Abstract
Chemical modifications are found on almost all RNAs and affect their coding and noncoding functions. The identification of m6A on mRNA and its important role in gene regulation stimulated the field to investigate whether additional modifications are present on mRNAs. Indeed, modifications including m1A, m5C, m7G, 2′-OMe, and Ψ were detected. However, since their abundances are low and tools used for their corroboration are often not well characterized, their physiological relevance remains largely elusive. Antibodies targeting modified nucleotides are often used but have limitations such as low affinity or specificity. Moreover, they are not always well characterized and due to the low abundance of the modification, particularly on mRNAs, generated data sets might resemble noise rather than specific modification patterns. Therefore, it is critical that the affinity and specificity is rigorously tested using complementary approaches. Here, we provide an experimental toolbox that allows for testing antibody performance prior to their use.
Keywords: monoclonal antibodies, affinity, m6A, m5C, validation, base modifications
INTRODUCTION
RNA molecules are composed of nucleotides carrying the four bases adenine (A), guanine (G), cytosine (C), and uracil (U). Soon after the discovery of RNAs with noncoding functions such as transfer RNAs (tRNAs) or ribosomal RNAs (rRNAs), it became evident that RNAs can be heavily modified and these modifications are important for their structures and functions (Littlefield and Dunn 1958; Bergquist and Matthews 1962). Bases can be chemically modified to gain or lose specific biophysical properties. Such modifications may, for example, lead to changes in RNA base-pairing or RNA folding (Motorin and Helm 2011; Polikanov et al. 2015). In addition, some modifications could generate binding platforms for specialized RNA binding proteins (RBPs). A prominent example for modified bases is pseudouridine (Ψ), which is present in rRNA, tRNA, and also mRNA (Zhao et al. 2017). The modification reactions are catalyzed by specialized RNA–protein complexes (RNPs) containing box H/ACA small nucleolar RNAs (snoRNAs) and the associated catalytic subunit dyskerin (snoRNPs) (Matera et al. 2007).
The recent developments in RNA sequencing technologies revealed that modifications are widespread and found in almost all RNAs including mRNAs (Helm and Motorin 2017). An example for an abundant modification found in mRNAs is N6-methyl-Adenosine (m6A) (Dominissini et al. 2012; Meyer et al. 2012). This modification is generated on mRNAs by the METTL3-METTL14 enzyme complex (Batista et al. 2014; Liu et al. 2014; Meyer and Jaffrey 2017). A multitude of different m6A methylation patterns have been reported and thus many cellular functions are associated with this modification (Fu et al. 2014; Maity and Das 2016). For example, m6A is enriched around stop codons, on 3′-UTRs and in large exons (Ke et al. 2015; Yue et al. 2015). In the nucleus, m6A modification accelerates turnover of modified transcripts but seems to be dispensable for splicing (Ke et al. 2017). In contrast, it has been reported that hnRNPG binds RNA polymerase II and m6A modifications on nascent pre-mRNAs leading to changes in splicing patterns (Zhou et al. 2019). In the cytoplasm, m6A promotes cap-independent translation initiation by direct recruitment of initiation factors (Meyer et al. 2015; Coots et al. 2017; Yang et al. 2017).
In addition to m6A, a number of other mRNA modifications have been reported. M1A (N1-methyladenosine) has recently been identified on cytosolic and mitochondrial mRNA but probably at low frequency (Li et al. 2017; Safra et al. 2017). Ψ has been profiled and found that mRNAs also carry this modification (Carlile et al. 2014; Lovejoy et al. 2014; Schwartz et al. 2014; Li et al. 2015). M5C (5-methylcytosine), a common modification found on DNA, has been reported on mRNAs as well (Squires et al. 2012; Amort et al. 2017; Legrand et al. 2017; Huang et al. 2019) and this phenomenon appears to be conserved in Archaea (Edelheit et al. 2013). In addition, noncoding RNAs have also been found to contain m5C modifications (Hussain et al. 2013; Trixl and Lusser 2019). Most of these m5C studies utilized bisulfite-sequencing protocols, which are widely used for studying DNA m5C modifications and found very few to thousands of methylated sites on mRNAs. Other studies used m5C-specific antibodies to validate bisulfite-sequencing results. However, available antibodies were often selected for DNA specificity and their applicability for single stranded RNA is unclear.
It is likely that the abovementioned examples are more the tip of the iceberg rather than a complete picture of mRNA base modifications. M6A is the best-studied mRNA modification to date and this is due to the rather high abundance and the availability of antibodies against this modification. Some of them have been developed a long time ago and proved to be invaluable tools for the analysis of m6A on mRNA (e.g., Bringmann and Luhrmann 1987). Antibodies against other base modifications have been generated but low modification abundance as well as a lack of thorough validation led to rather vague results in RNA-seq experiments (Grozhik et al. 2019; Helm et al. 2019). Thus, a rigorous validation of base-specific antibodies for each individual assay that is applied is a critical prerequisite for the generation of conclusive and trustable data (Feederle and Schepers 2017).
To help solving this fundamental problem, we developed a panel of assays for antibody validation. In addition, we generated our own monoclonal antibodies against m6A, m5C, m26A (N6, N6-dimethyladenosine), and Ψ to prove the applicability of our validation and testing pipeline (Erlanger and Beiser 1964). We used a number of biochemical and biophysical assays and measurements ranging from Kd and enrichment factor determination to immunofluorescence and FACS analysis. Our study provides a detailed characterization platform for antibodies against base modifications helping to define quality standards for these widely used research tools. (See Table 1)
TABLE 1.
Summary of antibody validation results
RESULTS
Generation of monoclonal antibodies against modified nucleosides
For the establishment and testing of different validation methods, large quantities of antibodies are required. Therefore, we set out to generate our own monoclonal antibodies against specific base modifications, which were validated during the course of this study. We coupled individual nucleosides to a carrier protein and used the conjugate for immunization. The association to a carrier is necessary, as single nucleosides alone are too small to produce an efficient immune response. Ovalbumin (OVA) was used as an appropriate immunogenic carrier protein for immunization (Plescia and Braun 1967). To couple the modified nucleosides m6A, m5C, m26A, and Ψ, a modified Erlanger–Beiser protocol was applied (Fig. 1A, see Materials and Methods for details). The first chemical reaction oxidizes and opens the ribose ring between the 2′ and 3′ position. An amino group of a lysine side chain, for example, can now efficiently react with the nucleoside leading to covalent coupling to the protein (Fig. 1B). To validate and estimate coupling efficiencies, photometric analyses were performed (Fig. 1C). OVA alone peaks at a wavelength of about 280 nm (Fig. 1C, blue lines in all panels). Generally, the nucleoside-OVA conjugation shifts the OVA peak (purple lines) toward the free nucleosides (red lines). Coupling of m6A (purple graph, panel I) and Ψ (purple graph, panel II) shifted the peaks toward the peak of m6A or Ψ alone (Fig. 1C, red graphs). For m5C and m26A (panels III and IV) shifts were less pronounced since the individual components peak at a rather small window. Based on the peak intensities, coupling efficiencies were estimated to ∼20% (data not shown). Similar results were obtained for coupling of the nucleosides to bovine serum albumin (BSA) as carrier protein (data not shown).
FIGURE 1.
Synthesis of antigens for immunization: Coupling of nucleosides to ovalbumin. (A) Scheme for the conjugation reaction of the nucleosides to ovalbumin using the lysine NH2-group as reactive group. After oxidative coupling, the nucleoside no longer has RNA-specific properties, as the ribose ring is destroyed in the course of the reaction. (B) Schematic model of the coupling reaction of nucleoside (red) to ovalbumin (blue) to gain the conjugate (purple). (C) UV spectra of the coupled conjugates (purple) versus free ovalbumin (blue) and free nucleosides (red) are shown. The first spectrum shows m6A coupling followed by the spectra of m5C, pseudouridine (Ψ), and of m26A. (D) Outline of the generation of monoclonal antibodies against modified bases. Hybridoma cells were tested by detection and capture ELISAs using BSA-coupled nucleosides or biotinylated RNA–DNA-oligo hybrids.
OVA-conjugated nucleosides carrying m5C, m6A, m26A, and Ψ, were used to immunize rats and mice (Fig. 1D). Six to eight weeks after primary immunization, one boost injection was given and the immunized animals were sacrificed 3 d later. After fusion of splenic B cells and the myeloma cell line, all cells were evenly distributed in 96-well plates and hybridoma cells were selected. All outgrowing hybridoma cells were first screened in ELISA experiments for the expression of IgG antibodies specifically binding the respective modified nucleoside either as BSA-conjugates or as biotinylated oligonucleotides (Fig. 1D, detection ELISA I/II). Capture ELISA experiments using BSA-conjugated nucleosides were performed to identify those supernatants containing IgG antibodies that are potentially useful for immunoprecipitation experiments (Fig. 1D, capture ELISA). Unmodified oligonucleotides were included in all screenings as negative controls. Monoclonal hybridoma cell lines directed against m6A, m5C, m26A, and Ψ were established and used for further validation studies. (See Table 1.)
Determination of dissociation constants (Kd)
In order to estimate the general performance of the antibodies in functional assays, we determined their affinities to free nucleosides (Fig. 2). Equimolar mixtures of modified and unmodified nucleosides were incubated with the respective antibody. The mixture was subsequently centrifuged through a filter allowing molecules <10 kDa passing through. This procedure separates antibody-bound from -unbound nucleosides, which can be used for Kd estimations (Fig. 2A). Input samples and filtrates were further analyzed and quantified by HPLC. Kd values were calculated via Scatchard plots (Fig. 2B; Supplemental Fig. 1) and a binding model was generated using data fitting (Fig. 2C–F; Supplemental Fig. 1; see Material and Methods for experimental details and Kd calculations). Using this method, we estimated the Kd of the α-m6A clone 9B7 to 0.55 µM, clone 11D11 to 0.59 µM and clone 13G2 to 1.92 µM (Fig. 2G). We next tested antibody clones against m5C and identified affinities in a similar range with clone 32E2 showing the highest affinity of 0.39 µM (Fig. 2D,G). Also, α-m26A clone 60G3 had a rather high affinity to the modified nucleoside (Fig. 2E,G), while two clones directed against Ψ (26H5 and 27C5) showed only moderate affinities (Fig. 2F,G). Our data therefore suggest that most of the tested antibodies have reasonably high affinity in solution (Fig. 2G) at least to their nucleoside antigen and might be useful tools for further functional work.
FIGURE 2.
Determination of KD-values of antibodies against base modifications. (A) Schematic outline of the experiment to determine KD values of the antibodies. An equimolar mixture of modified and unmodified nucleotides was incubated with the respective antibodies tested and centrifugated through a filter with a cutoff, allowing free nucleotides but not nucleoside-antibody complexes to pass through. HPLC quantification of bound and unbound nucleosides were used for KD estimation. (B) Example for a Scatchard plot for antibody α- m6A 9B7. (C) Binding model for antibody m6A 9B7 shows the slow convergence toward saturation of the antibody binding capacity for the binding to m6A nucleosides (gray squares). The black triangles depict the binding to m26A nucleosides. (D) Binding model of antibody m5C 32E2, showing binding to m5C nucleosides (squares) and to m3C (triangles). (E) Estimation of the KD-value for antibody m26A 60G3, using a binding model based on m26A (squares) or m6A nucleoside binding (triangles). (F) The binding model for α-Ψ antibody 27C8 was performed as described using Ψ nucleosides. For additional Scatchard plots and binding models for the antibody KD-values listed in Figure 2G, see Supplemental Figure 1. (G) Overview of the KD-values of the different tested antibody clones against m6A, m5C, Ψ, and m26A. The last row indicates the respective nucleoside that was used for estimating the KD-value.
“Dot blot” analysis
A common strategy to screen large numbers of hybridoma clones are western blots against antigens spotted onto nitrocellulose membranes—a method commonly referred to as “dot blots.” BSA-conjugated nucleosides or oligonucleotides were immobilized and incubated with the respective antibodies. Methylene blue staining served as loading control (Fig. 3A). Indeed, all tested clones (anti-m6A 9B7; anti-m5C 32E2; anti-Ψ 27C8 and anti-m26A 60G3) recognized their modified base epitopes while the unmodified base was not detected (Fig. 3B). To evaluate whether antibodies recognize modified bases in a more natural environment, we modified the dot blot approach and spotted modified and unmodified oligonucleotides onto a nylon membrane and incubated it with the antibodies (Fig. 3C). A widely used commercially available antibody against m6A (Synaptic Systems) was included for comparison. Although nylon membranes generated stronger antibody background, the tested hybridoma clones readily detected the modified but not the unmodified oligonucleotide with the exception of anti-Ψ 27C8, which did not work in this assay. This further underscores the need of different validation approaches to estimate antibody performance.
FIGURE 3.
Antibody specificity against base modifications using dot blot assays. (A) Scheme of the dot blot experimental setup. Unmodified/modified BSA conjugates or oligonucleotides (m5C or C as examples) were spotted on a nylon membrane. After cross-linking and blocking, the membrane was incubated with the primary and secondary antibodies. Methylene blue stains nucleic acids and was used as a loading control. (B) Dot blot experiments, for which modified and unmodified BSA-nucleoside conjugate was used to assess the specificity of the antibodies α-m6A 9B7, α-m5C 32E2, α-Ψ 27C8, and α-m26A 60G3. For m6A, also the m26A-BSA conjugate was spotted to investigate specificity between the two very similar modifications. (C) Dot blot experiments using a modified and a nonmodified 12-mer RNA oligonucleotide as negative control. The antibody staining is shown on the left and the methylene blue staining as a loading control on the right. The dot blots for the antibodies α-m6A 9B7 and Synaptic Systems, α-m5C 32E2, α-Ψ 27C8, and α-m26A 60G3, are depicted.
Determination of modified RNA enrichment
Although antibodies against RNA modifications are widely used in genome-wide profiling studies, it is often not known how specific they enrich modified RNAs compared to their unmodified counterparts adding uncertainty to many of these experiments. Therefore, we established two assays allowing for the determination of enrichment factors of modification-specific antibodies (Fig. 4). In a first approach, we used chemically synthesized oligonucleotides containing either modified or unmodified bases (m6A, m5C, Ψ, m26A). RNAs were 32P-labeled, immunoprecipitated using the modification-specific antibodies and enrichments of modified compared to unmodified oligonucleotides were determined (Fig. 4B–F). The α-m6A clone 9B7 enriched m6A-modified RNA by approximately fivefold and is highly specific for m6A (Fig. 4B, left panel). The widely used commercially available m6A-specific polyclonal antibody from Synaptic Systems showed moderate cross-reactivity with m5C and m26A in these experiments (Fig. 4B, right panel). Furthermore, we tested whether the affinity of the antibodies differ when m6A is within its natural RACH motif or when total RNA is added to simulate RIP experiments under realistic conditions (Supplemental Fig. 3A). Indeed, under these conditions, both the m6A-specific antibody 9B7 and the one from Synaptic Systems efficiently enriched modified RNA. The α-m5C clone 32E2 highly specifically enriched the m5C-modified RNA, other tested modifications were not recognized (Fig. 4C). m5C-specific antibodies from Diagenode or Cell Signaling only moderately enriched m5C-containing RNAs, most likely due to the fact that they were raised against modified DNA (Fig. 4D). A similar experiment was performed with the α-Ψ antibody 27C8 (Fig. 4E). The antibody enriched Ψ-containing RNA by approximately fourfold but seems to enrich m5C even more. It is therefore not specific enough and the use of this antibody is clearly limited. Finally, we analyzed the m26A-specific antibody clone 60G3 (Fig. 4F). This antibody enriches m26A-modified RNA eightfold and does not cross-react with any other modification that we tested. Of note, even the structurally highly similar m6A modification seems to be discriminated by this antibody.
FIGURE 4.
Qualitative enrichment of modified vs. nonmodified RNAs using quantitative immunoprecipitation and a thin-layer chromatography-based assay. (A) Schematic overview of the experimental setup. Modified (m5C as example) and unmodified or differently modified (depicted as X) chemically synthesized oligonucleotides of 12 nt in length were radioactively 5′ labeled. Oligonucleotides were subjected to immunoprecipitation using the indicated antibodies. After washing, the radioactive signals (cpm-values) of the immunoprecipitated RNAs as well as the input samples were measured using a scintillation detector (Cerenkov measurement), and enrichment factors were calculated. (B–F) Depiction of the enrichment of the antibodies α-m6A 9B7, polyclonal α-m6A (Synaptic Systems), α-m5C 32E2, α-m5C (Diagenode), α-m5C (Cell Signaling), α-Ψ 27C8, and α-m26A 60G3 by using the RNA-IP-based approach, shown in A. (B) Results for the m6A antibodies 9B7 and polyclonal Synaptic Systems, (C) the enrichment with antibody α-m5C 32E2, (D) enrichment of α-m5C from Diagenode or Cell Signaling, (E) of antibody clone Ψ 27C8, and (F) depicts the enrichment of the antibody α-m26A 60G3. Experiments were conducted in triplicate. (G) Workflow for enrichment factor using in vitro transcription (ivt), antibody enrichment, and thin layer chromatography (TLC). Ivt with modified (m5CTP as example) and radiolabeled NTPs and immunoprecipitation, the RNA was hydrolyzed with the nuclease P1 and analyzed by TLC. (H) Evaluation of m6A IP-TLC experiments as described above using 1% (upper panel) or 50% (lower panel) m6ATP for the ivt and analysis of the monoclonal m6A antibody clone 9B7 and the polyclonal m6A-antibody from Synaptic Systems. The light gray bars show the normalized signal intensities, corresponding to the precipitated unmodified RNA; the dark gray bars show the same for the precipitated modified RNAs. Experiments were conducted in triplicate. (I) Evaluation of m5C IP-TLC experiments using 1% (upper panel) and 50% (lower panel) m5CTP for the ivt and analysis of m5C antibody clone 32E2 and commercially available m5C-specific antibodies from Diagenode and Cell Signaling. Experiments were conducted in triplicate. (J) Determination of the enrichment factors of antibody α-Ψ 27C8 based on the RNA-IP and TLC experiments. For these experiments, 1% (upper panel) and 50% (lower panel) ΨTP was used for the ivt. TLCs and further evaluations of other antibodies that we tested can be found in Supplemental Figure 2.
In addition to the RIP experiments described above, we further developed a second approach to determine enrichment factors. In two in vitro transcription reactions, either the modified or the unmodified nucleotide was added (Slama et al. 2019). The modified RNA was transcribed in the presence of α-32P-UTP and the unmodified was labeled with α-32P-ATP. Both RNAs were mixed in different ratios and used for immunoprecipitation experiments using the modification-specific antibodies. After washing, the immunoprecipitated RNAs were fully hydrolyzed and the nucleotides were separated by thin layer chromatography (Fig. 4G). Since α-32P-UMP can be separated from α-32P-AMP, the ratio between the two signals can be quantified and used for calculating the enrichment factor of the modified compared to the unmodified RNA. Using this approach, we tested α-m6A 9B7, α-m5C 32E2 and α-Ψ 27C8, because these modified nucleotides can be incorporated by T7 polymerase-mediated in vitro transcription. We tested two different conditions with 1% or 50% modified nucleotides being added to the in vitro transcription reaction. The α-m6A 9B7 antibody enriched the modified RNA moderately by 1.5-fold (Fig. 4H), which is similar to the enrichment factor of 1.5 to twofold by the commercial antibody from Synaptic Systems (Fig. 4H, upper panel). When 50% modified RNA were used, up to eightfold enrichment is achieved compared to unmodified RNA (Fig. 4H, lower panel). We also tested two other α-m6A antibody clones (11D11 and 13G2) and both displayed similar enrichment factors (Supplemental Fig. 2A,B). We next tested α-m5C 32E2 and determined a specific enrichment of more than fourfold, when 1% of the RNA is modified (Fig. 4I, upper panel). Two commercial antibodies (Diagenode, Cell Signaling) show only mild enrichment. However, when 50% modified RNA was applied, all three tested antibodies enriched modified RNA (Fig. 4I, lower panel; Supplemental Fig. 2C,D). Finally, we analyzed anti-Ψ clone 27C8 and did not find a specific enrichment of modified versus unmodified RNA in both conditions (Fig. 4J; Supplemental Fig. 2E). This is consistent with the poor performance of the antibody in assays described before and demonstrates that our measurements are specific and reproducible in different approaches. In conclusion, our quantitative data allows for an estimation of antibody performance in solution reminiscent to conditions in genome-wide profiling experiments.
Binding to endogenous modified RNAs
Modification-specific antibodies are mainly used for RNA-profiling studies. Therefore, it is important to validate binding to endogenous RNAs carrying known modifications. To test this, we investigated whether the tested modification-specific antibodies generally bind to endogenous RNAs by cross-linking them to their RNA targets using UV light irradiation (Fig. 5A). Such an UV cross-linking step is for example crucial in miCLIP experiments (Linder et al. 2015). The anti-m6A antibody clone 9B7, the commercial polyclonal antibody (Synaptic Systems), m5C-specific clone 32E2, the m5C-specific commercial monoclonal antibody clone 33D3 (Diagenode), the anti-Ψ antibody clone 27C8 and m26A-specific clone 60G3 were incubated with total RNA isolated from HEK 293 cells and UV cross-linked. The RNA was partially digested, immunoprecipitates were radioactively labeled at the 5′ end and transferred onto a nitrocellulose membrane (Fig. 5B). Indeed, all antibodies tested except the m5C-specific antibody clone 33D3 (Diagenode) were efficiently cross-linked and immunoprecipitated, while no signals were observed in non-cross-linked control reactions indicating that the antibodies bind and are efficiently cross-linked to endogenous RNA targets. However, cross-linking efficiencies differ between the antibodies possibly reflecting different modification levels or antibody affinities.
FIGURE 5.
Immunoprecipitation of endogenous modified RNAs. (A) Overview of the workflow for cross-linking modification-specific antibodies to endogenous RNAs. Fragmented total RNA from HEK293T cells was cross-linked to the antibody of interest using 254 nm UV light. RNA was immunoprecipitated, radiolabeled, and the RNA-antibody complexes were analyzed by SDS-PAGE. As a negative control, a nonirradiated setup was used. (B) Autoradiographs of the cross-linking experiments described in A using m6A antibody clones 9B7, Synaptic Systems (I), m5C antibody clone 32E2 and the commercial antibody 33D3 (Diagenode) (II), α-Ψ antibody clone 27C8 (III) and m26A antibody clone 60G3 (IV). (C) Binding competition assays between endogenous RNAs and free nucleosides. Specific antibodies were used for immunoprecipitation from 2 µg total HEK293T cell RNA and 75 ng GFP mRNA as a negative control spike-in. To test for specificity, 100 µM of the respective modified (e.g., m5C) and unmodified nucleoside (e.g., C) were added to the immunoprecipitation as competitor. The immunoprecipitated RNA was separated on an RNA gel and blotted onto a nylon membrane. A probe against the 18S or 5.8S rRNA and a probe against the GFP mRNA were used for detection. (D) Northern blots for endogenous 18S and 5.8S rRNAs as well as GFP mRNA spike-ins. The antibodies α-m6A 9B7, α-m5C 32E2, α-Ψ 27C8, and α-m26A 60G3 were used. Input samples (200 ng total RNA and 75 ng GFP mRNA) are shown in lanes 1 and immunoprecipitates using an IgG control antibody used as a negative control in lanes 2. The upper panels show the signals of the 18S/5.8S rRNA; the lower ones for the spiked-in a GFP mRNA. Lanes 3 show signals for untreated IP experiments, lanes 4 depict the signals of an IP competed with modified and lanes 5 with unmodified nucleosides. (E) Competition titration experiment of antibodies α-m6A 9B7, α-m5C 32E2, and α-Ψ 27C8. Different concentrations ranging from 10 to 100 µM of modified and unmodified nucleosides were added to RNA-IP experiments. For detection, probes against 18S and 5.8S rRNA were used. (F) Nucleoside-mediated elution of antibody-bound endogenous 18S rRNA. (G) Northern blots of immunoprecipitation experiments using antibodies α-m6A 9B7 and α-m5C 32E2. RNA either extracted from beads (lanes 2–5) or from specifically eluted supernatants (lanes 6–9) were analyzed by northern blotting. Lane 1 shows the input of the immunoprecipitation experiments.
All four modifications, against which antibodies were established, are present in rRNA (Cecchini and Miassod 1979). Thus, we next asked whether these antibodies also specifically immunoprecipitate rRNA. Antibodies were coupled to beads and incubated with total RNA isolated from HeLa cells (Fig. 5C). For specificity control, in vitro transcribed GFP mRNA was added to the mixture. After washing, the immunoprecipitated RNA was analyzed by northern blotting using probes against 18S and 5.8S rRNA, respectively. Antibodies α-m6A 9B7, α-m5C 32E2, and α-m26A 60G3 efficiently immunoprecipitated 18S rRNA, while α-Ψ 27C8 enriched 5.8S rRNA albeit less efficient (Fig. 5D, lanes 3 of each panel). IgG subtype-specific control antibodies confirmed the specificity of the analyzed antibodies (Fig. 5D, lanes 2). These data highlight that endogenous target RNAs are bound by the tested antibodies and thus might be suitable for profiling experiments.
Since we found that the anti-m6A antibodies 9B7 and 19B7 were efficiently cross-linked to endogenous RNAs by UV irradiation (Fig. 5A,B), we performed miCLIP assays to determine m6A modification of endogenous mRNA. We defined a gene set of 17,158 peaks from MEF cells, which harbored at least one peak in each of the three replicates when performing miCLIP experiments with a commercial antibody (Abcam) (Supplemental Fig. 3B). These genes represented 65.6%, 75.5%, or 69.1% of the peaks in the three different replicates, indicating high reproducibility. We then analyzed whether our antibodies also pulled-down m6A targets enriched by the Abcam antibody. Performing miCLIP with either 9B7 or 19B7 antibodies revealed a similar distribution of peaks over individual mRNAs like Cdk9 as the commercial antibody and displayed a typical enrichment of signals in the 3′ UTR. In addition, C to T transition mutations occurred on the m6A DRACH motif on mRNAs like Slc2a1 for all three antibodies tested (Supplemental Fig. 3C,D). Moreover, about 5671 peaks were similarly identified by both of our newly generated anti-m6A antibodies, and this overlap represented 32.7% or 25.9% of peaks with one m6A peak in the samples generated with 9B7 or 19B7 antibodies (Supplemental Fig. 3C). Collectively, these data indicate that the monoclonal antibodies can be used for CLIP experiments to determine the targets and positions of m6A modifications.
Competition and elution experiments using modified and unmodified nucleosides
To further test for antibody specificity, competition experiments with the respective epitopes were performed. For α-m6A 9B7, α-m5C 32E2, and α-m26A 60G3, the modified nucleotide efficiently competed with 18S rRNA binding (Fig. 5D, lanes 4). Competition with Ψ, however, inhibited binding of α-Ψ 27C8 to 5.8S rRNA rather weakly (Fig. 5D). The unmodified nucleotides did not show any competition with α-m6A 9B7, α-m5C 32E2, and α-Ψ 27C8. For α-m26A 60G3, a mild reduction of 18S rRNA binding was observed when the unmodified nucleotide was added (Fig. 5D). Thus, these data suggest that except for α-Ψ 27C8, antibodies α-m6A 9B7, α-m5C 32E2, and α-m26A 60G3 bind their modified nucleotide targets specifically. Lower affinity and specificity of α-Ψ 27C8 is consistent with our previous results. To receive a more comprehensive picture of nucleotide competition and to assess optimal conditions for nucleoside-mediated elution, we performed titration experiments using nucleotide concentrations ranging from 10 nM to 100 µM and performed experiments as described above (Fig. 5E). Consistently, 18S rRNA binding was strongly reduced when a concentration of 1 µM modified nucleotide was added to the washing buffer. As expected, unmodified nucleotides did not inhibit binding (Fig. 5E). As observed in the experiments before, competition using α-Ψ 27C8 was less efficient and binding to the 18S rRNA was not completely lost even when 100 µM nucleotide concentrations are used (Fig. 5E).
To increase specificity in immunoprecipitation experiments, bound factors can be eluted by an excess of the antigen. To test whether this strategy is applicable to modification-specific antibodies as well, we immunoprecipitated the 18S rRNA from total RNA samples using α-m6A 9B7 and α-m5C 32E2 (Fig. 5F,G). We incubated the immunoprecipitation reactions with 250 and 500 µM modified nucleosides and analyzed the eluates by northern blotting. Indeed, an excess of both, m6A and m5C efficiently eluted the 18S rRNA from affinity beads, while the unmodified nucleosides had a much weaker or no effect (Fig. 5G, lanes 5, 9). Taken together, binding of α-m6A 9B7 and α-m5C 32E2 antibodies to 18S rRNA is efficiently competed by the respective modified nucleosides and bound RNAs can be efficiently eluted.
Detection of modified nucleic acids by immunofluorescence
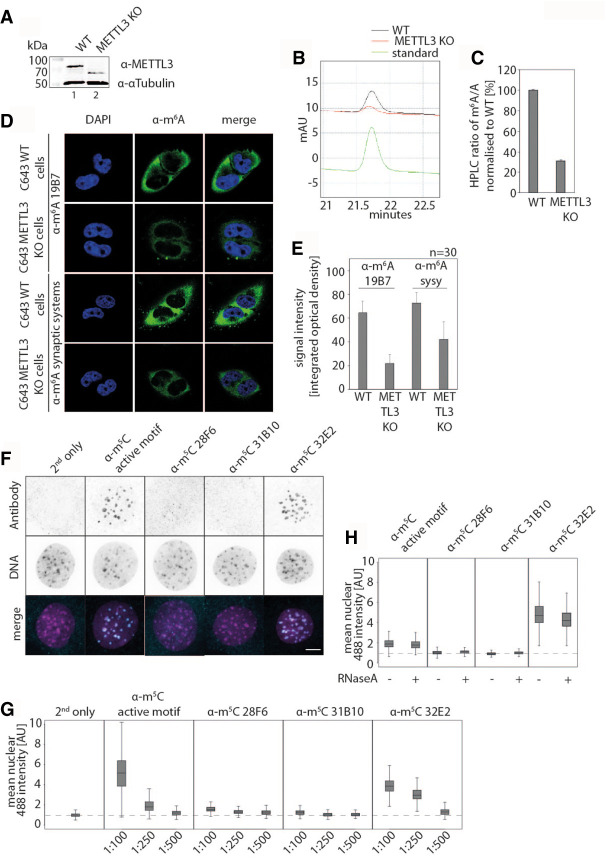
Antibodies are widely used for the visualization of intracellular localization patterns of their antigens. Therefore, we tested whether modification-specific antibodies are useful for immunofluorescence applications as well (Fig. 6). In order to assess signal specificity for anti-m6A antibodies, we knocked out METTL3, the enzyme that generates m6A on mRNAs, from C643 cells and compared the signals and wild-type (wt) cells. METTL3 knock out was confirmed by western blotting (Fig. 6A). As expected, full digestion of poly(A)-selected RNAs from these cells and subsequent identification of m6A nucleotides by HPLC analysis shows strong m6A reduction in METTL3 knock out cells (Fig. 6B,C). The remaining signal most likely originates from rRNA contaminations. Of note, a less abundant shorter band is detected on western blots, which could be a truncated METTL3 (Fig. 6A). However, since m6A levels are strongly reduced, we assume that such a putative truncated version is most likely nonfunctional. We next performed confocal microscopy-based immunofluorescence studies using the anti-m6A clones as well as the commercial anti-m6A antibody (Synaptic Systems) for comparison (Fig. 6D, clone 19B7 is shown as example). Both antibodies strongly stained the cytoplasm of wt C643 cells while nuclear signals were not detected. METTL3 knock out C643 cells, however, show a markedly reduced cytoplasmic signal suggesting that both antibodies recognize m6A-modified RNAs in immunofluorescence experiments. Noncoding RNAs such as rRNAs or snRNAs contain m6A modifications but these modifications are independent of METTL3 function and thus the signal was only reduced by ∼40% as revealed by signal intensity measurements (Fig. 6E). Using an alternative staining protocol optimized for staining chromatin-associated factors and modifications, the m6A clone 19B7 showed a specific cytoplasmic staining, while other clones did not show specific signals (Supplemental Fig. 4A–C).
FIGURE 6.
Immunofluorescence stainings of C643 wild-type (WT) and METTL3 knockout (KO) cell lines using α-m6A antibodies and immunofluorescences of MTF cells stained with α-m5C clone 32E2 detecting genomic m5C. (A) Western blot analysis of C643 WT and METTL3 KO cell lysates, probed with antibodies against METTL3 and α-Tubulin as a loading control. (B) Verification of m6A levels in the mRNA of C643 cells of WT compared to METTL3 KO by HPLC measurements. (C) Integrated values from B were normalized to WT and are given in percentages. (D) Immunofluorescences of WT and METTL3 KO cells. The cells were stained with α-m6A 19B7 (upper part) and polyclonal α-m6A Synaptic Systems (lower part). The DAPI staining (blue) shows the nuclei of the cells; in green, the m6A signals are shown. The right panel displays merged stainings. (E) Quantification of the signal intensities of the immunofluorescence staining of WT and METTL3 KO cells shown in A. For quantification, 30 cells each were analyzed. (F) Exemplary pseudo-colored maximum confocal Z-projections of RNaseA treated mouse tail fibroblasts (MTFs), stained with the indicated m5C antibodies diluted 1:250. An Alexa Fluor 488 conjugated secondary antibody was used to visualize the primary antibodies. DNA was stained with DAPI (scale bar, 10 µm). (G) Boxplots showing the normalized mean nuclear Alexa Fluor 488 intensities. Nuclear levels were normalized against cells stained without any primary antibody (“second only”). The outcome of three tested antibody dilutions, 1:100, 1:250, 1:500, is shown (n > 15,000). The dashed line indicates the median of “second only” cells. (H) Boxplots showing the normalized mean nuclear levels of Alexa Fluor 488 in MTF cells treated with or without RNaseA and stained with an antibody dilution of 1:250 (n > 40,000). Signal levels of Alexa Fluor 488 were normalized against control cells that were only stained with the secondary antibody. The dashed line indicates the median of cells, stained without primary antibody.
M5C is a very abundant modification on DNA and it is often found at promoter regions. It is enriched in distinct areas referred to as CpG islands and associated with epigenetic gene regulation processes (Jones 2012). We therefore tested whether α-m5C antibodies also recognize modified DNA in immunofluorescence stainings (Fig. 6F–H). Mouse-tail fibroblasts were permeabilized, treated with RNase A to test DNA specificity and stained with m5C-specific antibodies (Fig. 6F; Ludwig et al. 2017). Both, the widely used commercial monoclonal antibody 33D3 (Active Motif) and our clone 32E2 detected distinct nuclear foci, which colocalized with DAPI-dense DNA structures (Fig. 6F, middle and lower panels). These supra-chromosomal structures are termed chromocenters and correspond to clusters of constitutive pericentromeric heterochromatin that are composed by tandem repeats of m5C enriched major satellite DNA (Casas-Delucchi et al. 2012). These data indicate that both clones recognize m5C-modified DNA in immunostaining experiments. The clones α-m5C 28F6 and 31B10, however, did not show specific signals suggesting that these antibodies are RNA-specific or not sensitive enough to allow the detection of modified DNA in immunofluorescence studies. We further tested different antibody dilutions and quantified the nuclear signal relative to the negative control (Fig. 6G, dashed line, “second only”: secondary antibody only). The signal is lost when the antibody is linearly diluted suggesting an ideal dilution of 1:100 in such assays. Propidium iodide treatment, which stains not only DNA but also RNA showed a moderate reduction of the nuclear signal when RNase A treatment was performed. The mild reduction shows that a small portion of the nuclear nucleic acid is indeed RNA (Supplemental Fig. 4D). We next compared RNase A-treated with nontreated cells (Fig. 6H). A dilution of 1:250 was chosen to avoid unspecific signals due to high antibody concentrations. Consistently with our previous results, RNase A treatment does not affect nuclear staining indicating that the two α-m5C clones 33D3 (Active Motif) and 32E2 (this work) are DNA-specific under these experimental conditions. Since at least clone 32E2 detects efficiently modified RNA molecules, our results suggest that m5C-modified RNAs are mainly found in the cytoplasm of these cells.
Taken together, modification-specific antibodies can be used for immunofluorescence as well, as exemplified by a number of antibodies that we tested.
m6A detection in cell sorting experiments
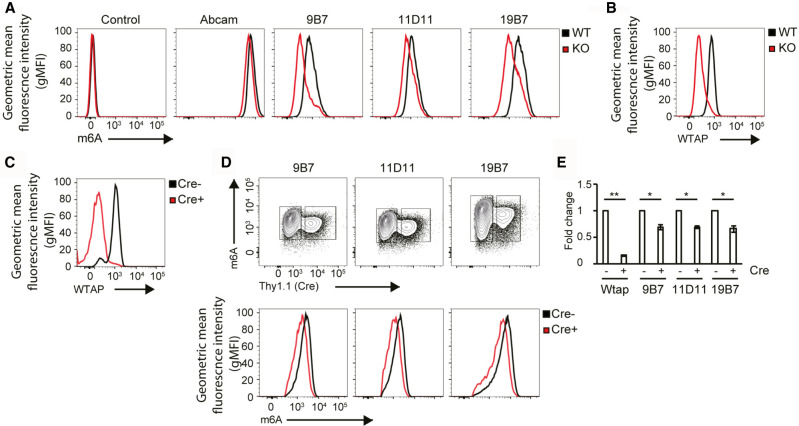
In previous experiments, we have provided methods to validate antibody performance in solution und thus we asked whether we could also use such antibodies for fluorescence-activated cell sorting (FACS) experiments. As an example, we used m6A-specific antibodies since knock out cell lines of the writer machinery are available and can be used for comparison (Fig. 7). Flow cytometry analysis of wt mouse embryonic fibroblasts (MEFs) revealed that m6A-modified RNA was robustly detected using anti-m6A antibody clones 9B7, 11D11 and 19B7, as they also determined decreased m6A abundance in Wtap-deficient MEF cells (Fig. 7A, red overlay). Wtap is an essential component of the m6A writer complex and its expression was indeed lost in knockout cells as assessed by FACS with anti-Wtap antibodies (Fig. 7B, red overlay). Of note, a commercially available antibody (Abcam) did not distinguish between wt and WTAP knock out cells. To rule out clonal variation that may arise during drug-selection of MEF cell lines, we investigated whether these antibodies also specifically recognized m6A-modified RNAs after acute depletion of Wtap. As shown in Figure 7C, the expression of Wtap was drastically reduced after retroviral transduction expressing the Cre recombinase, marked by coexpression of Thy1.1 in Wtapflox/flox MEF cells (Fig. 7C). Consistently, all monoclonal antibodies recognized the difference of m6A levels between Thy1.1+ (i.e., Cre-expressing, Wtap-deficient cells) and the Thy1.1− (WT cells) (Fig. 7D,E). In summary, base modification-specific antibodies can in general be used for FACS experiments.
FIGURE 7.
(A) Flow cytometry comparison of MEF cells stained with purified anti-m6A-specific rat monoclonal antibodies or polyclonal commercial anti-m6A antibodies (Abcam). Staining with a secondary anti-rat antibody served as a negative control. Data are representative of three independent experiments. (B,C) Deletion of Wtap in Wtap−/− MEF cells after puromycin selection (B) or acute deletion via Cre transduction of MEF cells at Day 4 (C) was confirmed via anti-Wtap staining. (D,E) Flow cytometry analysis of m6A and Thy1.1 (Cre) in Cre-transduced MEF cells Day 7 is shown as contour plot (D, upper panel) indicating the gates used to overlay m6A levels that are present in the Thy1.1 positive (D, lower panel, red) and Thy1.1 negative (D, lower panel, black) cells. (E) The fold change of the geometric mean fluorescence intensity (gMFI) of Wtap (C) or m6A (D, lower panel) is shown.
DISCUSSION
Base-specific antibodies are widely used for the detection of modified RNAs and have been vital for the tremendous progress made in the analysis of m6A functions. The success of m6A-specific antibodies fostered the development of antibodies against other modifications. However, such modifications appear to be much less abundant on mRNAs and the used antibodies are often only partially validated. Therefore, not always reliable and sometimes even conflicting results were produced. These developments contributed to the view that antibody-based profiling of RNA modifications might be generally error-prone (Helm et al. 2019). Moreover, antibody-independent approaches have been developed for individual modifications and these methods also produced distinct results. For example, bisulfite-sequencing approaches were performed for m5C modifications and led to very different results with mRNA targets ranging from very few to thousands (Squires et al. 2012; Edelheit et al. 2013; Hussain et al. 2013; Amort et al. 2017; Legrand et al. 2017; Huang et al. 2019). In addition, bulky chemical adducts are linked to specific modifications acting as roadblocks in PCR reactions and thus defining the position of the modification. For example, CMC-addition to Ψ has been developed but highly varying data sets were reported (Zaringhalam and Papavasiliou 2016; Zhou et al. 2018). To validate these results, independent methods are required and therefore we set out to provide example strategies and methods for characterizing modification-specific antibodies. We established monoclonal antibodies that specifically bind m6A, m26A, m5C, and Ψ modifications to obtain larger amounts of antibodies for testing since commercial antibodies are rather cost-intensive and could therefore not be included in all experiments.
A main shortcoming of available antibodies is that affinities and enrichment factors are often unknown. We therefore developed a method to define these key numbers. We found that all antibody clones, except antibodies against Ψ, bound free nucleosides in the low µM range (Fig. 2G). However, it is important to note that these numbers might differ in the context of longer RNA molecules. Since the antibodies discriminate between modified and unmodified bases, it is likely that bases will also be efficiently bound when embedded into larger RNAs but affinities might differ. Another informative number for estimating antibody performance is the enrichment factor indicating “fold enrichment” of modified over unmodified RNAs (Fig. 4). In two different assays, we found that a commercially available antibody directed against m6A (Synaptic Systems) as well as antibody clones developed in our laboratory enrich modified RNAs between 1.5- and sixfold. Our m5C-specific antibody enriched RNA fourfold in a TLC-based assay (Fig. 4I) and more than 100-fold in an independent RNA-IP-based approach with chemically synthesized RNA oligonucleotides (Fig. 4D). Of note, commercial antibodies enriched m5C-modified RNA only marginally. These and other antibodies were mainly generated to detected m5C in DNA. Thus, they may detect additional structural aspects of double stranded DNA, which are not present in short single-stranded RNA fragments. Nevertheless, the α-m5C antibody clone 32E2 also recognized DNA in immunofluorescence staining experiments (Fig. 6F) while the other established α-m5C antibody clones did not stain DNA foci. In summary, for obtaining solid and reproducible results, it is important that affinities and efficiencies are considered when antibodies are combined with RNA-seq approaches.
Immunofluorescence staining with m6A antibodies detected m6A RNAs in the cytoplasm and the signal was reduced in METTL3 knock out cells (Fig. 6A–E). Since m6A modifications on noncoding RNAs such as rRNAs or snRNAs are independent of METTL3, such a pattern is expected. However, it was unexpected that no nuclear signal was detected (Fig. 6D) because the nuclear U6 snRNA, for example, carries one m6A modification (Bohnsack and Sloan 2018). Furthermore, several YTH domain reader proteins are localized to the nucleus suggesting nuclear m6A-modified RNAs are also present in this cellular compartment. Several scenarios explaining the lack of nuclear m6A signals can be envisioned. First, the YTH domain covers the m6A base and thus the epitope is not accessible for antibodies. Second, nuclear RNAs containing m6A modifications are rather unstable and therefore of low abundance and not visible since m6A might tag for rapid degradation. Third, the modified U6 snRNA could be deeply buried into the spliceosome and again the single modified base might not be accessible for antibodies. It is further conceivable that these aspects are also relevant for the cytoplasmic signal and the actual level of modified RNA in the cytoplasm might be even higher.
In summary, we generated and thoroughly validated a number of monoclonal antibodies. The antibody clones 9B7 (m6A), 32E2 (m5C), and 60G3 (m26A) are suitable for various applications such as RIP, immunofluorescence approaches, CLIP and FACS. However, we also emphasize that each individual base-specific antibody needs to be optimized regarding the experimental conditions and it is difficult to generalize such conditions in a single protocol. Concentration, dilution, salt conditions are very important and each antibody has to be optimized for the individual experimental settings. We strongly recommend that antibody conditions and performance in a number of different assays as exemplified here, should be assessed prior to usage and this should become a prerequisite rather than an option for publication.
MATERIALS AND METHODS
Coupling of the nucleosides to ovalbumin
The coupling protocol of nucleosides to ovalbumin is based on the method described by (Erlanger and Beiser 1964). An amount of 25 mg of the nucleoside was dissolved in 1.25 mL 0.1 M NaIO4 and incubated for 20 min at room temperature. An amount of 75 µL 1 M ethylene glycol was added and incubated for another 5 min at room temperature. An amount of 50 mg of ovalbumin was dissolved in 5 mL H2O. The pH was adjusted to 9 using a 5% K2CO3 solution. After adding the oxidized nucleoside to the ovalbumin solution, the mixture was stirred for 45 min, keeping the pH constantly at 9. A freshly prepared reduction solution (75 mg NaBH4/5 mL H2O) was then added to the conjugate and incubated overnight at room temperature. Using 1 M formic acid, the pH was reduced to 5–6 and incubated for another hour at room temperature. After adjusting the pH to 8.5 using 1 M NH3, the solution was gel filtrated on a Superose 12 column (GE Healthcare) in 0.2 M ammonium formate, pH 8.5. For analysis, the absorption of a defined amount of the conjugate was measured via UV spectroscopy. The molar ratio of bound nucleoside per carrier protein was estimated by measuring the absorbance of the conjugate at 5 different wavelengths (250, 260, 270, 280, and 290 nm) and fitting the measured data to the corresponding calculated absorption values. The spectrum of the conjugate was assumed to be the sum of nucleoside and carrier protein spectra. The absorbance was calculated by the extinction coefficients at the different wavelengths and the composition of the constituents. To obtain the “best fit”-composition of nucleoside and carrier protein, a grid search with a resolution of 0.1 µg was conducted using the sum-of-squares of the differences between measured and calculated absorption values as fit indicator.
Generation of monoclonal antibodies recognizing m5C, m6A, and pseudouridine (ψ) and ELISA screening
Approximately 50 µg of modified nucleobases coupled to ovalbumin (OVA) were dissolved in PBS, emulsified in an equal volume of incomplete Freund's adjuvant (Sigma) supplemented with 5 nmol CpG oligonucleotides (TIB Molbiol, Berlin), and injected both intraperitoneally (i.p.) and subcutaneously (s.c.) into Wistar rats and C57BL/6N mice. After 6 to 8 wk, a boost with 50 µg of nucleobase-conjugated OVA without Freund's adjuvant was given 3 d before fusion. Fusion of the myeloma cell line P3X63-Ag8.653 with splenic B cells of immunized rat or mouse was performed according to standard procedures (Kohler and Milstein 1975). P3X63-Ag8.653 cells were cultured at 37°C in a humidified 5% CO2 incubator in standard medium RPMI 1640 (Sigma/GIBCO), supplemented with 1% glutamine, 1% nonessential amino acids, 1% sodium pyruvate, 1% penicillin/streptomycin (Sigma), and 2.5% FCS (PAN). Hybridoma cells were cultured in standard RPMI 1640 medium supplemented with 20% FCS and 2% HT supplement (Life Technologies). Hybridoma supernatants were positively tested in a solid-phase enzyme-linked immunoassay (ELISA) using the corresponding modified nucleobase coupled to BSA and negatively tested on nonmodified nucleobase also coupled to BSA. To identify m6A-specific hybridoma clones, 96-well polystyrene plates were coated with m6A-conjugated BSA overnight at room temperature (m6A-BSA: 2.5 µg/mL). In parallel, 96-well plates were coated with m26A and A (conc.: 2.5 µg/mL). To identify m5C-specific antibodies, screening plates were coated with m5C (positive screen) and C (negative screen), for screening plates were coated with Ψ (positive screen) or C and U (negative screens). After coating, plates were washed once with PBS, unbound sites were blocked with 2% FCS in PBS for 20 min. After washing off unbound nucleobase BSA-conjugates, hybridoma supernatants (1:10 diluted) were added and incubated for 30 min. After another wash with PBS, plates were incubated for 30 min with a mix of HRP-coupled subclass-specific mouse anti-rat or rat anti-mouse secondary antibodies. After five washes with PBS, TMB substrate (1 Step Ultra TMB-ELISA; ThermoFisher Scientific Inc.) was added and the absorbance was measured at 650 nm with a microplate reader (Tecan). To determine the specific antibody subclasses in a second validation after expansion of positive clones, respective BSA-nucleobase conjugates were coated onto 96-well polystyrene plates as described above, incubated with the hybridoma supernatants and then detected with HRP-coupled monoclonal mouse or rat antibodies specific for the different IgG subclasses of rat or mouse, respectively. Selected hybridoma cells of positively tested supernatants specific for m6A (13G2 rat IgG2a, 11D11 rat IgG1, 9B7 rat IgG1), m5C (31B10 mouse IgG1/λ, 28F6 mouse IgG2b, 23E2 mouse IgG2c/λ), m26A (60G3 rat IgG2a), and Ψ (26H5 mouse igG2b, 27C8 mouse IgG2b) were cloned at least twice by limiting dilution.
Capture ELISA
Subclass-specific antibodies were coated onto microplates (200 mM Na2CO2 pH 9.5, 12 h, at 4°C). The following concentrations of subclass-specific antibodies were used: mouse IgG1 and IgG2a: 3 µg/mL; mouse Ig2b and rat IgG1: 5 µg/mL; rat IgG2a: 20 µg/mL. The plates were blocked with 2% FCS in PBS. Hybridoma supernatants were incubated for 30 min at room temperature. After washing with PBS, the respective OVA-conjugated modified nucleotide was added at a concentration of 2.5 µg/mL and incubated for 30 min at room temperature. After incubation with a HRP-conjugated polyclonal ovalbumin-specific antibody and several PBS washes, TMB substrate was added and absorbance was detected with a microplate reader (Tecan).
Determination of the KD of the antibodies
For the determination of the antibody bound fraction (BF) of a modified nucleoside, six samples of an equimolar mixture of the modified nucleoside with an internal standard (mostly the unmodified nucleoside) were prepared at six different concentrations (concentrations ranged from 75 to 250 µM). In order to maintain a constant ratio between the amount of the modified nucleoside and the internal standard, the dilutions were prepared out of a premixed stock solution with a concentration of 0.5 mM for each nucleoside. A volume of 20 µL of each of these mixtures were pipetted to 100 µL PBS (=Input sample) and to 100 µL PBS containing exactly the same amount of antibody for each sample (∼150 µg) leading to initial nucleoside concentrations in the range of 12.5 to 41.7 µM. After incubation of the mixtures for 2 h at 4°C, the unbound nucleosides of the antibody-containing samples were separated by centrifugation (2 min, 14,000g) using a 10 kDa cut-off spin filter (Roti-Spin MINI, Roth). A volume of 40 µL of each input and filtrate-sample was then applied to the HPLC with one replicate (see HPLC analysis of nucleosides). The two peaks of each chromatogram were integrated. The peak area of the modified nucleoside normalized to the peak area of the internal standard eventually gave the normalized peak areas of the modified nucleoside in the input sample (nucleoside input, NI) and in the filtrate of the antibody sample (nucleoside-antibody, NA) at the various concentrations. The antibody-bound fraction (BF) of the modified nucleoside can then be calculated by: BF = (NI − NA)/NI, which can be used to derive the concentrations of bound ([AbN]) and free nucleoside ([N]) from the initial nucleoside concentration ([N0]): [AbN] = BF × [N0] and [N] = (1 − BF) × [N0]. To get a first estimation of the values for KD and the maximal concentration of binding sites of the antibody ([Bmax]), the ratio [AbN]/[N] was plotted against [AbN] to obtain a Scatchard plot (Scatchard 1949). From this, the KD (dissociation constant) was estimated using the negative reciprocal value of the slope of the resulting regression line. The maximal concentration of binding sites is represented by the intersection point of the regression line with the x-axis. These estimates were then used to describe the binding with the following model:
To enhance the accuracy of the model-parameters, the measured data points were fitted with nonlinear regression, whereby the residual sum-of-squares between model and measured data points was minimized using Excel solver. Finally, the 95%-confidence limits of the model parameters were determined via the model comparison-approach (F-test).
HPLC analysis of nucleosides
The nucleosides were resolved on a Hypercarb-column (5 µm, 100 × 2.1; Thermo Scientific) using the HPLC-system “WellChrom” from Knauer, equipped with Pump K-1001, Diode Array Detector K-2800, column oven and a Vacuum Degasser from Techlab GmbH (Germany). The experiments were done, detecting at wavelengths ranging from 260 to 280 nm. The resulting chromatograms were analyzed with the software ChromGate Client/Server Vers. 3.1.7. Depending on the particular nucleosides, three different gradients (purinfast2, pyrimidinfast2, pyrimidin50%ACN) of the buffers A (50 mM NH4CH3CO2, pH 5.0), B (20% 50 mM NH4CH3CO2, pH 5.0/80% acetonitrile) and C (50% acetonitrile) were applied at different temperatures (25°C, 55°C) and flow rates (0.2 mL/min, 0.25 mL/min). For details see the gradient tables in the Supplemental Information. For quantifying the percentage changes of the nucleoside concentrations between different samples, an equimolar amount of an internal standard (mostly the unmodified nucleoside) was added to the solution of the modified nucleoside. The peak area of the modified nucleoside was then normalized to the internal standard to correct for loading errors and/or unspecific binding during processing.
Dot blot
A total of 8 µg of RNA-oligo (Dharmacon) were spotted on a nylon membrane. The RNA was EDC [1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride] cross-linked to the membrane. When using BSA-nucleotide conjugates, 20 µg was spotted, and the membrane was baked at 80°C for 1 h to cross-link. In both cases, the membrane was blocked in 1× TBS-T (150 mM NaCl, 10 mM Tris, pH 8.0, 0.1% Tween) containing 5% skimmed milk for 1 h at 4°C. The first antibody (hybridoma) was diluted 1:5 in a 5% skimmed milk solution in TBS-T and incubated overnight at 4°C, shaking. The secondary antibody (α-mouse and α-rat [Licor]) was diluted 1:10,000 in TBS-T containing 5% skimmed milk and incubated for 1 h. The documentation was conducted using the Odyssey scanner system (LI-COR Biosciences).
RNA immunoprecipitation
Total RNA from HEK293T cells was isolated using TRIzol (Thermo Fisher Scientific). Several titration experiments have been conducted for the optimization of the RNA immunoprecipitations. Thus, wide ranges of RNA, antibody and buffer concentrations and amounts are given in this protocol. Two different protocol setups for the RNA immunoprecipitations were tested for these antibodies. For a part of the experiments, 0.1–100 µg of the respective purified antibody were coupled overnight at 4°C to 35 µL Protein G Sepharose beads (GE Healthcare). The coupled beads were washed thrice in RNA-IP buffer (150–750 mM LiCl, 0.5% NP-40, 10 mM Tris-HCl, pH 7.5) or NET buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 5 mM EDTA, 0.5% NP-40, 10% glycerol). Using 0.1–100 µg of the total RNA (∼1 µg/µL) or 0.5–1 nmol of the RNA-oligos (ACGCGUm6ACUUGA, ACGCGUAm5CUUGA, AGCCUACCΨACUCAG, AGCCUACCm26ACUCAG; Dharmacon), the immunoprecipitation was conducted for 2 h in 0.5–1 mL RNA-IP buffer. For the other setup, the antibody was incubated with the RNA in RNA-IP buffer for 2 h. Protein G Sepharose beads were added and incubated for an additional 2 h. For the nucleotide-competition assay, 5 µM–5 mM nucleotide (end concentration) was added and incubated for one additional hour. The setups were then washed once each with RNA-IP buffer, wash buffer I (RNA-IP buffer with 300–1000 mM LiCl) and wash buffer II (RNA-IP buffer with 450–1500 mM LiCl) or with NET buffer and twice with NET wash buffer (NET buffer with an additional 150 mM NaCl). For isolation of the RNA from the beads, either TRIzol was used or the immune precipitated RNA was eluted from the beads by adding 250–500 µM nucleoside in 150 µL RIP buffer to the beads and incubating another 1 h at 4°C, shaking. The RNA was precipitated using TRIzol afterwards.
In vitro transcription with 32P-labeled and modified NTPs, immune precipitation, and digestion
DNA oligos with different lengths (880, 100, and 50 bp) were in vitro transcribed in four different setups. For the first two transcriptions, modified NTPs of interest in different ratios of the total NTPs were used. In one of them, additional 32P-UTP was added. The control setups were transcribed using unmodified NTPs and for the radioactive sample, 32P-ATP was used. The four different samples were then DNase-digested and purified using the MEGAclear Transcription Clean-Up Kit (Ambion). For the cold samples, the concentration was determined using a NanoDrop Photometer, for the hot samples, the cpm-values were determined using the Cerenkov setting at a Scintillation-counter. The different RNA-solutions were then mixed to obtain equal cpm-values as well as same amounts (1.25–8 µg) for the modified and unmodified samples. These setups were then used for immune precipitation (for protocol: see RNA Immunoprecipitation). The precipitated RNA was digested using Nuclease P1 overnight at 37°C. The single nucleotides were afterwards analyzed via 1D thin layer chromatography.
Thin layer chromatography (TLC)
The digestion with nuclease P1 and the TLC was conducted as described earlier (e.g., Grosjean et al. 2004). The digested RNA was spotted on a TLC-plate which ran in 66% isobutyric acid and 1% conc. ammonia for 3–4 h. After drying, the signals were detected by exposure to a screen and scanning using a Phospho-imager (PMI, Bio-Rad).
Northern blot
Northern blot analysis was carried out as described earlier (Pall and Hamilton 2008). Briefly, the RNA was complemented with a 2× RNA loading dye (formamide with bromophenol blue and xylene blue) and separated on 6%–12% Urea gels (Rotiphorese, Roth) and semi-dry blotted onto a nylon membrane. The RNA was then either EDC cross-linked to the membrane and/or cross-linked via UV, depending on the size of the RNA of interest and hybridized overnight at 50°C. 32P-labeled 5′-GACGCTCAGACAGGCGTAGCCC-3′ was used for 5.8 S rRNA and 5′-CATGCATGGCTTAATCTTTGAGACAAGC-3′ for 18 S rRNA detection. To detect GFP mRNA, a probe with the following sequence was used: 5′-CCTTGAAGAAGATGGTGC-3′. The blot was then washed twice with wash buffer I (5× SSC, 1% SDS) and once with wash buffer II (1× SSC, 1% SDS). Signals were detected and documented using a Phospho-imager (PMI, Bio-Rad).
miCLIP analysis
The miCLIP experiments were executed largely following the protocol of (Grozhik et al. 2017). The fragmentation of the total RNA was performed with ZnCl2 at 94°C for 5 min as it is described in (Dominissini et al. 2013). The fragmented total RNA was incubated with the antibody of interest for 2 h, rotating and afterwards UV-cross-linked, using 254 nm and 150 mJ/cm2. Using protein G dynabeads (Invitrogen), the cross-linked RNA was precipitated for 2 h while rotating. After several washing steps, the RNA, attached to the antibody and beads was dephosphorylated and 5′-radiolabeled with γ-32P-ATP. The beads were then resuspended in SDS-loading dye for elution. After SDS-PAGE, the gel was wet-blotted onto a nitrocellulose membrane at 90 V for 90 min. The radioactive signals were then detected using a Phospho-imager (PMI, Bio-Rad).
Western blot analysis
For performing western blot analysis, proteins were transferred onto a nitrocellulose membrane (GE Healthcare) using Towbin blotting buffer (192 mM glycine, 25 mM Tris/HCl pH 8.6, 20% methanol). Membranes were blocked in 1× TBST (150 mM NaCl, 10 mM Tris/HCl pH 8, 0.1% Tween 20) containing 5% skimmed milk for 1 h at 4 °C. After incubation with first and secondary antibody, the membrane was washed three times with 1× TBST. α-αTubulin (mouse, Sigma, clone DM1A) and α-METTL3 (rabbit, Proteintech Europe) were used as primary and α-mouse and α-rabbit (Licor) were used as secondary antibodies. The documentation was conducted using the Odyssey scanner system (LI-COR Biosciences).
Immunofluorescence staining of human cells
C643 wild-type (WT) and METTL3 knockout (KO) cells were seeded on coverslips. The immunofluorescence experiments were conducted as described previously (Schraivogel et al. 2015). In short, fixation was performed, using 3.7% PFA in PBS and stopped with 7.5 mg/mL glycine in PBS. Permeabilization was done with 0.2% Triton-X 100 in PBS. After blocking in 1% BSA in PBS with 0.05% Triton X-100, the first and secondary antibodies were incubated in blocking buffer. After incubation with the antibodies, cells were mounted using Prolong Gold containing DAPI (Thermo Fisher Scientific–Life Technologies). Confocal microscopy was done on a TCSSP8 (Leica Microsystems) equipped with acousto-optical beam splitter, 405 nm laser (for DAPI) and argon laser (488 nm for α-rat and α-rabbit Alexa 488 [Invitrogen]). Signal intensity was quantified using ImageJ (Wayne Rasband, NIH).
Immunostaining and fluorescence of MTF cells
Mouse tail fibroblasts MTF-line3 (Guy et al. 2001) were seeded on gelatin coated glass coverslips 1 d prior immunostaining. To test the ability of the different antibody clones to detect genomic 5-methylcytosine, a previously described immunofluorescence staining protocol (Ludwig et al. 2017) was used. In brief, cells were fixed for 10 min with 3.7% formaldehyde, permeabilized for 20 min with 0.5% Triton X-100 and incubated with ice-cold methanol for 5 min. Afterwards, cells were incubated with or without 10 µg/mL RNaseA and blocked in 0.2% fish skin gelatin each for 30 min at 37°C. Cells were incubated with the primary antibodies for 70 min at 37°C. The primary antibody mix contained 2% BSA, 1× DNase I Buffer (10 mM Tris-HCl, 2.5 mM MgCl2, 0.5 mM CaCl2), 25 U/mL DNase I (Sigma Aldrich) and the respective mouse anti-m5C clones, 28F6, 31B10, and 32E2 or the commercially available clone 33D3 (Active Motif). All clones were tested in dilutions of 1:100, 1:250 and 1:500 with an assumed concentration of 1 µg/µL. As control, samples were incubated with primary antibody mix without antibody (“2° only”). After incubation with the primary antibody, cells were washed three times with PBS-TE (0.01% Tween, 1 mM EDTA) and incubated with the secondary Alexa Fluor 488 conjugated goat anti-mouse-IgG antibody (1:250, The Jackson Laboratory) for 45 min at room-temperature. After washing with PBS-T (0.01% Tween), cells were counterstained with DAPI (1 µg/mL, Sigma Aldrich) and mounted in Moviol (Sigma Aldrich). To control for RNaseA activity, cells were treated as described for the immunostaining, with the difference that all antibodies in the mix were omitted and instead of DAPI, cells were stained with propidium iodide (1 µg/mL). The respective signals were quantified by imaging cells with the Operetta high-content screening system (PerkinElmer), equipped with a Xenon fiber optic light source, a 20× long/0.45 NA objective, and 360–400, 460–490, and 520–550 nm excitation—as well as 410–480, 500–550, and 560-630 emission filters. Nuclei were identified by their DAPI or propidium iodide signal and in DAPI and m5C stained nuclei the respective Alexa Fluor 488 signal was calculated. Intensities of stained nuclei were normalized by dividing all values by the mean nuclear Alexa Fluor 488 intensity of the “2° only” control. Normalized nuclear Alexa Fluor 488 values and mean nuclear propidium iodide values were plotted using R Studio. Also, statistical significance was tested with R Studio. Confocal Z stacks of immunostained cells were acquired with an Ultra-View VoX spinning disc microscope (PerkinElmer, UK) controlled by Volocity 6.3 (PerkinElmer) and equipped with a 60×/1.45 NA Planapochromat oil immersion objective (voxel size, 0.12 × 0.12 × 0.5 µm; Nikon) and a cooled 14-bit CCD camera (cat. no. C9100-50, Hamamatsu Photonics K.K.). Z-stacks were assembled into maximum Z-projections using ImageJ.
Cell culture of MEF cells
Mouse embryonic fibroblast (MEF) cells were cultured in Dulbecco's modified Eagle's medium (DMEM, GIBCO) supplemented with 10% (v/v) fetal bovine serum (FBS), 1000 U/mL penicillin–streptomycin (GIBCO), and 10 mM HEPES, pH 7.4 (GIBCO) at 37°C in 10% CO2. To establish WTAP−/− MEF cells, retrovirus (mock or Cre) infection of MEFs was performed as described previously. Two days after virus infection, cells were diluted following trypsination and cultured in the presence of puromycin (1 μg/mL) for an dditional 3 d to select infected cell populations.
FACS sample preparation and analysis
After harvesting MEF WT and WTAP KO cells, they were washed with PBS. Cells were fixed with of 2% Formaldehyde in PBS for 15 min at RT. Subsequently, the cells were permeabilized with 0.5% saponin buffer in PBS. The first antibody was applied for 90 min at RT in 0.5% Saponin buffer in PBS. Along the generated α-m6A antibody clones, the α-m6A antibody from Abcam (ab151230) was applied. After washing in PBS, the cells were incubated for 30 min at RT with the second antibody (Alex647-conjugated goat α-rat IgG, BioLegend, poly4054 or rabbit IgG, Invitrogen) in 0.5% Saponin buffer in PBS. For detection of the biotinylated antibodies, APC-conjugated streptavidin was applied. To stain WTAP, cells were fixed and permeabilized by using the Foxp3/Transcription Factor staining buffer set (eBioscience) according to manufacturer's instructions. Anti-WTAP antibody (Proteintech, 60188) and FITC-conjugated goat antibody α-mouse IgG (BD Biosciences) were applied. After staining, cells were acquired on a FACS Canto II (BD Biosciences) device and samples were analyzed with FlowJo software.
SUPPLEMENTAL MATERIAL
Supplemental material is available for this article.
ACKNOWLEDGMENTS
We thank S. Ammon and C. Friederich for technical support. This work was supported by the Deutsche Forschungsgemeinschaft (DFG) grant SPP 1784/1 and 2 and the Bavarian Systems-Biology Network (BioSysNet) to G.M.; and by DFG grant CA 198/10-1 to M.C.C.
Footnotes
Article is online at http://www.rnajournal.org/cgi/doi/10.1261/rna.076026.120.
REFERENCES
- Amort T, Rieder D, Wille A, Khokhlova-Cubberley D, Riml C, Trixl L, Jia XY, Micura R, Lusser A. 2017. Distinct 5-methylcytosine profiles in poly(A) RNA from mouse embryonic stem cells and brain. Genome Biol 18: 1 10.1186/s13059-016-1139-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista PJ, Molinie B, Wang J, Qu K, Zhang J, Li L, Bouley DM, Lujan E, Haddad B, Daneshvar K, et al. 2014. m6A RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell 15: 707–719. 10.1016/j.stem.2014.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergquist PL, Matthews RE. 1962. Occurrence and distribution of methylated purines in the ribonucleic acids of subcellular fractions. Biochem J 85: 305–313. 10.1042/bj0850305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnsack MT, Sloan KE. 2018. Modifications in small nuclear RNAs and their roles in spliceosome assembly and function. Biol Chem 399: 1265–1276. 10.1515/hsz-2018-0205 [DOI] [PubMed] [Google Scholar]
- Bringmann P, Luhrmann R. 1987. Antibodies specific for N6-methyladenosine react with intact snRNPs U2 and U4/U6. FEBS Lett 213: 309–315. 10.1016/0014-5793(87)81512-0 [DOI] [PubMed] [Google Scholar]
- Carlile TM, Rojas-Duran MF, Zinshteyn B, Shin H, Bartoli KM, Gilbert WV. 2014. Pseudouridine profiling reveals regulated mRNA pseudouridylation in yeast and human cells. Nature 515: 143–146. 10.1038/nature13802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas-Delucchi CS, van Bemmel JG, Haase S, Herce HD, Nowak D, Meilinger D, Stear JH, Leonhardt H, Cardoso MC. 2012. Histone hypoacetylation is required to maintain late replication timing of constitutive heterochromatin. Nucleic Acids Res 40: 159–169. 10.1093/nar/gkr723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchini JP, Miassod R. 1979. Studies on the methylation of cytoplasmic ribosomal RNA from cultured higher plant cells. Eur J Biochem 98: 203–214. 10.1111/j.1432-1033.1979.tb13178.x [DOI] [PubMed] [Google Scholar]
- Coots RA, Liu XM, Mao Y, Dong L, Zhou J, Wan J, Zhang X, Qian SB. 2017. m6A facilitates eIF4F-independent mRNA translation. Mol Cell 68: 504–514 e507. 10.1016/j.molcel.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K, Jacob-Hirsch J, Amariglio N, Kupiec M, et al. 2012. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 485: 201–206. 10.1038/nature11112 [DOI] [PubMed] [Google Scholar]
- Dominissini D, Moshitch-Moshkovitz S, Salmon-Divon M, Amariglio N, Rechavi G. 2013. Transcriptome-wide mapping of N6-methyladenosine by m6A-seq based on immunocapturing and massively parallel sequencing. Nat Protoc 8: 176–189. 10.1038/nprot.2012.148 [DOI] [PubMed] [Google Scholar]
- Edelheit S, Schwartz S, Mumbach MR, Wurtzel O, Sorek R. 2013. Transcriptome-wide mapping of 5-methylcytidine RNA modifications in bacteria, archaea, and yeast reveals m5C within archaeal mRNAs. PLoS Genet 9: e1003602 10.1371/journal.pgen.1003602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlanger BF, Beiser SM. 1964. Antibodies specific for ribonucleosides and ribonucleotides and their reaction with DNA. Proc Natl Acad Sci 52: 68–74. 10.1073/pnas.52.1.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feederle R, Schepers A. 2017. Antibodies specific for nucleic acid modifications. RNA Biol 14: 1089–1098. 10.1080/15476286.2017.1295905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Dominissini D, Rechavi G, He C. 2014. Gene expression regulation mediated through reversible m6A RNA methylation. Nat Rev Genet 15: 293–306. 10.1038/nrg3724 [DOI] [PubMed] [Google Scholar]
- Grosjean H, Keith G, Droogmans L. 2004. Detection and quantification of modified nucleotides in RNA using thin-layer chromatography. Methods Mol Biol 265: 357–391. 10.1385/1-59259-775-0:357 [DOI] [PubMed] [Google Scholar]
- Grozhik AV, Linder B, Olarerin-George AO, Jaffrey SR. 2017. Mapping m6A at individual-nucleotide resolution using crosslinking and immunoprecipitation (miCLIP). Methods Mol Biol 1562: 55–78. 10.1007/978-1-4939-6807-7_5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozhik AV, Olarerin-George AO, Sindelar M, Li X, Gross SS, Jaffrey SR. 2019. Antibody cross-reactivity accounts for widespread appearance of m1A in 5′UTRs. Nat Commun 10: 5126 10.1038/s41467-019-13146-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J, Hendrich B, Holmes M, Martin JE, Bird A. 2001. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet 27: 322–326. 10.1038/85899 [DOI] [PubMed] [Google Scholar]
- Helm M, Motorin Y. 2017. Detecting RNA modifications in the epitranscriptome: predict and validate. Nat Rev Genet 18: 275–291. 10.1038/nrg.2016.169 [DOI] [PubMed] [Google Scholar]
- Helm M, Lyko F, Motorin Y. 2019. Limited antibody specificity compromises epitranscriptomic analyses. Nat Commun 10: 5669 10.1038/s41467-019-13684-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T, Chen W, Liu J, Gu N, Zhang R. 2019. Genome-wide identification of mRNA 5-methylcytosine in mammals. Nat Struct Mol Biol 26: 380–388. 10.1038/s41594-019-0218-x [DOI] [PubMed] [Google Scholar]
- Hussain S, Sajini AA, Blanco S, Dietmann S, Lombard P, Sugimoto Y, Paramor M, Gleeson JG, Odom DT, Ule J, et al. 2013. NSun2-mediated cytosine-5 methylation of vault noncoding RNA determines its processing into regulatory small RNAs. Cell Rep 4: 255–261. 10.1016/j.celrep.2013.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PA. 2012. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet 13: 484–492. 10.1038/nrg3230 [DOI] [PubMed] [Google Scholar]
- Ke S, Alemu EA, Mertens C, Gantman EC, Fak JJ, Mele A, Haripal B, Zucker-Scharff I, Moore MJ, Park CY, et al. 2015. A majority of m6A residues are in the last exons, allowing the potential for 3′UTR regulation. Genes Dev 29: 2037–2053. 10.1101/gad.269415.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke S, Pandya-Jones A, Saito Y, Fak JJ, Vagbo CB, Geula S, Hanna JH, Black DL, Darnell JE Jr,Darnell RB. 2017. m6A mRNA modifications are deposited in nascent pre-mRNA and are not required for splicing but do specify cytoplasmic turnover. Genes Dev 31: 990–1006. 10.1101/gad.301036.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler G, Milstein C. 1975. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256: 495–497. 10.1038/256495a0 [DOI] [PubMed] [Google Scholar]
- Legrand C, Tuorto F, Hartmann M, Liebers R, Jacob D, Helm M, Lyko F. 2017. Statistically robust methylation calling for whole-transcriptome bisulfite sequencing reveals distinct methylation patterns for mouse RNAs. Genome Res 27: 1589–1596. 10.1101/gr.210666.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhu P, Ma S, Song J, Bai J, Sun F, Yi C. 2015. Chemical pulldown reveals dynamic pseudouridylation of the mammalian transcriptome. Nat Chem Biol 11: 592–597. 10.1038/nchembio.1836 [DOI] [PubMed] [Google Scholar]
- Li X, Xiong X, Zhang M, Wang K, Chen Y, Zhou J, Mao Y, Lv J, Yi D, Chen XW, et al. 2017. Base-resolution mapping reveals distinct m1A methylome in nuclear- and mitochondrial-encoded transcripts. Mol Cell 68: 993–1005 e1009. 10.1016/j.molcel.2017.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder B, Grozhik AV, Olarerin-George AO, Meydan C, Mason CE, Jaffrey SR. 2015. Single-nucleotide-resolution mapping of m6A and m6Am throughout the transcriptome. Nat Methods 12: 767–772. 10.1038/nmeth.3453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlefield JW, Dunn DB. 1958. Natural occurrence of thymine and three methylated adenine bases in several ribonucleic acids. Nature 181: 254–255. 10.1038/181254a0 [DOI] [PubMed] [Google Scholar]
- Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, Jia G, Yu M, Lu Z, Deng X, et al. 2014. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol 10: 93–95. 10.1038/nchembio.1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy AF, Riordan DP, Brown PO. 2014. Transcriptome-wide mapping of pseudouridines: pseudouridine synthases modify specific mRNAs in S. cerevisiae. PLoS ONE 9: e110799 10.1371/journal.pone.0110799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig AK, Zhang P, Hastert FD, Meyer S, Rausch C, Herce HD, Muller U, Lehmkuhl A, Hellmann I, Trummer C, et al. 2017. Binding of MBD proteins to DNA blocks Tet1 function thereby modulating transcriptional noise. Nucleic Acids Res 45: 2438–2457. 10.1093/nar/gkw1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maity A, Das B. 2016. N6-methyladenosine modification in mRNA: machinery, function and implications for health and diseases. FEBS J 283: 1607–1630. 10.1111/febs.13614 [DOI] [PubMed] [Google Scholar]
- Matera AG, Terns RM, Terns MP. 2007. Non-coding RNAs: lessons from the small nuclear and small nucleolar RNAs. Nat Rev Mol Cell Biol 8: 209–220. 10.1038/nrm2124 [DOI] [PubMed] [Google Scholar]
- Meyer KD, Jaffrey SR. 2017. Rethinking m6A readers, writers, and erasers. Annu Rev Cell Dev Biol 33: 319–342. 10.1146/annurev-cellbio-100616-060758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. 2012. Comprehensive analysis of mRNA methylation reveals enrichment in 3′UTRs and near stop codons. Cell 149: 1635–1646. 10.1016/j.cell.2012.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer KD, Patil DP, Zhou J, Zinoviev A, Skabkin MA, Elemento O, Pestova TV, Qian SB, Jaffrey SR. 2015. 5′UTR m6A promotes cap-independent translation. Cell 163: 999–1010. 10.1016/j.cell.2015.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motorin Y, Helm M. 2011. RNA nucleotide methylation. Wiley Interdiscip Rev RNA 2: 611–631. 10.1002/wrna.79 [DOI] [PubMed] [Google Scholar]
- Pall GS, Hamilton AJ. 2008. Improved northern blot method for enhanced detection of small RNA. Nat Protoc 3: 1077–1084. 10.1038/nprot.2008.67 [DOI] [PubMed] [Google Scholar]
- Plescia OJ, Braun W. 1967. Nucleic acids as antigens. Adv Immunol 6: 231–252. 10.1016/S0065-2776(08)60523-4 [DOI] [PubMed] [Google Scholar]
- Polikanov YS, Melnikov SV, Soll D, Steitz TA. 2015. Structural insights into the role of rRNA modifications in protein synthesis and ribosome assembly. Nat Struct Mol Biol 22: 342–344. 10.1038/nsmb.2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safra M, Sas-Chen A, Nir R, Winkler R, Nachshon A, Bar-Yaacov D, Erlacher M, Rossmanith W, Stern-Ginossar N, Schwartz S. 2017. The m1A landscape on cytosolic and mitochondrial mRNA at single-base resolution. Nature 551: 251–255. 10.1038/nature24456 [DOI] [PubMed] [Google Scholar]
- Scatchard G. 1949. Equilibrium in non-electrolyte mixtures. Chem Rev 44: 7–35. 10.1021/cr60137a002 [DOI] [PubMed] [Google Scholar]
- Schraivogel D, Schindler SG, Danner J, Kremmer E, Pfaff J, Hannus S, Depping R, Meister G. 2015. Importin-β facilitates nuclear import of human GW proteins and balances cytoplasmic gene silencing protein levels. Nucleic Acids Res 43: 7447–7461. 10.1093/nar/gkv705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S, Bernstein DA, Mumbach MR, Jovanovic M, Herbst RH, Leon-Ricardo BX, Engreitz JM, Guttman M, Satija R, Lander ES, et al. 2014. Transcriptome-wide mapping reveals widespread dynamic-regulated pseudouridylation of ncRNA and mRNA. Cell 159: 148–162. 10.1016/j.cell.2014.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slama K, Galliot A, Weichmann F, Hertler J, Feederle R, Meister G, Helm M. 2019. Determination of enrichment factors for modified RNA in MeRIP experiments. Methods 156: 102–109. 10.1016/j.ymeth.2018.10.020 [DOI] [PubMed] [Google Scholar]
- Squires JE, Patel HR, Nousch M, Sibbritt T, Humphreys DT, Parker BJ, Suter CM, Preiss T. 2012. Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Res 40: 5023–5033. 10.1093/nar/gks144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trixl L, Lusser A. 2019. The dynamic RNA modification 5-methylcytosine and its emerging role as an epitranscriptomic mark. Wiley Interdiscip Rev RNA 10: e1510 10.1002/wrna.1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Fan X, Mao M, Song X, Wu P, Zhang Y, Jin Y, Yang Y, Chen LL, Wang Y, et al. 2017. Extensive translation of circular RNAs driven by N6-methyladenosine. Cell Res 27: 626–641. 10.1038/cr.2017.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y, Liu J, He C. 2015. RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation. Genes Dev 29: 1343–1355. 10.1101/gad.262766.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaringhalam M, Papavasiliou FN. 2016. Pseudouridylation meets next-generation sequencing. Methods 107: 63–72. 10.1016/j.ymeth.2016.03.001 [DOI] [PubMed] [Google Scholar]
- Zhao BS, Roundtree IA, He C. 2017. Post-transcriptional gene regulation by mRNA modifications. Nat Rev Mol Cell Biol 18: 31–42. 10.1038/nrm.2016.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou KI, Clark WC, Pan DW, Eckwahl MJ, Dai Q, Pan T. 2018. Pseudouridines have context-dependent mutation and stop rates in high-throughput sequencing. RNA Biol 15: 892–900. 10.1080/15476286.2018.1462654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou KI, Shi H, Lyu R, Wylder AC, Matuszek Z, Pan JN, He C, Parisien M, Pan T. 2019. Regulation of co-transcriptional pre-mRNA splicing by m6A through the low-complexity protein hnRNPG. Mol Cell 76: 70–81.e79. 10.1016/j.molcel.2019.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]