Abstract
Objectives
The purpose of this study was to estimate prevalence of exposure to environmental tobacco smoke and other environmental toxins in dogs with primary lung tumors and to assess association between exposure and lung tumor development. We hypothesized that secondhand smoke exposure is associated with the development of primary lung tumors in dogs.
Methods
A case-control study was conducted and an owner survey developed to collect data related to patient characteristics, general health care, and environmental exposures. Dogs diagnosed with primary lung carcinomas served as the case group. Dogs diagnosed with mast cell tumors served as control group 1 and dogs diagnosed with neurologic disease served as control group 2. Bivariate and multivariate statistical analyses were conducted to evaluate associations between the diagnosis of primary lung tumor and patient and environmental exposure variables
Results
A total of 1,178 owner surveys were mailed and 470 surveys were returned and included in statistical analysis including 135 cases, 169 dogs in control group 1, and 166 dogs in control group 2. An association between exposure to second-hand smoke and prevalence of primary lung cancer was not identified in this study.
Clinical Significance
Second-hand smoke is associated with primary lung cancer in people but a definitive association has not been found in dogs. The results of this study suggest that tobacco smoke exposure may not be associated with primary lung cancer development in dogs or that study limitations precluded detection of an association in this study population.
Keywords: tobacco, canine, carcinoma, case-control, epidemiology
INTRODUCTION:
Lung cancer in people accounts for approximately 13% of all cancer diagnoses and is responsible for more deaths than any other cancer (American Cancer Society 2015, Dela Cruz et al. 2011). Cigarette smoking is the most important risk factor, such that the risk of cancer increases with both quantity and duration of smoking. Exposure to radon gas and certain building materials are also implicated in the development of lung cancer. Other risk factors include environmental exposure to asbestos, metals (chromium, cadmium, arsenic), radiation, and air pollution (American Cancer Society 2015, Dela Cruz et al. 2011). Genetic susceptibility also plays a role in the development of lung cancer especially for disease occurring in younger patients. Inhalation of secondhand smoke is known to cause lung cancer in nonsmokers, and 3,000 lung cancer deaths per year are ascribed to secondhand smoke exposure (American Cancer Society 2015, Dela Cruz et al. 2011). It is estimated that living with a smoker increases a nonsmoker’s lung cancer risk by 20 to 30 percent (Dela Cruz et al. 2011, U.S. Department of Health and Human Services 2006).
In comparison to lung cancer in people, primary lung cancer in dogs is rare representing only 1% of all canine cancers (Dorn et al. 1968). Attempts to link exposure to secondhand cigarette smoking to lung cancer have not shown a convincing positive etiologic association in dogs despite the fact that dogs encounter environmental exposures to secondhand smoke (Reif et al. 1992, Coggins 2007). Evaluation of the literature reveals only one epidemiological study attempting to link secondhand smoke exposure to lung cancer in dogs. In the publication by Reif et al., a weak association was found between exposure to one smoker in the home and the development of lung cancer in dogs (1992). Further increase in risk associated with more than one smoker in the home was not found, nor was a significant trend observed for increasing number of packs of cigarettes smoked per day or an exposure index based on number of smokers in each household, packs smoked per day, or the proportion of time the dog spent within the home. The study was a case-controlled survey assessment from 1985-1987 from two veterinary teaching hospitals. A population of confirmed canine lung cancer cases and a population of unmatched controls with other forms of cancer without proven relation to cigarette smoke exposure in people were evaluated via questionnaire. The final sample sizes included 51 cases and 83 controls. The crude odds ratio for exposure to environmental smoke was 1.5 (95% confidence interval 0.7-3.0) and with adjustment rose to 1.6 (95% confidence interval 0.7-3.7) (Reif et al. 1992). This study was limited by small sample sizes, difficulties in measuring exposure amounts, and imprecise risk estimates with final conclusions recommending further epidemiologic investigation in order to understand the effect of environmental tobacco smoke effects on the development of lung tumors in dogs.
The purpose of this study was to estimate prevalence of exposure to environmental tobacco smoke in a cohort of dogs with primary lung tumors and to assess association between exposure and lung tumor development. This study also aimed to assess possible associations between lung tumor diagnosis and exposure to other environmental toxins. We hypothesized that secondhand smoke exposure is associated with the development of primary lung tumors in dogs.
MATERIALS AND METHODS:
A case-control study was conducted with cases and 2 control groups selected from dogs evaluated at the University of California, Davis, or The University of Tennessee, Knoxville from January 2002-May 2012. The institutional review boards at the University of California and The University of Tennessee reviewed and approved the questionnaire used in this study. Dogs were eligible for inclusion in the case group if they had a histopathologic or postmortem diagnosis of a primary lung tumor including pulmonary, bronchogenic, alveolar, bronchoalveolar, or bronchiolar carcinoma, adenocarcinoma, histiocytic sarcoma, or squamous cell carcinoma. Dogs were excluded from the case group if review of the medical record identified the presence of any concurrent neoplasia including neoplasia that could have spread to lungs or if the cellular diagnosis was equivocal for metastatic rather than primary pulmonary neoplasia.
Control group 1 consisted of dogs with a histopathologic, cytologic, or postmortem diagnosis of cutaneous mast cell tumor and complete medical records indicating absence of concurrent neoplasia. Control group 2 consisted of dogs with a diagnosis of neurologic-associated disease including intervertebral disk disease or myelopathy and complete medical records indicating absence of concurrent neoplastic disease. Control populations were selected to allow comparison to both cancer and non-cancer controls believed to have tobacco smoke exposure representative of the general population of older dogs presenting to teaching hospitals.
A survey was developed with similar format to previous surveys such as The California Health Interview Survey, The United States Census Survey (2000), and The Behavioral Risk Factor Surveillance System Questionnaire (2011). The owners of eligible dogs (case and controls) were sent a three-page survey specifically requesting information about their pet for the 5 years prior to diagnosis. Questions related to breed included hair length, nose length, body size, and reproductive status. General health care was evaluated with questions inquiring about diet, flea products, bathing frequency, vaccination status, and respiratory clinical signs (i.e., cough, wheeze, difficulty breathing). Environmental exposures were evaluated based on location of the home (i.e., suburban, urban, rural, farm), county, known presence of toxins (i.e., lead paint, radon, asbestos, mold), source of heating and cooling, and number of rooms. Questions also aimed to identify the presence of nearby industrial companies (within 5 miles) and garbage/hazardous waste disposal centers.
The survey intended to quantify the amount of exposure to environmental toxins including tobacco smoke, candles/incense, aerosols, and herbicides/pesticides/fertilizers. Questions pertaining to exposure to passive smoke included evaluation of the number of smokers in the household (a smoker was defined as a person smoking at least once per day most days of the week during any part of the five years prior to the pet’s diagnosis), the number of days per week smoking occurred in the household, whether smoking was allowed inside the home, types of tobacco products used (i.e., cigarettes, cigars, pipe), number of cigarettes smoked per 24 hours (i.e. 0, 1-19, >20), number of cigarettes smoked inside the home per 24 hours (i.e., 0, 1-19, >20), how far the dog was from those smoking inside the home (i.e., lap, next to, same room, not present).
Letters and surveys were sent to owners of eligible cases and controls acknowledging a previous diagnosis of a primary lung tumor, mast cell tumor, or a neurologic condition and an explanation of the general purpose of the study without revelation of the objectives. Included was a diagram depicting clarification of nose lengths. Owners were provided an enclosed postage paid return envelope. Owners who had not responded by mail within 2 months were sent a second copy of the introduction letter and survey.
Statistical Analysis
A power analysis was performed to determine the approximate number of dogs needed in each group to detect an odds ratio of 1.6 as found by Reif et al. at the 0.05 level (2-sided) with 80% power (1992). Based on the assumption that 15% of respondents in the control groups would be smokers based on current smoking prevalence, initial targets were set to obtain results from 374 cases and 747 control dogs (divided equally between the 2 control groups).
Initial data collection included dogs with all primary lung tumor histologies, but final statistical analysis excluded dogs with histiocytic sarcomas or squamous cell carcinomas due to low numbers. If returned questionnaires had unanswered questions, those cases or controls were excluded from statistical analysis of the specific variable not answered. Bivariate and multivariate analyses were performed by a biostatistician (SS) using commercial statistical softwarea. Data were summarized in each group as mean and standard deviation for age, and as frequencies and percentages for all other variables, which were categorical. Breeds were not evaluated separately, but dogs were grouped by size (small, medium or large) and nose length (short, average, or long) as reported by owners. In bivariate analyses, the three groups were compared using analysis of variance (ANOVA) for age and chi-square tests for all other variables. In addition, dogs in the two states were compared with respect to smoke exposure in the sample as a whole and within each group using chi-square tests. Multivariate analysis evaluated associations between the diagnosis of primary lung tumor and any dog characteristic that was identified as significant at the 0.05 level by bivariate analysis as well as target variables related to tobacco smoke exposure, controlling for state (California or Tennessee). Case group data were compared to both control groups combined in multivariable logistic regression models. Because measures of smoke exposure were expected to be highly correlated, the effect of each smoke exposure variable was assessed separately using 2 models, one with main effects only and another that also included an interaction between nose length and smoke exposure; the area under the ROC curve (AUROC) was computed to assess concordance between observed values and predicted probabilities. Significance was defined as a p value less than 0.05.
RESULTS:
A total of 1,178 dogs were identified through medical record search that met the inclusion criteria including 384 cases, 432 mast cell tumor controls (control group 1), and 362 neurologic controls (control group 2). Surveys were mailed to owners of all of these dogs and percentages of surveys returned included 44% from the case group (170), 39% from control group 1 (169), and 46% from control group 2 (166). Twenty-seven dogs with pulmonary histiocytic sarcoma with owners that completed surveys were excluded from statistical analysis due to concerns that the disease etiology may be different than lung carcinomas. Eight dogs with squamous cell carcinoma with owners that completed surveys were also excluded from the case group due to low numbers. The final case group used in statistical analysis consisted of 135 dogs with primary lung carcinoma or adenocarcinoma. Age data were normally distributed and mean age was 11.0 +/− 2.5 years for cases, 8.5 +/− 2.8 years for control group 1 and 8.3 +/− 3.2 years for control group 2. Bivariate analysis identified a significant difference in age with cases on average 2.5 years older than control group 1 (p<.0001) and 2.7 years older than control group 2 (p<.0001). In addition, there were significant differences with respect to nose length (p=0.016): control group 1 dogs were more likely to have short/average noses (92%) compared to cases or control group 2 (83% each). There were also significant differences by size (p<.0001), with 60% of control group 1, 41% of cases, and 26% of control group 2 classified as large dogs.
Breed distribution of lung tumor cases was as follows: mixed breed (41), Australian Shepherd (7), Boxer (7), Labrador (6), Golden Retriever (5), Bichon Frise (4), Cocker Spaniel (4), Shetland Sheepdog (4), West Highland White Terrier (4), Standard Poodle (4) Weimaraner (4) and 3 or fewer dogs of 33 additional breeds. Breed distribution of control group 1 was as follows: mixed breed (44), Labrador Retriever (30), Boxer (12), Golden Retriever (9), Boston Terrier (7), Pug (6), Australian Shepherd (5), Beagle (4), Cocker Spaniel (4), and 3 or fewer dogs of 30 additional breeds. Breed distribution of control group 2 was as follows: Dachshund (33), Mixed breed (27), Miniature Dachshund (7), German Shepherd (7), Labrador (6), Miniature Schnauzer (5), Beagle (4), Chihuahua (4), Pembroke Welsh Corgi (4), and 3 or fewer dogs of 43 additional breeds.
Overall, any secondhand tobacco smoke exposure was reported in 20.9% of returned questionnaires (cases and controls combined). Evaluation of exposure to tobacco smoke by state revealed higher secondhand smoke exposure in State 2 than in State 1 with similar results found in case and control groups (Figure 1). Overall, 15.9% of all dogs (cases and controls) had any tobacco smoke exposure in California and 32.8% had any tobacco smoke exposure in Tennessee (p<.0001). When evaluating data related to potential environmental toxins by bivariate analysis, cases were found to be less likely than control group 1 or control group 2 dogs to have had lead paint in the home (1% vs. 9% and 6%, respectively, p=0.022), and control group 1 dogs were more likely than cases or control group 2 dogs to have had mould in the home (9% vs. 4% and 2%, respectively, p=0.0066). There were no significant associations between case-control status and exposure to smoke regardless of number of smokers, number of cigarettes smoked per day or whether owners smoked inside the house (Table 1). Analysis of tobacco smoke exposure revealed that both control groups had a higher frequency of exposure to secondhand smoke than cases but these differences were not significant. A daily smoker in the household was reported in 13% of dogs in the case group (n=17), 18% of dogs in control group 1 (n=30), and 21% of dogs in control group 2 (n=35). No other significant findings were found based on case-control status and any other household or neighborhood exposures on bivariate analysis.
Figure 1.
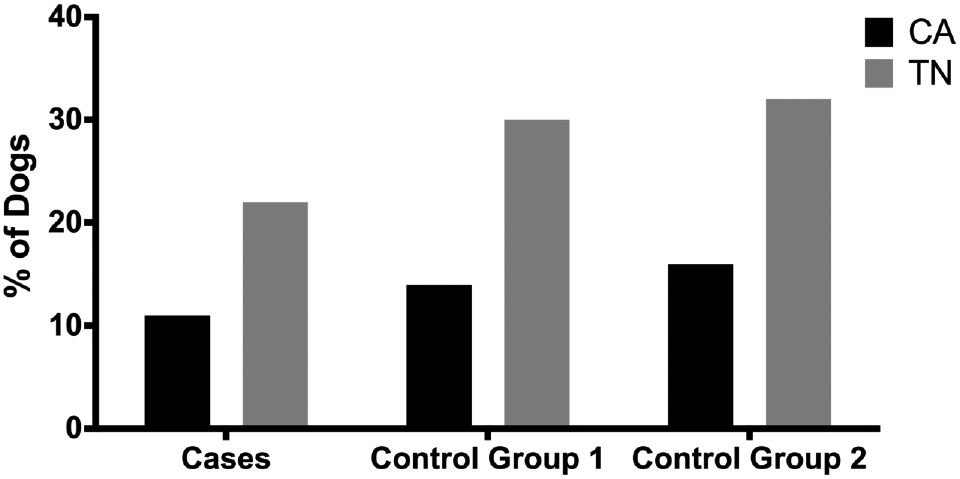
Reported smoke exposure by state in cases and control groups. Dogs in Tennessee had significantly more smoke exposure than dogs in California.
Table 1.
Bivariate analysis comparing patient and exposure variables between lung cancer cases, control group 1 (mast cell tumor controls), and control group 2 (neurologic controls).
| Characteristic | Cases (n=135) n (%) or mean (SD) |
Control Group 1 (n=169) n (%) or mean (SD) |
Control Group 2 (n=166) n (%)or mean (SD) |
p-value |
|---|---|---|---|---|
| Age (years) | 11.0 (2.5) | 8.5 (2.8) | 8.3 (3.2) | <.0001 |
| Size | <.0001 | |||
| Small | 38 (28) | 27 (16) | 95 (57) | |
| Medium | 41 (31) | 41 (24) | 28 (17) | |
| Large | 55 (41) | 100 (60) | 43 (26) | |
| Nose Length | 0.016 | |||
| Short/Average | 111 (83) | 155 (92) | 137 (83) | |
| Long | 23 (17) | 13 (8) | 29 (17) | |
| Reproductive Status | 0.22 | |||
| Intact | 6 (4) | 16 (10) | 11 (7) | |
| Spay/Neuter | 129 (96) | 152 (90) | 155 (93) | |
| Environment | ||||
| Lead Paint | 2 (1) | 15 (9) | 10 (6) | 0.022 |
| Asbestos | 3 (2) | 7 (4) | 8 (5) | 0.49 |
| Mold | 6 (4) | 16 (9) | 3 (2) | 0.0066 |
| Daily smoker in household | 17 (13) | 30 (18) | 35 (21) | 0.13 |
| Number of smokers | 0.17 | |||
| 0 | 114 (87) | 136 (83) | 121 (77) | |
| 1 | 11 (8) | 21 (13) | 29 (18) | |
| 2 or more | 6 (5) | 7 (4) | 8 (5) | |
| Smoking inside the home | 12 (9) | 18 (11) | 28 (17) | 0.065 |
| Number of cigarettes smoked inside per 24 hours | 0.50 | |||
| 0 | 122 (93) | 151 (90) | 134 (88) | |
| 1-19 | 8 (6) | 11 (7) | 12 (8) | |
| 20 or more | 1 (1) | 5 (3) | 6 (4) | |
| Exposures | ||||
| Incense/Candles | 19 (14) | 35 (21) | 26 (16) | 0.27 |
| Aerosols | 20 (15) | 23 (14) | 23 (14) | 0.96 |
| Herbicides | 1 (1) | 3 (2) | 2 (1) | 0.72 |
| Pesticides | 6 (5) | 7 (4) | 1 (1) | 0.083 |
| Fertilizers | 5 (4) | 7 (4) | 2 (1) | 0.25 |
| Wood shavings | 18 (14) | 23 (14) | 11 (7) | 0.084 |
Note: p-value is for ANOVA (age) or chi-square test (all other variables);SD=standard deviation
In multivariable logistic regression models (Table 2), cases remained more likely than controls to be older (OR=1.4, 95% CI 1.3-1.5, p<0.0001) and cases were less likely to have an average/short nose (OR=0.5, 95% CI 0.3-0.9, p=0.03). The OR for any smokers in the household was 0.8 (95% CI 0.4-1.4, p=0.38) and for any tobacco smoke inside the house was 0.7 (95% CI 0.3-1.6, p=0.41). Interactions between nose length and both smoke exposure variables were not statistically significant (smokers in the household: p=0.86, smoke inside the house: p=0.34; data not shown).
Table 2.
Multivariate analysis results indicating that lung cancer cases were older than the combined control group (mast cell tumor and neurologic controls) and lung cancer cases were less likely to have an average/short nose than the combined control group.
| Model 1 (n=448) |
Model 2 (n=448) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Cases n |
Controls n |
Odds Ratio* |
95% Cl | p | Odds Ratio* | 95% Cl | p |
| State | 0.94 | 0.88 | ||||||
| 1 | 99 | 244 | 1.0 | 0.6-1.8 | 1.0 | 0.6-1.9 | ||
| 2 (ref) | 25 | 80 | 1.0 | 1.0 | ||||
| Age (per year) | 124 | 324 | 1.4 | 1.3-1.5 | <.0001 | 1.4 | 1.3-1.5 | <.0001 |
| Size | 0.26 | 0.27 | ||||||
| Small (ref) | 36 | 119 | 1.0 | 1.0 | ||||
| Medium | 38 | 67 | 1.6 | 0.9-3.0 | 1.6 | 0.9-3.0 | ||
| Large | 50 | 138 | 1.2 | 0.7-2.0 | 1.1 | 0.7-2.0 | ||
| Nose Length | 0.035 | 0.034 | ||||||
| Short/Average | 103 | 282 | 0.5 | 0.3-1.0 | 0.5 | 0.3-0.9 | ||
| Long (ref) | 21 | 42 | 1.0 | 1.0 | ||||
| Daily smoker in household | 0.23 | |||||||
| Yes | 15 | 62 | 0.7 | 0.3-1.3 | NA | |||
| No (ref) | 109 | 262 | 1.0 | |||||
| Smoking inside the home | 0.41 | |||||||
| Yes | 11 | 44 | NA | 0.7 | 0.3-1.6 | |||
| No (ref) | 113 | 280 | 1.0 | |||||
Adjusted for all variables in model.
Note: CI=confidence interval; ref=referent level; NA=not applicable (not in model);
area under the ROC curve (AUROC)=0.77 for both models
DISCUSSION:
Our understanding of exposure to environmental toxins and its association with the development of disease is critical for the advancement of both animal and human health. Primary lung cancer in dogs is a rare disease and little is known about factors involved in its development (Withrow et al 2013). Previous studies evaluating the development of primary lung tumors in the dog have shown a possible association with exposure to tobacco products. However, these studies often used direct smoke exposure including exposure to levels greater than would occur passively (Coggins CR 2007, Hammond et al. 1971, Van der Vaart et al. 2004). Only one previous study has evaluated the association between primary lung tumor development in dogs and passive exposure to secondhand smoke (Reif et al. 1992). That study identified a weak association between the development of primary lung tumors and exposure to second hand smoke but had low enough case and control numbers to prompt further investigation. Given these findings, our study intended to further investigate associations between lung tumor diagnoses and exposure to secondhand smoke and other environmental factors in pet dogs in a larger population. Using a similar survey approach, we did not find a statistically significant relationship between exposure to second hand smoke and the development of primary lung tumors in dogs.
This study also aimed to estimate prevalence of exposure to environmental tobacco smoke in cases and controls. Secondhand tobacco smoke exposure was reported in 20.9% of returned questionnaires and this is consistent with data indicating that 22.5% of adult Americans were cigarette smokers in 2002 and 18.1% were smokers in 2012 (Husten et al. 2004, Agaku et al. 2014). Exposure was reported in 15.9% of dogs in California consistent with adult cigarette smoking frequencies in that state of 16.4% in 2002 and 12.6% in 2012 (Bombard et al. 2004, CDC STATE 2017). Exposure was reported in 32.8% of dogs in Tennessee, which is slightly higher than adult cigarette smoking frequencies in that state of 27.8% in 2002 and 24.9% in 2012 (Bombard et al. 2004, CDC STATE 2017). This higher frequency could have been caused by smaller case and control numbers from State 2 or by neighboring states within the referral base with even higher reported adult smoking frequencies during these years (Bombard et al. 2004, CDC STATE 2017).
One possible reason for these findings may lie in the differences in lung cancer histology between dogs and humans. In people, lung cancer has been described as a heterogeneous disease clinically, biologically, histologically, and molecularly (Dela Cruz et al. 2011, Khuder 2001, Travis 2015). Two main classifications of lung cancer are described, non-small cell lung cancer (80-85% of cases) and small cell lung cancer (15-20% of cases). Non-small cell lung cancers include adenocarcinoma, squamous cell carcinoma, and large cell carcinoma. Small cell carcinoma is classified separately and is believed to originate from neuroendocrine cells of the bronchus (Dela Cruz et al. 2011, Khuder 2001, Travis 2015). All types of lung cancer have been associated with smoking in people, but small cell carcinoma has the strongest association and the majority of never smokers (adults who have never smoked or have smoked <100 cigarettes in his/her lifetime) develop adenocarcinomas, with bronchioloalveolar carcinomas being most common in female never smokers (Dela Cruz et al. 2011, Khuder 2001, Yang 2011). Lung cancers in dogs consist primarily of adenocarcinomas or alveolar carcinomas and small cell lung cancer has not been described (Ogilvie et al. 1989, Moulton et al. 1981). The fact that dogs overwhelmingly develop a form of lung cancer that is less likely to be smoke induced in people may suggest that secondhand tobacco smoke does not play a significant role in tumor development. Alternatively, it is possible that tobacco smoke only plays a role in the development of some histologic types in dogs, but the data from this study was not able to detect this association due to small numbers of certain histologic subtypes. Further research into lung cancer subtyping in dogs and a possible association with tobacco smoke exposure is warranted.
Another possible reason for the findings in this study is type II error resulting in an inability to detect an association between tobacco smoke and lung cancer. Despite utilizing caseload at two academic institutions over a 10-year period, case and control numbers were just slightly higher than what was estimated necessary in power analysis before accounting for data loss due to survey non-response rates. Also, the power analysis used the relatively high OR of 1.6 suggested in previous research, but the OR for lung cancer development and second hand smoke exposure in people is approximately 1.28 (Hori, 2016). This lower OR would require even higher numbers of cases and controls to detect. This underscores the fact that primary lung tumors in dogs are rare, which limits our ability to perform large-scale epidemiologic studies. Although it was clear early on that this study would be underpowered, the research was continued because only one publication on this topic exists and results are often quoted. Dissemination of study results was deemed important given how little research has been conducted on this topic in veterinary medicine to date. This study had 2.6 times more cases and 4 times more controls than the previous publication that suggested a possible association between tobacco smoke and lung cancer in dogs (Reif et al. 1992). Also, the 95% CI of the OR for having any smokers in the household and for any smoking inside the house ended below the OR of 1.6 that was found in the Reif paper (1992). Increasing the sample size would be expected to result in narrower confidence intervals, but, based on the current data, it is unlikely that results would shift significantly. Nonetheless, it is possible that a significant association between lung cancer development and exposure to tobacco smoke went undetected in our study and further studies with a larger number of cases and controls could be beneficial.
Study results also found an association between lung tumor diagnosis and nose length where dogs with lung tumors were less likely to have short/average noses than control dogs. This finding was likely due to the selection of dogs with mast cell tumors as a control group and the fact that brachycephalic breeds are known to be predisposed to MCT and, thus, were highly represented in control group 1 (Villamil et al. 2011, Dobson 2013). This result should be interpreted cautiously because study methodology was not ideal to assess breed or nose length and its impact on lung cancer development in dogs. Nonetheless, it is possible that there is a true association between dolichocephalia and lung cancer diagnosis in dogs possibly due to tobacco smoke particle collection in larger nasal passages resulting in chronic airway exposure over time. Alternatively, dogs of different breed groups and nose lengths may have different genetic characteristics that predispose or reduce risk of lung cancer development. A previous study in dogs found an association between sinonasal cancer and tobacco smoke in dolichocephalic dogs, but not brachycephalic and mesocephalic dogs (Reif et al. 1998). In contrast, the lung cancer study by Reif et al. found an association between lung cancer in dogs with tobacco smoke exposure only in brachycephalic and mesochephalic dogs (1992). With these conflicting results, further study into the role of breed, nose length, and genetics in environmental toxin exposure in dogs is warranted.
This study has significant limitations that affect interpretation of results. One limitation is with the inherent differences between the case and control populations studied. The control populations were chosen because they were thought to be representative of a population of older dogs presenting to the teaching hospitals similar to dogs diagnosed with lung cancer. Differences in age, breed, and nose length were found between cases and controls, however, and it is possible that differences in environmental exposures also existed between groups. Although age and nose length were controlled for in statistical analysis, these differences may still have affected results. Future studies should attempt to utilize more similar control populations. Another limitation of this study is the possible inclusion of some dogs with pulmonary metastatic neoplasia in the case group. Cases were excluded if medical record review revealed evidence of extra-pulmonary neoplasia or if histology was consistent with or equivocal for metastasis. Nonetheless, staging tests were not uniform due to the retrospective nature of this study and cases of metastasis from non-respiratory neoplasms may have been misclassified as primary lung tumors.
Another difficulty was encountered when analyzing survey data in attempts to objectively quantify tobacco smoke exposure. The survey included questions about number of smokers, type of tobacco products, number of cigarettes, smoking inside the home, and distance between dogs and smokers, but survey replies were highly variable and made grouping cases and controls by exposure categories challenging. As a result, case numbers were low in some groups and only major exposure categories were evaluable, which may have hindered analysis. Difficulty in quantifying exposure is a common problem in studies of environmental exposures in all species. Future studies may benefit from research into potential biomarkers of tobacco smoke exposure, which may be more easily quantified.
Possible confounding factors also that likely influenced study results include recall bias and response bias. The inclusion of diagnosis dates of study between 2002-2012 with recall period of 5 years prior to diagnosis resulted in a significant time lapse in some cases before questionnaire completion, which may have influenced the accuracy of recall of certain factors. Owners of cases may also have been more likely to recall tobacco smoke exposure than controls due to the known association between tobacco smoke and lung cancer in people and this could have dramatically affected results. Further, only 39-46% of questionnaires were returned and it is possible that owners of dogs with lung cancer who had a history of tobacco use may have been less likely to respond due to guilt or other factors. Human studies have found that surveys of health compromising behaviors are likely to underestimate the prevalence of those behaviors and the same may be true of pet owners (Meiklejohn et al. 2012). This form of response bias may have affected the study’s ability to test the hypothesis and could explain the lower, but non-significant, rates of tobacco smoke exposure reported in the case group compared to both control groups. The survey questions were designed in a way meant to distract from a specific interest in tobacco smoke exposure; however, several tobacco related questions were included and response bias resulting in differential misclassification likely occurred to some degree. Because owners may know about the association between tobacco smoke and lung cancer in people, they may have been more likely to report tobacco smoke exposure than owners of controls because of a desire to know a cause for their pet’s cancer or less likely to report tobacco smoke due to guilt that their actions may have contributed to cancer development. These biases, while inherent to studies involving patient or owner reporting, limit the ability to draw conclusions from this study.
In conclusion, an association was not found between exposure to second-hand smoke and primary lung cancer diagnosis in dogs in this study. The results of this study suggest that tobacco smoke exposure may not be associated with primary lung cancer development in dogs or that study limitations precluded detection of an association in this study population. It is also possible that smoke exposure plays a role in a subgroup of dogs with certain histologic types of lung cancer and further study with a larger number of dogs is necessary. Further evaluation of environmental exposures in conjunction with tumor histology, immunohistochemistry, and genetic analysis should be investigated in a prospective study in order to achieve the most information about tumor development and associated factors.
Supplementary Material
ACKNOWLEDGEMENTS:
This work was done at the University of California, Davis and the University of Tennessee, Knoxville. This work was supported by a grant from the American Humane Association.
Footnotes
No conflicts of interest have been declared.
Supporting Information: A copy of the survey distributed to pet owners for this study.
Survey available in PDF form only.
SAS Statistical Software, version 9.3
REFERENCES
- Agaku IT, King BA, Dube SR (2014) Current cigarette smoking among adults—United States, 2005-2012. Centers for Disease Control and Prevention Morbidity and Mortality Weekly Report 63,29–34. [PMC free article] [PubMed] [Google Scholar]
- American Cancer Society. (2015) Cancer Facts & Figures 2015. Atlanta: American Cancer Society. [Google Scholar]
- Bombard J, Trosclair A, Schooley M, et al. (2004) State-specific prevalence of current cigarette smoking among adults—United States, 2002. Centers for Disease Control Morbidity and Mortality Weekly Report 52,1277–80. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention State Tobacco Activities Tracking and Evaluation System (CDC STATE System) (2017) https://www.cdc.gov/statesystem/ [accessed 9 August 2017]
- Cheng L, Alexander RE, MacLennan GT, et al. (2012) Molecular pathology of lung cancer: key to personalized medicine. Modern Pathology 25,347–69. [DOI] [PubMed] [Google Scholar]
- Coggins CR (2007) An updated review of inhalation studies with cigarette smoke in laboratory animals. International Journal of Toxicology 26,331–8. [DOI] [PubMed] [Google Scholar]
- Dela Cruz CS, Tanoue LT, Matthay RA (2011) Lung cancer: Epidemiology, etiology, and prevention. Clinics in Chest Med 32,605–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson JM (2013) Breed-predispositions to cancer in pedigree dogs.” ISRN Veterinary Science 2013:941275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn CR, Taylor DO, Schneider R, et al. (1968) Survey of animal neoplasms in Alameda and Contra Costa Counties, California. II. Cancer morbidity in dogs and cats from Alameda County. Journal of the National Cancer Institute 40,307–318. [PubMed] [Google Scholar]
- Hammond EC, Auerbach O, Kirman D, et al. (1971) Effects of cigarette smoking on dogs. CA: A Cancer Journal for Clinicians; 21,78–94. [Google Scholar]
- Hori M, Tanaka H, Wakai K, et al. (2016) Secondhand smoke exposure and risk of lung cancer in Japan: a systematic review and meta-analysis of epidemiologic studies. Japanese Journal of Clinical Oncology 46,942–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husten C, Jackson K (2004) Cigarette smoking among adults—United States, 2002. Centers for Disease Control Morbidity and Mortality Weekly Report 53,427–31. [PubMed] [Google Scholar]
- Khuder SA (2001) Effect of cigarette smoking on major histological types of lung cancer: a meta-analysis. Lung Cancer 31,139–48. [DOI] [PubMed] [Google Scholar]
- Meiklejohn J, Connor J, Kypri K (2012) The effect of low survey response rates on estimates of alcohol consumption in a general population survey. PLoS One 7,e35527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton JE, von Tscharner C, Schneider R (1981) Classification of lung carcinomas in the dog and cat. Veterinary Pathology 18,513–28. [DOI] [PubMed] [Google Scholar]
- Ogilvie GK, Hascheck WM, Withrow SJ, et al. (1989) Classification of primary lung tumors in dogs: 210 cases (1975–1985). Journal of the American Veterinary Medical Association 195,106–8. [PubMed] [Google Scholar]
- Rebhun RB, Cult WTN (2013) Pulmonary Neoplasia In: Small Animal Clinical Oncology. 5th edn. Eds Withrow SJ, Vail DM, and Page RL R.L. Elsevier Saunders St. Louis; pp 453–459. [Google Scholar]
- Reif JS, Bruns C, Lower KS (1998) Cancer of the nasal cavity and paranasal sinuses and exposure to environmental tobacco smoke in pet dogs. American Journal of Epidemiology 147,488–92. [DOI] [PubMed] [Google Scholar]
- Reif JS, Dunn K, Ogilvie GK, et al. (1992) Passive smoking and canine lung cancer risk. American Journal of Epidemiology 135,234–9. [DOI] [PubMed] [Google Scholar]
- Travis WD, Brambilla E, Nicholson AG, et al. on Behalf of the WHO Panel. (2015) The 2015 World Health Organization classification of lung tumors. Journal of Thoracic Oncology 10,1243–60. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. (2006) The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. [Google Scholar]
- Van der Vaart H, Postma DS, Timens W, et al. (2004) Acute effects of cigarette smoke on inflammation and oxidative stress: a review. Thorax 59,713–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villamil JA, Henry CJ, Bryan JN (2011) Identification of the most common cutaneous neoplasms in dogs and evaluation of breed and age distributions for selected neoplasms. Journal of the American Veterinary Medical Association 239,960–65. [DOI] [PubMed] [Google Scholar]
- Yang P (2011) Lung cancer in never smokers. Seminars in Respiratory Critical Care Medicine 32,10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


