Abstract
Background:
For advanced stage non–small cell lung cancer (NSCLC), chemotherapy and chemoradiation are the primary treatments. Although surgery in these patients is associated with improved survival, the effect of selection bias is poorly defined. Our objective was to characterize selection bias and identify potential surgery candidates by constructing a surgical selection score (SSS).
Methods:
Patients with clinical stage IIIA, IIIB, or IV NSCLC were identified in the National Cancer Data Base from 1998–2012. Logistic regression was used to develop the SSS based on clinical characteristics. Estimated area under the receiver operating characteristic curve (AUROC) was used to assess discrimination performance of the SSS. Kaplan-Meier analysis was used to compare patients with similar SSS.
Results:
300,572 patients with stage IIIA, IIIB or IV NSCLC without missing data were identified; 6% (18,701) had surgery. The surgical cohort was 57% (10,650) stage IIIA, 19% (3,483) stage IIIB, and 24% (4,568) stage IV. The AUROCs from the best-fit logistic regression model in the training and validation sets were not significantly different; 0.83 (0.82–0.83, 95% CI) and 0.83 (0.82–0.83, 95% CI). The range of SSS is 43–1141. As expected, SSS is a good predictor of survival. Within each quartile of SSS, patients in the surgical group had significantly longer survival than non-surgical patients (p<0.001).
Conclusions:
A prediction model for selection of patients for surgery was created. Once validated and prospectively tested, this model may be used to identify patients who may benefit from surgical intervention.
Introduction
For patients with advanced stage non-small cell lung cancer (stages IIIA, IIIB and IV), despite the introduction of new systemic therapies, 5-year survival remains very poor.[1] Treatment approaches for these patients are very heterogeneous, and a small proportion of these patients undergo surgery either alone or in combination with other modalities.[1] Overall, in the setting of poor survival outcomes as well as the potential morbidity of invasive surgery, curative-intent treatments such as surgical resection are not the primary focus of multimodality therapy since the goals of care remain disease control and palliation.[2]
However, the inclusion of surgery into the treatment strategy for advanced stage NSCLC has been reproducibly associated with improvements in survival.[3–8] Using data from population-based and institutional databases, we and others have demonstrated significantly longer cancer-specific and overall survival in advanced NSCLC patients treated with surgery (alone or in combination) when compared to non-surgical modalities, including chemoradiation, chemotherapy alone, radiation alone, and no-treatment (p<0.001).[3–6] Similarly, in a propensity-matched analysis using data from the National Cancer Data Base (NCDB), stage IIIB patients treated with chemotherapy, radiation and surgery had a median survival of 28.9 months compared to 17.2 months for patients treated with chemoradiation without surgery (p<0.001).[4]
A routine criticism of these analyses is that selection bias (even with advanced statistical techniques like propensity matching) remains an important confounding factor potentially accounting for superior survival outcomes observed for surgical patients. [4,5,9] This selection bias may reflect the influence of treatment-related variables which are not independently associated with the outcome variable of interest. Yet, despite an understanding of surgical selection bias as a concept, the measurable impact of surgical selection bias on outcomes has been difficult to quantify. In part because of limited data on clinico-pathological characteristics related to surgery selection, particularly in advanced stage NSCLC. Our objective was to quantify the factors influencing selection of advanced stage NSCLC patients for surgery by generating a predictive model (Surgical selection score – SSS). We hypothesized that the SSS would be independently associated with increased overall survival (OS).
Methods
This study received a determination letter from the University of California, Davis Institutional Review Board. We queried the NCDB for cases of biopsy-proven NSCLC from 1998 – 2012. The NCDB is a joint program of the Commission on Cancer (CoC) and the American Cancer Society (ACS). Data from the NCDB represent 1,500 CoC- accredited facilities including over 70% of all newly diagnosed cancer cases in the United States. These data are used to track treatments and outcomes as well as provide quality-related performance measures.[10]
Patients with stage IIIA, IIIB and IV NSCLC with histologic data available were included. The study cohort is summarized in Figure 1. Standard patient, tumor, and treatment data were extracted and categorized as appropriate. Surgical operations included wedge resection, sublobar resection, lobectomy, bilobectomy, and pneumonectomy. Patient comorbidities were assessed using the Charlson comorbidity index, described by Deyo et al[11]. Additional categorical variables examined included clinical tumor group, clinical tumor size, clinical node group, clinical M group, histology, age, sex, race, income, education, insurance status, and treatment facility. Age was categorized by percentile (10th, 25th, 50th, 75th, 90th). Income categories were defined as follows: low <$38,000; middle >$38,000–47,999, and high >$48,000. Education categories were defined by the percent of adults in the patients’ zip code who did not graduate from high school: low ≥13%; middle =7- to <13%, and high <7%. Patients with an additional cancer diagnosis, missing TNM stage group, stage I or II, or missing Charlson Index or other demographic data were excluded.
Figure 1.
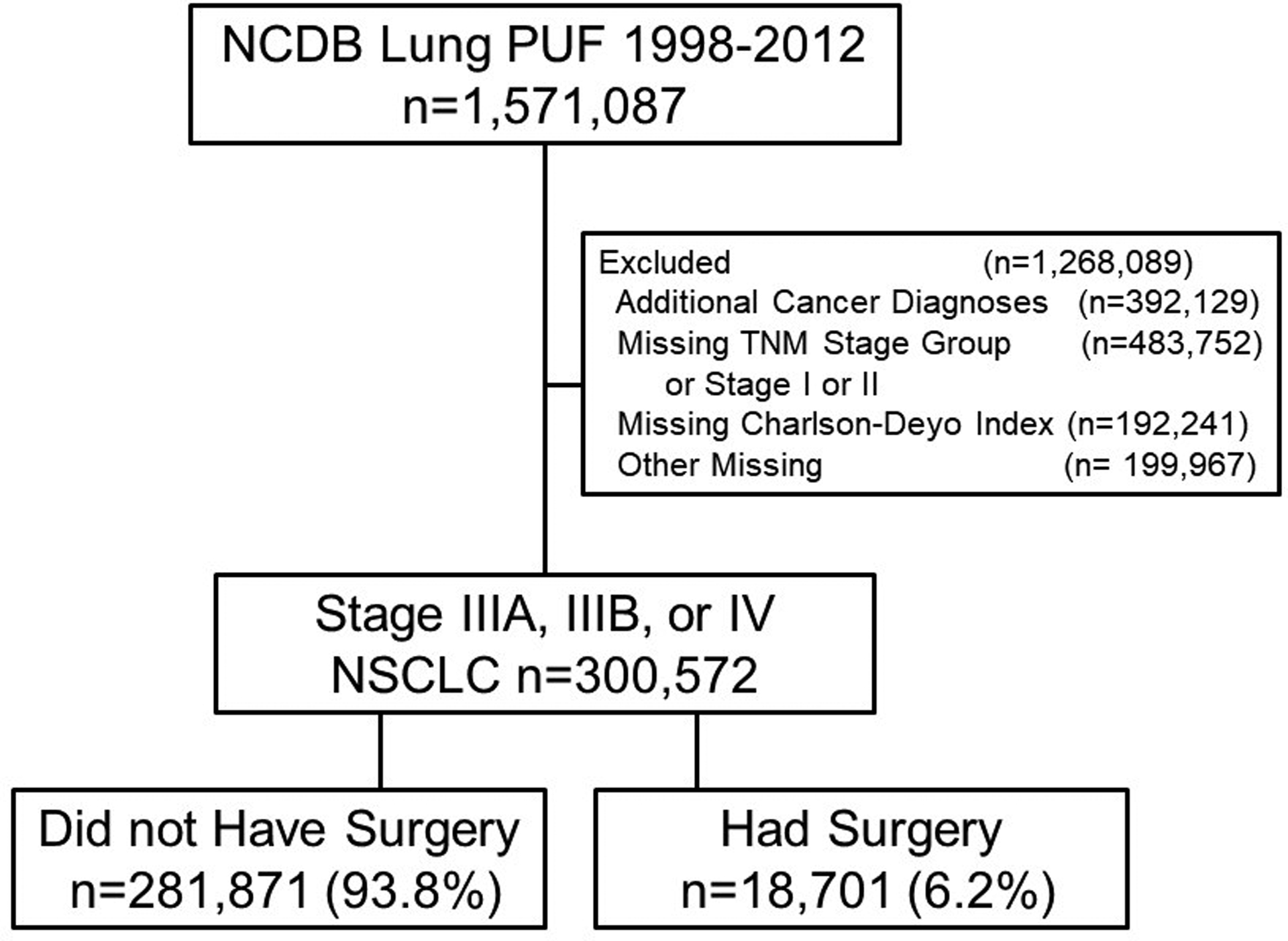
Cohort of Stage IIIA, IIIB, and IV patients from the National Cancer Database
Statistical Analyses
Categorical variables were compared using chi-square tests to determine differences between the treatment groups (Table 1). Logistic regression was used to create the surgical selection score (SSS) using models containing increasing numbers of clinical and tumor characteristics as shown in Figure 2, with the outcome variable representing inclusion of surgery in the treatment regimen.[12] The models were developed in a nested fashion, with a simple model initially and then complexity increased as clinical factors were added. The SSS was developed on a training dataset and was validated using a separate validation data set. These training and validation sets were generated using stratified randomization to maintain the proportion of surgical patients in each dataset. The entire cohort was split 50/50 into two sets, one for training and one for validation. This process was repeated with multiple random seeds and similar results were obtained. The ability of each model to predict selection for surgery was assessed and validated by calculating the area under the receiver operating characteristic curve (AUROC).[13] The equality of the AUROCs from the training and validation sets were compared using the chi square test.. The final model was determined using the Bayesian information criterion (BIC).[14]
Table 1.
Patients with Advanced Stage NSCLC in NCDB from 1998–2012, surgically treated compared to non-surgically treated using Chi-Square tests.
| Variable | Nonsurgical Patients N= 281,871 (94%) | Surgical Patients N= 18,701 (6%) | p-value |
|---|---|---|---|
| AJCC Stage Group | |||
| IIIA | 47,525 (16.9) | 10,650 (56.9) | P<0.0001 |
| IIIB | 51,603 (18.3) | 3,483 (18.6) | |
| IV | 182,743 (64.8) | 4,568 (24.4) | |
| Histology | |||
| NSCLC | 83,826 (29.7) | 3,184 (17.0) | |
| Squamous Cell Ca | 75,487 (26.8) | 6,171 (33) | |
| Adenocarcinoma | 122,558 (43.5) | 9,346 (49.9) | |
| Tumor Size | |||
| 1a | 26,654 (9.5) | 2,945 (15.8) | P<0.0001 |
| 1b | 42,176 (14.9) | 3,590 (19.2) | |
| 2a | 91,140 (32.3) | 5,735 (30.7) | |
| 2b | 64,377 (22.8) | 3,480 (18.6) | |
| 3 | 57,524 (20.4) | 2,951 (15.8) | |
| AJCC T Status | |||
| 1 | 36,946 (13.11) | 3,875 (20.7) | P<0.0001 |
| 2 | 82,382 (29.2) | 6,273 (33.5) | |
| 3 | 39,958 (14.2) | 3,277 (17.5) | |
| 4 | 97,944 (34.8) | 4,458 (23.8) | |
| X | 24,641 (8.7) | 818 (4.4) | |
| AJCC N Status | |||
| 0 | 44,503 (15.8) | 4,117 (22) | P<0.0001 |
| 1 | 23,775 (8.4) | 2,430 (12.9) | |
| 2 | 131,743 (46.7) | 10,201 (54.6) | |
| 3 | 50,664 (17.9) | 850 (4.6) | |
| X | 31,186 (11.1) | 1,103 (5.9) | |
| AJCC M Status | |||
| 0 | 103,413 (36.7) | 14,269 (76.3) | P<0.0001 |
| 1 | 133,452 (47.4) | 3,530 (18.9) | |
| 1A | 11,226 (3.9) | 331 (1.8) | |
| 1B | 33,780 (11.9) | 571 (3.1) | |
| Charlson Index | |||
| 0 | 176,275 (62.5) | 10,978 (59.7) | P<0.0001 |
| 1 | 73,277 (26) | 5,743 (30.8) | |
| 2 | 32,319 (11.5) | 1,980 (10.6) | |
| Age | |||
| 0–52 | 27,384 (9.7) | 2,592 (13.9) | P<0.0001 |
| 52–59 | 40,071 (14.2) | 3,350 (17.9) | |
| 59–67 | 65,500 (23.2) | 5,122 (27.4) | |
| 67–75 | 71,840 (25.5) | 4,675 (24.5) | |
| 75–81 | 44,609 (15.8) | 2,170 (11.6) | |
| >81 | 32,467 (11.5) | 702 (3.8) | |
| Race | |||
| White | 236,598 (83.9) | 16,321 (87.3) | P<0.0001 |
| Black | 36,736 (13) | 1,847 (9.9) | |
| Other | 8,537 (3.0) | 533 (2.9) | |
| Facility Type | |||
| Community Cancer Program | 38,445 (13.6) | 1,906 (10.2) | P<0.0001 |
| Comprehensive Community Cancer Program | 160,635 (56.9) | 10,022 (53.6) | |
| Academic/Research Program | 82,791 (29.4) | 6773 (36.2) | |
| Insurance | |||
| Not Insured | 13,691 (4.9) | 578 (3.1) | P<0.0001 |
| Private Insurance | 84,181 (29.9) | 7915 (42.3) | |
| Medicaid | 21,701 (7.7) | 1,224 (6.6) | |
| Medicare | 158,253 (56.1) | 8,737 (46.7) | |
| Other Government | 4,045 (1.4) | 247 (1.3) | |
| Income | |||
| Low | 59,661 (21.2) | 3,478 (18.6) | P<0.0001 |
| Middle | 76,594 (27.2) | 4,757 (25.4) | |
| High | 145,616 (51.7) | 10,466 (55.9) |
Figure 2.
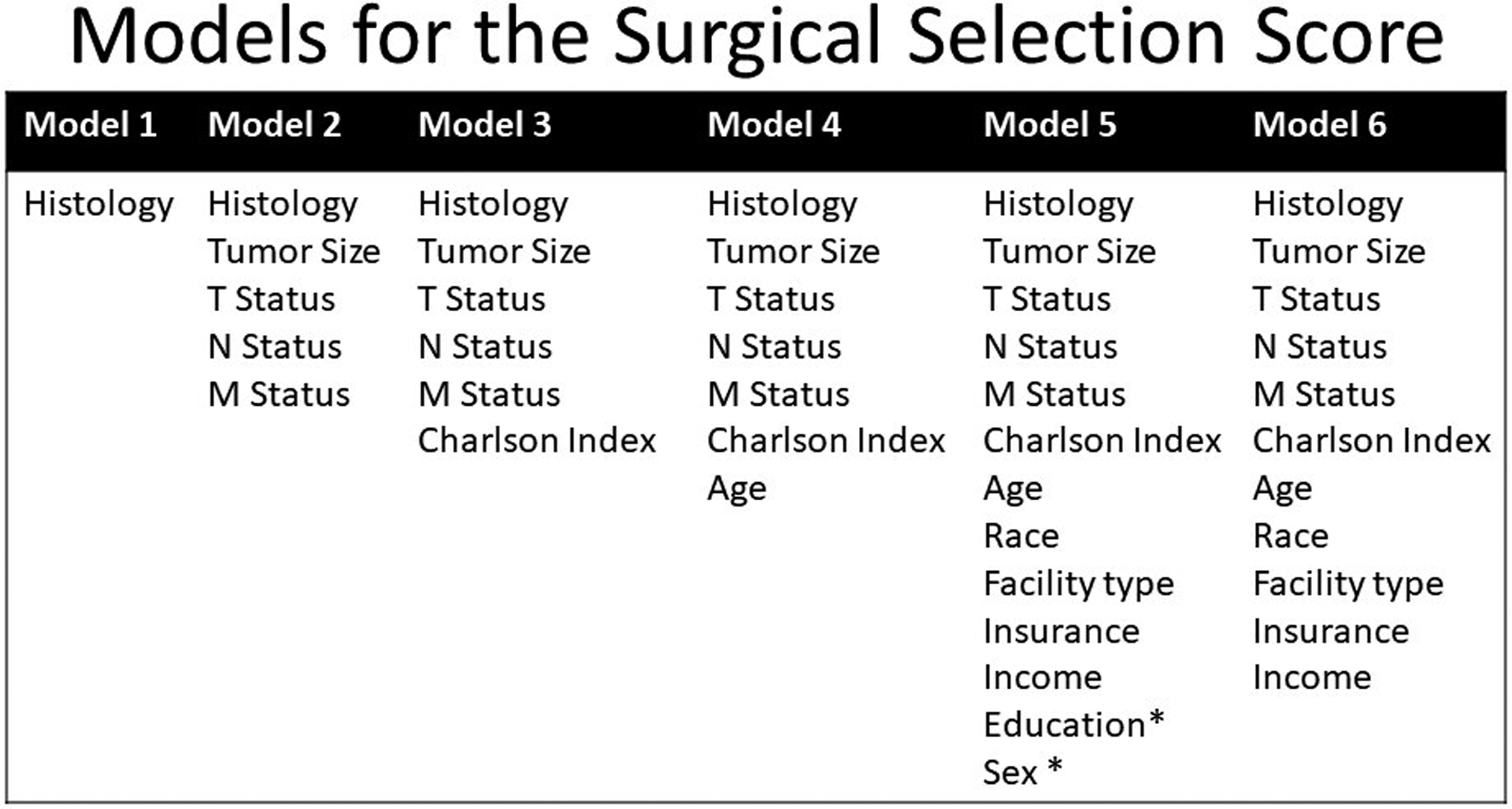
Logistic regression models used to develop the Surgical Selection Score. * represents variables without significant predictive ability for selection for surgery. The final model was model 6.
The SSS was created by multiplying the logarithm of the odds ratios from the logistic regression model by 100 and adding the total, to generate one numeric score for each patient. The probability of undergoing surgical therapy was calculated for the entire cohort and separately by stage. OS functions were estimated using Kaplan-Meier method within treatment groups. Log-rank tests were conducted to examine whether the differences in OS between the treatment groups were statistically significant within quartiles of the SSS. P-values less than 0.05 were considered statistically significant. All statistical analyses were conducted using SAS for Windows, version 9.4 (SAS Institute Cary, NC).
Results
300,572 patients with biopsy-proven stage IIIA, IIIB or IV NSCLC were identified without missing data. 18,701 (6.2%) of these patients had surgery as part of their treatment regimen (Figure 3). In the surgical cohort, the most common treatment regimen was chemotherapy, radiation and surgery. In the nonsurgical cohort, the most common treatment regimen was chemotherapy and radiation. The surgical cohort was 57% (10,650) stage IIIA, 19% (3,483) stage IIIB, and 24% (4,568) stage IV. As expected, the surgical cohort differed from the nonsurgical cohort for all variables (Table 1).
Figure 3.
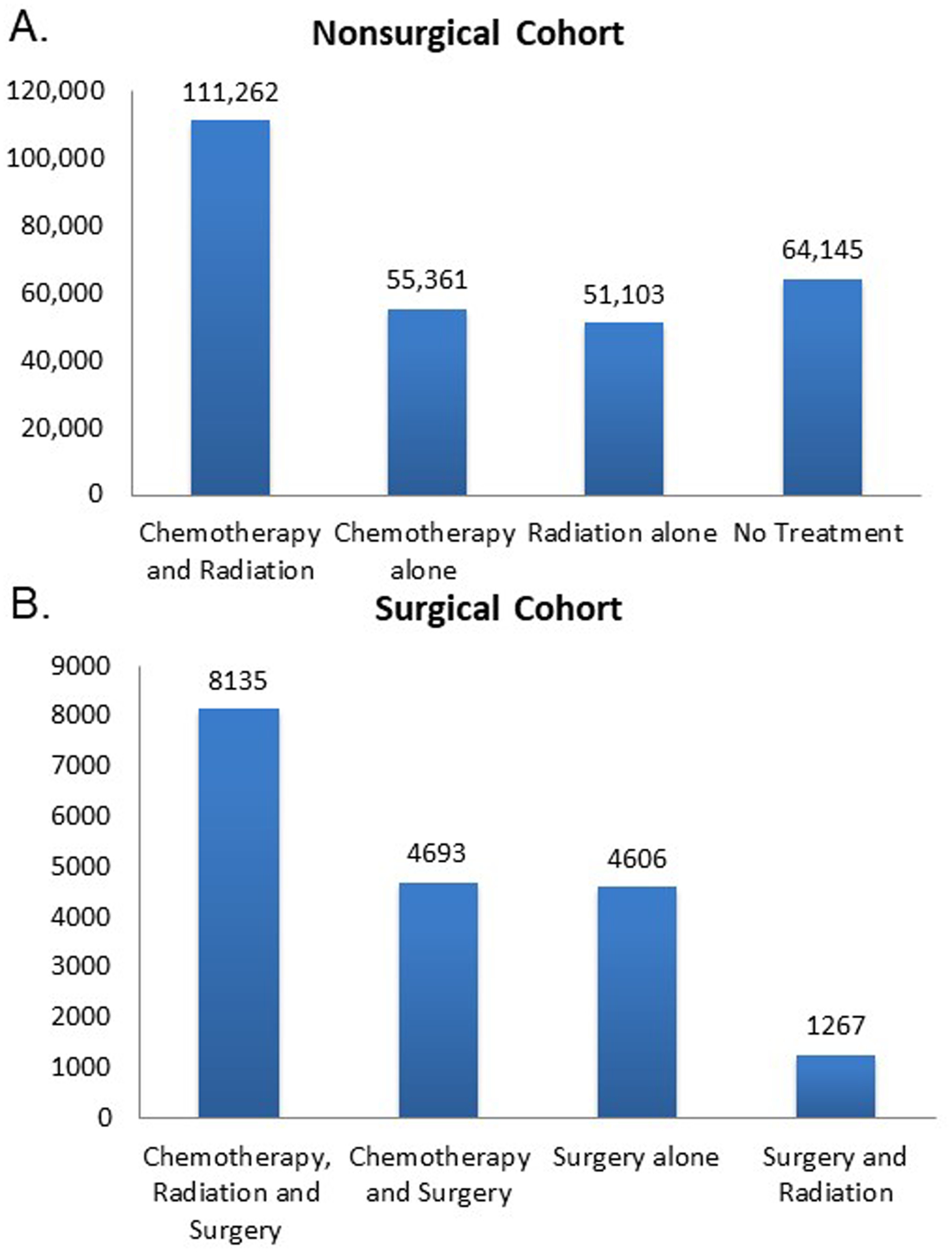
Distribution of treatment regimens in surgically and nonsurgically treated advanced stage NSCLC patients. The nonsurgical cohort is 94% of the total and the surgical cohort is 6%.
The AUROCs from the regression models are shown in Table 2. For all of the models, the AUROCs in the training and validation sets were not significantly different. Model 6, which includes histology, tumor size, AJCC tumor status, AJCC nodal status, AJCC metastatic status, Charlson Deyo Index, age, race, facility type, insurance type and income, was chosen as the final model since it had the lowest Bayesian information criterion (BIC), indicating that it was the best performing model (Table 3). Similar results were generated using different random seeds to split the dataset into different training and validation sets. The factors that most heavily influenced the decision to select a patient for surgery were AJCC metastatic status, AJCC nodal status and patient age (Table 3).
Table 2.
Area Under the Receiver Operating Characteristic Curve for each logistic regression model with goodness of fit assessment.
| Model | TrainSet AUROC | 95% CI | ValidSet AUROC | 95% CI | Chi Square Test | p-value | TrainSet BIC | ValidSet BIC |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.77 | 0.76–0.77 | 0.77 | 0.76–0.77 | 0.14 | 0.71 | 61119 | 61043 |
| 2 | 0.81 | 0.80–0.81 | 0.81 | 0.81–0.81 | 0.36 | 0.55 | 58448 | 58522 |
| 3 | 0.81 | 0.80–0.81 | 0.81 | 0.81–0.81 | 0.35 | 0.56 | 58409 | 58470 |
| 4 | 0.82 | 0.82–0.83 | 0.83 | 0.82–0.83 | 0.14 | 0.71 | 56965 | 57053 |
| 5 | 0.83 | 0.83–0.83 | 0.83 | 0.83–0.83 | 0.06 | 0.81 | 56414 | 56471 |
| 6 | 0.83 | 0.83–0.83 | 0.83 | 0.83–0.83 | 0.05 | 0.82 | 56381 | 56436 |
TrainSet =Training Set, ValidSet=Validation Set AUROC = Area Under Receiver Operating Characteristic Curve, 95% CI= 95% confidence interval, BIC= Bayesian information criterion.
Table 3.
Association between Surgical Treatment and Patient Variables for the model creating the Surgical Selection Score (SSS).
| Variable | Odds ratio (95% CI) | Sample patient SSS* | Variable | Odds ratio (95% CI) | Sample patient SSS* |
|---|---|---|---|---|---|
| Clinical T Status | Charlson Index | ||||
| 1 | 2.8 (2.6–3.0) | 0 | Reference | 0 | |
| 2 | 3.1 (3.0–3.3) | 1 | 1.3 (1.3–1.3) | ||
| 3 | 2.4 (2.3–2.6) | 2 | 1.0 (1.0–1.1) | ||
| 4 | Reference | 0 | Race | ||
| X | 1.2 (1.1–1.3) | White | 1.4 (1.3–1.5) | ||
| Tumor Size | Other | 1.4 (1.2–1.5) | |||
| T1a | 2.2 (2.1–2.3) | Black | Reference | 0 | |
| T1b | 1.7 (1.6–1.8) | Insurance Status | |||
| T2a | 1.2 (1.1–1.2) | Private | 2.0 (1.9–2.2) | ||
| T2b | 1.0 (1.0–1.1) | Medicare | 1.6 (1.4–1.7) | ||
| T3 | Reference | 0 | Medicaid | 1.2 (1.1–1.4) | |
| Clinical Nodal Status | Other Government | 1.2 (1.0–1.4) | |||
| 0 | 14.3 (13.2–15.5) | 266 | Not Insured | Reference | 0 |
| 1 | 11.4 (10.4–12.4) | Income | |||
| 2 | 4.7 (4.4–5.1) | High | 1.2 (1.1–1.2) | ||
| 3 | Reference | Middle | 1.0 (1.0–1.1) | ||
| X | 5.7 (5.2–6.4) | Low | Reference | 0 | |
| Clinical M Status | Facility Type | ||||
| 0 | 15.6 (14.3–17.1) | 275 | Academic/Research Program | 1.7 (1.6–1.8) | |
| 1 | 1.9 (1.7–2.1) | Compreh. Com. Cancer Program | 1.2 (1.2–1.3) | ||
| 1A | 2.1 (1.8–2.4) | Community Cancer Program | Reference | 0 | |
| 1B | Reference | ||||
| Histology | |||||
| Squamous Cell Carcinoma | 1.8 (1.7–1.9) | ||||
| Adenocarcinoma | 2.2 (2.1–2.3) | ||||
| NSCLC, NOS | Reference | 0 | |||
| Age group | |||||
| <52 | 6.1 (5.6–6.7) | 181 | |||
| 52–59 | 4.9 (4.5–5.4) | ||||
| 59–67 | 4.1 (3.8–4.5) | ||||
| 67–75 | 3.4 (3.1–3.7) | ||||
| 75–81 | 2.3 (2.1–2.5) | ||||
| >81 | Reference | ||||
| Surgical Selection Score | 722 |
The SSS was created by multiplying the logarithm of the odds ratios by 100 and adding the total. The sample SSS is provided for a 50 year old, black man with Stage IIIA (T4N0M0) NSCLC NOS, with Charlson Index =0 and belonging to the reference group for all remaining factors. His SSS is 722 and estimated probability of surgical treatment is 4.5%. (Sample SSS calculation for the patient described: ((ln 14.3 × 100) + (ln 15.6 × 100) + (ln 6.1 × 100)) = 722).
Over the entire cohort, SSS had a bell-shaped distribution with range 43–1141, and likelihood of surgery increased sharply in each stage at higher SSS (Figure 4). As expected, patients with stage IIIA disease had higher SSS and higher probability of surgery (19.5%) than patients with stage IIIB or stage IV disease. Additionally, SSS was a good predictor of OS, with patients with the highest SSS surviving longest (Figure 5). Importantly, within each quartile of SSS, patients in the surgical group also had significantly longer survival compared to non-surgical patients (p<0.001). For patients in the lowest quartile of SSS, the median survival time (MST) was 9.1 (95% CI 8.1–10.2) months in surgical patients vs. 4.2 (95% CI 4.2–4.3) months in nonsurgical patients (p<0.0001). In the highest quartile of SSS, MST was 35.7 (95% CI 34.6–36.9) months for surgical patients compared to 12.5 (95% CI 12.4–12.7) months for nonsurgical patients (p<0.0001).
Figure 4.
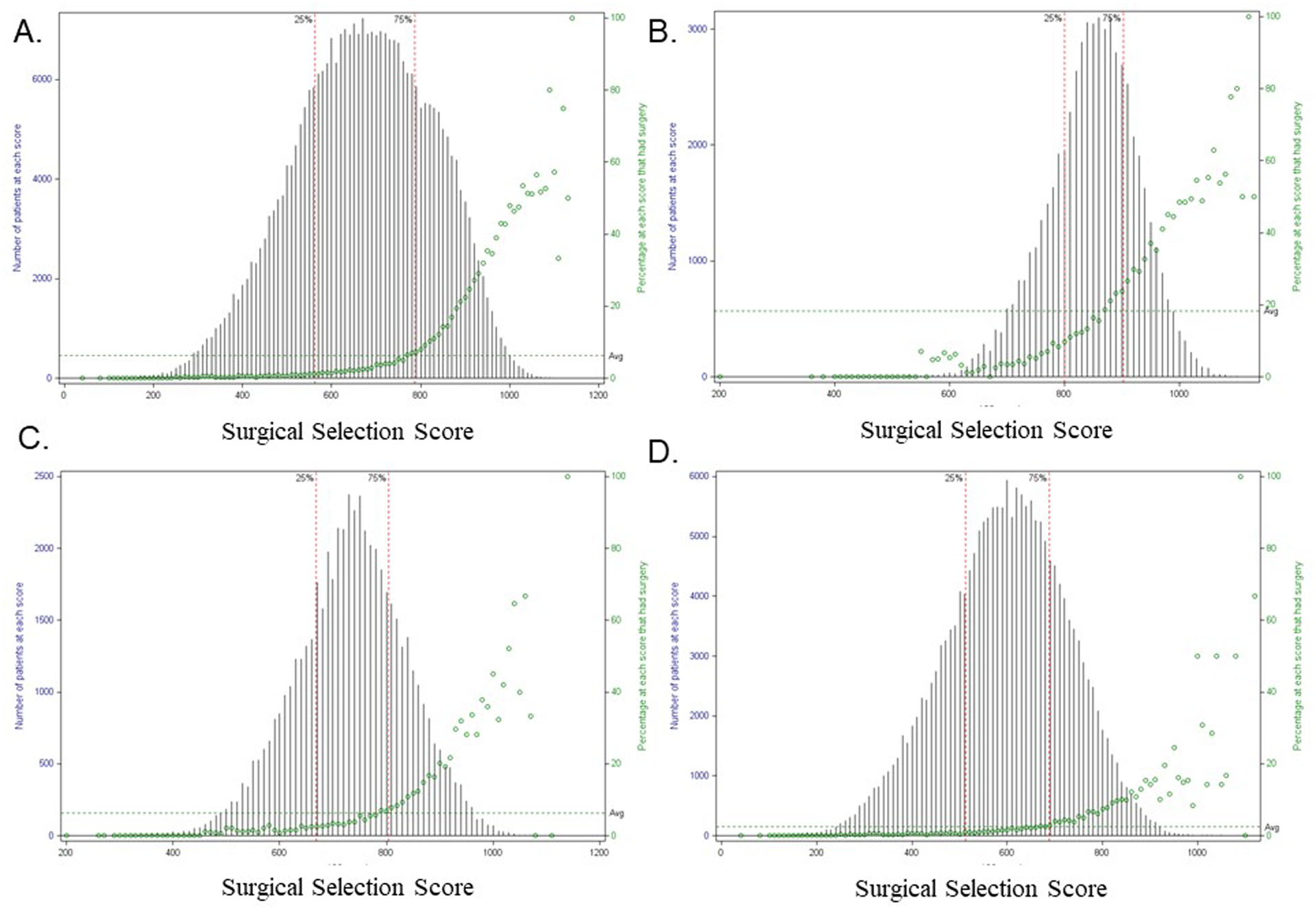
Distribution of Surgical Selection Score and probability of undergoing surgery by Stage. A. Entire cohort. B. Stage IIIA. C. Stage IIIB. D. Stage IV.
Figure 5.
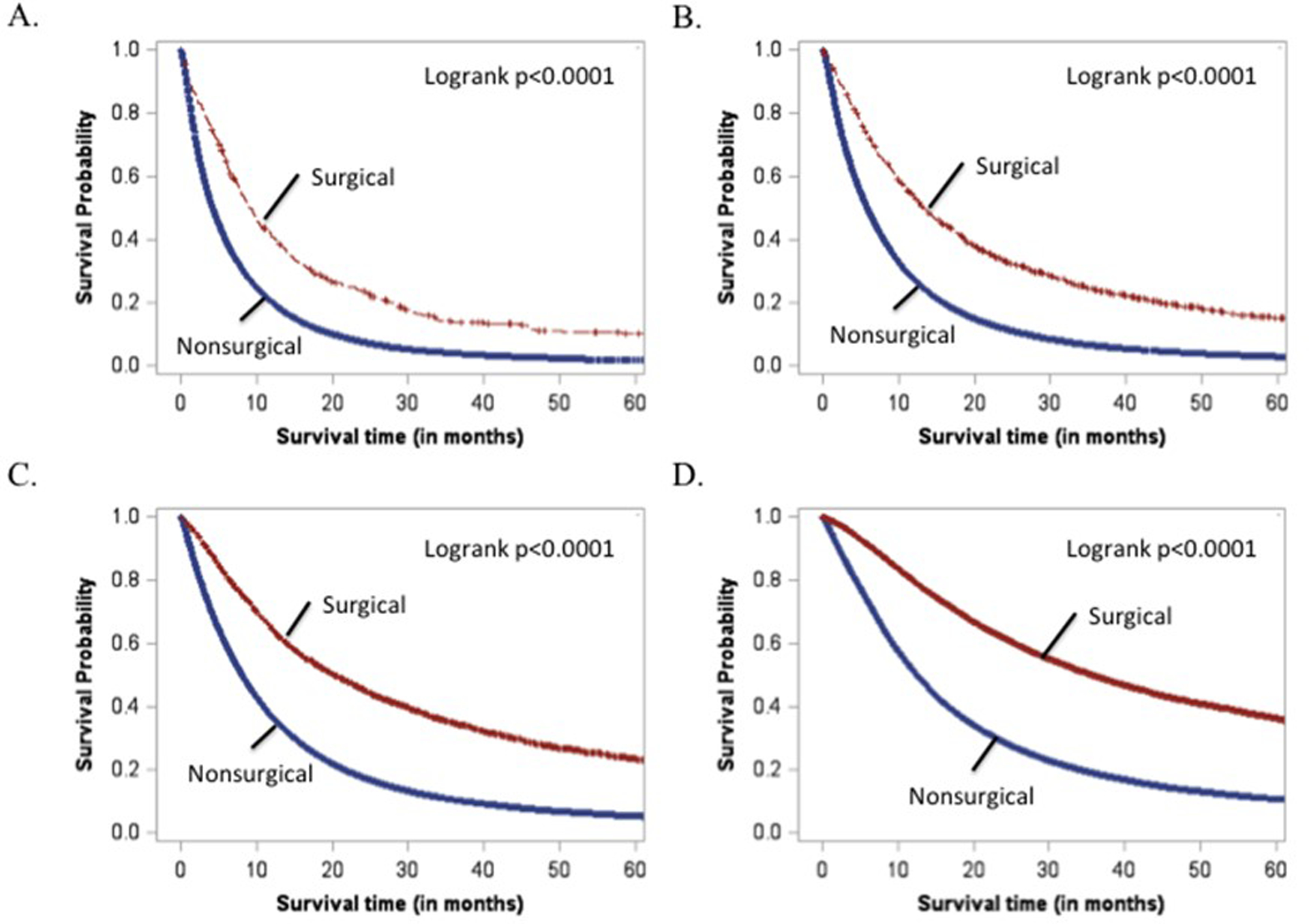
Kaplan-Meier analysis of patients stratified by treatment group. A. Lowest SSS quartile B. 2nd SSS quartile C. 3rd SSS quartile D. 4th SSS quartile.
Comment
This is the first study to develop a quantitative model predictive of selection for surgical treatment for patients with advanced stage NSCLC. Using detailed clinical variables, readily available at the time of treatment decision making for advanced stage NSCLC patients, we developed the SSS that predicts the use of surgery for these patients and found evidence that a therapeutic effect of surgery remains after accounting for this SSS. Survival for advanced stage NSCLC is only 4%, and outcomes are generally governed by response to systemic therapy.[1] Yet, the inclusion of surgery into multimodality treatment regimens has been reproducibly associated with improved outcomes.[3–8,15] A recurrent criticism of these studies is that the observation of improved survival with surgery is attributable to selection bias for patients with favorable characteristics (fewer comorbidities, lower volume disease) rather the surgical treatment. Yet, it is possible that there is a therapeutic effect of surgery, which remains obscured by the selection bias, and that selection bias represents a component part rather than the sum total of the improved outcomes observed in surgically-treated patients.
Overall, AJCC metastatic status, AJCC nodal status and age were the strongest predictors of patient selection for surgery in the SSS. Intentionally, AJCC stage group (as a single variable) was not used in the SSS. This will allow for use of the SSS in patients whose stage group may be in question at the time of treatment decisions. Our findings are not surprising as these factors are known to favorably influence prognosis, reinforcing the concept that patients with an expectation of greater survival are offered higher risk treatments. In fact, the independent relationship between lower metastatic status, nodal status, and younger age with longer survival is well established in both clinical trials and previously created prediction models.[7,15–17]
For example, in a pooled analysis of patients from North Central Cancer Treatment Group Trials, a prediction model for survival and time to progression (TTP) was developed for stage IIIB and IV patients which was developed to screen patients for benefit from phase II clinical trials.[17] High white blood cell count, anemia, decreased performance status, body mass index <18.5, and stage IV disease were predictive of worse OS and TTP. Although these individual-level clinical factors are not currently available in the NCDB, this model was developed in only 1,053 patients and did not include stage IIIA patients in contrast to the SSS. A second prediction model of survival for patients with NSCLC was developed by Schild et al. In this model, quality of life, age, performance status, primary tumor diameter, nodal status, distant metastases and smoking cessation were significant prognostic factors for OS.[16] These authors found longer survival in patients with higher scores on their prediction model, but the model was not used to assess for likelihood of receiving surgery or the impact of surgery on outcomes after controlling for prognostic factors.
In our study, stratification of patients by SSS score not only demonstrates longer survival in patients with higher SSS scores, but also demonstrates superior OS for patients receiving surgery compared to non-surgical patients after controlling for SSS. Notably, we observed 2 – 3 fold higher MST for surgical patients within all quartiles of SSS. Although these results are similar to other studies which have demonstrated improved survival for advanced stage NSCLC patients who have surgery included in their treatment regimens, this is the first study to derive and quantitate a SSS which allows for a comparison of both selection bias on survival outcomes as well as survival outcomes among patients who have similar probabilities of receiving surgery.[4–6]
A key strength of this study is the use of detailed clinical information from both operative and nonoperative cohorts using a robust and statistically powerful sample. This approach avoids potential pitfalls of highly curated specialty or institutional databases which may provide granular data but are limited by institutional and referral biases which may limit the generalizability of their findings.[10,18]
We recognize that there are many prediction models that have been developed to predict outcomes for NSCLC patients, including: survival after resection, pathologic N2 disease, postoperative morbidity and recurrence, and selection of proper candidates for postoperative radiation with pathologic N2 disease. However, no studies have used selection for surgical therapy as their primary outcome.[19–23].
As with other studies using administrative/registry datasets such as the NCDB, it is important to acknowledge limitations. A principle limitation of our study is that the intent for which surgery was performed was not specified in the dataset. For this reason, we prospectively limited the surgical procedures we included in the analysis to those, which would have likely been undertaken for curative intent and/or improved oncologic outcome rather than palliation or diagnosis. However, despite this inclusion criterion, it is possible that some diagnostic procedures were analyzed. Given that outcomes remained superior in the surgical cohort, we do not think that this potential bias was a major determinant of outcomes since the null hypothesis was nevertheless rejected. Additionally, the NCDB does not currently capture data on patient smoking status. Although smoking status is a predictor of perioperative morbidity/mortality and influences surgical decision-making, we do not believe that this is a major factor in surgical selection, particularly if smoking cessation can be achieved. This is an important question for future research.[24,25] Although the SSS has potential application to guide therapeutic decision-making, further work is also needed before it can be applied in this fashion.
In conclusion, we have created a surgical selection score which predicts with high accuracy the likelihood of undergoing surgery in advanced NSCLC patients. When stratified by SSS, we demonstrate that patients undergoing surgery have superior survival within all quartiles of the SSS. Once further validated using additional datasets and prospectively tested in clinical settings, we believe this model can be utilized to identify advanced stage NSCLCpatients who will likely benefit from consideration for surgical intervention as part of multimodality therapy.
Funding sources:
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR000002. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
This work was directly supported by the Department of Surgery Outcomes Research Group.
The American Cancer Society Institutional Research Grant (ACS IRG-95-125-13).
Footnotes
Meeting Affiliation: STS Annual Meeting 2017, Houston, TX
References
- [1].SEER Stat Fact Sheets: Lung and Bronchus Cancer. Surveillance, Epidemiol End Results Progr Cancer Stat 2016. http://seer.cancer.gov/statfacts/html/lungb.html (accessed December 26, 2016). [Google Scholar]
- [2].NCCN Guidelines for NSCLC n.d http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (accessed January 1, 2016). [Google Scholar]
- [3].David EA, Canter RJ, Chen Y, Cooke DT, Cress R, Chen Y, et al. Surgical Management of Advanced Stage NSCLC is Decreasing but Remains Associated with Improved Survival. Ann Thorac Surg 2016. doi: 10.1016/j.athoracsur.2016.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bott MJ, Patel AP, Crabtree TD, Morgensztern D, Robinson CG, Colditz GA, et al. Role for Surgical Resection in the Multidisciplinary Treatment of Stage IIIB Non-Small Cell Lung Cancer. Ann Thorac Surg 2015;99:1921–8. doi: 10.1016/j.athoracsur.2015.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Patel AP, Crabtree TD, Bell JM, Guthrie TJ, Robinson CG, Morgensztern D, et al. National patterns of care and outcomes after combined modality therapy for stage IIIA non-small-cell lung cancer. J Thorac Oncol 2014;9:612–21. doi: 10.1097/jto.0000000000000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Herskovic A, Chitti B, Christos P, Wernicke AG, Parashar B. Addition of Surgery After Radiation Significantly Improves Survival in Stage IIIB Non-small Cell Lung Cancer: A Population-Based Analysis. World J Surg 2016. doi: 10.1007/s00268-016-3764-y. [DOI] [PubMed] [Google Scholar]
- [7].Albain KS, Swann RS, Rusch VW, Turrisi AT, Shepherd FA, Smith C, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379–86. doi: 10.1016/S0140-6736(09)60737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gomez DR, Blumenschein GR, Lee JJ, Hernandez M, Ye R, Camidge DR, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol 2016;0:578–83. doi: 10.1016/S1470-2045(16)30532-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Samson P, Patel A, Robinson CG, Morgensztern D, Chang SH, Colditz GA, et al. The Role of Surgical Resection in Stage IIIA Non-Small Cell Lung Cancer: A Decision and Cost-Effectiveness Analysis. Ann Thorac Surg 2015. doi: 10.1016/j.athoracsur.2015.05.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bilimoria K, Stewart A, Winchester D, Ko C. The National Cancer Data Base: A Powerful Initiative to Improve Cancer Care in the United States. Ann Surg Oncol 2008;15:683–90. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- [12].Connelly CR, Laird A, Barton JS, Fischer PE, Krishnaswami S, Schreiber M a., et al. A Clinical Tool for the Prediction of Venous Thromboembolism in Pediatric Trauma Patients. JAMA Surg 2015;97239:1. doi: 10.1001/jamasurg.2015.2670. [DOI] [PubMed] [Google Scholar]
- [13].Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation 2007;115:928–35. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]
- [14].Schwarz G Estimating the dimension of a model. Ann Stat 1978;6:461–4. doi: 10.1214/aos/1176344136. [DOI] [Google Scholar]
- [15].Ziel E, Hermann G, Sen N, Bonomi P, Liptay MJ, Fidler MJ, et al. Survival Benefit of Surgery after Chemoradiotherapy for Stage III (N0–2) Non–Small-Cell Lung Cancer Is Dependent on Pathologic Nodal Response. J Thorac Oncol 2015;10:1475–80. doi: 10.1097/JTO.0000000000000639. [DOI] [PubMed] [Google Scholar]
- [16].Schild SE, Tan AD, Wampfler JA, Ross HJ, Yang P, Sloan JA. A new scoring system for predicting survival in patients with non-small cell lung cancer. Cancer Med 2015;4:1334–43. doi: 10.1002/cam4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mandrekar SJ, Schild SE, Hillman SL, Allen KL, Marks RS, Mailliard JA, et al. A prognostic model for advanced stage nonsmall cell lung cancer: Pooled analysis of north central cancer treatment group trials. Cancer 2006;107:781–92. doi: 10.1002/cncr.22049. [DOI] [PubMed] [Google Scholar]
- [18].Jacobs JP, Shahian DM, Prager RL, Edwards FH, McDonald D, Han JM, et al. The Society of Thoracic Surgeons National Database 2016 Annual Report. Ann Thorac Surg 2016;102:1790–7. doi: 10.1016/j.athoracsur.2016.10.015. [DOI] [PubMed] [Google Scholar]
- [19].Birim O, Kappetein AP, Waleboer M, Puvimanasinghe JP, Eijkemans MJ, Steyerberg EW, et al. Long-term survival after non-small cell lung cancer surgery: development and validation of a prognostic model with a preoperative and postoperative mode. J Thorac Cardiovasc Surg 2006;132:491–8. doi: 10.1016/j.jtcvs.2006.04.010. [DOI] [PubMed] [Google Scholar]
- [20].Farjah F, Lou F, Sima C, Rusch VW, Rizk NP. A prediction model for pathologic N2 disease in lung cancer patients with a negative mediastinum by positron emission tomography. J Thorac Oncol 2013;8:1170–80. doi: 10.1097/JTO.0b013e3182992421. [DOI] [PubMed] [Google Scholar]
- [21].Liang W, Zhang L, Jiang G, Wang Q, Liu L, Liu D, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol 2015;33:861–9. doi: 10.1200/JCO.2014.56.6661. [DOI] [PubMed] [Google Scholar]
- [22].Zhang Y, Sun Y, Xiang J, Zhang Y, Hu H, Chen H. A clinicopathologic prediction model for postoperative recurrence in stage Ia non-small cell lung cancer. J Thorac Cardiovasc Surg 2014;148:1193–9. doi: 10.1016/j.jtcvs.2014.02.064. [DOI] [PubMed] [Google Scholar]
- [23].Hui Z, Dai H, Liang J, Lv J, Zhou Z, Feng Q, et al. Selection of proper candidates with resected pathological stage IIIA-N2 non-small cell lung cancer for postoperative radiotherapy. Thorac Cancer 2015;6:346–53. doi: 10.1111/1759-7714.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Marino KA, Little MA, Bursac Z, Sullivan JL, Klesges R, Weksler B. Operating on Patients Who Smoke: A Survey of??Thoracic Surgeons in the United States. Ann Thorac Surg 2016;102:911–6. doi: 10.1016/j.athoracsur.2016.03.076. [DOI] [PubMed] [Google Scholar]
- [25].Mason DP, Subramanian S, Nowicki ER, Grab JD, Murthy SC, Rice TW, et al. Impact of Smoking Cessation Before Resection of Lung Cancer: A Society of Thoracic Surgeons General Thoracic Surgery Database Study. Ann Thorac Surg 2009;88:362–71. doi: 10.1016/j.athoracsur.2009.04.035. [DOI] [PubMed] [Google Scholar]


