To the Editor:
Acute intermittent hypoxia (AIH) is a novel treatment to enhance respiratory and nonrespiratory motor function after chronic incomplete spinal cord injury (SCI) (1–3). Despite promising findings, uncertainties in optimal AIH delivery to a heterogeneous population of persons with SCI remain a challenge to clinical translation. Beneficial AIH responses are variable between individuals, and we lack useful biomarkers to determine which individuals benefit most from treatment. AIH-induced functional benefits rely on mechanisms of AIH-induced neuroplasticity. Multiple factors modify AIH-induced plasticity in rodent models, including intermittent hypoxia preconditioning and systemic inflammation, among others (4, 5). One factor not accounted for in human AIH trials, to date, is the incidence of sleep-disordered breathing (SDB). Many individuals with SCI exhibit mild to moderate SDB (6), leading to extended periods of nocturnal high-dose intermittent hypoxia. Because chronic intermittent hypoxia (CIH) elicits both neuroplasticity and inflammation (5), SDB after SCI may contribute to between-person variations in response to AIH therapy.
Here, we report associations between SDB and AIH-induced motor recovery in individuals with chronic incomplete SCI. Specifically, we conducted a blinded continuing analysis in 20 participants who performed baseline sleep assessments and one of three clinical trials with similar experimental designs (1–3), including treatment methods, randomization (AIH/sham treatment), blindness, and washout period. Participants who missed an evaluation session (n = 5), were enrolled in more than one of these studies (n = 5) (in which case, we evaluated only the first study completed), or had <4 hours of sleep data duration (n = 8) were not included. Participants performed five consecutive (daily) AIH breathing treatment sessions (or a single-day session; n = 3). A manually controlled air delivery system (Hypoxico, Inc.) was used with 15 sequences of 60–90 seconds of low-oxygen breathing (AIH; FiO2 = 0.10 ± 0.02) or room air (sham; FiO2 = 0.21 ± 0.02) alternated with 60-second intervals of breathing room air. We compiled the apnea–hypopnea index, lowest O2 pulse saturation (average pulse oxygen saturation [SpO2] nadir), inspiratory flow limitations, snoring counts, and pulse frequency (ApneaLink; Resmed Corp.) and quantified associations between these metrics and AIH-induced improvements in motor function. We computed effect sizes to standardize analyses across trials with different functional outcome measures (limb strength, walking ability, or hand function). Within-subject Cohen’s d scores corresponded with a participant’s effect size after treatment and were calculated as follows: Cohen’s d = [average change from baseline with AIH] − [average changes from baseline with sham]/baseline pooled SD (7).
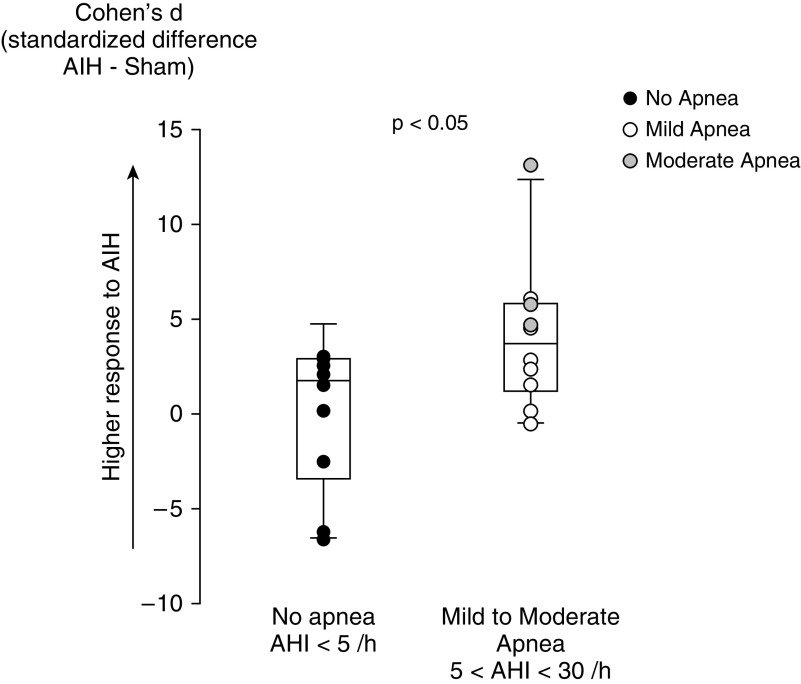
Mild to moderate sleep apnea (5 < apnea–hypopnea index <30/h) occurred in 50% of study participants. The participants with mild to moderate apnea (n = 10) had the same characteristics as those without apnea (n = 10, Table 1). Participants with mild to moderate SDB demonstrated greater motor gains with AIH therapy (Cohen’s d median: 3.6; range: −0.5 to 13.6) versus those without apnea (1.7; range: −6.6 to 4.9; P = 0.043) (Figure 1). Cohen’s d positively correlated with the oxygen desaturation index (ODI-4%) (r = 0.74; P < 0.0001) and negatively correlated with the average SpO2 nadir (r = −0.69; P = 0.001). Moreover, the time spent during sleep with SpO2 below 90% also correlated to Cohen’s d (r = 0.51; P = 0.024); in a multiple regression analysis (r = 0.73; P = 0.002), ODI-4% was an independent predictor of Cohen’s d (P = 0.007), whereas time spent during sleep with SpO2 below 90% was not (P = 0.56), suggesting that the AIH response mostly related to repetitive nocturnal O2 desaturations. Conversely, pulse frequency (sympathetic activity) or snoring events were not related to AIH response. Finally, Cohen’s d did not correlate with SpO2 during AIH therapy (first and fifth sessions) (r < 0.30; P > 0.25).
Table 1.
Patient Characteristics, Sleep Parameters, and Functional Gain after AIH Therapy (Cohen’s d)
| No Apnea (n = 10) | Mild to Moderate Apnea (n = 10) | P Value* | Mild Apnea 5 ≤ AHI < 15 (n = 7) | Moderate Apnea 15 < AHI < 30 (n = 3) | |
|---|---|---|---|---|---|
| AHI, no./h† | 1.5 (0–4) | 9.0 (6–29) | <0.001 | 6.5 (6–10) | 23 (17–29) |
| Cohen’s d | 0.2 ± 4.0 | 4.2 ± 3.8 | 0.043 | 2.4 ± 2.3 | 7.8 ± 4.6 |
| Age, yr | 46 ± 14 | 45 ± 8 | 0.90 | 42 ± 15 | 53 ± 14 |
| Level of injury† | C5 (C2–T4) | C5 (C4–T8) | 0.26 | C5 (C5–T8) | C5 (C4–T3) |
| Time since injury, yr† | 7 (3–38) | 7 (3–25) | 0.64 | 7.5 (6–18) | 11 (7–24) |
| BMI, kg/m2† | 20 (19–30) | 24 (20–33) | 0.23 | 24 (20–25) | 28 (24–33) |
| Sleep parameters | |||||
| Evaluation duration, h/night | 6 ± 2 | 7 ± 2 | 0.42 | 6 ± 2 | 8 ± 1 |
| Apnea index, no./h† | 0 (0–2.5) | 2.5 (0.5–27) | 0.001 | 1.5 (0.5–4.3) | 14 (0.5–27) |
| Hypopnea index, no./h | 1.1 ± 0.9 | 5.6 ± 2.1 | <0.001 | 5.1 ± 1.0 | 5.6 ± 3.7 |
| Oxygen desaturation index, no./h† | 1 (0–6) | 10 (4–34) | <0.001 | 8.5 (4–13) | 23 (4–34) |
| Average SpO2, % | 93 ± 4 | 91 ± 4 | 0.048 | 93 ± 2 | 89 ± 7 |
| Average SpO2 nadir, % | 91 ± 5 | 84 ± 6 | 0.005 | 87 ± 2 | 76 ± 3 |
| Time with SpO2 ≤ 90%, min† | 2 (0–36) | 67 (1–137) | 0.002 | 48 (1–110) | 85 (1–137) |
| Average pulse frequency, beats/min | 65 ± 9 | 65 ± 10 | 0.95 | 65 ± 12 | 65 ± 6 |
| Highest pulse frequency, beats/min | 130 ± 41 | 114 ± 32 | 0.77 | 102 ± 29 | 140 ± 42 |
| Snoring events, no./h | 578 ± 673 | 653 ± 760 | 0.47 | 353 ± 411 | 1,353 ± 1,023 |
Definition of abbreviations: AHI = apnea–hypopnea index; AIH = acute intermittent hypoxia; BMI = body mass index; SpO2 = pulse oxygen saturation.
Data are mean ± SD except where otherwise noted.
Comparison between “no apnea” and “apnea” (mild to moderate apnea) (t test or Mann-Whitney rank test comparisons).
Median (range) for nonparametric variables.
Figure 1.
Effect of sleep-disordered breathing (as assessed by the apnea–hypopnea index [AHI]) on the response to acute intermittent hypoxia (AIH) therapy. Shown are individual values and box plot (median, 10th, 25th, 75th, and 90th percentiles with error bar) of Cohen’s d in individuals with incomplete spinal cord injury and sleep apnea (mild to moderate apnea 5 < AHI < 30/h) or no apnea (AHI < 5/h).
Our results demonstrated that mild to moderate SDB is associated with more significant AIH-induced therapeutic benefits in people with chronic incomplete SCI. SDB (and associated CIH) may precondition AIH therapy, enabling larger responses to subsequent AIH treatments as reflected in improved AIH-induced performance gains. The dose–response between nocturnal O2 desaturation and AIH-induced motor gains supports the hypothesis that a minimum hypoxia severity is necessary to induce motor adaptations (4). Based on available literature in rodent models, this effect may arise via CIH-preconditioning effects on carotid body chemoreceptors, enabling long-term sensory facilitation in carotid sinus nerve activity and amplification of chemoafferent integration within the central nervous system (8). However, given the lack of correlation between SpO2 during AIH therapy (an inverse indicator of respiratory chemoreflex) and AIH-induced motor gains, it seems unlikely that chemosensory plasticity would explain our results. Alternatively, spinal mechanisms may enhance the capacity for AIH-induced motor plasticity (4). For example, repetitive serotonin receptor activation may increase BDNF (brain-derived neurotrophic factor) protein synthesis and BDNF/TrkB (tropomyosin receptor kinase B) signaling, causing the functional synaptic plasticity that underlies beneficial effects of therapeutic AIH (9). In humans, reminder doses of three times/week maintain increases in walking speed and endurance for up to 5 weeks after 1 week of daily AIH (10). The independent effect of the repetitive occurrence of sleep apnea (ODI) on functional gain would support this hypothesis. Hence, plasticity in somatic motor output could result from cumulative repeated CIH/AIH.
Our results also suggest that SDB may serve as a “biomarker” that indicates the persons most likely to benefit from AIH therapy. It would be interesting to know if SDB itself (via mild to moderate CIH) is directly associated with better functional outcomes during normal rehabilitative therapeutics. However, because severity of hypoxia (dosage) and repetitive intermittent hypoxia are key components of metaplasticity, mild to moderate amounts of CIH may more likely precondition AIH therapy (4).
Participants accepted into this study did not have severe SDB; more severe SDB may lead to deleterious proinflammatory responses that inhibit AIH therapy–induced gains (5). Given the potential benefits of mild or modest CIH versus the known pathological impact of obstructive and central SDB on health, this study does not guide whether individuals with SCI need SDB treatment. Understanding the history of hypoxic exposures merits further consideration when evaluating therapeutic AIH dosing and efficiency.
Overall, our study demonstrates that AIH therapy–induced functional gains are more robust in people with chronic incomplete SCI and mild to moderate SDB. Repetitive nocturnal desaturation may precondition spared neural circuitry involved in functional tasks, enabling them to respond to subsequent AIH therapy more positively. If confirmed, these findings strongly suggest a need to refine AIH delivery protocols and that assessment of SDB provides valuable insight into individual responsiveness to AIH therapy. Further larger and dedicated studies are needed to confirm our findings and pursue the mechanisms by which mild to moderate SDB may precondition AIH therapy and favor motor recovery in SCI.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the physical therapists and physicians who supported functional tests and therapies at Emory University’s Department of Rehabilitation Medicine in Atlanta, GA, the Shepherd Center in Atlanta, GA, and the Shirley Ryan Ability Lab in Chicago, IL. The authors thank all study participants.
Footnotes
Supported by the U.S. Department of Defense Spinal Cord Injury Research Program grant W81XWH-15-2-0045 and the NIH National Institute of Child Health and Human Development grant R01HD081274.
Originally Published in Press as DOI: 10.1164/rccm.202002-0245LE on May 5, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Trumbower RD, Jayaraman A, Mitchell GS, Rymer WZ. Exposure to acute intermittent hypoxia augments somatic motor function in humans with incomplete spinal cord injury. Neurorehabil Neural Repair. 2012;26:163–172. doi: 10.1177/1545968311412055. [DOI] [PubMed] [Google Scholar]
- 2.Hayes HB, Jayaraman A, Herrmann M, Mitchell GS, Rymer WZ, Trumbower RD. Daily intermittent hypoxia enhances walking after chronic spinal cord injury: a randomized trial. Neurology. 2014;82:104–113. doi: 10.1212/01.WNL.0000437416.34298.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trumbower RD, Hayes HB, Mitchell GS, Wolf SL, Stahl VA. Effects of acute intermittent hypoxia on hand use after spinal cord trauma: a preliminary study. Neurology. 2017;89:1904–1907. doi: 10.1212/WNL.0000000000004596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fields DP, Mitchell GS. Spinal metaplasticity in respiratory motor control. Front Neural Circuits. 2015;9:2. doi: 10.3389/fncir.2015.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huxtable AG, Smith SM, Peterson TJ, Watters JJ, Mitchell GS. Intermittent hypoxia-induced spinal inflammation impairs respiratory motor plasticity by a spinal p38 MAP kinase-dependent mechanism. J Neurosci. 2015;35:6871–6880. doi: 10.1523/JNEUROSCI.4539-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sankari A, Vaughan S, Bascom A, Martin JL, Badr MS. Sleep-disordered breathing and spinal cord injury: a state-of-the-art review. Chest. 2019;155:438–445. doi: 10.1016/j.chest.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen J. Statistical power analysis for the behavioral sciences. New York, NY: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 8.Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci USA. 2003;100:10073–10078. doi: 10.1073/pnas.1734109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lovett-Barr MR, Satriotomo I, Muir GD, Wilkerson JE, Hoffman MS, Vinit S, et al. Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury. J Neurosci. 2012;32:3591–3600. doi: 10.1523/JNEUROSCI.2908-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navarrete-Opazo A, Alcayaga J, Sepúlveda O, Rojas E, Astudillo C. Repetitive intermittent hypoxia and locomotor training enhances walking function in incomplete spinal cord injury subjects: a randomized, triple-blind, placebo-controlled clinical trial. J Neurotrauma. 2017;34:1803–1812. doi: 10.1089/neu.2016.4478. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.