Abstract
Integrated immunometabolic responses link dietary intake, energy utilization, and storage to immune regulation of tissue function and is therefore essential for the maintenance and restoration of homeostasis. Adipose-resident leukocytes have non-traditional immunological functions that regulate organismal metabolism by controlling insulin action, lipolysis, and mitochondrial respiration to control the usage of substrates for production of heat versus ATP. Energetically expensive vital functions such as immunological responses might have thus evolved to respond accordingly to dietary surplus and deficit of macronutrient intake. Here, we review the interaction of dietary intake of macronutrients and their metabolism with the immune system. We discuss immunometabolic checkpoints that promote healthspan and highlight how dietary fate and regulation of glucose, fat, and protein metabolism might affect immunity.
Dietary intake and metabolic fate of carbohydrates, fats, and proteins have a profound effect on immune cell function. Lee and Dixit review the current understanding of the bi-directional communication between immune and metabolic systems and reveal potential opportunities for harnessing immunometabolic checkpoints in health and disease.
Introduction
The hominids that were destined to become humans evolved with larger and intensive energetic demands for brain function. Host survival thus required mechanisms that balance the energetic costs of essential functions such as successful immune response against infections and tissue repair. Accordingly, humans have developed an integrated immunometabolic response (IIMR) that involves sensing of nutrient balance by neuronal (sympathetic and sensory innervation) and humoral signals (e.g., hormones like insulin, incretins, FGF21, GDF15, ghrelin, and leptin) between the hypothalamus and peripheral tissues to allow the host to prioritize storage and/or utilize substrates for tissue growth, maintenance, and immune responses. The evolutionary pressure to maintain healthy adiposity concurrently with tissue-protective inflammatory responses to injuries and infections are important drivers of IIMR. It has been hypothesized that because energy-rich nutrients have been scarce throughout most of human evolution, dominant genetic pathways evolved to favor increasing intake of calorie-rich diets and storage of energy as triglycerides in adipose tissue. Within this evolutionary pressure to store calories, consumption of energy-rich diets in the modern world has given rise to obesity. Excessive adiposity or obesity with a body mass index (BMI) of > 30, is a multisystem disorder resulting from chronic caloric intake and perturbation of IIMR. A large body of literature suggests that altered IIMR in obesity causes a chronic proinflammatory state that is linked to diseases such as type 2 diabetes, non-alcoholic steatohepatitis, and cardiovascular disease (Christ et al., 2019). In addition, obesity impairs adaptive immune responses and is a major risk factor for mortality and morbidity from H1N1 influenza (Van Kerkhove et al., 2011).
In opposition to obesity, negative energy balance without malnutrition is induced by restriction of calories and is one of the most effective means for lifespan and healthspan extension in multiple species (Anderson et al., 2017; Weindruch and Sohal, 1997). Caloric restriction (CR) limits glucose availability, and therefore the host must engage fatty acid oxidation to generate ATP. The effector immune functions that rely on glucose as their primary substrates are most likely modulated by such dietary interventions, and these interventions could be promising avenues to prevent or treat diseases that involve hyperinflammatory responses. It is recognized that severe reduction in nutrient and energy intake might cause tradeoffs in non-essential functions. As posited by the disposable soma theory, this involves diversion of resources from host growth and reproduction toward cellular maintenance, and when nutrients become available again, energetically costly pathways dedicated for host defense can be re-engaged (Kirkwood et al., 2000). Which scenarios induce such a tradeoff and whether the immune system is indeed dispensable when energy is limiting are open questions. However, emerging evidence suggests that immune response to infectious challenges is maintained during moderate dietary restriction (Collins et al., 2019; Jordan et al., 2019; Nagai et al., 2019) and is boosted when carbohydrate deficient, high-fat diet (HFD) intake induces ketogenesis (Goldberg et al., 2019). Surprisingly, restriction of proteins, including the restriction of essential amino acid methionine in adult life extends lifespan in rodents by almost 40%–50% (Orentreich et al., 1993; Solon-Biet et al., 2014). The biochemical metabolic pathways engaged by such dietary alterations could hold the keys to identifying as of yet unknown mechanisms that could be harnessed to control reparative responses, inflammation, and immune surveillance. In this review, we discuss the interaction of dietary intake of macronutrients and their metabolic fate on immune system including adipose-tissue-resident leukocytes in regulation of inflammation and organismal metabolism.
Immune Defense in Diet-Induced Obesity
Modern diets are rich in saturated fats and processed carbohydrates, such as high fructose corn syrup, and are deficient in fiber, vitamins, and minerals, while containing high levels of salt. These diets are a leading cause of the emergence of obesity-associated chronic diseases, the majority of which are linked to chronic inflammation (Bray et al., 2017). A vast number of studies have described a predominant role of inflammation, originating from visceral adipose tissue and lipotoxicity, in engaging innate immune cells that lead to development of type 2 diabetes through their production of tumor necros factor-α (TNF-α), interleukin-1β (IL-1β), and IL-6 (Donath and Shoelson, 2011; Hotamisligil, 2006; Saltiel and Olefsky, 2017). There is however, also increasing evidence of obesity as an independent risk factor for dysregulated adaptive immune response against various infections. The retrospective analyses after the 2009 H1N1 pandemic across the globe revealed obesity to be co-morbid with influenza in nearly one-third of hospitalized patients with increased fatality after infection (Van Kerkhove et al., 2011). The mechanisms by which obesity disrupts immune response and recovery from influenza are manifold and represent the effect of chronic metabolic perturbation on multiple cell types. Diet-induced obese (DIO) mice have reduced amounts of H1N1-specific antibodies as well as lower neutralization response during influenza infection (Honce et al., 2020). Whether obesity affects germinal center reactions, class-switching of antibodies, or the B cell repertoire remains unknown. There are data that suggest that obesity restricts T cell receptor (TCR) repertoire diversity (Yang et al., 2010) and decreases the number of T cell receptor excision circles (TRECs) in the blood of obese and diabetic individuals, suggesting reduced thymic generation of naive T cells (Yang et al., 2009b).
In addition, the differentiation of naive T cells (CD4 and CD8) into an effector state upon antigen recognition is dependent on glucose and glutamine (Jacobs et al., 2008; Michalek et al., 2011; Pearce et al., 2009; Wang et al., 2011). It is thus likely that hyperglycemia and dyslipidemia seen in obesity affects T cell immune activation against influenza infection. Interestingly, HFD feeding expands γδ T cells in the lungs that are IL-17 competent but lack a tissue-protective transcriptional profile and fail to confer protection against influenza (Goldberg et al., 2019). Whether altered lipid versus glucose metabolism drives dysfunctional γδ T responses in obesity remains to be characterized.
Furthermore, effective memory T cell response relies on efficient transition from glycolytic to a more oxidative metabolism (Pearce et al., 2009), although this distinction might not be binary in vivo. Recent studies using 13C-based stable isotope labeling techniques in T cells highlight that glucose use by CD8+ cells is dynamically regulated during the course of an immune response to Listeria infection and identified that glucose-dependent serine biosynthesis as a key metabolic program for optimal T cell expansion in vivo (Ma et al., 2019). How diet-induced obesity affects the metabolic reprogramming of T cells in vivo in bacterial or viral infection requires further investigation. Initial analyses suggest that indeed altered glycolytic and oxidative respiration in memory T cells of obese animals is a likely mechanism influencing influenza disease susceptibility (Rebeles et al., 2019). In addition, Mauro et al. (2017) found that metabolic stress induced by saturated fatty acids affects T cell differentiation and causes preferential trafficking of CD4+ T cells to non-lymphoid effector sites in obesity. Palmitate drove CD4+ T cells to acquire a specific CD44hiCCR7loCD62LloCXCR3+LFA1+ pro-inflammatory dysfunctional phenotype via the activation of a PI3K p110δ-Akt-dependent pathway (Mauro et al., 2017). Interestingly, adipose tissue serves as a reservoir of memory T cells specific to several viruses including but not limited to lymphocytic choriomeningitis virus (LCMV) and HIV (Damouche et al., 2015), as well as bacteria like Yersenia and protozoa like Toxoplasma (Han et al., 2017). It is possible that antigen-specific T cells might enter the adipose tissue to potentially resolve the infection but then remain as a memory population distinct from memory T cells in classical sites of immune response. Formal studies would be required to test the hypothesis that the adipose microenvironment shapes the specific transcriptional signatures of memory phenotype that is compromised in obesity. It is, however, known that in obese mice, upon re-challenge to infection, adipose memory T cells upregulate lipases and cause severe disease including calcification of adipose tissue, pancreatitis, and reduced survival (Misumi et al., 2019). Concernedly, data from the COVID-19 pandemic has identified obesity and diabetes as a major risk factor in disease severity and mortality (Drucker, 2020) (Box 1 ). Given that in the US, over one third of adults are obese, urgent research efforts are needed to determine immunoregulatory mechanisms that affect immune response, disease tolerance, treatment, and potential vaccination failure in this large high-risk population.
Box 1. Immunometabolic Regulators of COVID-19 Severity.
In addition to old age, metabolic syndrome, diabetes, and obesity are independent risk factors for increased severe acute respiratory syndrome coronavirus 2 (SARS-COV-2)-induced mortality and morbidity. Given the prevalence of obesity is 10% among younger adults aged 20-39, 45% among adults aged 40–59 years, and 43% among older adults aged 60 and over (Hales et al., 2020), there is urgent need for studies that define the mechanism of aberrant immune response that promotes predisposition to infection and disease. Recent analyses show that 47% of cases of COVID-19 hospitalization represented obese patients with BMI >30. Moreover, 85% of patients above the BMI of 35 developed hyperinflammatory response in lungs that required mechanical ventilation to boost oxygen saturation (Simonnet et al., 2020). Macrophage activation and neutrophil influx mediate a hyperinflammatory response in the lungs in COVID-19 (Zhou et al., 2020). Given obesity’s known effects on increasing inflammation, future studies will be needed to determine the immunological mechanism of diet-driven complications of SARS-COV-2 viral infection. Of note, NLRP3 inflammasome, a myeloid-cell-expressed multiprotein complex that senses pathogen-associated molecular patterns (PAMPS) and danger-associated molecular patterns (DAMPS) to cause IL-1β and IL-18 release, is activated in obesity, type-2 diabetes, atherosclerosis, and aging (Camell et al., 2015; Goldberg and Dixit, 2015). There is increasing evidence that SARS-COV-2 infection activates the NLRP3 inflammasome (Siu et al., 2019) with increased levels of IL-18 and lactate dehydrogenase (LDH) because of inflammasome-mediated pyroptotic cell death (Lucas et al., 2020; Zhou et al., 2020). Interestingly, SARS-COV-2 open reading frame 3a (ORF3a) and ORF8b activates the NLRP3 inflammasome (Siu et al., 2019) by inducing ER stress and lysosomal damage (Shi et al., 2019), mechanisms that are independently shown to be elevated in obesity. It is now known that increased glycolysis, which also induces transcriptional upregulation of inflammasome machinery, causes aberrant activation of myeloid cells, and worsens COVID-19 (Codo et al., 2020). This raises the question whether dietary approaches that mimic a glucoprivic state by feeding of a low carbohydrate, high-fat diet, which necessitates the switch from glycolysis to production of ketone metabolites, can be employed to stave off COVID-19. Indeed, ketone bodies inhibits the NLRP3 inflammasome (Youm et al., 2015) and ketogenic diet protects against influenza-induced lethality in mice by expanding γδ T cells (Goldberg et al., 2019). Thus, raising the possibility that harnessing specific immunometabolic checkpoints, such as glycolytic-to-ketogenic switch maybe relevant to several infections, including SARS-COV-2.
Adipose Tissue as an Immunological Organ
Of the metabolic organs responding in the IIMR during chronic positive energy balance (Figure 1 ), adipose tissue has been best characterized and strongly contributes to the pathogenesis of obesity. Obese adipose contains approximately 2 to 5 million stromal-vascular cells per gram, of which approximately 60%–70% are immune cells. In severe obesity, adipose tissue can expand to constitute up to 30%–50% of total body mass (Kanneganti and Dixit, 2012). Thus, the adipose tissue represents an underappreciated immunological organ that harbors distinct subpopulations of immune cells that localize to specific niches to perform potentially unique functions.
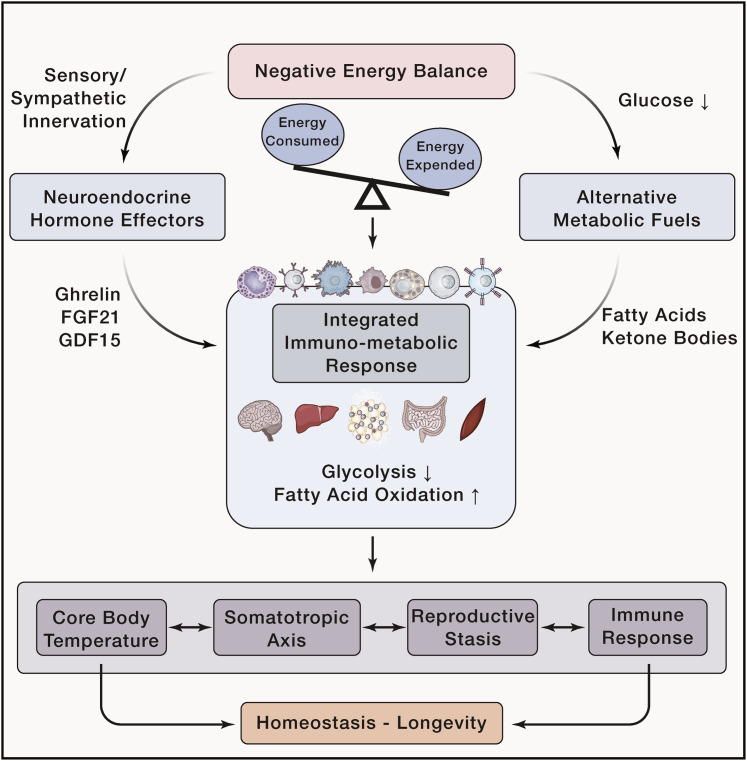
Figure 1.
Negative Energy Balance Drives Integrated Immunometabolic Responses to Counterbalance Homeostatic Processes to Promote Longevity Lower Disease Burden
Negative energy balance is achieved when energy consumed is less than energy expended. This drives neuroendocrine hormone effectors such as ghrelin, FGF21, and GDF15, and the usage of alternative metabolic fuels such as fatty acids and ketone bodies. These signals get integrated by organs such as the brain, liver, adipose tissue, gut, and skeletal muscle with the immune system to promote metabolic reprogramming that prioritizes fatty acid oxidation over glycolysis. These changes adjust the balance of normal physiological processes such as regulation of body temperature, growth, reproduction, and immune response in a way that promotes longevity and health.
Even before it was recognized that immune function could be regulated by cellular metabolism (Buck et al., 2015; Frauwirth et al., 2002; O’Neill et al., 2016), the discovery that obesity-induced insulin resistance was directly caused by elevated TNF levels in adipose tissues launched the field of immunometabolism, pioneering the association of the immune system with metabolic processes (Hotamisligil et al., 1993). Although, initially thought to be derived from adipocytes, later studies clarified that accumulation of macrophages in the adipose were the main drivers of inflammatory cytokine production (Lumeng et al., 2007; Weisberg et al., 2003). It has now been elucidated that during obesity, in addition to macrophages, there are large changes in the adipose immune landscape as certain cells are recruited and activated while other cell types are depleted and replaced, leading to a more pro-inflammatory environment that drives type 2 diabetes and chronic disease progression (Figure 2 ). Mechanistically, pro-inflammatory cytokines such as TNF, IL-6, and IL-1β have been shown to block insulin receptor signaling through inhibition of insulin receptor substrates (IRSs), by increasing their serine phosphorylation (Hotamisligil et al., 1996; Werner et al., 2004). Conversely, gain of function of inhibitor of nuclear factor-κB kinase (IKKβ) in the liver was found to reduce endoplasmic reticulum (ER) stress by elevated X-box binding protein 1 (XBP1) activity leading to an actual improvement in insulin sensitivity (Liu et al., 2016). Additional studies are needed to determine the delicate balance of chronic pro- and anti-inflammatory cytokine signaling required to repair and restore metabolic homeostasis.
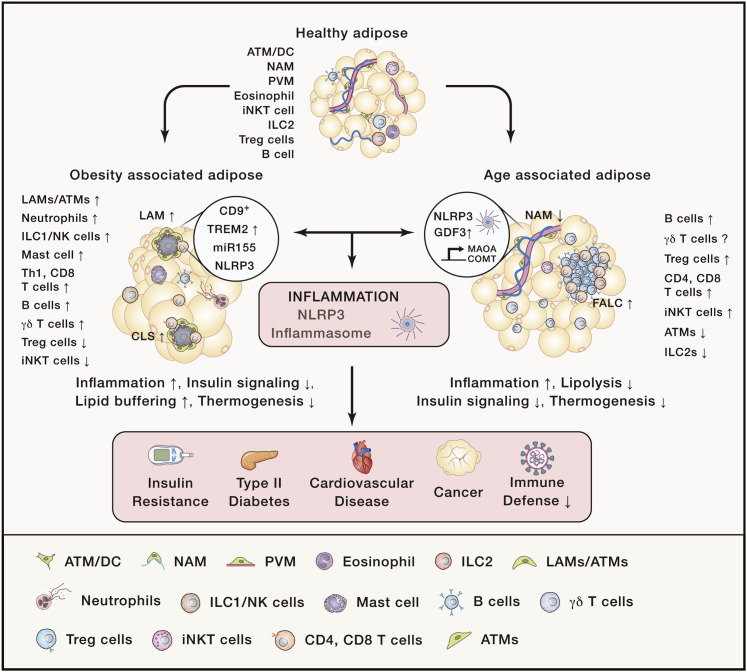
Figure 2.
Obesity and Aging Lead to Distinct Changes in the Immune Profile of Adipose Tissue to Drive Inflammation and Pathology
Healthy, lean adipose tissue is associated with anti-inflammatory immune subsets, such as CD206-expressing macrophage subsets (ATMs, NAMs, and PVMs), IL-4-expressing eosinophils, IL-10-expressing iNKT cells, B cells, Treg cells, and IL-5-expressing ILC2s. Energy imbalance leads to a disruption of immune and metabolic homeostasis, leading to an infiltration of inflammatory immune cells such as IL-6- and TNF-α-producing LAMs, elastase-producing neutrophils, IgG2C-producing B cells, degranulating mast cells, IFN-γ-producing CD4 T cells, IL-17-producing γδ T cells, and cytotoxic CD8 T cells. Accumulation of danger-associated molecular patterns (DAMPs) such as free fatty acids (FAs), oxidized LDL (oxLDL), cholesterol crystals, and islet amyloid polypeptides (IAPP) in obesity further drives the activation of NLRP3 inflammasomes. These changes in immune-mediated inflammation drive pathologies such as insulin resistance, type II diabetes, cardiovascular disease, and cancer while weakening immune defense against pathogens, leading to higher risk for premature death. Unlike obesity, which is characterized by an accumulation CLSs, where immune cells such as T cells and CD9+ macrophages associated with increased lipid processing and inflammation accumulate to deal with dying adipocytes, aging causes a reduction of adipose tissue macrophages that increase GDF3 and catecholamine degradation enzymes such as MAOA and COMT to promote lipolysis resistance. Aging also leads to an increase of FALCs, where B cells and T cells accumulate and contribute to dysregulated adipose homeostasis such as reduced insulin signaling and thermogenesis. Although γδ T cells increase with obesity, iNKT cells and Treg cells increase with age and decrease with obesity, and although no marked changes in eosinophils are reported in aged adipose, obesity is associated with significant decreases in eosinophil populations. These differences in immune profiles and immune lymphoid structures during aging and obesity suggest that distinct mechanisms contribute to the tissue dysfunction and pathologies associated with each condition.
Adipose Tissue Macrophages in Obesity
It is now understood that macrophages are resident immune cells found in almost every tissue and play key roles in maintaining homeostasis (Lavin et al., 2014; Okabe and Medzhitov, 2014). In healthy adipose tissue, macrophages are thought to constitute about 10% of the hematopoietic cell composition. However, it is possible that enzymatic digestion protocols of adipose tissue are not releasing all macrophages from their tissue niches and thus current analyses could be underestimating the total quantity of cells residing in fat. During obesity, an infiltration of monocytes and macrophages, local proliferation, reduced egress, and increased cell longevity leads to a further accumulation of macrophages (Amano et al., 2014; Hill et al., 2015; Nagareddy et al., 2014; Oh et al., 2012) so that more than 50% of the cells in the fat depot can be made up of macrophages (Lumeng et al., 2007; Weisberg et al., 2003).
Pro-inflammatory adipose tissue macrophages (ATMs) drive obesity-induced insulin resistance by activating kinases such as IκB Kinase (IKK) and c-Jun N-terminal kinase (JNK) (Arkan et al., 2005; Han et al., 2013; Solinas et al., 2007). ATMs that accumulate at crown-like structures (CLSs) further secrete exosomes containing miR155 that act on adipocytes to block insulin signaling (Ying et al., 2017). Ablation of pro-inflammatory CD11c+ ATMs during DIO reduces local and systemic pro-inflammatory cytokine amounts, reduces CLSs and infiltrating macrophages, and leads to rapid normalization of insulin sensitivity, thus highlighting the role of inflammatory ATMs in driving metabolic disease (Patsouris et al., 2008). However, ATMs can be divided into various populations of diverse lineages and functionality that respond dynamically to local metabolic cues within their unique niches. Although these macrophage subsets are still being defined, careful examination of the transcriptional profiles of different ATM groups have clarified that there are numerous ways to define macrophage subsets and most infiltrating macrophages in adipose tissue in chronic inflammation do not fit a simple M1/M2 polarization model (Camell et al., 2017; Kratz et al., 2014).
In lean adipose tissue, macrophages are a mixed heterogeneous population of yolk-sac-derived, self-maintaining macrophages and bone marrow (BM)-derived monocytes and macrophages that are continuously replenished (Amano et al., 2014; Hassnain Waqas et al., 2017; Schulz et al., 2012; Silva et al., 2019). Silva et al. (2019) have recently described four different groups of lean visceral ATMs. The group of macrophages that make up the majority of ATMs in lean adipose tissue express high amounts of CD206 and are CD11b+MHCII+Tim4+. Through BM chimera experiments, these macrophages were found to be largely tissue resident with minimal replenishment from the periphery (Amano et al., 2014; Silva et al., 2019). Surprisingly, these macrophages were found to be laden with lipids even in the lean state (Silva et al., 2019). One of the roles of macrophages in lean adipose is to clear dead adipocytes. ATMs form CLSs around dying adipocytes to clear the dying cell and lipids in a contained manner (Murano et al., 2008). Because adipocytes are too large to fully be engulfed by macrophages, ATMs release acidic exosomes laden with lysosomal enzymes that can take up and degrade large adipocyte fragments, which can then be taken up by the macrophages in a process called exophagy (Haka et al., 2016). This process allows for macrophages to internalize large amounts of lipids. During obesity, this process becomes increasingly overburdened because there is an increase in the number of dying adipocytes and CLSs. The accumulation of macrophages in response to an HFD is mostly attributed to an influx of peripheral BM-derived monocytes that quickly differentiate and proliferate locally (Amano et al., 2014; Hill et al., 2018; Jaitin et al., 2019; Nagareddy et al., 2014; Oh et al., 2012; Silva et al., 2019). The macrophages that accumulate with obesity are also heterogeneous and further studies might clarify the specific roles of each subset in disease progression (Hill et al., 2018; Jaitin et al., 2019).
Most of the infiltrating macrophages are found at CLSs and proliferate locally. They are largely CD9+ and are enriched with signatures for lipid metabolism, lysosomal biosynthesis, and phagocytosis (Hill et al., 2018; Jaitin et al., 2019; Xu et al., 2013). Transcriptomic profiling has identified that CD9+ macrophages that accumulate with diet-induced obesity also express CD163 and Trem2. Because of their association with enlarged adipocytes at CLSs, these macrophages have been named lipid-associated macrophages, or LAMs (Jaitin et al., 2019). Depletion of TREM2+ LAMs led to reduced lipid uptake by macrophages and increased adipocyte hypertrophy and weight gain and a worsening of glucose homeostasis, challenging the idea that infiltrating ATMs during obesity purely cause disease progression, but also play critical protective roles in the adaptation to metabolic stresses that occur during obesity (Jaitin et al., 2019).
Adipose tissue is highly vascularized. As adipocytes increase in size and number via hypertrophy and hyperplasia to store excess calories as triglycerides, hypoxia ensues as the diffusion limit of oxygen is reached. Pockets of local hypoxia leads to angiogenesis to meet the oxygen demands required for hypertrophy and increased mitochondrial respiration. Lyve1+ macrophages express MMP-7, MMP-9, and MMP-12 to remodel the extracellular matrix (ECM) and promote angiogenesis (Cho et al., 2007). In lean adipose, macrophages are found tightly wound around blood vessels as a subset of perivascular macrophages, or PVMs (Hilgendorf et al., 2019; Silva et al., 2019). These self-maintaining PVMs that are in direct contact with the vasculature make up a large proportion of macrophages in healthy adipose and express high amounts of CD206 (Silva et al., 2019). Surprisingly, PVMs store lipid droplets even in the lean state, and rapidly take up dextran and ovalbumin (OVA) from the circulation, suggesting that they are constantly surveilling and buffering lipids within the adipose circulation and might play important roles in presenting antigens and maintaining T cells within the adipose tissue (Silva et al., 2019). During obesity, PVMs change in morphology from an elongated shape to a rounded shape and have a reduction in their capacity to take up dextran, suggesting that their homeostatic functions are diminished in response to HFD (Silva et al., 2019). Given that PVMs might play important roles as antigen-presenting cells (APCs), how this might affect the interaction of PVMs with other immune populations during DIO remains to be elucidated.
Adipose tissue is also densely innervated by both sympathetic and sensory nerves, which are important in controlling lipolysis and thermogenesis (Bartness et al., 2014; Chi et al., 2018; Slavin and Ballard, 1978). Density of innervation varies among different fat depots, with brown adipose tissue (BAT) having the most, then subcutaneous adipose tissues (SFAT), and various visceral adipose tissues such as the mesenteric adipose and gonadal adipose tissues. Release of catecholamines such as norepinephrine (NE) by sympathetic nerves is essential for hydrolysis of triglycerides into fatty acids, a process called lipolysis that provides substrates for energetic and synthetic purposes. (Bartness et al., 2010). Interestingly, macrophages also reside on nerves in the adipose tissue as nerve-associated macrophages, or NAMs (Camell et al., 2017; Pirzgalska et al., 2017; Wolf et al., 2017). Although a significant portion of brown adipose NAMs are self-maintaining, long-lived resident macrophages, white adipose NAMs are more frequently replenished by BM-derived macrophages (Pirzgalska et al., 2017; Wolf et al., 2017). NAMs lack tyrosine hydroxylase and therefore do not produce catecholamines (Fischer et al., 2017; Pirzgalska et al., 2017; Spadaro et al., 2017). Instead, they express the canonical catecholamine transporter, Slc6a2, allowing for the uptake of catecholamines from the local environment and express catecholamine degradation enzymes such as monoamine oxidase A (MAOA) and catechol-o-methyl transferase (COMT) to control local availability of catecholamines (Camell et al., 2017; Pirzgalska et al., 2017). Furthermore, the expression of MAOA and COMT in NAMs is upregulated by NLR family pyrin domain containing 3 (NLRP3)-mediated inflammation during obesity and aging, leading to increased catecholamine degradation by NAMs to contribute to metabolic dysregulation (Camell et al., 2017). How NAMs integrate metabolic and immune sensing in adipose tissue in response to different diets is not well understood and is likely to reveal important non-traditional functions of macrophages in maintaining tissue homeostasis.
Effect of Diet-Induced Obesity on Adipose Leukocytes
Besides macrophages, many other immune cell types have been described in adipose tissue and in the context of obesity. Adipose tissue is relatively enriched with innate immune cells such as eosinophils, innate lymphoid cells (ILCs), invariate natural killer T (iNKT) cells, and γδ T cells and have been well described in other reviews (Kane and Lynch, 2019; Mraz and Haluzik, 2014). In brief, mast cells, ILC1s, γδ T cells, and neutrophils all increase in adipose tissue with obesity and are associated with inflammation. Mast cells increase in the visceral adipose tissue (VAT), particularly around CLSs and actively degranulate to release TNF-α, IL-6, and interferon-γ (IFN-γ) to promote obesity and diabetes (Altintas et al., 2011; Liu et al., 2009). Recently, Zhang et al. (2019) have demonstrated that dietary cholesterol leads to activation of mast cells, suggesting mast cells might be directly sensing and responding to dysregulated metabolites. Neutrophils are transiently increased during obesity and are one of the earliest cells to infiltrate adipose tissue and secrete elastase to contribute to pathology (Elgazar-Carmon et al., 2008; Talukdar et al., 2012). ILC1s or NK cells were found to contribute to obesity by producing IL-2 and IFN-γ to promote M1 skewing in macrophages, and displayed higher killing activity toward M2-like macrophages over M1-like macrophages (Boulenouar et al., 2017; O’Sullivan et al., 2016). In humans, CD1c+CD11c+CD83+ dendritic cells (DCs) increase in obese adipose and induce Th17 differentiation (Bertola et al., 2012).
γδ T cells are tissue resident and increase in proportion to fat mass during diet-induced obesity (Mehta et al., 2015). Mice lacking γδ T cells have reduced accumulation of pro-inflammatory macrophages and significant reductions in systemic insulin resistance, suggesting that γδ T cells play a role in driving the pro-inflammatory response during diet-induced obesity (Mehta et al., 2015). However, γδ T cells might also serve homeostatic functions in healthy adipose tissue given that IL-17A production by γδ T cells stimulates the secretion of IL-33 in stromal cells to maintain the expansion of Treg cells in adipose tissue during development (Kohlgruber et al., 2018). Conversely, iNKT cells, ILC2s, and eosinophils are reduced in the adipose tissue during obesity and their presence is associated with a reduction in metabolic inflammation, increased insulin signaling and promotion of thermogenesis (Lynch et al., 2016; Lynch et al., 2009; Molofsky et al., 2015; Wu et al., 2011).
Besides innate immune cells, B and T cells also become dysregulated during obesity. B cells in healthy adipose tissue are thought to express IL-10 to negatively control adipose tissue inflammation (Nishimura et al., 2013). During obesity, although adipose B are fewer than T cells, their overall numbers are increased and contribute to insulin resistance by secreting autoreactive, pathogenic immunoglobulin G (IgG) antibodies and cytokines that drive inflammatory macrophages, Th17 polarization, and CD8+ T cell cytotoxicity (DeFuria et al., 2013; Duffaut et al., 2009; Winer et al., 2011). Cytotoxic CD8 T cells increase with an HFD even before macrophages accumulate (Rausch et al., 2008) and along with increased Th1 cells contribute to insulin resistance during obesity (Nishimura et al., 2009; Pacifico et al., 2006; Winer et al., 2009). The expansion of T cells before infiltration of peripheral monocytes and macrophages occurs through antigen expression of resident ATMs, especially within CLSs, but the increase in T cells might not be exclusively through clonal expansion during obesity (Morris et al., 2013). Regulatory T cells and iNKT cells largely contribute to the homeostasis of adipose tissue and produce anti-inflammatory factors such as IL-10, IL-4, and transforming growth factor-β (TGF-β) to promote metabolic homeostasis (Feuerer et al., 2009; Lynch et al., 2012; Schipper et al., 2012). During obesity, both Treg cells and iNKT cells are reduced in adipose tissue (Feuerer et al., 2009; Lynch et al., 2009). How many of these immune cells are sensing metabolic dysregulation and macronutrient availability remains to be established.
Immune Cells Regulate Thermogenesis
Maintenance of core body temperature in homeotherms through heat production is a high-priority metabolic event that is crucial for survival. The invaginations of the inner mitochondrial membrane that forms cristae are the sites of oxidative phosphorylation (OXPHOS) complexes that generate ATP (Kozak and Harper, 2000). In addition to ATP synthesis, mitochondrial OXPHOS generates heat as an energy byproduct. To enhance adaptive thermogenesis, adipocytes upregulate the expression of uncoupling protein 1 (UCP1) that forms pores in the inner mitochondrial membrane to allow protons to pass through without producing ATP (Kozak and Harper, 2000). Therefore, in the uncoupled mitochondria, the rate of OXPHOS is significantly boosted and releases heat instead of ATP. Although UCP1 was long thought to be the primary mediator of non-shivering thermogenesis, the discovery that UCP1−/− mice are able to adapt to long-term cold exposure uncovered the possibility of alternative thermogenic mechanisms independent of UCP1 (Ukropec et al., 2006). More recently, studies have revealed that other mechanisms such as futile creatine cycling, sarco/endoplasmic reticulum Ca2+-ATPase (SERCA)-mediated calcium cycling, and UCP3 can also contribute to fat thermogenesis (Ikeda et al., 2017; Kazak et al., 2015; Riley et al., 2016).
Macrophages control thermogenesis by controlling the bioavailability of catecholamines. Inhibition of MAOA in macrophages increases NE content in adipose tissue, leading to increased lipolysis and thermogenesis by upregulation of UCP1 (Camell et al., 2017). Moreover, ILC2s, eosinophils, iNKT, and γδ T cells have also been implicated in increasing thermogenesis and upregulating UCP1 (Brestoff et al., 2015; Hu et al., 2020; Lynch et al., 2016; Qiu et al., 2014). Notably, fibroblast growth factor 21 (FGF21), which gets induced during dietary restriction, can promote the expression of C-C motif chemokine ligand 11 (CCL11) by adipocytes to recruit eosinophils, suggesting that immune cells are actively regulated during adaptive adipose tissue remodeling (Huang et al., 2017). Future studies are needed to determine whether immune cells directly affect UCP1 and alternative thermogenic pathways, differentiation of de novo brown or beige adipocytes, or regulate the sympathetic nervous system to control heat production. Identification of such mechanisms could reveal strategies to divert the fate of dietary lipids from storage to heat production through increased mitochondrial uncoupling, thus reducing metabolic diseases emanating from lipotoxicity and obesity.
Inflammation and Adipose Tissue Fibrosis
Metabolic perturbations such as diet-induced obesity or dietary restriction lead to rapid remodeling of the adipose tissue, where alterations in ECM proteins play an important role in maintaining adipose tissue homeostasis. During obesity, there are dynamic changes in the deposition of ECM components such as collagens, fibrillins, proteoglycans, and specific matrix metalloproteases. Although obesity is associated with an excessive accumulation of hypoxia-driven fibrosis that directly leads to poor metabolic output (Halberg et al., 2009), the regulation and interaction of the adipose ECM and the immune system is still being established. Chronic inflammation had been thought to be the main driver of fibrosis and metabolic dysfunction during obesity, but studies have revealed that fibrosis might precede and occur independently of tissue inflammation (Halberg et al., 2009). Furthermore, repression of fibrosis alone can improve metabolic parameters without largely affecting inflammation (Hasegawa et al., 2018; Khan et al., 2009). However, immune cells might contribute to the continued pathologic fibrosis during chronic obesity. Wang and colleagues have demonstrated that IFN-γ-producing ILC1s can increase TGF-β output by macrophages to increase fibrosis, whereas sensing of dying adipocytes and hypoxia by Mincle and hypoxia-inducible factor 1- α (HIF1-α) in macrophages within CLSs can further drive adipose tissue fibrosis (Henegar et al., 2008; Tanaka et al., 2014; Wang et al., 2019). Recent studies suggest that Mincle might potentially be sensing cholesterol moieties released by dying adipocytes, given that human Mincle can bind crystalline cholesterol and Mincle was shown to sense cholesterol sulfate in mouse skin (Kiyotake et al., 2015; Kostarnoy et al., 2017). Further understanding of how different ECM components interact with the immune system to mediate adipose homeostasis and pathology remains to be elucidated.
Obesity Is Not Accelerated Aging of the Adipose Resident Immune System
Both obesity and aging are characterized by NLRP3 inflammasome-driven, low-grade chronic inflammation and metabolic dysregulation. Despite these shared similarities, obesity is not an accelerated model of aging. Although aging is commonly associated with visceral obesity, there are distinct immunological changes that underlie the pathological disease progression of each condition (Figure 2).
In adipose tissue, obesity leads to an increase in most of the defined macrophage subsets (Jaitin et al., 2019; Silva et al., 2019). During aging, however, the opposite trend is observed where macrophage numbers modestly decline (Camell et al., 2017; Lumeng et al., 2011). Age-associated VAT ATMs are enriched for NLRP3-dependent senescence and catecholamine catabolism gene signatures, with growth differentiation factor 3 (GDF3) being the most highly upregulated gene with age (Camell et al., 2017). Although an accumulation of senescent pre-adipocytes, endothelial cells, and T cells have been described with age in adipose tissue, these signatures have yet to be highlighted in highly proliferative obese ATMs (Salvestrini et al., 2019). Careful investigation of how these newly defined subsets of macrophages and their niches change with age might help identify therapeutic targets in ameliorating metabolic pathogenesis in aging.
In contrast to the reduction of innate macrophage populations with aging, adaptive B and T cells increase in fat with age (Camell et al., 2019; Lumeng et al., 2011). Although there is also an increase in T and B cells in obese adipose tissue, during aging these cells accumulate in specific niches called fat-associated lymphoid clusters (FALCs). Therefore, FALCs increase their size and number with age (Lumeng et al., 2011). FALCs are a form of non-classical lymphoid structures that are not encapsulated like traditional lymph nodes but can form germinal centers and respond to immunological challenges (Cruz-Migoni and Caamaño, 2016). Although sterile peritoneal inflammation induced by zymosan increases the size and numbers of FALCs in visceral adipose depots, an increase in FALC formation is not associated with inflamed obese fat (Bénezéch et al., 2015). During aging, there is an NLRP3-dependent accumulation of adipose-tissue-resident B cells within FALCs that produce inflammatory mediators such as IL-1β and CCL2. Depletion of adipose B cells in aged mice improves lipolytic signaling and insulin sensitivity, identifying aged adipose B cells as important mediators of age-associated insulin resistance, lipolysis resistance, and adipose dysfunction (Camell et al., 2019).
Treg cells play important regulatory roles in protecting metabolic homeostasis in the adipose of young animals and their dramatic reduction during obesity contributes to pathology (Feuerer et al., 2009). Conversely, Treg cells accumulate in adipose with age (Bapat et al., 2015; Lumeng et al., 2011). Unlike young Treg cells, the accumulation of aged adipose Treg cells drive insulin resistance and depletion of Treg cells in aged animals improves metabolic health (Bapat et al., 2015). However, when aged animals are challenged with an HFD to induce obesity, metabolic homeostasis is not maintained by depletion of Treg cells, confirming that distinct mechanisms drive the pathophysiology of age- and obesity-associated insulin resistance (Bapat et al., 2015).
Other adipose-resident immune cells, which have been characterized in healthy and obese adipose tissue, still require additional examination in the context of aging. Although eosinophils, which are associated with promoting homeostasis in healthy adipose, are decreased in obese fat, there seem to be no substantial changes during aging (Bapat et al., 2015; Wu et al., 2011). Innate T lymphocytes such as iNKT cells are decreased during obesity, whereas iNKTs significantly increase with age, and although γδT cells increase during obesity in the visceral adipose tissue, whether they are altered during aging still remains to be elucidated (Mehta et al., 2015). Surprisingly, ILC2s, which reside in visceral adipose tissues, including in FALCs, and produce IL-5 and IL-13 to recruit and maintain eosinophils, are also reduced with aging (Molofsky et al., 2015; Moro and Koyasu, 2010). In fact, many of the age-associated immunological changes that drive metabolic pathology predominantly occur within the visceral adipose. Although immune composition remains to be characterized within the BAT, the depletion of macrophages, and accumulation of FALCs and Treg cells with age occur mainly in the visceral adipose depots. Similarly, during obesity, changes in pro-inflammatory subsets such as macrophages are much stronger in visceral adipose depots than in subcutaneous adipose tissue (Weisberg et al., 2003).These depot-specific immune perturbations most likely drive tissue differences given that visceral adiposity is strongly associated with increased risk for metabolic dysfunction and disease, whereas SFAT has been considered to be more beneficial by acting as a metabolic sink that can buffer daily influx of nutrients to prevent ectopic lipid storage in visceral organs (Chusyd et al., 2016). As the immune system within adipose continues to be explored, it is becoming clear there are complex crosstalk between different immune cell types. How these homeostatic interactions become dysregulated during obesity and aging, and their specific tissue niches, remain to be carefully investigated.
Dietary Protein and Amino Acid Restriction
Although the mechanisms of CR are not completely understood, protein quality and amino acid composition of diet have been more strongly associated with metabolic and age-associated health. Independently of caloric intake, low-protein and high-carbohydrate diets were found to have the strongest extension of lifespan (Solon-Biet et al., 2014). In addition to dietary protein restriction (PR), some studies suggest it is the restriction of essential amino acids (EAAs) that drive beneficial effects, given that supplementing dietary restriction with EAAs but not non-essential amino acids were sufficient to reverse prolongevity effects (Grandison et al., 2009; Yoshida et al., 2018). Restriction of specific EAAs, such as the restriction of sulfur-containing amino acids (SAAs), methionine and cysteine, or methionine restriction (MR), and deficiency of the branched chain amino acids, leucine, isoleucine, and valine, or branched chain amino acid restriction (BCAAR), have been extensively characterized to increase metabolic homeostasis and promote healthy aging (Fontana et al., 2016; Lee et al., 2016; Solon-Biet et al., 2014). These dietary interventions commonly lead to reduced adiposity and inflammation, increased insulin sensitivity, and lipolytic gene signatures, and reduction of lipogenesis in adipose tissue and liver (Kitada et al., 2019). Although much less characterized, restriction of tryptophan can also lead to increased lifespan and increases recovery to cold stress, suggesting an increase in thermogenic capacity (Segall and Timiras, 1975, 1976; Zapata et al., 2018). Surprisingly, these studies indicate that deficiencies of specific essential amino acids drive remodeling and programming of metabolic tissues to promote health. Despite numerous studies investigating the mechanisms driving increased longevity during these dietary interventions, alterations to the immune system and their role in tissue reprogramming have been lacking.
One of the pathways that are induced with CR, PR, and amino acid restriction, is the transsulfuration pathway (TSP) (Hine et al., 2015; Mitchell et al., 2016). The TSP involves the metabolism of SAAs and involves the catabolism of methionine to generate intermediates such as the methyl donor S-adenosylmethionine (SAM), S-adenosylhomocysteine (SAH), homocysteine, and cystathionine. The TSP also allows for the generation of cysteine through the action of cystathionine-γ lyase (CSE), which can further provide important metabolites and byproducts such as glutathione, pyruvate, and hydrogen sulfide (H2S) (Figure 3 ). Some TSP metabolites such as SAM and homocysteine are increased with obesity and aging and have been implicated with inflammation and disease risk (Elshorbagy et al., 2013; Obata and Miura, 2015; Rodríguez et al., 2006; Vayá et al., 2012).
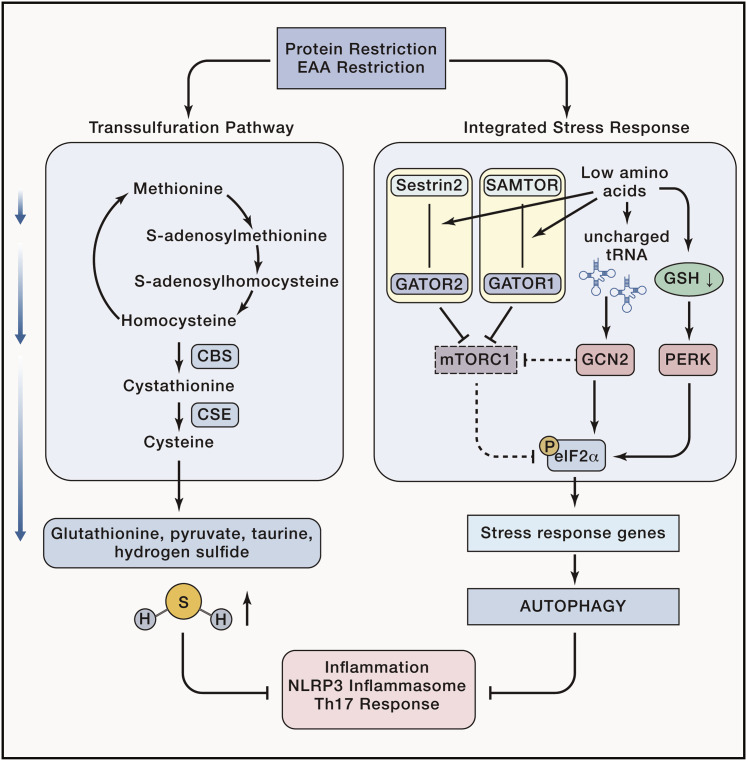
Figure 3.
Protein and Essential Amino Acid Restriction Induces the Transsulfuration Pathway and the Integrated Stress Response to Regulate Immune Response
Induction of the transsulfuration pathway leads to production of byproducts such as hydrogen sulfide, which can reduce NLRP3 inflammasome-mediated inflammation and promote and maintain regulatory T cell differentiation. Low amino acid availability leads to the interaction of amino acid sensors such as Sestrin 2 and SAMTOR with GATOR2 and GATOR1, respectively, to restrict mTORC1 activation. Furthermore, sensing of uncharged amino acids by GCN2 and lack of glutathione by PERK further inhibits mTORC1 and leads to the activation of eIF2α, stress response genes, and autophagy, inhibiting NLRP3 inflammasome-mediated inflammation and Th17 responses.
Another exciting, relevant byproduct of the TSP is H2S. H2S is a gaseous signaling molecule produced by three mammalian enzymes, CSE, cystathionine-β synthase (CBS), and mercaptopyruvate sulfurtransferase (MPST), but some studies have shown that H2S might be produced via non-enzymatic reactions (Glorieux et al., 2020). Of note, lifespan extension induced by CR in Drosophila was abolished when treated with the inhibitor of CSE, propargylglycine (PPG) (Kabil et al., 2011). Overexpression of another TSP enzyme, CBS, and treatment with H2S increased median lifespan in C. elegans and yeast, respectively, highlighting TSP-mediated H2S production as an important mediator of dietary benefits (Hine et al., 2015). Interestingly, a role for H2S in immune responses is emerging. In T cells, H2S is needed for the differentiation of regulatory T cells by promoting demethylation at the Foxp3 locus by Tet1 and Tet2 (Yang et al., 2015). An anti-inflammatory role for H2S has been implicated by its action on nuclear factor kappa B (NFκB) to regulate its transcriptional activity in macrophages (Sen et al., 2012) and its action on antioxidant gene regulation (Yang et al., 2013). These studies highlight that H2S might generally promote a more anti-inflammatory phenotype in immune cells. In myeloid cells, H2S can act specifically to inhibit inflammasome activation (Castelblanco et al., 2018; Lin et al., 2018), which might act as an important mechanism to reduce inflammation and initiate tissue remodeling during dietary interventions. However, studies with H2S have been largely limited to in vitro studies using H2S donors, inhibitors, or whole-body deletions of TSP enzymes, particularly CSE. CBS deficiency in mice leads to neonatal lethality (Gupta et al., 2009), further complicating the investigation of the role of H2S in immune cells. These complications have led to numerous contradictory reports of the action of H2S on immune cells, such as the action of H2S in regulating leukocyte adherence and tissue infiltration or NLRP3 activation (Basic et al., 2017; Spiller et al., 2010; Zanardo et al., 2006). Cell-specific and tissue-specific genetic deletion could allow for careful dissection of the role of the TSP in modulating immune response in physiological contexts.
Another pathway that is increased during protein and amino acid restriction is autophagy mediated by the integrated stress response. The integrated stress response allows for adaptive responses to various stresses such as starvation and ER stress. Stressors are sensed by four different sensors: general control nonderepressible 2 (GCN2), protein kinase R (PKR), heme-regulated inhibitor (HRI), and PKR-like endoplasmic reticulum kinase (PERK) (Pakos-Zebrucka et al., 2016). GCN2 senses amino acid depletion by sensing the accumulation of uncharged transfer RNAs (tRNAs) (Gallinetti et al., 2013). HRI recognizes heme deficiency, and PKR senses viral infection through activation by double-stranded RNA (Pakos-Zebrucka et al., 2016). Finally, PERK is activated by ER stress (Pakos-Zebrucka et al., 2016). Activation of these sensors are integrated by the activation and phosphorylation of eukaryotic initiation factor 2ɑ (eIF2ɑ), leading to global translational arrest (Pakos-Zebrucka et al., 2016). One central component of the integrated stress response is autophagy (Kroemer et al., 2010). In addition to GCN2 sensing of uncharged tRNAs, depletion of EAAs leads to interaction of their specific sensors such as SAMTOR (a SAM sensor), sestrin 2 (a leucine sensor), and CASTOR (an arginine sensor) with GATOR1 or GATOR2 to prevent mammalian target of rapamycin complex 1 (mTORC1) activation and promote autophagy (Kim and Guan, 2019) (Figure 3). Interestingly, autophagy and amino acid sensing also regulates immune function. It is now well established that autophagy inhibits inflammasome activation (Sun et al., 2017). In a model of colitis, PR and leucine restriction were able to reduce inflammation through the inhibition of NLRP3 inflammasome-mediated inflammation and reduction of Th17 differentiation. This response was dependent on amino acid sensing through GCN2 (Ravindran et al., 2016). Furthermore, treatment with halofuginone, which activates starvation responses by inhibiting prolyl tRNA charging, also inhibits Th17 cell differentiation (Sundrud et al., 2009). These studies highlight the induction of amino acid starvation responses in immune cells as potential strategies against inflammation-driven pathological states such as obesity, aging, and autoimmunity.
Although these different dietary amino acid restrictions might be inducing some common pathways, specific amino acids might target different pathways. Unlike leucine or BCAAR diets, MR also induces hepatic FGF21 and lipolytic gene signatures independently of GCN2 (Lees et al., 2017). Instead, MR-specific hepatic changes are driven by PERK and glutathione depletion (Wanders et al., 2016). Thus, different mechanisms might drive adaptations to specific amino acid deficiencies in tissues and immune cells (Figure 3). Despite numerous studies investigating the broad metabolic adaptations that occur during PR, insight into how immune cell function is being altered has been lacking. A plethora of early studies have investigated immune defense in the context of protein malnutrition, suggesting alterations in both macrophage and lymphocytic responses to infection. However, these studies were conducted prior to the classification of broad immune subsets and advancement of technological tools, and they need to be revisited to properly understand how the immune system might be responding during PR without malnutrition in the context of both tissue adaptation and immune defense.
Integrated Immunometabolic Response to Carbohydrate and Caloric Restriction
In an event of low glucose availability, such as food restriction, the limited glycogen reserves in the liver and muscle cannot sustain non-essential metabolic demand. Instead, triglycerides undergo fatty acid oxidation, ketogenesis, and ketolysis to support ATP production.
It is known that long-term CR lowers inflammation and protects against thymic lipoatrophy and maintains T cell repertoire during aging (Yang et al., 2009a). Intriguingly, however, CR causes a major redistribution of circulating leukocytes. A two-year multicenter randomized controlled trial where healthy humans achieved approximately 14% CR (CALERIE-II) provided evidence that CR reduces the number of circulating lymphocytes and monocytes without compromising vaccine responses or increasing susceptibility to infections (Meydani et al., 2016). Similarly, studies from Jordan et al. (2019) found that 19 h of fasting in humans led to reduction of monocytes and DCs in blood but instead increased retention of pro-inflammatory Ly6Chi monocytes in the BM. In agreement with prior studies (De Rosa et al., 2015), fasting reduced systemic inflammation in a mouse model of multiple sclerosis. Importantly, monocytes could still respond and control Listeria monocytogenes infection during fasting in a peroxisome proliferator-activated receptor α (PPARα)-dependent manner (Jordan et al., 2019). Similarly, negative energy-balance-mediated reduction in mTOR also led to CXCL13-driven homing of naive B cells from Peyer’s patches to BM. Moreover, Collins et al. (2019) found that mice subjected to 50% dietary restriction for 3 weeks caused a loss of peripheral memory CD8 T cells and their surprising accumulation in the BM. Markedly, this redistribution of B and memory T cells led to enhanced protection against secondary bacterial infection (Collins et al., 2019). It is intriguing that during energy deficit, memory T cells do not prefer adipose tissue that is undergoing increased lipolysis with abundant fatty acids as energy substrates and home instead to BM. CR’s salutary effects on the immune system and control of inflammation require further characterization. From the current evidence, switch from glycolysis to fatty acid oxidation appears to be an important immunometabolic checkpoint affected by CR. For example, production of ketone bodies like β-hydroxybutyrate downstream of fatty acid oxidation, inhibits NLRP3 inflammasome-mediated inflammation and protects against influenza infection by expanding tissue-protective γδ T cells in lung and adipose tissue (Goldberg et al., 2019). Whether a low-carbohydrate, high-fat ketogenic diet that increases fatty acid oxidation and beta-hydroxybutyrate (BHB) can be harnessed to protect against inflammatory damage, as in COVID-19 immunopathology, requires careful testing. The endogenous immunometabolic mediators that drive CR salutary effects thus offer a promising avenue to regulate inflammation and enhance healthspan.
Concluding Remarks
Significant progress over the last decade has firmly established that reciprocal interactions between immune and metabolic systems are needed for the host to maintain homeostasis. Immunometabolism, not only encompasses immune-cell-intrinsic regulation of glycolysis, tricarboxylic acid (TCA) cycle, pentose phosphate pathway, and fatty acid metabolism to affect inflammation and effector functions, but also entails immune regulation of systemic whole-body metabolism and coupling of positive and negative energy balance to successful immune response. As outlined in this review, the integrated immunometabolic response is in fact vital for regulation of organismal metabolism and host defense. A major influencer of IIMR is the diet. It is evident that metabolic fate of fat, protein, and carbohydrates is important for immunologic function. However, how macronutrient intake elicits specific pathways in a host to either induce disease or promote health and longevity is only beginning to be understood. In addition, both immune response and metabolism are regulated by the autonomic nervous system, which are not under our conscious control. The neural regulation of immunometabolism, by which the autonomic nervous system integrates the central nervous system, immune system, and metabolic organs to maintain essential functions and prepare the host for surmounting stressful challenges, is not well understood. Thus, study of neuroimmunometabolism could reveal new mechanisms to promote health and understand disease. Identification of immune cells like macrophages in sympathetic nerve niches in adipose tissue represents one mode of neuroimmunometabolic crosstalk that controls inflammation and lipolysis setpoints. These advances have set the stage for the next era of immunometabolism studies where regulation of diet-driven metabolic checkpoints are likely to be revealed and potentially implemented in clinical practice to promote health.
Acknowledgments
A.L. is supported by the Gruber Science Fellowship and the NSF GRFP. The Dixit lab is supported in part by NIH grants P01AG051459 and AR070811, and the Cure Alzheimer's Fund. Figures were created by using biorender.com.
References
- Altintas M.M., Azad A., Nayer B., Contreras G., Zaias J., Faul C., Reiser J., Nayer A. Mast cells, macrophages, and crown-like structures distinguish subcutaneous from visceral fat in mice. J. Lipid Res. 2011;52:480–488. doi: 10.1194/jlr.M011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano S.U., Cohen J.L., Vangala P., Tencerova M., Nicoloro S.M., Yawe J.C., Shen Y., Czech M.P., Aouadi M. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 2014;19:162–171. doi: 10.1016/j.cmet.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R.M., Le Couteur D.G., de Cabo R. Caloric Restriction Research: New Perspectives on the Biology of Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2017;73:1–3. doi: 10.1093/gerona/glx212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkan M.C., Hevener A.L., Greten F.R., Maeda S., Li Z.W., Long J.M., Wynshaw-Boris A., Poli G., Olefsky J., Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat. Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- Bapat S.P., Myoung Suh J., Fang S., Liu S., Zhang Y., Cheng A., Zhou C., Liang Y., LeBlanc M., Liddle C., et al. Depletion of fat-resident Treg cells prevents age-associated insulin resistance. Nature. 2015;528:137–141. doi: 10.1038/nature16151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartness T.J., Shrestha Y.B., Vaughan C.H., Schwartz G.J., Song C.K. Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Mol. Cell. Endocrinol. 2010;318:34–43. doi: 10.1016/j.mce.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartness T.J., Liu Y., Shrestha Y.B., Ryu V. Neural innervation of white adipose tissue and the control of lipolysis. Front. Neuroendocrinol. 2014;35:473–493. doi: 10.1016/j.yfrne.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basic A., Alizadehgharib S., Dahlén G., Dahlgren U. Hydrogen sulfide exposure induces NLRP3 inflammasome-dependent IL-1β and IL-18 secretion in human mononuclear leukocytes in vitro. Clin. Exp. Dent. Res. 2017;3:115–120. doi: 10.1002/cre2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bénézech C., Luu N.T., Walker J.A., Kruglov A.A., Loo Y., Nakamura K., Zhang Y., Nayar S., Jones L.H., Flores-Langarica A., et al. Inflammation-induced formation of fat-associated lymphoid clusters. Nat. Immunol. 2015;16:819–828. doi: 10.1038/ni.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertola A., Ciucci T., Rousseau D., Bourlier V., Duffaut C., Bonnafous S., Blin-Wakkach C., Anty R., Iannelli A., Gugenheim J., et al. Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes. 2012;61:2238–2247. doi: 10.2337/db11-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulenouar S., Michelet X., Duquette D., Alvarez D., Hogan A.E., Dold C., O’Connor D., Stutte S., Tavakkoli A., Winters D., et al. Adipose Type One Innate Lymphoid Cells Regulate Macrophage Homeostasis through Targeted Cytotoxicity. Immunity. 2017;46:273–286. doi: 10.1016/j.immuni.2017.01.008. [DOI] [PubMed] [Google Scholar]
- Bray G.A., Kim K.K., Wilding J.P.H., World Obesity F., World Obesity Federation Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes. Rev. 2017;18:715–723. doi: 10.1111/obr.12551. [DOI] [PubMed] [Google Scholar]
- Brestoff J.R., Kim B.S., Saenz S.A., Stine R.R., Monticelli L.A., Sonnenberg G.F., Thome J.J., Farber D.L., Lutfy K., Seale P., Artis D. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519:242–246. doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck M.D., O’Sullivan D., Pearce E.L. T cell metabolism drives immunity. J. Exp. Med. 2015;212:1345–1360. doi: 10.1084/jem.20151159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camell C., Goldberg E., Dixit V.D. Regulation of Nlrp3 inflammasome by dietary metabolites. Semin. Immunol. 2015;27:334–342. doi: 10.1016/j.smim.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camell C.D., Sander J., Spadaro O., Lee A., Nguyen K.Y., Wing A., Goldberg E.L., Youm Y.H., Brown C.W., Elsworth J., et al. Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature. 2017;550:119–123. doi: 10.1038/nature24022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camell C.D., Gunther P., Lee A., Goldberg E.L., Spadaro O., Youm Y.H., Bartke A., Hubbard G.B., Ikeno Y., Ruddle N.H., et al. Aging Induces an Nlrp3 Inflammasome-Dependent Expansion of Adipose B Cells That Impairs Metabolic Homeostasis. Cell Metab. 2019;30:1024–1039. doi: 10.1016/j.cmet.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelblanco M., Lugrin J., Ehirchiou D., Nasi S., Ishii I., So A., Martinon F., Busso N. Hydrogen sulfide inhibits NLRP3 inflammasome activation and reduces cytokine production both in vitro and in a mouse model of inflammation. J. Biol. Chem. 2018;293:2546–2557. doi: 10.1074/jbc.M117.806869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi J., Wu Z., Choi C.H.J., Nguyen L., Tegegne S., Ackerman S.E., Crane A., Marchildon F., Tessier-Lavigne M., Cohen P. Three-Dimensional Adipose Tissue Imaging Reveals Regional Variation in Beige Fat Biogenesis and PRDM16-Dependent Sympathetic Neurite Density. Cell Metab. 2018;27:226–236. doi: 10.1016/j.cmet.2017.12.011. [DOI] [PubMed] [Google Scholar]
- Cho C.H., Koh Y.J., Han J., Sung H.K., Jong Lee H., Morisada T., Schwendener R.A., Brekken R.A., Kang G., Oike Y., et al. Angiogenic role of LYVE-1-positive macrophages in adipose tissue. Circ. Res. 2007;100:e47–e57. doi: 10.1161/01.RES.0000259564.92792.93. [DOI] [PubMed] [Google Scholar]
- Christ A., Lauterbach M., Latz E. Western Diet and the Immune System: An Inflammatory Connection. Immunity. 2019;51:794–811. doi: 10.1016/j.immuni.2019.09.020. [DOI] [PubMed] [Google Scholar]
- Chusyd D.E., Wang D., Huffman D.M., Nagy T.R. Relationships between Rodent White Adipose Fat Pads and Human White Adipose Fat Depots. Front. Nutr. 2016;3:10. doi: 10.3389/fnut.2016.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codo A.C., Davanzo G.G., Monteiro L.B., de Souza G.F., Muraro S.P., Virgilio-da-Silva J.V., Prodonoff J.S., Carregari V.C., de Biagi Junior C.A.O., Crunfli F., et al. Elevated Glucose Levels Favor SARS-CoV-2 Infection and Monocyte Response through a HIF-1α/Glycolysis-Dependent Axis. Cell Metab. 2020 doi: 10.1016/j.cmet.2020.07.007. [DOI] [Google Scholar]
- Collins N., Han S.J., Enamorado M., Link V.M., Huang B., Moseman E.A., Kishton R.J., Shannon J.P., Dixit D., Schwab S.R., et al. The Bone Marrow Protects and Optimizes Immunological Memory during Dietary Restriction. Cell. 2019;178:1088–1101. doi: 10.1016/j.cell.2019.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Migoni S., Caamaño J. Fat-Associated Lymphoid Clusters in Inflammation and Immunity. Front. Immunol. 2016;7:612. doi: 10.3389/fimmu.2016.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damouche A., Lazure T., Avettand-Fènoël V., Huot N., Dejucq-Rainsford N., Satie A.P., Mélard A., David L., Gommet C., Ghosn J., et al. Adipose Tissue Is a Neglected Viral Reservoir and an Inflammatory Site during Chronic HIV and SIV Infection. PLoS Pathog. 2015;11:e1005153. doi: 10.1371/journal.ppat.1005153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa V., Galgani M., Santopaolo M., Colamatteo A., Laccetti R., Matarese G. Nutritional control of immunity: Balancing the metabolic requirements with an appropriate immune function. Semin. Immunol. 2015;27:300–309. doi: 10.1016/j.smim.2015.10.001. [DOI] [PubMed] [Google Scholar]
- DeFuria J., Belkina A.C., Jagannathan-Bogdan M., Snyder-Cappione J., Carr J.D., Nersesova Y.R., Markham D., Strissel K.J., Watkins A.A., Zhu M., et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc. Natl. Acad. Sci. USA. 2013;110:5133–5138. doi: 10.1073/pnas.1215840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donath M.Y., Shoelson S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- Drucker D.J. Coronavirus infections and type 2 diabetes-shared pathways with therapeutic implications. Endocr. Rev. 2020;41:bnaa011. doi: 10.1210/endrev/bnaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffaut C., Galitzky J., Lafontan M., Bouloumié A. Unexpected trafficking of immune cells within the adipose tissue during the onset of obesity. Biochem. Biophys. Res. Commun. 2009;384:482–485. doi: 10.1016/j.bbrc.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Elgazar-Carmon V., Rudich A., Hadad N., Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J. Lipid Res. 2008;49:1894–1903. doi: 10.1194/jlr.M800132-JLR200. [DOI] [PubMed] [Google Scholar]
- Elshorbagy A.K., Nijpels G., Valdivia-Garcia M., Stehouwer C.D., Ocke M., Refsum H., Dekker J.M. S-adenosylmethionine is associated with fat mass and truncal adiposity in older adults. J. Nutr. 2013;143:1982–1988. doi: 10.3945/jn.113.179192. [DOI] [PubMed] [Google Scholar]
- Feuerer M., Herrero L., Cipolletta D., Naaz A., Wong J., Nayer A., Lee J., Goldfine A.B., Benoist C., Shoelson S., Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer K., Ruiz H.H., Jhun K., Finan B., Oberlin D.J., van der Heide V., Kalinovich A.V., Petrovic N., Wolf Y., Clemmensen C., et al. Alternatively activated macrophages do not synthesize catecholamines or contribute to adipose tissue adaptive thermogenesis. Nat. Med. 2017;23:623–630. doi: 10.1038/nm.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L., Cummings N.E., Arriola Apelo S.I., Neuman J.C., Kasza I., Schmidt B.A., Cava E., Spelta F., Tosti V., Syed F.A., et al. Decreased Consumption of Branched-Chain Amino Acids Improves Metabolic Health. Cell Rep. 2016;16:520–530. doi: 10.1016/j.celrep.2016.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frauwirth K.A., Riley J.L., Harris M.H., Parry R.V., Rathmell J.C., Plas D.R., Elstrom R.L., June C.H., Thompson C.B. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- Gallinetti J., Harputlugil E., Mitchell J.R. Amino acid sensing in dietary-restriction-mediated longevity: roles of signal-transducing kinases GCN2 and TOR. Biochem. J. 2013;449:1–10. doi: 10.1042/BJ20121098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glorieux G., Gryp T., Perna A. Gut-Derived Metabolites and Their Role in Immune Dysfunction in Chronic Kidney Disease. Toxins (Basel) 2020;12:245. doi: 10.3390/toxins12040245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg E.L., Dixit V.D. Drivers of age-related inflammation and strategies for healthspan extension. Immunol. Rev. 2015;265:63–74. doi: 10.1111/imr.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg E.L., Molony R.D., Kudo E., Sidorov S., Kong Y., Dixit V.D., Iwasaki A. Ketogenic diet activates protective γδ T cell responses against influenza virus infection. Sci. Immunol. 2019;4:eaav2026. doi: 10.1126/sciimmunol.aav2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandison R.C., Piper M.D., Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 2009;462:1061–1064. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Kühnisch J., Mustafa A., Lhotak S., Schlachterman A., Slifker M.J., Klein-Szanto A., High K.A., Austin R.C., Kruger W.D. Mouse models of cystathionine beta-synthase deficiency reveal significant threshold effects of hyperhomocysteinemia. FASEB J. 2009;23:883–893. doi: 10.1096/fj.08-120584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haka A.S., Barbosa-Lorenzi V.C., Lee H.J., Falcone D.J., Hudis C.A., Dannenberg A.J., Maxfield F.R. Exocytosis of macrophage lysosomes leads to digestion of apoptotic adipocytes and foam cell formation. J. Lipid Res. 2016;57:980–992. doi: 10.1194/jlr.M064089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberg N., Khan T., Trujillo M.E., Wernstedt-Asterholm I., Attie A.D., Sherwani S., Wang Z.V., Landskroner-Eiger S., Dineen S., Magalang U.J., et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol. Cell. Biol. 2009;29:4467–4483. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales C.M., Carroll M.D., Fryar C.D., Ogden C.L. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017-2018. NCHS Data Brief. 2020;360:1–8. [PubMed] [Google Scholar]
- Han M.S., Jung D.Y., Morel C., Lakhani S.A., Kim J.K., Flavell R.A., Davis R.J. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013;339:218–222. doi: 10.1126/science.1227568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S.J., Glatman Zaretsky A., Andrade-Oliveira V., Collins N., Dzutsev A., Shaik J., Morais da Fonseca D., Harrison O.J., Tamoutounour S., Byrd A.L., et al. White Adipose Tissue Is a Reservoir for Memory T Cells and Promotes Protective Memory Responses to Infection. Immunity. 2017;47:1154–1168. doi: 10.1016/j.immuni.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa Y., Ikeda K., Chen Y., Alba D.L., Stifler D., Shinoda K., Hosono T., Maretich P., Yang Y., Ishigaki Y., et al. Repression of Adipose Tissue Fibrosis through a PRDM16-GTF2IRD1 Complex Improves Systemic Glucose Homeostasis. Cell Metab. 2018;27:180–194. doi: 10.1016/j.cmet.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassnain Waqas S.F., Noble A., Hoang A.C., Ampem G., Popp M., Strauß S., Guille M., Röszer T. Adipose tissue macrophages develop from bone marrow-independent progenitors in Xenopus laevis and mouse. J. Leukoc. Biol. 2017;102:845–855. doi: 10.1189/jlb.1A0317-082RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henegar C., Tordjman J., Achard V., Lacasa D., Cremer I., Guerre-Millo M., Poitou C., Basdevant A., Stich V., Viguerie N., et al. Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity. Genome Biol. 2008;9:R14. doi: 10.1186/gb-2008-9-1-r14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgendorf K.I., Johnson C.T., Mezger A., Rice S.L., Norris A.M., Demeter J., Greenleaf W.J., Reiter J.F., Kopinke D., Jackson P.K. Omega-3 Fatty Acids Activate Ciliary FFAR4 to Control Adipogenesis. Cell. 2019;179:1289–1305. doi: 10.1016/j.cell.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A.A., Anderson-Baucum E.K., Kennedy A.J., Webb C.D., Yull F.E., Hasty A.H. Activation of NF-κB drives the enhanced survival of adipose tissue macrophages in an obesogenic environment. Mol. Metab. 2015;4:665–677. doi: 10.1016/j.molmet.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D.A., Lim H.W., Kim Y.H., Ho W.Y., Foong Y.H., Nelson V.L., Nguyen H.C.B., Chegireddy K., Kim J., Habertheuer A., et al. Distinct macrophage populations direct inflammatory versus physiological changes in adipose tissue. Proc. Natl. Acad. Sci. USA. 2018;115:E5096–E5105. doi: 10.1073/pnas.1802611115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hine C., Harputlugil E., Zhang Y., Ruckenstuhl C., Lee B.C., Brace L., Longchamp A., Treviño-Villarreal J.H., Mejia P., Ozaki C.K., et al. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell. 2015;160:132–144. doi: 10.1016/j.cell.2014.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honce R., Karlsson E.A., Wohlgemuth N., Estrada L.D., Meliopoulos V.A., Yao J., Schultz-Cherry S. Obesity-Related Microenvironment Promotes Emergence of Virulent Influenza Virus Strains. MBio. 2020;11:e03341-19. doi: 10.1128/mBio.03341-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Hotamisligil G.S., Shargill N.S., Spiegelman B.M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Hotamisligil G.S., Peraldi P., Budavari A., Ellis R., White M.F., Spiegelman B.M. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- Hu B., Jin C., Zeng X., Resch J.M., Jedrychowski M.P., Yang Z., Desai B.N., Banks A.S., Lowell B.B., Mathis D., Spiegelman B.M. γδ T cells and adipocyte IL-17RC control fat innervation and thermogenesis. Nature. 2020;578:610–614. doi: 10.1038/s41586-020-2028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Zhong L., Lee J.T.H., Zhang J., Wu D., Geng L., Wang Y., Wong C.M., Xu A. The FGF21-CCL11 Axis Mediates Beiging of White Adipose Tissues by Coupling Sympathetic Nervous System to Type 2 Immunity. Cell Metab. 2017;26:493–508. doi: 10.1016/j.cmet.2017.08.003. [DOI] [PubMed] [Google Scholar]
- Ikeda K., Kang Q., Yoneshiro T., Camporez J.P., Maki H., Homma M., Shinoda K., Chen Y., Lu X., Maretich P., et al. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat. Med. 2017;23:1454–1465. doi: 10.1038/nm.4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S.R., Herman C.E., Maciver N.J., Wofford J.A., Wieman H.L., Hammen J.J., Rathmell J.C. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J. Immunol. 2008;180:4476–4486. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaitin D.A., Adlung L., Thaiss C.A., Weiner A., Li B., Descamps H., Lundgren P., Bleriot C., Liu Z., Deczkowska A., et al. Lipid-Associated Macrophages Control Metabolic Homeostasis in a Trem2-Dependent Manner. Cell. 2019;178:686–698. doi: 10.1016/j.cell.2019.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan S., Tung N., Casanova-Acebes M., Chang C., Cantoni C., Zhang D., Wirtz T.H., Naik S., Rose S.A., Brocker C.N., et al. Dietary Intake Regulates the Circulating Inflammatory Monocyte Pool. Cell. 2019;178:1102–1114. doi: 10.1016/j.cell.2019.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabil H., Kabil O., Banerjee R., Harshman L.G., Pletcher S.D. Increased transsulfuration mediates longevity and dietary restriction in Drosophila. Proc. Natl. Acad. Sci. USA. 2011;108:16831–16836. doi: 10.1073/pnas.1102008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane H., Lynch L. Innate Immune Control of Adipose Tissue Homeostasis. Trends Immunol. 2019;40:857–872. doi: 10.1016/j.it.2019.07.006. [DOI] [PubMed] [Google Scholar]
- Kanneganti T.D., Dixit V.D. Immunological complications of obesity. Nat. Immunol. 2012;13:707–712. doi: 10.1038/ni.2343. [DOI] [PubMed] [Google Scholar]
- Kazak L., Chouchani E.T., Jedrychowski M.P., Erickson B.K., Shinoda K., Cohen P., Vetrivelan R., Lu G.Z., Laznik-Bogoslavski D., Hasenfuss S.C., et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell. 2015;163:643–655. doi: 10.1016/j.cell.2015.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan T., Muise E.S., Iyengar P., Wang Z.V., Chandalia M., Abate N., Zhang B.B., Bonaldo P., Chua S., Scherer P.E. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol. Cell. Biol. 2009;29:1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Guan K.L. mTOR as a central hub of nutrient signalling and cell growth. Nat. Cell Biol. 2019;21:63–71. doi: 10.1038/s41556-018-0205-1. [DOI] [PubMed] [Google Scholar]
- Kirkwood T.L., Kapahi P., Shanley D.P. Evolution, stress, and longevity. J. Anat. 2000;197:587–590. doi: 10.1046/j.1469-7580.2000.19740587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada M., Ogura Y., Monno I., Koya D. The impact of dietary protein intake on longevity and metabolic health. EBioMedicine. 2019;43:632–640. doi: 10.1016/j.ebiom.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyotake R., Oh-Hora M., Ishikawa E., Miyamoto T., Ishibashi T., Yamasaki S. Human Mincle Binds to Cholesterol Crystals and Triggers Innate Immune Responses. J. Biol. Chem. 2015;290:25322–25332. doi: 10.1074/jbc.M115.645234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlgruber A.C., Gal-Oz S.T., LaMarche N.M., Shimazaki M., Duquette D., Koay H.F., Nguyen H.N., Mina A.I., Paras T., Tavakkoli A., et al. γδ T cells producing interleukin-17A regulate adipose regulatory T cell homeostasis and thermogenesis. Nat. Immunol. 2018;19:464–474. doi: 10.1038/s41590-018-0094-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostarnoy A.V., Gancheva P.G., Lepenies B., Tukhvatulin A.I., Dzharullaeva A.S., Polyakov N.B., Grumov D.A., Egorova D.A., Kulibin A.Y., Bobrov M.A., et al. Receptor Mincle promotes skin allergies and is capable of recognizing cholesterol sulfate. Proc. Natl. Acad. Sci. USA. 2017;114:E2758–E2765. doi: 10.1073/pnas.1611665114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak L.P., Harper M.E. Mitochondrial uncoupling proteins in energy expenditure. Annu. Rev. Nutr. 2000;20:339–363. doi: 10.1146/annurev.nutr.20.1.339. [DOI] [PubMed] [Google Scholar]
- Kratz M., Coats B.R., Hisert K.B., Hagman D., Mutskov V., Peris E., Schoenfelt K.Q., Kuzma J.N., Larson I., Billing P.S., et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014;20:614–625. doi: 10.1016/j.cmet.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G., Mariño G., Levine B. Autophagy and the integrated stress response. Mol. Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin Y., Winter D., Blecher-Gonen R., David E., Keren-Shaul H., Merad M., Jung S., Amit I. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159:1312–1326. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.C., Watkins S.M., Lorenzo C., Wagenknecht L.E., Il’yasova D., Chen Y.D., Haffner S.M., Hanley A.J. Branched-Chain Amino Acids and Insulin Metabolism: The Insulin Resistance Atherosclerosis Study (IRAS) Diabetes Care. 2016;39:582–588. doi: 10.2337/dc15-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees E.K., Banks R., Cook C., Hill S., Morrice N., Grant L., Mody N., Delibegovic M. Direct comparison of methionine restriction with leucine restriction on the metabolic health of C57BL/6J mice. Sci. Rep. 2017;7:9977. doi: 10.1038/s41598-017-10381-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z., Altaf N., Li C., Chen M., Pan L., Wang D., Xie L., Zheng Y., Fu H., Han Y., Ji Y. Hydrogen sulfide attenuates oxidative stress-induced NLRP3 inflammasome activation via S-sulfhydrating c-Jun at Cys269 in macrophages. Biochim Biophys Acta Mol Basis Dis. 2018;1864:2890–2900. doi: 10.1016/j.bbadis.2018.05.023. [DOI] [PubMed] [Google Scholar]
- Liu J., Divoux A., Sun J., Zhang J., Clément K., Glickman J.N., Sukhova G.K., Wolters P.J., Du J., Gorgun C.Z., et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat. Med. 2009;15:940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Ibi D., Taniguchi K., Lee J., Herrema H., Akosman B., Mucka P., Salazar Hernandez M.A., Uyar M.F., Park S.W., et al. Inflammation Improves Glucose Homeostasis through IKKbeta-XBP1s Interaction. Cell. 2016;167:1052–1066. doi: 10.1016/j.cell.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas C., Wong P., Klein J., Castro T.B.R., Silva J., Sundaram M., Ellingson M.K., Mao T., Oh J.E., Israelow B., et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020 doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng C.N., Deyoung S.M., Bodzin J.L., Saltiel A.R. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- Lumeng C.N., Liu J., Geletka L., Delaney C., Delproposto J., Desai A., Oatmen K., Martinez-Santibanez G., Julius A., Garg S., Yung R.L. Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue. J. Immunol. 2011;187:6208–6216. doi: 10.4049/jimmunol.1102188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch L., O’Shea D., Winter D.C., Geoghegan J., Doherty D.G., O’Farrelly C. Invariant NKT cells and CD1d(+) cells amass in human omentum and are depleted in patients with cancer and obesity. Eur. J. Immunol. 2009;39:1893–1901. doi: 10.1002/eji.200939349. [DOI] [PubMed] [Google Scholar]
- Lynch L., Nowak M., Varghese B., Clark J., Hogan A.E., Toxavidis V., Balk S.P., O’Shea D., O’Farrelly C., Exley M.A. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity. 2012;37:574–587. doi: 10.1016/j.immuni.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch L., Hogan A.E., Duquette D., Lester C., Banks A., LeClair K., Cohen D.E., Ghosh A., Lu B., Corrigan M., et al. iNKT Cells Induce FGF21 for Thermogenesis and Are Required for Maximal Weight Loss in GLP1 Therapy. Cell Metab. 2016;24:510–519. doi: 10.1016/j.cmet.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma E.H., Verway M.J., Johnson R.M., Roy D.G., Steadman M., Hayes S., Williams K.S., Sheldon R.D., Samborska B., Kosinski P.A., et al. Metabolic Profiling Using Stable Isotope Tracing Reveals Distinct Patterns of Glucose Utilization by Physiologically Activated CD8+ T Cells. Immunity. 2019;51:856–870. doi: 10.1016/j.immuni.2019.09.003. [DOI] [PubMed] [Google Scholar]
- Mauro C., Smith J., Cucchi D., Coe D., Fu H., Bonacina F., Baragetti A., Cermenati G., Caruso D., Mitro N., et al. Obesity-Induced Metabolic Stress Leads to Biased Effector Memory CD4+ T Cell Differentiation via PI3K p110δ-Akt-Mediated Signals. Cell Metab. 2017;25:593–609. doi: 10.1016/j.cmet.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., Nuotio-Antar A.M., Smith C.W. γδ T cells promote inflammation and insulin resistance during high fat diet-induced obesity in mice. J. Leukoc. Biol. 2015;97:121–134. doi: 10.1189/jlb.3A0414-211RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meydani S.N., Das S.K., Pieper C.F., Lewis M.R., Klein S., Dixit V.D., Gupta A.K., Villareal D.T., Bhapkar M., Huang M., et al. Long-term moderate calorie restriction inhibits inflammation without impairing cell-mediated immunity: a randomized controlled trial in non-obese humans. Aging (Albany N.Y.) 2016;8:1416–1431. doi: 10.18632/aging.100994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek R.D., Gerriets V.A., Jacobs S.R., Macintyre A.N., MacIver N.J., Mason E.F., Sullivan S.A., Nichols A.G., Rathmell J.C. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misumi I., Cook K.D., Mitchell J.E., Lund M.M., Vick S.C., Lee R.H., Uchimura T., Bergmeier W., Mieczkowski P., Pardo-Manuel de Villena F., et al. Identification of a Locus in Mice that Regulates the Collateral Damage and Lethality of Virus Infection. Cell Rep. 2019;27:1387–1396. doi: 10.1016/j.celrep.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell S.J., Madrigal-Matute J., Scheibye-Knudsen M., Fang E., Aon M., González-Reyes J.A., Cortassa S., Kaushik S., Gonzalez-Freire M., Patel B., et al. Effects of Sex, Strain, and Energy Intake on Hallmarks of Aging in Mice. Cell Metab. 2016;23:1093–1112. doi: 10.1016/j.cmet.2016.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky A.B., Van Gool F., Liang H.E., Van Dyken S.J., Nussbaum J.C., Lee J., Bluestone J.A., Locksley R.M. Interleukin-33 and Interferon-γ Counter-Regulate Group 2 Innate Lymphoid Cell Activation during Immune Perturbation. Immunity. 2015;43:161–174. doi: 10.1016/j.immuni.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro K., Koyasu S. Innate production of Th2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. J. Immunol. 2010;463:184. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- Morris D.L., Cho K.W., Delproposto J.L., Oatmen K.E., Geletka L.M., Martinez-Santibanez G., Singer K., Lumeng C.N. Adipose tissue macrophages function as antigen-presenting cells and regulate adipose tissue CD4+ T cells in mice. Diabetes. 2013;62:2762–2772. doi: 10.2337/db12-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mraz M., Haluzik M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J. Endocrinol. 2014;222:R113–R127. doi: 10.1530/JOE-14-0283. [DOI] [PubMed] [Google Scholar]
- Murano I., Barbatelli G., Parisani V., Latini C., Muzzonigro G., Castellucci M., Cinti S. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J. Lipid Res. 2008;49:1562–1568. doi: 10.1194/jlr.M800019-JLR200. [DOI] [PubMed] [Google Scholar]
- Nagai M., Noguchi R., Takahashi D., Morikawa T., Koshida K., Komiyama S., Ishihara N., Yamada T., Kawamura Y.I., Muroi K., et al. Fasting-Refeeding Impacts Immune Cell Dynamics and Mucosal Immune Responses. Cell. 2019;178:1072–1087. doi: 10.1016/j.cell.2019.07.047. [DOI] [PubMed] [Google Scholar]
- Nagareddy P.R., Kraakman M., Masters S.L., Stirzaker R.A., Gorman D.J., Grant R.W., Dragoljevic D., Hong E.S., Abdel-Latif A., Smyth S.S., et al. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab. 2014;19:821–835. doi: 10.1016/j.cmet.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura S., Manabe I., Nagasaki M., Eto K., Yamashita H., Ohsugi M., Otsu M., Hara K., Ueki K., Sugiura S., et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- Nishimura S., Manabe I., Takaki S., Nagasaki M., Otsu M., Yamashita H., Sugita J., Yoshimura K., Eto K., Komuro I., et al. Adipose Natural Regulatory B Cells Negatively Control Adipose Tissue Inflammation. Cell Metab. 2013;18:759–766. doi: 10.1016/j.cmet.2013.09.017. [DOI] [PubMed] [Google Scholar]
- O’Neill L.A., Kishton R.J., Rathmell J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 2016;16:553–565. doi: 10.1038/nri.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan T.E., Rapp M., Fan X., Weizman O.E., Bhardwaj P., Adams N.M., Walzer T., Dannenberg A.J., Sun J.C. Adipose-Resident Group 1 Innate Lymphoid Cells Promote Obesity-Associated Insulin Resistance. Immunity. 2016;45:428–441. doi: 10.1016/j.immuni.2016.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata F., Miura M. Enhancing S-adenosyl-methionine catabolism extends Drosophila lifespan. Nat. Commun. 2015;6:8332. doi: 10.1038/ncomms9332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh D.Y., Morinaga H., Talukdar S., Bae E.J., Olefsky J.M. Increased macrophage migration into adipose tissue in obese mice. Diabetes. 2012;61:346–354. doi: 10.2337/db11-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe Y., Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;157:832–844. doi: 10.1016/j.cell.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orentreich N., Matias J.R., DeFelice A., Zimmerman J.A. Low methionine ingestion by rats extends life span. J. Nutr. 1993;123:269–274. doi: 10.1093/jn/123.2.269. [DOI] [PubMed] [Google Scholar]
- Pacifico L., Di Renzo L., Anania C., Osborn J.F., Ippoliti F., Schiavo E., Chiesa C. Increased T-helper interferon-gamma-secreting cells in obese children. Eur. J. Endocrinol. 2006;154:691–697. doi: 10.1530/eje.1.02138. [DOI] [PubMed] [Google Scholar]
- Pakos-Zebrucka K., Koryga I., Mnich K., Ljujic M., Samali A., Gorman A.M. The integrated stress response. EMBO Rep. 2016;17:1374–1395. doi: 10.15252/embr.201642195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsouris D., Li P.P., Thapar D., Chapman J., Olefsky J.M., Neels J.G. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–309. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce E.L., Walsh M.C., Cejas P.J., Harms G.M., Shen H., Wang L.S., Jones R.G., Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirzgalska R.M., Seixas E., Seidman J.S., Link V.M., Sánchez N.M., Mahú I., Mendes R., Gres V., Kubasova N., Morris I., et al. Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat. Med. 2017;23:1309–1318. doi: 10.1038/nm.4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y., Nguyen K.D., Odegaard J.I., Cui X., Tian X., Locksley R.M., Palmiter R.D., Chawla A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157:1292–1308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch M.E., Weisberg S., Vardhana P., Tortoriello D.V. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int. J. Obes. 2008;32:451–463. doi: 10.1038/sj.ijo.0803744. [DOI] [PubMed] [Google Scholar]
- Ravindran R., Loebbermann J., Nakaya H.I., Khan N., Ma H., Gama L., Machiah D.K., Lawson B., Hakimpour P., Wang Y.C., et al. The amino acid sensor GCN2 controls gut inflammation by inhibiting inflammasome activation. Nature. 2016;531:523–527. doi: 10.1038/nature17186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeles J., Green W.D., Alwarawrah Y., Nichols A.G., Eisner W., Danzaki K., MacIver N.J., Beck M.A. Obesity-Induced Changes in T-Cell Metabolism Are Associated With Impaired Memory T-Cell Response to Influenza and Are Not Reversed With Weight Loss. J. Infect. Dis. 2019;219:1652–1661. doi: 10.1093/infdis/jiy700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley C.L., Dao C., Kenaston M.A., Muto L., Kohno S., Nowinski S.M., Solmonson A.D., Pfeiffer M., Sack M.N., Lu Z., et al. The complementary and divergent roles of uncoupling proteins 1 and 3 in thermoregulation. J. Physiol. 2016;594:7455–7464. doi: 10.1113/JP272971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez J.J., Santolaria F., Martínez-Riera A., González-Reimers E., de la Vega Prieto M.J., Valls M.R., Gaspar M.R. Clinical significance of homocysteine in elderly hospitalized patients. Metabolism. 2006;55:620–627. doi: 10.1016/j.metabol.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Saltiel A.R., Olefsky J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Invest. 2017;127:1–4. doi: 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvestrini V., Sell C., Lorenzini A. Obesity May Accelerate the Aging Process. Front. Endocrinol. (Lausanne) 2019;10:266. doi: 10.3389/fendo.2019.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipper H.S., Rakhshandehroo M., van de Graaf S.F., Venken K., Koppen A., Stienstra R., Prop S., Meerding J., Hamers N., Besra G., et al. Natural killer T cells in adipose tissue prevent insulin resistance. J. Clin. Invest. 2012;122:3343–3354. doi: 10.1172/JCI62739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz C., Gomez Perdiguero E., Chorro L., Szabo-Rogers H., Cagnard N., Kierdorf K., Prinz M., Wu B., Jacobsen S.E., Pollard J.W., et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- Segall P.E., Timiras P.S. Age-related changes in the thermoregulatory capacity of tryptophan-deficient rats. Fed. Proc. 1975;34:83–85. [PubMed] [Google Scholar]
- Segall P.E., Timiras P.S. Patho-physiologic findings after chronic tryptophan deficiency in rats: a model for delayed growth and aging. Mech. Ageing Dev. 1976;5:109–124. doi: 10.1016/0047-6374(76)90012-9. [DOI] [PubMed] [Google Scholar]
- Sen N., Paul B.D., Gadalla M.M., Mustafa A.K., Sen T., Xu R., Kim S., Snyder S.H. Hydrogen sulfide-linked sulfhydration of NF-κB mediates its antiapoptotic actions. Mol. Cell. 2012;45:13–24. doi: 10.1016/j.molcel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C.S., Nabar N.R., Huang N.N., Kehrl J.H. SARS-Coronavirus Open Reading Frame-8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Discov. 2019;5:101. doi: 10.1038/s41420-019-0181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva H.M., Báfica A., Rodrigues-Luiz G.F., Chi J., Santos P.D.A., Reis B.S., Hoytema van Konijnenburg D.P., Crane A., Arifa R.D.N., Martin P., et al. Vasculature-associated fat macrophages readily adapt to inflammatory and metabolic challenges. J. Exp. Med. 2019;216:786–806. doi: 10.1084/jem.20181049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A., Labreuche J., Mathieu D., Pattou F., Jourdain M., et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28:1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu K.L., Yuen K.S., Castaño-Rodriguez C., Ye Z.W., Yeung M.L., Fung S.Y., Yuan S., Chan C.P., Yuen K.Y., Enjuanes L., Jin D.Y. Severe acute respiratory syndrome coronavirus ORF3a protein activates the NLRP3 inflammasome by promoting TRAF3-dependent ubiquitination of ASC. FASEB J. 2019;33:8865–8877. doi: 10.1096/fj.201802418R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavin B.G., Ballard K.W. Morphological studies on the adrenergic innervation of white adipose tissue. Anat. Rec. 1978;191:377–389. doi: 10.1002/ar.1091910310. [DOI] [PubMed] [Google Scholar]
- Solinas G., Vilcu C., Neels J.G., Bandyopadhyay G.K., Luo J.L., Naugler W., Grivennikov S., Wynshaw-Boris A., Scadeng M., Olefsky J.M., Karin M. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6:386–397. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Solon-Biet S.M., McMahon A.C., Ballard J.W., Ruohonen K., Wu L.E., Cogger V.C., Warren A., Huang X., Pichaud N., Melvin R.G., et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014;19:418–430. doi: 10.1016/j.cmet.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadaro O., Camell C.D., Bosurgi L., Nguyen K.Y., Youm Y.H., Rothlin C.V., Dixit V.D. IGF1 Shapes Macrophage Activation in Response to Immunometabolic Challenge. Cell Rep. 2017;19:225–234. doi: 10.1016/j.celrep.2017.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller F., Orrico M.I., Nascimento D.C., Czaikoski P.G., Souto F.O., Alves-Filho J.C., Freitas A., Carlos D., Montenegro M.F., Neto A.F., et al. Hydrogen sulfide improves neutrophil migration and survival in sepsis via K+ATP channel activation. Am. J. Respir. Crit. Care Med. 2010;182:360–368. doi: 10.1164/rccm.200907-1145OC. [DOI] [PubMed] [Google Scholar]
- Sun Q., Fan J., Billiar T.R., Scott M.J. Inflammasome and autophagy regulation - a two-way street. Mol. Med. 2017;23:188–195. doi: 10.2119/molmed.2017.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundrud M.S., Koralov S.B., Feuerer M., Calado D.P., Kozhaya A.E., Rhule-Smith A., Lefebvre R.E., Unutmaz D., Mazitschek R., Waldner H., et al. Halofuginone inhibits TH17 cell differentiation by activating the amino acid starvation response. Science. 2009;324:1334–1338. doi: 10.1126/science.1172638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukdar S., Oh D.Y., Bandyopadhyay G., Li D., Xu J., McNelis J., Lu M., Li P., Yan Q., Zhu Y., et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat. Med. 2012;18:1407–1412. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Ikeda K., Suganami T., Komiya C., Ochi K., Shirakawa I., Hamaguchi M., Nishimura S., Manabe I., Matsuda T., et al. Macrophage-inducible C-type lectin underlies obesity-induced adipose tissue fibrosis. Nat. Commun. 2014;5:4982. doi: 10.1038/ncomms5982. [DOI] [PubMed] [Google Scholar]
- Ukropec J., Anunciado R.P., Ravussin Y., Hulver M.W., Kozak L.P. UCP1-independent thermogenesis in white adipose tissue of cold-acclimated Ucp1-/- mice. J. Biol. Chem. 2006;281:31894–31908. doi: 10.1074/jbc.M606114200. [DOI] [PubMed] [Google Scholar]
- Van Kerkhove M.D., Vandemaele K.A., Shinde V., Jaramillo-Gutierrez G., Koukounari A., Donnelly C.A., Carlino L.O., Owen R., Paterson B., Pelletier L., et al. WHO Working Group for Risk Factors for Severe H1N1pdm Infection Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med. 2011;8:e1001053. doi: 10.1371/journal.pmed.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vayá A., Rivera L., Hernández-Mijares A., de la Fuente M., Solá E., Romagnoli M., Alis R., Laiz B. Homocysteine levels in morbidly obese patients: its association with waist circumference and insulin resistance. Clin. Hemorheol. Microcirc. 2012;52:49–56. doi: 10.3233/CH-2012-1544. [DOI] [PubMed] [Google Scholar]
- Wanders D., Stone K.P., Forney L.A., Cortez C.C., Dille K.N., Simon J., Xu M., Hotard E.C., Nikonorova I.A., Pettit A.P., et al. Role of GCN2-Independent Signaling Through a Noncanonical PERK/NRF2 Pathway in the Physiological Responses to Dietary Methionine Restriction. Diabetes. 2016;65:1499–1510. doi: 10.2337/db15-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Dillon C.P., Shi L.Z., Milasta S., Carter R., Finkelstein D., McCormick L.L., Fitzgerald P., Chi H., Munger J., Green D.R. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35:871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Shen L., Sun X., Liu F., Feng W., Jiang C., Chu X., Ye X., Jiang C., Wang Y., et al. Adipose group 1 innate lymphoid cells promote adipose tissue fibrosis and diabetes in obesity. Nat. Commun. 2019;10:3254. doi: 10.1038/s41467-019-11270-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R., Sohal R.S. Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N. Engl. J. Med. 1997;337:986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner E.D., Lee J., Hansen L., Yuan M., Shoelson S.E. Insulin resistance due to phosphorylation of insulin receptor substrate-1 at serine 302. J. Biol. Chem. 2004;279:35298–35305. doi: 10.1074/jbc.M405203200. [DOI] [PubMed] [Google Scholar]
- Winer S., Chan Y., Paltser G., Truong D., Tsui H., Bahrami J., Dorfman R., Wang Y., Zielenski J., Mastronardi F., et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat. Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer D.A., Winer S., Shen L., Wadia P.P., Yantha J., Paltser G., Tsui H., Wu P., Davidson M.G., Alonso M.N., et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat. Med. 2011;17:610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf Y., Boura-Halfon S., Cortese N., Haimon Z., Sar Shalom H., Kuperman Y., Kalchenko V., Brandis A., David E., Segal-Hayoun Y., et al. Brown-adipose-tissue macrophages control tissue innervation and homeostatic energy expenditure. Nat. Immunol. 2017;18:665–674. doi: 10.1038/ni.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Molofsky A.B., Liang H.E., Ricardo-Gonzalez R.R., Jouihan H.A., Bando J.K., Chawla A., Locksley R.M. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Grijalva A., Skowronski A., van Eijk M., Serlie M.J., Ferrante A.W., Jr. Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab. 2013;18:816–830. doi: 10.1016/j.cmet.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Youm Y.H., Dixit V.D. Inhibition of thymic adipogenesis by caloric restriction is coupled with reduction in age-related thymic involution. J. Immunol. 2009;183:3040–3052. doi: 10.4049/jimmunol.0900562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Youm Y.H., Vandanmagsar B., Rood J., Kumar K.G., Butler A.A., Dixit V.D. Obesity accelerates thymic aging. Blood. 2009;114:3803–3812. doi: 10.1182/blood-2009-03-213595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Youm Y.H., Vandanmagsar B., Ravussin A., Gimble J.M., Greenway F., Stephens J.M., Mynatt R.L., Dixit V.D. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J. Immunol. 2010;185:1836–1845. doi: 10.4049/jimmunol.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G., Zhao K., Ju Y., Mani S., Cao Q., Puukila S., Khaper N., Wu L., Wang R. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid. Redox Signal. 2013;18:1906–1919. doi: 10.1089/ars.2012.4645. [DOI] [PubMed] [Google Scholar]
- Yang R., Qu C., Zhou Y., Konkel J.E., Shi S., Liu Y., Chen C., Liu S., Liu D., Chen Y., et al. Hydrogen Sulfide Promotes Tet1- and Tet2-Mediated Foxp3 Demethylation to Drive Regulatory T Cell Differentiation and Maintain Immune Homeostasis. Immunity. 2015;43:251–263. doi: 10.1016/j.immuni.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying W., Riopel M., Bandyopadhyay G., Dong Y., Birmingham A., Seo J.B., Ofrecio J.M., Wollam J., Hernandez-Carretero A., Fu W., et al. Adipose Tissue Macrophage-Derived Exosomal miRNAs Can Modulate In Vivo and In Vitro Insulin Sensitivity. Cell. 2017;171:372–384. doi: 10.1016/j.cell.2017.08.035. [DOI] [PubMed] [Google Scholar]
- Yoshida S., Yamahara K., Kume S., Koya D., Yasuda-Yamahara M., Takeda N., Osawa N., Chin-Kanasaki M., Adachi Y., Nagao K., et al. Role of dietary amino acid balance in diet restriction-mediated lifespan extension, renoprotection, and muscle weakness in aged mice. Aging Cell. 2018;17:e12796. doi: 10.1111/acel.12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youm Y.H., Nguyen K.Y., Grant R.W., Goldberg E.L., Bodogai M., Kim D., D’Agostino D., Planavsky N., Lupfer C., Kanneganti T.D., et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015;21:263–269. doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanardo R.C., Brancaleone V., Distrutti E., Fiorucci S., Cirino G., Wallace J.L. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20:2118–2120. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- Zapata R.C., Singh A., Ajdari N.M., Chelikani P.K. Dietary Tryptophan Restriction Dose-Dependently Modulates Energy Balance, Gut Hormones, and Microbiota in Obesity-Prone Rats. Obesity (Silver Spring) 2018;26:730–739. doi: 10.1002/oby.22136. [DOI] [PubMed] [Google Scholar]
- Zhang X., Huang Q., Wang X., Deng Z., Li J., Yan X., Jauhiainen M., Metso J., Libby P., Liu J., Shi G.P. Dietary cholesterol is essential to mast cell activation and associated obesity and diabetes in mice. Biochim Biophys Acta Mol Basis Dis. 2019;1865:1690–1700. doi: 10.1016/j.bbadis.2019.04.006. [DOI] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]