absolute blood eosinophil count in subjects with CSU, from 608 publications retrieved from database searches following duplicate removal, 51 were screened for full-text review, which includes 5 papers from citation analysis (Table 7). Of these, 24 did not meet the outlined eligibility criteria, yielding 27 full texts from 13 countries that were included for analysis (Fig. 1d). 15 studies included information regarding the discontinuation of systemic corticosteroids prior to the commencement of the trial. One study, Anuradha et al. stopped systemic corticosteroids for 4 weeks and the mean eosinophil % was 4.2% (n = 60), while the median was 364 106/L [23]. Another study, Dakhale et al., stopped systemic corticosteroids for 8 weeks and the mean eosinophil % was 6.8% (n = 64) [24]. The absolute eosinophil count was not provided. Gomez-Vera et al., excluded all patients who responded to normal dose antihistamines and had a history of asthma or allergic rhinitis, anaphylactic reactions, and patients using systemic or topical corticosteroids but only reported the median eosinophil count 0.170 × 103/μL (IQR 0.100–0.300) [25]. Chang et al. didn’t state if subjects had taken corticosteroids or not and only included CSU subjects 1 to 18 years old (17/60 under 7 years old). In the 54 CSU subjects with lab work available, 13% (7/54) had elevated eosinophils [26].
Table 7.
Summary of studies measuring blood eosinophil % and absolute eosinophil count in CSU subjects
| Reference (author, year) | Country | Definition of CSU in weeks | Treatment | Number of CSU patients undergoing eosinophil testing | Mean blood eosinophil % (SD) | Median blood eosinophil % (IQR) | Mean absolute eosinophil count | Median absolute eosinophil count (IQR) | Number of antihistamine (AH) and/or systemic corticosteroid (SC)-free days |
|---|---|---|---|---|---|---|---|---|---|
| Toyoda, 1996 [113] | United States | – | None stated | 30 | – | – | 164.3 (SE 54.6) | – | 28 (SC) |
| Di Lorenzo, 1996 [114] | Italy | > 8–12 | None stated | 13 | – | – | 270 (SD 90) | – | 2 (AH), SC not allowed |
| Kim, 1997 [115] | Korea | – | None stated | 285 | – | – | 184.4 (SE 8.4) | – | 28 (SC) |
| Grattan, 2003 [116] | United Kingdom | – | Loratadine & Prednisolone | 7 | – | – | 121 (SE 13) | – | 7 (AH), 28 (SC) |
| Garmendia, 2004 [117] | Venezuela | > 6 | Ceterizine & Loratadine | 32 | – | – | 146 (SD 132) | – | 5 (AH), 3 (SC) |
| Anuradha, 2010 [23] | India | > 6 | Loratadine | 30 | 4.1 (1.6) | – | 357 (SD 176) | – | 3 (AH), 28 (SC) |
| Levocetirizine | 30 | 4.2 (1.4) | – | 368 (SD 131) | – | 3 (AH), 28 (SC) | |||
| Boonpiyathad, 2014 [118] | Thailand | > 6 | Vitamin D | 60 | – | – | 127 (SD 50) | – | AH allowed |
| Dakhale, 2014 [24] | India | > 6 | Cetirizine | 31 | 6.9 (1.9) | – | – | – | 7 (AH), 56 (SC) |
| Rupatadine | 33 | 6.7 (1.5) | – | – | – | 7 (AH), 56 (SC) | |||
| Woo, 2015 [119] | Korea | > 6 | None stated | 72 | 2.6 (1.8) | – | 202 (SD 239) | – | Not stated |
| Dakhale, 2016 [50] | India | > 6 | Rupatadine | 30 | 6.9 (1.8) | – | – | – | 7 (AH), 56 (SC) |
| Olopatadine | 30 | 6.8 (1.6) | – | – | – | 7 (AH), 56 (SC) | |||
| Gomez-Vera, 2016 [25] | Mexico | > 6 | AH, Omalizumab | 76 | – | – | – | 170 (100–300) | Not allowed |
| Wardhana, 2017 [120] | Bali | > 6 | None stated | 25 | – | – | 176 (SD 120) | – | Not stated |
| Aitella, 2018 [121] | Italy | > 6 | None stated | 50 | – | – | 199 (SD 100) | 190 (110–268) | 7 (AH) |
| Cildag, 2018 [122] | Turkey | > 6 | Omalizumab | 41 | – | – | – | 160 (80–245) | Not stated |
| Türk, 2018 [87] | Turkey | > 6 | Omalizumab | 25 | – | 1.7 (0.9-2.8) | – | 130 (79–195) | AH, SC allowed |
| Acer, 2019 [123] | Turkey | > 6 | Omalizumab | 106 | – | – | 150 (SD 170) | – | Not stated |
| Akdogan, 2019 [124] | Turkey | > 6 | Omalizumab | 74 | 1.9 (1.9) | – | – | – | Not stated |
| Kolkhir, 2019 [125] | Germany | > 6 | None stated | 1613 | – | – | – | 150 (80–230) | Allowed |
| Nazik, 2019 [126] | Turkey | – | Omalizumab | 136 | 2.1 (2.0) | – | – | – | AH, SC allowed |
| Oliver, 2019 [127] | United States | > 6 | None stated | 23 | 2.5 (0.4) | – | – | – | AH allowed, SC not allowed |
| Tamer, 2020 [128] | Turkey | > 6 | Omalizumab | 60 | 2.5 (3.0) | – | 250 (SD 89) | – | SC not allowed |
| Pediatrics studies | |||||||||
| Jirapongsananuruk, 2010 [129] | Thailand | > 6 | None stated | 94 | – | – | 256 | – | 7 (AH) |
| Chang, 2013 [26] | Taiwan | > 6 | None stated | 54 | – | 2.0 | – | – | Not stated |
| Chansakulporn, 2014 [130] | Thailand | > 6 | None stated | 92 | – | – | – | 259 | 7 (AH) |
| Cavkaytar, 2015 [131] | Turkey | > 6 | None stated | 68 | – | 1.4 (0.8–2.2) | – | 100 (100–200) | 8 (AH) |
| Uysal, 2016 [132] | Turkey | > 6 | Desloratadine | 92 | – | – | – | 180 (80–290) | Allowed |
| Lee, 2017 [133] | Korea | > 6 | None stated | 57 | 3.1 (1.6) | – | – | – | Not stated |
IQR interquartile range, SC systemic corticosteroid, SE standard error, SD standard deviation
Combining the nine studies that assessed mean eosinophil counts in CSU subjects, the mean blood eosinophil percentage was 4.2% (SEM 0.6), which is similar to the normal of 4% (Fig. 9a). However, there were a large number of studies that measured eosinophils but allowed systemic corticosteroids (SC) and/or antihistamines (AH), which could have resulted in reduced counts. In three studies that withheld SC for ≥ 28 days and AH for ≥ 3 days prior to testing, the mean blood eosinophil percentage was 5.9% (SEM 0.6), compared to 2.5% (SEM 0.2) in six studies that only withheld SC for ≤ 7 days. Four weeks of treatment with loratadine resulted in 10% reduction while a 26% reduction was measured with cetirizine. Dakhale found a 16% reduction with 6 weeks of cetirizine and 31% reduction with 6 weeks treatment with rupatadine.
Fig. 9.
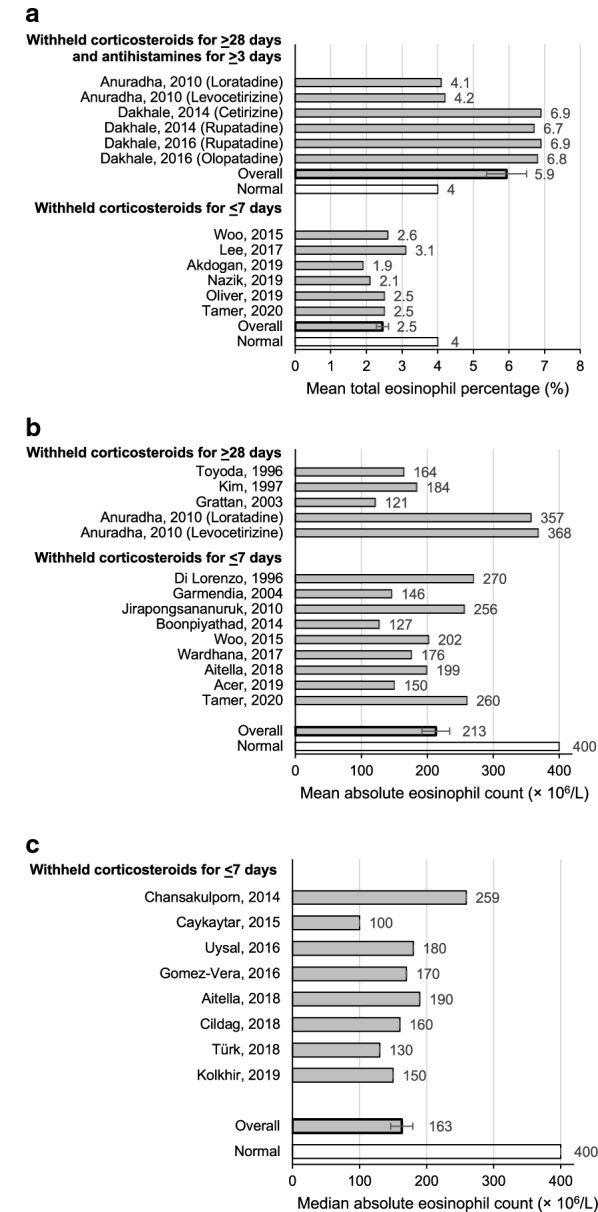
a Mean total eosinophil % in CSU populations undergoing treatment with omalizumab or H1-antihistamines that withheld corticosteroids for 28 days or longer and antihistamines for at least 3 days compared to studies that withheld corticosteroids for at least 7 days. b Mean absolute eosinophil count in CSU populations undergoing treatment with omalizumab or H1-antihistamines from studies that withheld systemic corticosteroids for 28 days or longer compared to studies that withheld systemic corticosteroids for less than 7 days. c Median absolute eosinophil count in CSU populations undergoing treatment with omalizumab or H1-antihistamines that withheld corticosteroids for less than 7 days
13 studies that evaluated mean absolute blood eosinophil counts in CSU subjects reported an average mean count of 213 L (SEM 21.0) (Fig. 9b). In four studies that withheld SC for 28 or more days prior to testing, the average mean absolute eosinophil count was 239 L (SEM 51.5). Conversely, in 9 studies that either allowed corticosteroids or did not define the length of time corticosteroid use was stopped for prior to testing, the average mean count was 198 L (SEM 17.8). Finally, eight studies reported an overall average median absolute blood eosinophil count of 163 L (SEM 16.6) (Fig. 9c).
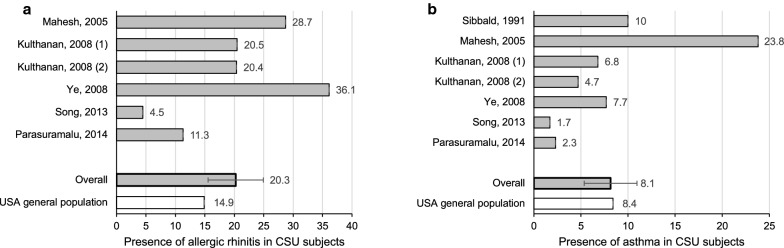
The prevalence of positive personal history of other atopic diseases was identified in seven studies assessing positive SPTs to aeroallergens in patients with CSU, with AR (20.3%) (SEM 4.7) and asthma (8.1%) (SEM 2.8) being the most common (Fig. 10a, b). This is greater than the prevalence of AR (14.9%) [27], but not asthma (8.4%) [28] in the USA general population.
Fig. 10.
a Presence of allergic rhinitis in CSU subjects compared to the USA general population. b Presence of asthma in CSU subjects compared to the USA general population
Discussion
This is the first systematic review of the evaluation of T2 markers of allergic inflammation in CSU subjects.
An elevated tIgE is defined as above 137 kU/L, which represents the upper 90th percentile in a general population [22]. CSU subjects had significantly elevated mean tIgE levels in all studies, with an average of 238 kU/L. Higher levels of tIgE in CSU subjects may indicate the presence of specific IgE to allergens, including aeroallergens.
We found the presence of positive SPT results to single or multiple aeroallergens in subjects with CSU was common, ranging from 2.3 to 67.9% in 21 studies. The ratio of multiple positive aeroallergen SPT in the CSU subjects was on average, considerably higher than the control groups (34.2% compared to 13.6%, p = 0.047), respectively. CSU subjects were 3.1 times more likely to have a positive aeroallergen SPT (95% CI 1.7–5.8, p = 0.0002) and 6.1 times more likely to have a positive perennial allergen HDM SPT (95% CI 3.7–9.9, p < 0.00001) than controls. In all 18 studies, an average of 1 in 4 CSU subjects had a positive SPT to HDM. Of the four studies that included control populations, approximately 1 in 4 CSU subjects had a positive SPT to HDM, compared to only 1 in 50 controls. As specific levels of IgE can increase with inhalant allergen exposure, SPT is important in identifying IgE sensitization to specific antigens. There are various treatment options that reduce aeroallergen specific IgE production, thus decreasing the likelihood of stimulating antibodies to IgE and its receptor. For instance, HDM sublingual immunotherapy treatment may act to effectively reduce HDM rhinitis and asthma [29]. Additionally, there is some evidence that allergen-specific immunotherapy [30] as well as the use of inhaled corticosteroids [31] promotes a long-term reduction of IgE levels to specific antigens and subsequent alleviation of symptoms.
The presence of aeroallergen positive SPT in CSU subjects was common; however, sensitization was not present in all CSU subjects. Non-sedating anti-H1 antihistamines remain the mainstay of initial intervention for treatment of CSU. H1 antihistamines can block SPT responses and decrease skin reactivity for upwards of 5 days, leading to false negative results. Thus, it is possible the rate of positive SPTs is actually higher than reported. Only 8 studies clearly stated that they discontinued use of antihistamine medications prior to proceeding with skin prick testing and reported the number of days of temporary discontinuation; however, of these eight studies, four withheld antihistamines for 3 days or less, which may not have been sufficient. For those studies that did not indicate the number of antihistamine-free days, the risk for an increase in false negative tests is elevated.
While omalizumab can suppress the SPT response for up to 6 months [32], no study indicated the discontinuation of omalizumab in their trials; although this was unlikely to have been important in the studies reviewed.
Oral corticosteroids are also commonly used to treat CSU [2]. While short-term oral corticosteroids may not alter the results of SPT [33], the effects of long-term treatment are not known. In a recent study evaluating the efficacy of omalizumab in the treatment of 68 subjects with severe CSU, 45.6% of subjects were reported to have an open prescription for systemic glucocorticoids at the time of treatment initiation. All of these subjects had required treatment with H1-antihistamines and oral glucocorticoids at some stage of their disease [34]. Similar patient demographics have been noted in the ASTERIA I trials where 50% of patients with CSU had been previously treated with corticosteroids [35]. In another study, it was reported that 57.9% of CSU patients had required treatment with systemic steroids prior to initiating omalizumab treatment. All patients had a medication history of H1-antihistamines, with 15.8% of the study population taking four times the standard dose on study day 1.
Consequently, in the present review, there is a high possibility that a significant percentage of subjects with CSU were undergoing treatment with oral corticosteroids. However, an evaluation of the prevalence of AR (20.3%, normal 14.9%) and asthma (8.1%, normal 8.4%) in CSU subjects revealed no considerable difference to the normal. The use of antihistamines and immunosuppressant medications, such as prednisone and ciclosporine A may mask AR and asthma symptoms, contributing to an under-diagnosis of these atopic conditions in CSU. Inhaled and intranasal steroids may be safer, less expensive and more effective therapies for AR and asthma. Because symptoms are masked by various CSU treatments, perhaps this may be a factor as to why skin testing is not perceived as relevant. That being said, most IgE is bound to the high affinity receptor on the mast cell and skin testing can determine if this is present [36].
Underrecognized asthma and AR could be important when considering patient safety, particularly if systemic steroids are withdrawn, while the urticaria is controlled with omalizumab. Hence, it may be important to consider inhalant sensitization in these patients. In the Xolair Treatment Efficacy of Longer Duration in Chronic Idiopathic Urticaria (XTEND-CIU) study, two anaphylactic episodes were reported whereby both patients were admitted to the emergency department with wheezing. The first patient had a history of mild, intermittent asthma while the second patient had a history of AR [37]. Therefore, skin testing and clinical suspicion of AR and asthma in these patients with CSU accompanied by assessment for inhalant allergen sensitivity may be important in reducing the risk of adverse reactions, particularly as omalizumab may have a systemic steroid sparing effect.
It has been demonstrated that the rate of superantigen producing Staphylococcus aureus colonization in the nasal cavity of patients with perennial AR was significantly elevated at 22% compared to healthy controls (6.7%) [38]. Similarly, patients with CRSwNP are also very likely to have frequent Staphylococcus aureus colonization in the nasal passages, which can cross airway epithelial barriers and induce elevated inflammation severity and vascular permeability [39]. Recent literature suggests the elevated presence of specific IgE to SEB in AR and CRSwNP patients, which may amplify mucosal inflammation and initiate a type 2 allergic response [18]. As IgE to SE is common in CSU patients [40], if T2 activity was controlled, complications involving SE and IgE to SEB in the pathogenesis of airway inflammation would be reduced, possibly indirectly leading to a reduction in CSU symptoms.
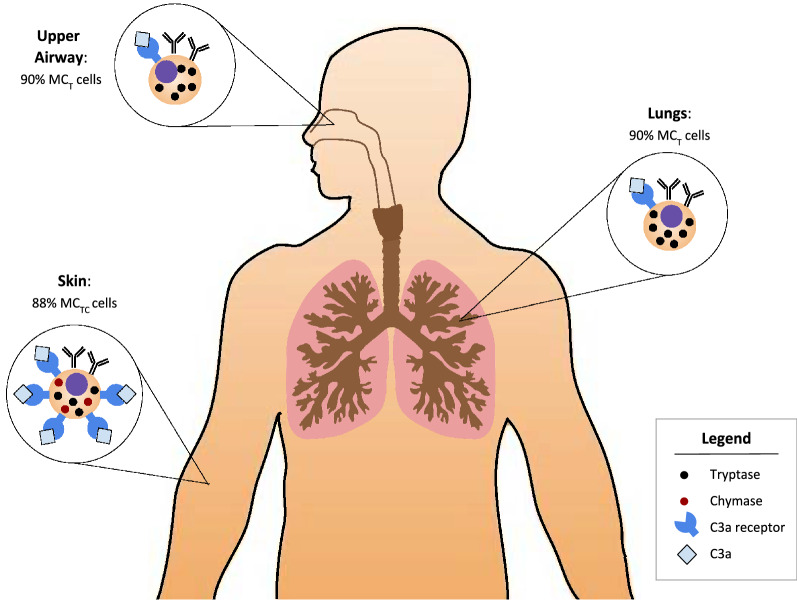
Approximately 90% of mast cells in dispersed lung tissue have been found to be of the MCT (tryptase only) phenotype, while 88% of mast cells in dermal tissue were MTC (tryptase and chymase) [41]. Airway epithelial cells, macrophages in lung tissue, and skin mast cells all produce anaphylatoxin C3a, which acts upon mast cells to cause degranulation [42, 43], and express its respective receptor, C3aR (Fig. 11). C3a and C5a complement factors are both upregulated in the lungs of subjects with allergic asthma [44]. While MCTC cells found mainly in the skin are responsive to C3a, MCT cells found mainly in the lung are not [45]. CSU may result from C3a being formed in areas of allergic inflammation such as the sinus, nose or lung. This may explain why CSU patients generally do not go on to have anaphylaxis, as C3a causes non-cytotoxic subacute degranulation of mast cells not through IgE cross-linking, and C5a has been found to upregulate the effect of IgG on cutaneous mast cells in CSU [46, 47].
Fig. 11.
Chymase/tryptase mast cell type by tissue site
In addition, thrombin can induce the generation of C5a in the absence of C3, bypassing the first stage of the complement cascade [48]. Recently, Zhu et al. outlined the concept of the interaction of the complement and coagulation systems in CSU [49]. They found increased levels of C5a and D-dimer in patients with active CSU, but C3 was not decreased [48].
Our search retrieved 4 studies that found increased mean absolute peripheral blood eosinophil counts in CSU subjects where systemic corticosteroids were withheld for at least 4 weeks compared to 9 studies that did not hold corticosteroids for 4 weeks or longer. Other studies that withheld corticosteroids for at least 4 weeks found a mean absolute eosinophil count of 239 L, which was not elevated. Where corticosteroids weren’t held, the mean eosinophil percentage, mean absolute eosinophil count and median absolute eosinophil count were not elevated. Studies did find that antihistamines reduced peripheral blood eosinophil counts in CSU subjects which may help explain these findings [23, 24, 50].
Three different anti-IL5 therapies given to patients with severe eosinophilic asthma and CSU have shown a benefit for symptoms of CSU. Mepolizumab was given to one patient, who experienced complete clearing of urticarial wheals 3 months after the first dose [14]. Subsequent discontinuation of mepolizumab prompted a relapse of CSU. Reslizumab was given to a patient with severe eosinophilic asthma, who had immediate improvement in her urticaria following treatment [15] Finally, benralizumab was given to a patient with severe asthma and symptomatic dermographism; the dermographism was markedly improved after the first injection [16]. After 3 months, the results of provocation tests for dermographism were negative. Mepolizumab and benralizumab are currently under evaluation for CSU (ClinicalTrials.gov Identifiers: NCT03183024, NCT03494881, respectively). In addition, dupilumab has also been described to help CSU in a series of 6 patients - 4 with atopic dermatitis and 2 with atopic dermatitis and asthma. It is also undergoing clinical trials in CSU subjects (ClinicalTrials.gov Identifiers: NCT04180488, NCT03749135) [17]. This demonstrates that initiating treatment of T2 inflammation with anti-IL5 or an agent which blocks IL4 and IL13 for subjects with asthma and/or atopic dermatitis who also have CSU, CSU symptoms may be improved.
In analyzing the literature on the presence of anti FcεR1α, anti-IgE, anti-TPO autoantibodies in patients with CSU, we found that the detection of all three autoantibodies in CSU patients were all significantly elevated in comparison to that of controls. In particular, CSU subjects were 6.5 times more likely to have IgG autoantibody against FcεR1α (95% CI 2.1–20.3, p = 0.001), 2.4 times more likely to have IgG anti-IgE antibody (95% CI 1.1–5.1, p = 0.03) and 5 times more likely anti-TPO antibody (95% CI 1.3–19.2, p = 0.02) than controls. These findings may indicate a potential autoimmune basis for the disease; however, further investigation is required to clarify the clinical significance of the presence of such antibodies in CSU and its association with disease activity. It is not clear if they could cause subclinical or partial degranulation of cutaneous mast cells, or if they just occur with T2 inflammation.
In this review, we found 21 studies where aeroallergen SPT was performed on CSU subjects, only 4 studies of which included a control group. One limitation of this study was the lack of studies including a healthy control group when performing SPTs for multiple aeroallergens or HDM in CSU subjects and healthy control groups. In addition, most studies did not indicate whether or not the use of antihistamines and systemic corticosteroids were appropriately discontinued, which may have resulted in more false negative SPT results. These treatments also may have masked underlying atopic conditions and may have reduced blood eosinophil counts. Two studies [51, 52] excluded subjects with atopic conditions entirely, which could have produced lower positive SPT results to aeroallergens. However, excluding these studies had minimal effect on the results observed and thus, were included in this review. Few studies evaluated blood eosinophil levels in CSU subjects off systemic steroids and antihistamines, but those that did demonstrated elevated levels in blood eosinophil percentage, but other studies did not find elevated absolute counts.
Conclusion
In conclusion, this review supports findings from numerous studies that CSU patients are more likely to have positive SPT for both aeroallergens and HDM specifically. This may point towards future therapeutic options in treating CSU. Elevated levels of aeroallergen sensitization, tIgE, and IgG autoantibody to FcεR1α in CSU subjects may indicate the importance of T2 inflammation in the pathogenesis of CSU. Further studies to determine whether or not specific allergen avoidance, desensitization or improved control of the mucosal allergic inflammation present in asthma and/or rhinitis has any benefit in the management of CSU in sensitized individuals may be warranted.
Acknowledgements
Not applicable.
Abbreviations
- CSU
Chronic spontaneous urticaria
- SPT
Skin prick test
- ASST
Autologous serum skin test
- IgE
Immunoglobulin E
- IgG
Immunoglobulin G
- FcεR1α
High-affinity receptor for the Fc region of immunoglobulin E
- anti-TPO
Anti-thyroid peroxidase
- HDM
House dust mite
- SE
Staphylococcal enterotoxin
- tIgE
Total serum IgE
- CRSwNP
Chronic rhinosinusitis with nasal polyps
- AR
Allergic rhinitis
Authors’ contributions
PKK conceived and designed the basis of the study. All authors conducted a peer review of the literature involving title/abstract/and full-text screening. MMW extracted the data from included articles. All authors contributed towards analyzing the extracted data and inputting data into analysis tools (i.e. forest plot program). Both authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
PKK declares competing interests as follows: personal fees from Novartis, personal fees from Aralez Pharmaceuticals, personal fees from Pediapharm, grants and personal fees from AstraZeneca, grants and personal fees from Shire, grants and personal fees from CSL Behring, grants from Green Cross, personal fees from Merck, personal fees from GlaxoSmithKline, personal fees from Teva Pharmaceuticals, personal fees from Mylan, personal fees from Stallergenes Greer, grants from Genentech, and personal fees from ALK, outside the submitted work. MMW declares that she has no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Melanie Mitsui Wong, Email: melaniewong19@gmail.com.
Paul Kevin Keith, Email: keithp@mcmaster.ca.
References
- 1.Urticaria Schaefer P. Evaluation and Treatment. Am Fam Physician. 2011;83:1078–1084. [PubMed] [Google Scholar]
- 2.Bernstein J, Lang D, Khan D, Craig T, Dreyfus D, Hsieh F, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133:1270–1277. doi: 10.1016/j.jaci.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 3.Kolkhir P, Church MK, Weller K, Metz M, Schmetzer O, Maurer M. Autoimmune chronic spontaneous urticaria: what we know and what we do not know. J Allergy Clin Immunol. 2017;139(1772–1781):e1. doi: 10.1016/j.jaci.2016.08.050. [DOI] [PubMed] [Google Scholar]
- 4.Borzova E, Grattan CEH. Urticaria, angioedema, and anaphylaxis. In: Clinical immunology: principles and practice. 4th ed. 2013.
- 5.Zuberbier T, Asero R, Bernstein J, Brzoza Z. The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy Eur J Allergy Clin Immunol. 2018;73:1393–1414. doi: 10.1111/all.13397. [DOI] [PubMed] [Google Scholar]
- 6.Okada H, Kuhn C, Feillet H, Bach JF. The “hygiene hypothesis” for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160:1–9. doi: 10.1111/j.1365-2249.2010.04139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rabin RL, Levinson AI. The nexus between atopic disease and autoimmunity: a review of the epidemiological and mechanistic literature. Clin Exp Immunol. 2008;153:19–30. doi: 10.1111/j.1365-2249.2008.03679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tedeschi A, Asero R. Asthma and autoimmunity: a complex but intriguing relation. Expert Rev Clin Immunol. 2008;4:767–776. doi: 10.1586/1744666X.4.6.767. [DOI] [PubMed] [Google Scholar]
- 9.Mukherjee M, Nair P. Autoimmune responses in severe asthma. Allergy Asthma Immunol Res. 2018;10:428–447. doi: 10.4168/aair.2018.10.5.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrer M, Kinét JP, Kaplan AP. Comparative studies of functional and binding assays for IgG anti-Fc(epsilon)RIalpha (alpha-subunit) in chronic urticaria. J Allergy Clin Immunol. 1998;101:672–676. doi: 10.1016/s0091-6749(98)70176-9. [DOI] [PubMed] [Google Scholar]
- 11.Fiebiger E, Maurer D, Holub H, Reininger B, Hartmann G. Serum IgG autoantibodies against the a-chain of FceRI: a selective marker for a distinct subset of chronic urticaria patients? J Clin Invest. 1995;96:2606–2612. doi: 10.1172/JCI118325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Vos G, Kravvariti E, Collins J, Tavdy A, Nazari R, Hudes G, et al. Increased allergic sensitization to mugwort in chronic urticaria. Dermatology. 2012;225:141–146. doi: 10.1159/000342356. [DOI] [PubMed] [Google Scholar]
- 13.Kasperska-Zajac A, Brzoza Z. Remission of chronic urticaria in the course of house dust mite immunotherapy-Mere coincidence or something more to it? Vaccine. 2009;27:7240–7241. doi: 10.1016/j.vaccine.2009.08.076. [DOI] [PubMed] [Google Scholar]
- 14.Magerl M, Terhorst D, Metz M, Altrichter S. Benefit of mepolizumab treatment in a patient with chronic spontaneous urticaria. J Ger Soc Dermatology. 2018;16:477–488. doi: 10.1111/ddg.13481. [DOI] [PubMed] [Google Scholar]
- 15.Deeks ED, Brusselle G. Reslizumab in eosinophilic asthma: a review. Drugs. 2017;77:777–784. doi: 10.1007/s40265-017-0740-2. [DOI] [PubMed] [Google Scholar]
- 16.Bergmann KC, Altrichter S, Maurer M. Benefit of benralizumab treatment in a patient with chronic symptomatic dermographism. J Eur Acad Dermatology Venereol. 2019;33:e413–e415. doi: 10.1111/jdv.15720. [DOI] [PubMed] [Google Scholar]
- 17.Lee JK, Simpson RS. Dupilumab as a novel therapy for difficult to treat chronic spontaneous urticaria. J Allergy Clin Immunol Pract. 2019;7(1659–1661):e1. doi: 10.1016/j.jaip.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 18.Cui X, Miao J, Lu H, Qi Q, Chen X, Xu J, et al. Serum levels of specific IgE to Staphylococcus aureus enterotoxins in patients with chronic rhinosinusitis. Exp Ther Med. 2015;9:1523–1527. doi: 10.3892/etm.2015.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nath A, Adityan B, Thappa B. Prick testing in chronic idiopathic urticaria: a report from a tertiary care centre in south India. Internet J Dermatol. 2007;6:1–5. [Google Scholar]
- 20.Parasuramalu B, Balaji R, Sharath Kumar B. Implication of pollen sensitivity among patients suffering from chronic urticaria: current scenario. Int J Heal Allied Sci. 2014;3:4–8. [Google Scholar]
- 21.Mounika K, Shivaswamy K. Autologous serum skin test and skin prick test in patients with chronic urticaria. Int J Res Dermatol. 2016;3:46–48. [Google Scholar]
- 22.Omenaas E, Bakke P, Elsayed S, Hanoa R, Gulsvik A. Total and specific serum IgE levels in adults: relationship to sex, age and environmental factors. Clin Exp Allergy. 1994;24:530–539. doi: 10.1111/j.1365-2222.1994.tb00950.x. [DOI] [PubMed] [Google Scholar]
- 23.Anuradha P, Maiti R, Jyothirmai J, Mujeebuddin O, Anuradha M. Loratadine versus levocetirizine in chronic idiopathic urticaria: a comparative study of efficacy and safety. Indian J Pharmacol. 2010;42:12–16. doi: 10.4103/0253-7613.62399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dakhale GN, Shinde AT, Mahatme MS, Hiware SK, Mishra DB, Mukhi JI, et al. Clinical effectiveness and safety of cetirizine versus rupatadine in chronic spontaneous urticaria: a randomized, double-blind, 6-week trial. Int J Dermatol. 2014;53:643–649. doi: 10.1111/ijd.12250. [DOI] [PubMed] [Google Scholar]
- 25.Gómez-Vera J, Gutiérrez-Ávila SA, Acosta-Gutiérrez DN, López-Tiro JJ, Bedolla-Barajas M. Omalizumab in the treatment of antihistamine-resistant chronic urticaria in adults. Ann Allergy Asthma Immunol. 2016;117:204–205. doi: 10.1016/j.anai.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Chang K-L, Yang Y-H, Yu H-H, Lee J-H, Wang L-C, Chiang B-L. Analysis of serum total IgE, specific IgE and eosinophils in children with acute and chronic urticaria. J Microbiol Immunol Infect. 2013;46:53–58. doi: 10.1016/j.jmii.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 27.Nathan RA, Meltzer EO, Selner JC, Storms W. Prevalence of allergic rhinitis in the United States. J Allergy Clin Immunol. 1997;99:S808–S814. [PubMed] [Google Scholar]
- 28.Loftus PA, Wise SK. Epidemiology of asthma. Curr Opin Otolaryngol Head Neck Surg. 2016;24:245–249. doi: 10.1097/MOO.0000000000000262. [DOI] [PubMed] [Google Scholar]
- 29.Lee JE, Choi YS, Kim MS, Han DH, Rhee CS, Lee CH, et al. Efficacy of sublingual immunotherapy with house dust mite extract in polyallergen sensitized patients with allergic rhinitis. Ann Allergy Asthma Immunol. 2011;107:79–84. doi: 10.1016/j.anai.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 30.Akdis CA, Akdis M. Mechanisms of allergen-specific immunotherapy and immune tolerance to allergens. World Allergy Organ J. 2015;8:1–12. doi: 10.1186/s40413-015-0063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnes PJ. Corticosteroids, IgE, and atopy. J Clin Invest. 2001;107:265–266. doi: 10.1172/JCI12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corren J, Shapiro G, Reimann J, Deniz Y, Wong D, Adelman D, et al. Allergen skin tests and free IgE levels during reduction and cessation of omalizumab therapy. J Allergy Clin Immunol. 2008;121:506–511. doi: 10.1016/j.jaci.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 33.Des Roches A, Paradis L, Bougeard YH, Godard P, Bousquet J, Chanez P. Long-term oral corticosteroid therapy does not alter the results of immediate-type allergy skin prick tests. J Allergy Clin Immunol. 1996;98:522–527. doi: 10.1016/s0091-6749(96)70085-4. [DOI] [PubMed] [Google Scholar]
- 34.Sussman G, Hébert J, Barron C, Bian J, Caron-Guay RM, Laflamme S, et al. Real-life experiences with omalizumab for the treatment of chronic urticaria. Ann Allergy Asthma Immunol. 2014;112:170–174. doi: 10.1016/j.anai.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Saini SS, Bindslev-Jensen C, Maurer M, Grob JJ, Bülbül Baskan E, Bradley MS, et al. Efficacy and safety of omalizumab in patients with chronic idiopathic/spontaneous urticaria who remain symptomatic on h 1 antihistamines: a randomized, placebo-controlled study. J Invest Dermatol. 2015;135:67–75. doi: 10.1038/jid.2014.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kianifar HR, Pourreza A, Jabbari Azad F, Yousefzadeh H, Masomi F. Sensitivity comparison of the skin prick test and serum and fecal radio allergosorbent test (RAST) in diagnosis of food allergy in children. Rep Biochem Mol Biol. 2016;4:98–103. [PMC free article] [PubMed] [Google Scholar]
- 37.Maurer M, Kaplan A, Rosén K, Holden M, Iqbal A, Trzaskoma BL, et al. The XTEND-CIU study: long-term use of omalizumab in chronic idiopathic urticaria. J Allergy Clin Immunol. 2018;141:1138–1139. doi: 10.1016/j.jaci.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 38.Shiomori T, Yoshida S, Miyamoto H, Makishima K. Relationship of nasal carriage of Staphylococcus aureus to pathogenesis of perennial allergic rhinitis. J Allergy Clin Immunol. 2000;105:449–454. doi: 10.1067/mai.2000.104256. [DOI] [PubMed] [Google Scholar]
- 39.Graefe H, Roebke C, Schäfer D, Meyer JE. Aspirin sensitivity and chronic rhinosinusitis with polyps: a fatal combination. J Allergy. 2012;2012:1–10. doi: 10.1155/2012/817910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Altrichter S, Hawro T, Liedtke M, Holtappels G, Bachert C, Skov PS, et al. In chronic spontaneous urticaria, IgE against staphylococcal enterotoxins is common and functional. Allergy Eur J Allergy Clin Immunol. 2018;112:170–174. doi: 10.1111/all.13381. [DOI] [PubMed] [Google Scholar]
- 41.Irani AA, Schechter NM, Craig SS, DeBlois G, Schwartz LB. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci USA. 1986;83:4464–4468. doi: 10.1073/pnas.83.12.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schäfer B, Piliponsky AM, Oka T, Song CH, Gerard NP, Gerard C, et al. Mast cell anaphylatoxin receptor expression can enhance IgE-dependent skin inflammation in mice. J Allergy Clin Immunol. 2013;13:541–548. doi: 10.1016/j.jaci.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ali H, Panettieri RA. Anaphylatoxin C3a receptors in asthma. Respir Res. 2005;6:19. doi: 10.1186/1465-9921-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krug N, Tschernig T, Erpenbeck VJ, Hohlfeld JM, Köhl J. Complement factors C3a and C5a are increased in bronchoalveolar lavage fluid after segmental allergen provocation in subjects with asthma. Am J Respir Crit Care Med. 2001;164:1841–1843. doi: 10.1164/ajrccm.164.10.2010096. [DOI] [PubMed] [Google Scholar]
- 45.Füreder W, Agis H, Willheim M, Bankl HC, Maier U, Kishi K, et al. Differential expression of complement receptors on human basophils and mast cells. Evidence for mast cell heterogeneity and CD88/C5aR expression on skin mast cells. J Immunol. 1995;155:3152–3160. [PubMed] [Google Scholar]
- 46.El-Lati SG, Dahinden CA, Church MK. Complement peptides C3a- and C5a-induced mediator release from dissociated human skin mast cells. J Invest Dermatol. 1994;102:803–806. doi: 10.1111/1523-1747.ep12378589. [DOI] [PubMed] [Google Scholar]
- 47.Kikuchi Y, Kaplan AP. A role for C5a in augmenting IgG-dependent histamine release from basophils in chronic urticaria. J Allergy Clin Immunol. 2002;109:114–118. doi: 10.1067/mai.2002.120954. [DOI] [PubMed] [Google Scholar]
- 48.Huber-Lang M, Sarma JV, Zetoune FS, Rittirsch D, Neff TA, McGuire SR, et al. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006;12:682–687. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- 49.Zhu H, Liang B, Li R, Li J, Lin L, Ma S, et al. Activation of coagulation, anti-coagulation, fibrinolysis and the complement system in patients with urticaria. Asian Pac J Allergy Immunol. 2013;31:43–50. [PubMed] [Google Scholar]
- 50.Dakhale GN, Wankhede SS, Mahatme MS, Hiware SK, Mishra DB, Dudhgaonkar SS. Comparison of efficacy, safety and cost-effectiveness of rupatadine and olopatadine in patients of chronic spontaneous urticaria: a randomized, double-blind, comparative, parallel group trial. Indian J Dermatol. 2016;61:63. doi: 10.4103/0019-5154.159621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caliskaner Z, Ozturk S, Turan M, Karaayvaz M. Skin test positivity to aeroallergens in the patients with chronic urticaria without allergic respiratory disease. J Investig Allergol Clin Immunol. 2004;14:50–54. [PubMed] [Google Scholar]
- 52.Bains P, Dogra A. Skin prick test in patients with chronic allergic skin disorders. Indian J Dermatol. 2015;60:159–164. doi: 10.4103/0019-5154.152513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sibbald G, Cheema A, Lozinski A, Tarlo S. Chronic urticaria: evaluation of the role of physical, immunologic, and other contributory factors. Int J Dermatol. 1991;30:381–386. doi: 10.1111/j.1365-4362.1991.tb03891.x. [DOI] [PubMed] [Google Scholar]
- 54.Liutu M, Kalimo K, Uksila J, Kalimo H. Etiologic aspects of chronic urticaria. Internet J Dermatology. 1998;37:515–519. doi: 10.1046/j.1365-4362.1998.00328.x. [DOI] [PubMed] [Google Scholar]
- 55.Ilondis G, Anogianakis G, Theofilogiannakos E, Trakatelli M. Skin prick tests in patients with chronic urticaria. Eur J Inflamm. 2005;3:33–36. [Google Scholar]
- 56.Kulthanan K, Jiamton S, Rutnin NO, Insawang M, Pinkaew S. Prevalence and relevance of the positivity of skin prick testing in patients with chronic urticaria. J Dermatol. 2008;35:330–335. doi: 10.1111/j.1346-8138.2008.00477.x. [DOI] [PubMed] [Google Scholar]
- 57.Ye YM, Hur GY, Park HJ, Kim SH, Kim HM, Park HS. Association of specific IgE to staphylococcal superantigens with the phenotype of chronic urticaria. J Korean Med Sci. 2008;23:845–851. doi: 10.3346/jkms.2008.23.5.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Daschner A, Rodero M, De Frutos C, Valls A, Cuéllar C. Chronic urticaria is associated with a differential helminth-arthropod- related atopy phenotype. J Dermatol. 2010;37:780–785. doi: 10.1111/j.1346-8138.2010.00869.x. [DOI] [PubMed] [Google Scholar]
- 59.Refaat M, Ossman E, Farres M, El-Khodeery M, Arafa N. Assessment of the role of aeroallergens in patients with chronic urticaria. Rev Française d’Allergologie. 2010;50:394–397. [Google Scholar]
- 60.Green BJ, Beezhold DH, Gallinger Z, Barron CS, Melvin R, Bledsoe TA, et al. Allergic sensitization in Canadian chronic rhinosinusitis patients. Allergy Asthma Clin Immunol. 2014;10:15. doi: 10.1186/1710-1492-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oncham S, Udomsubpayakul U, Laisuan W. Skin prick test reactivity to aeroallergens in adult allergy clinic in Thailand: a 12-year retrospective study. Asia Pac Allergy. 2018;8:e17. doi: 10.5415/apallergy.2018.8.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bilgir F, Özdemir RB, Değirmenci P, Dede B, Kırmaz C. Clinical features of patients with chronic urticaria and the results of the skin prick tests. Asthma Allergy Immunol. 2019;17:91–96. [Google Scholar]
- 63.Mounika K, Shivaswamy K. Skin prick test positivity to house dust mites (HDM) in patients with chronic urticaria. Int J Res Dermatol. 2017;3:7–9. [Google Scholar]
- 64.Mahesh PA, Kushalappa PA, Holla AD, Vedanthan PK. House dust mite sensitivity is a factor in chronic urticaria. Indian J Dermatol Venereol Leprol. 2005;71:99–101. doi: 10.4103/0378-6323.13993. [DOI] [PubMed] [Google Scholar]
- 65.Kulthanan K, Wachirakaphan C. Prevalence and clinical characteristics of chronic urticaria and positive skin prick testing to mites. Acta Derm Venereol. 2008;88:584–588. doi: 10.2340/00015555-0546. [DOI] [PubMed] [Google Scholar]
- 66.Geçer E, Erdem T. Aeroallergen prick skin test and autologous serum skin test results in patients with chronic urticaria and their comparison. Ann Dermatol. 2012;24:472–474. doi: 10.5021/ad.2012.24.4.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Song Z, Zhai Z, Zhong H, Zhou Z, Chen WC, Hao F. Evaluation of autologous serum skin test and skin prick test reactivity to house dust mite in patients with chronic spontaneous urticaria. PLoS ONE. 2013;8:e641642. doi: 10.1371/journal.pone.0064142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maurer M, Altrichter S, Bieber T, Biedermann T, Bräutigam M, Seyfried S, et al. Efficacy and safety of omalizumab in patients with chronic urticaria who exhibit IgE against thyroperoxidase. J Allergy Clin Immunol. 2011;128:202–209. doi: 10.1016/j.jaci.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 69.Saini S, Rosen KE, Hsieh H-J, Wong DA, Conner E, Kaplan A, et al. A randomized, placebo-controlled, dose-ranging study of single-dose omalizumab in patients with H1-antihistamine—refractory chronic idiopathic urticaria. J Allergy Clin Immunol. 2011;128:567–573. doi: 10.1016/j.jaci.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 70.Nam Y-H, Kim J-H, Jin HJ, Hwang E-K, Shin Y-S, Ye Y-M, et al. Effects of omalizumab treatment in patients with refractory chronic urticaria. Allergy Asthma Immunol Res. 2012;4:357–361. doi: 10.4168/aair.2012.4.6.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaplan A, Ledford D, Ashby M, Canvin J, Zazzali JL, Conner E, et al. Omalizumab in patients with symptomatic chronic idiopathic/spontaneous urticaria despite standard combination therapy. J Allergy Clin Immunol. 2013;132:101–109. doi: 10.1016/j.jaci.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 72.Labrador-Horrillo M, Valero A, Velasco M, Jáuregui I, Sastre J, Bartra J, et al. Efficacy of omalizumab in chronic spontaneous urticaria refractory to conventional therapy: analysis of 110 patients in real-life practice. Expert Opin Biol Ther. 2013;13:1225–1228. doi: 10.1517/14712598.2013.822484. [DOI] [PubMed] [Google Scholar]
- 73.Maurer M, Rosén K, Hsieh H-J, Saini S, Grattan C, Gimenéz-Arnau A, et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med. 2013;368:924–935. doi: 10.1056/NEJMoa1215372. [DOI] [PubMed] [Google Scholar]
- 74.Rottem M, Segal R, Kivity S, Shamshines L, Graif Y, Shalit M, et al. Omalizumab therapy for chronic spontaneous urticaria: the Israeli experience. Isr Med Assoc J. 2014;16:487–490. [PubMed] [Google Scholar]
- 75.Ensina L, Valle S, Juliani A, Galeane M. Omalizumab in chronic spontaneous urticaria: a Brazilian real-life experience. Int Arch Allergy Immunol. 2016;169:121–124. doi: 10.1159/000444985. [DOI] [PubMed] [Google Scholar]
- 76.Wilches P, Wilches P, Calderon JC, Cherrez A, Cherrez Ojeda I. Omalizumab for chronic urticaria in Latin America. World Allergy Organ J. 2016;9:1–5. doi: 10.1186/s40413-016-0127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Deza G, Bertolín-Colilla M, Pujol R, Curto-Barredo L, Soto D, García M, et al. Basophil FcεRI expression in chronic spontaneous urticaria: a potential immunological predictor of response to omalizumab therapy. Acta Derm Venereol. 2017;97:698–704. doi: 10.2340/00015555-2654. [DOI] [PubMed] [Google Scholar]
- 78.Gericke J, Metz M, Ohanyan T, Weller K, Altrichter S, Skov PS, et al. Serum autoreactivity predicts time to response to omalizumab therapy in chronic spontaneous urticaria. J Allergy Clin Immunol. 2017;139:1059–1061. doi: 10.1016/j.jaci.2016.07.047. [DOI] [PubMed] [Google Scholar]
- 79.Jörg L, Pecaric-Petkovic T, Reichenbach S, Coslovsky M, Stalder O, Pichler W, et al. Double-blind placebo-controlled trial of the effect of omalizumab on basophils in chronic urticaria patients. Clin Exp Allergy. 2018;48:196–204. doi: 10.1111/cea.13066. [DOI] [PubMed] [Google Scholar]
- 80.Metz M, Staubach P, Bauer A, Brehler R, Gericke J, Kangas M, et al. Clinical efficacy of omalizumab in chronic spontaneous urticaria is associated with a reduction of FcεRI-positive cells in the skin. Theranostics. 2017;7:1266–1276. doi: 10.7150/thno.18304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nettis E, Cegolon L, Di Leo E, Canonica WG, Detoraki A, Baiardini I, et al. Omalizumab in elderly patients with chronic spontaneous urticaria: an Italian real-life experience. Ann Allergy Asthma Immunol. 2018;120:318–323. doi: 10.1016/j.anai.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 82.Yang C-W, Cho Y-T, Chu C-Y. Efficacy of omalizumab treatment for patients with chronic idiopathic urticaria (CIU)/chronic spontaneous urticaria (CSU) in Taiwan. Dermatol Sin. 2017;35:182–186. [Google Scholar]
- 83.Bulur I, Baskan EB, Ozdemir M, Balevi A, Göncü EK, Altunay I, et al. The efficacy and safety of Omalizumab in refractory chronspontaneous urticaria: real-life experience in Turkey. Acta Dermatovenerol Alpina Pannonica Adriat. 2018;27:121–126. [PubMed] [Google Scholar]
- 84.Ertas R, Ozyurt K, Atasoy M, Hawro T, Maurer M. The clinical response to omalizumab in chronic spontaneous urticaria patients is linked to and predicted by IgE levels and their change. Allergy Eur J Allergy Clin Immunol. 2018;73:705–712. doi: 10.1111/all.13345. [DOI] [PubMed] [Google Scholar]
- 85.Ghazanfar MN, Holm JG, Thomsen SF. Effectiveness of omalizumab in chronic spontaneous urticaria assessed with patient-reported outcomes: a prospective study. J Eur Acad Dermatol Venereol. 2018;32:1761–1767. doi: 10.1111/jdv.15045. [DOI] [PubMed] [Google Scholar]
- 86.Hide M, Igarashi A, Yagami A, Chinuki Y. Efficacy and safety of omalizumab for the treatment of refractory chronic spontaneous urticaria in Japanese patients: subgroup analysis of the phase 3 POLARIS study. Allergol Int. 2018;67:243–252. doi: 10.1016/j.alit.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 87.Türk M, Yılmaz İ, Bahçecioğlu SN. Treatment and retreatment with omalizumab in chronic spontaneous urticaria: real life experience with twenty-five patients. Allergol Int. 2018;67:85–89. doi: 10.1016/j.alit.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 88.Hide M, Francis DM, Grattan C, Hakimi J, Kochan JP, Greaves MW. Autoantibodies against the high-affinity IgE receptor as a cause of histamine release in chronic urticaria. N Engl J Med. 1993;328:1599–1604. doi: 10.1056/NEJM199306033282204. [DOI] [PubMed] [Google Scholar]
- 89.Zweiman B, Valenzano M, Atkins P, Tanus T, Getsy J. Characteristics of histamine-releasing activity in the sera of patients with chronic idiopathic urticaria. J Allergy Clin Immunol. 1996;98:89–98. doi: 10.1016/s0091-6749(96)70230-0. [DOI] [PubMed] [Google Scholar]
- 90.Fiebiger E, Hammerschmid F, Stingl G, Maurer D. Anti-FcεRIα autoantibodies in autoimmune-mediated disorders. Identification of a structure-function relationship. J Clin Invest. 1998;101:243–251. doi: 10.1172/JCI511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zuberbier T, Henz BM, Fiebiger E, Maurer D, Stingl G. Anti-Fc(ε)RIα serum autoantibodies in different subtypes of urticaria. Allergy Eur J Allergy Clin Immunol. 2000;55:951–954. doi: 10.1034/j.1398-9995.2000.00445.x. [DOI] [PubMed] [Google Scholar]
- 92.Sabroe RA, Fiebiger E, Francis DM, Maurer D, Seed PT, Grattan CEH, et al. Classification of anti-FcεRI and anti-IgE autoantibodies in chronic idiopathic urticaria and correlation with disease severity. J Allergy Clin Immunol. 2002;110:492–499. doi: 10.1067/mai.2002.126782. [DOI] [PubMed] [Google Scholar]
- 93.Hidvégi B, Nagy E, Szabó T, Temesvári E, Marschalkó M, Kárpáti S, et al. Correlation between T-cell and mast cell activity in patients with chronic urticaria. Int Arch Allergy Immunol. 2003;132:177–182. doi: 10.1159/000073719. [DOI] [PubMed] [Google Scholar]
- 94.Eckman JA, Hamilton RG, Gober LM, Sterba PM, Saini SS. Basophil phenotypes in chronic idiopathic urticaria in relation to disease activity and autoantibodies. J Invest Dermatol. 2008;128:1956–1963. doi: 10.1038/jid.2008.55. [DOI] [PubMed] [Google Scholar]
- 95.Lee M-F, Lin T-M, Liu S-W, Chen Y-H. A Rapid method of detecting autoantibody against FcεRIα for chronic spontaneous urticaria. PLoS ONE. 2014;9:e109565. doi: 10.1371/journal.pone.0109565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ulambayar B, Chen YH, Ban GY, Lee JH, Jung CG, Yang EM, et al. Detection of circulating IgG autoantibody to FcεRIα in sera from chronic spontaneous urticaria patients. J Microbiol Immunol Infect. 2017;53:141–147. doi: 10.1016/j.jmii.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 97.Baioumy SA, Esawy MM, Shabana MA. Assessment of circulating FCεRIa in chronic spontaneous urticaria patients and its correlation with clinical and immunological variables. Immunobiology. 2018;223:807–811. doi: 10.1016/j.imbio.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 98.Schoepke N, Asero R, Ellrich A, Ferrer M, Gimenez-Arnau A, EH Grattan C, et al. Biomarkers and clinical characteristics of autoimmune chronic spontaneous urticaria: results of the PURIST Study. Allergy Eur J Allergy Clin Immunol. 2019;74:2427–2436. doi: 10.1111/all.13949. [DOI] [PubMed] [Google Scholar]
- 99.Asero R, Marzano AV, Ferrucci S, Lorini M, Carbonelli V, Cugno M. Co-occurrence of IgE and IgG autoantibodies in patients with chronic spontaneous urticaria. Clin Exp Immunol. 2020;200:242–249. doi: 10.1111/cei.13428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gruber BL, Baeza ML, Marchese MJ, Agnello V, Kaplan AP. Prevalence and functional role of anti-IgE autoantibodies in urticarial syndromes. J Invest Dermatol. 1988;90:213–217. doi: 10.1111/1523-1747.ep12462239. [DOI] [PubMed] [Google Scholar]
- 101.Tong LJ, Balakrishnan G, Kochan JP, Kinét JP, Kaplan AP. Assessment of autoimmunity in patients with chronic urticaria. J Allergy Clin Immunol. 1997;99:461–465. doi: 10.1016/s0091-6749(97)70071-x. [DOI] [PubMed] [Google Scholar]
- 102.Rojanapremsuk T, Kasprowicz S, Schafer E, Story R, Clarke MS, Walls T, et al. Clinicopathologic findings in (anti-FcepsilonR1alpha) autoimmune-related chronic urticaria. J Cutan Pathol. 2015;42:329–332. doi: 10.1111/cup.12471. [DOI] [PubMed] [Google Scholar]
- 103.Verneuil L, Leconte C, Ballet JJ, Coffin C, Laroche D, Izard JP, et al. Association between chronic urticaria and thyroid autoimmunity: a prospective study involving 99 patients. Dermatology. 2004;208:98–103. doi: 10.1159/000076480. [DOI] [PubMed] [Google Scholar]
- 104.Gangemi S, Saitta S, Lombardo G, Patafi M, Benvenga S. Serum thyroid autoantibodies in patients with idiopathic either acute or chronic urticaria. J Endocrinol Invest. 2009;32:107–110. doi: 10.1007/BF03345696. [DOI] [PubMed] [Google Scholar]
- 105.Nuzzo V, Tauchmanova L, Colasanti P, Zuccoli A, Colao A. Idiopathic chronic urticaria and thyroid autoimmunity: experience of a single center. Dermatoendocrinol. 2011;3:255–258. doi: 10.4161/derm.3.4.17066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Alpay A, Solak Tekin N, Tekin IÖ, Altinyazar HC, Koca R, Çnar S. Autologous serum skin test versus autologous plasma skin test in patients with chronic spontaneous urticaria. Dermatol Res Pract. 2013;2013:267278. doi: 10.1155/2013/267278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cho CB, Stutes SA, Altrich ML, Ardoin SP, Phillips G, Ogbogu PU. Autoantibodies in chronic idiopathic urticaria and non-urticarial systemic autoimmune disorders. Ann Allergy Asthma Immunol. 2013;110:29–33. doi: 10.1016/j.anai.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wan KS, Wu CS. The essential role of anti-thyroid antibodies in chronic idiopathic urticaria. Endocr Res. 2013;38:85–88. doi: 10.3109/07435800.2012.714432. [DOI] [PubMed] [Google Scholar]
- 109.Yadav S, Kanwar AJ, Parsad D, Minz RW. Chronic idiopathic urticaria and thyroid autoimmunity: perplexing association. Indian J Dermatol. 2013;58:325. doi: 10.4103/0019-5154.113932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim DH, Sung NH, Lee AY. Effect of levothyroxine treatment on clinical symptoms in hypothyroid patients with chronic urticaria and thyroid autoimmunity. Ann Dermatol. 2016;28:199–204. doi: 10.5021/ad.2016.28.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Czarnecka-Operacz M, Sadowska-Przytocka A, Jenerowicz D, Szeliga A, Adamski Z, Łacka K. Thyroid function and thyroid autoantibodies in patients with chronic spontaneous urticaria. Postep Dermatologii i Alergol. 2017;34:566–572. doi: 10.5114/ada.2017.72464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kasumagic-Halilovic E, Beslic N, Ovcina-Kurtovic N. Thyroid autoimmunity in patients with chronic urticaria. Med Arch. 2017;71:29–31. doi: 10.5455/medarh.2017.71.29-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Toyoda M, Maruyama T, Morohashi M, Bhawan J. Free eosinophil granules in urticaria: a correlation with the duration of wheals. Am J Dermatopathol. 1996;18:49–57. doi: 10.1097/00000372-199602000-00008. [DOI] [PubMed] [Google Scholar]
- 114.Di Lorenzo G, Mansueto P, Melluso M, Pellitteri ME, Di Salvo A, Caruso C, et al. Blood eosinophils and serum eosinophil cationic protein in patients with acute and chronic urticaria. Mediators Inflamm. 1996;5:113–115. doi: 10.1155/S0962935196000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim TY, Park HJ, Kim CW. Eosinophil cationic protein (ECP) level and its correlation with eosinophil number or IgE level of peripheral blood in patients with various skin diseases. J Dermatol Sci. 1997;15:89–94. doi: 10.1016/s0923-1811(97)00614-2. [DOI] [PubMed] [Google Scholar]
- 116.Grattan CEH, Dawn G, Gibbs S, Francis DM. Blood basophil numbers in chronic ordinary urticaria and healthy controls: diurnal variation, influence of loratadine and prednisolone and relationship to disease activity. Clin Exp Allergy. 2003;33:337–341. doi: 10.1046/j.1365-2222.2003.01589.x. [DOI] [PubMed] [Google Scholar]
- 117.Garmendia JV, Zabaleta M, Blanca I, Bianco NE, De Sanctis JB. Total and biologically active serum-soluble CD154 in patients with chronic idiopathic urticaria. Allergy Asthma Proc. 2004;25:121–125. [PubMed] [Google Scholar]
- 118.Boonpiyathad T, Pradubpongsa P, Sangasapaviriya A. Vitamin d supplements improve urticaria symptoms and quality of life in chronic spontaneous urticaria patients: a prospective case-control study. Dermato-Endocrinology. 2016;8:e983685. doi: 10.4161/derm.29727. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 119.Woo YR, Jung KE, Koo DW, Lee JS. Vitamin D as a marker for disease severity in chronic urticaria and its possible role in pathogenesis. Ann Dermatol. 2015;27(423):430. doi: 10.5021/ad.2015.27.4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wardhana M, Suryawati N, Ariana A. The effect of psychological stress on c-reactive protein and peripheral blood cells count in chronic idiopathic urticaria. Bali Med J. 2017;6:198–203. [Google Scholar]
- 121.Aitella E, De Bartolomeis F, Savoia A, Fabiani M, Romano M, Astarita C. The overlap syndrome of urticaria and gastroesophageal reflux disease. PLoS ONE. 2018;13:e0207602. doi: 10.1371/journal.pone.0207602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Çildaǧ S, Şentürk T. The effect of omalizumab treatment on IgE and other immunoglobulin levels in patients with chronic spontaneous urticaria and its association with treatment response. Postep Dermatologii i Alergol. 2018;35:316. doi: 10.5114/ada.2017.71422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Acer E, Kaya Erdogan H, Yüksel Çanakçı N, Saracoglu ZN. The effect of omalizumab on hematological and inflammatory parameters in patients with chronic spontaneous urticaria. Cutan Ocul Toxicol. 2019;38:5–8. doi: 10.1080/15569527.2018.1495227. [DOI] [PubMed] [Google Scholar]
- 124.Akdogan N, Demirel Ogut N, Dogan S, Atakan N. Long-term effects of omalizumab on peripheral blood cells and C-reactive protein levels in patients with chronic spontaneous urticaria. Dermatol Ther. 2019;32:e12966. doi: 10.1111/dth.12966. [DOI] [PubMed] [Google Scholar]
- 125.Kolkhir P, Church MK, Altrichter S, Skov PS, Hawro T, Frischbutter S, et al. Eosinopenia, in chronic spontaneous urticaria, is associated with high disease activity, autoimmunity, and poor response to treatment. J Allergy Clin Immunol Pract. 2020;8:318–325. doi: 10.1016/j.jaip.2019.08.025. [DOI] [PubMed] [Google Scholar]
- 126.Nazik H, Mülayim MK, Öztürk P. Omalizumab usage in chronic urticaria and atopic dermatitis: data from South-East province of Turkey. Postep Dermatologii i Alergol. 2019;36:734. doi: 10.5114/ada.2019.91424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Oliver ET, Chichester K, Devine K, Sterba PM, Wegner C, Vonakis BM, et al. Effects of an oral CRTh2 Antagonist (AZD1981) on eosinophil activity and symptoms in chronic spontaneous urticaria. Int Arch Allergy Immunol. 2019;179:21–30. doi: 10.1159/000496162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tamer F. Omalizumab does not lead to a distinct alteration in hematological parameters and complete blood count-derived inflammation biomarkers except for basophil count. Cutan Ocul Toxicol. 2020 doi: 10.1080/15569527.2020.1766483. [DOI] [PubMed] [Google Scholar]
- 129.Jirapongsananuruk O, Pongpreuksa S, Sangacharoenkit P, Visitsunthorn N, Vichyanond P. Identification of the etiologies of chronic urticaria in children: a prospective study of 94 patients. Pediatr Allergy Immunol. 2010;21:508–514. doi: 10.1111/j.1399-3038.2009.00912.x. [DOI] [PubMed] [Google Scholar]
- 130.Chansakulporn S, Pongpreuksa S, Sangacharoenkit P, Pacharn P, Visitsunthorn N, Vichyanond P, et al. The natural history of chronic urticaria in childhood: a prospective study. J Am Acad Dermatol. 2014;71:663–668. doi: 10.1016/j.jaad.2014.05.069. [DOI] [PubMed] [Google Scholar]
- 131.Cavkaytar O, Arik Yilmaz E, Buyuktiryaki B, Sekerel BE, Sackesen C, Soyer OU. Challenge-proven aspirin hypersensitivity in children with chronic spontaneous urticaria. Allergy Eur J Allergy Clin Immunol. 2015;70:153–160. doi: 10.1111/all.12539. [DOI] [PubMed] [Google Scholar]
- 132.Uysal P, Avcil S, Erge D. High-dose anti-histamine use and risk factors in children with urticaria. Turk Pediatr Ars. 2016;51:198. doi: 10.5152/TurkPediatriArs.2016.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee SJ, Ha EK, Jee HM, Lee KS, Lee SW, Kim MA, et al. Prevalence and risk factors of urticaria with a focus on chronic urticaria in children. Allergy, Asthma Immunol Res. 2017;9:212–219. doi: 10.4168/aair.2017.9.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.