A hypothesis is nothing but a hypothesis until proven. The fetal origins of disease hypothesis, formally the Developmental Origins of Health and Disease hypothesis, postulates that early life events may have a long-term impact on diseases and traits in adulthood (1). Such events, including environmental exposures, and developmental or pathophysiologic processes, may take place in utero, perinatally, or during childhood. Evidence is now accumulating that supports the Developmental Origins of Health and Disease hypothesis in that factors underpinning lung disease risk in adulthood act in early life (2–4).
In this context, Portas and colleagues (pp. 853–865) report in this issue of the Journal associations between lung developmental genes and adult lung function using the U.K. Biobank (5). They make use of lung development biology knowledge, selecting candidate genes to explore associations with lung function indices (Figure 1), rather than starting with an agnostic genome-wide association study (GWAS) analysis, currently a standard approach.
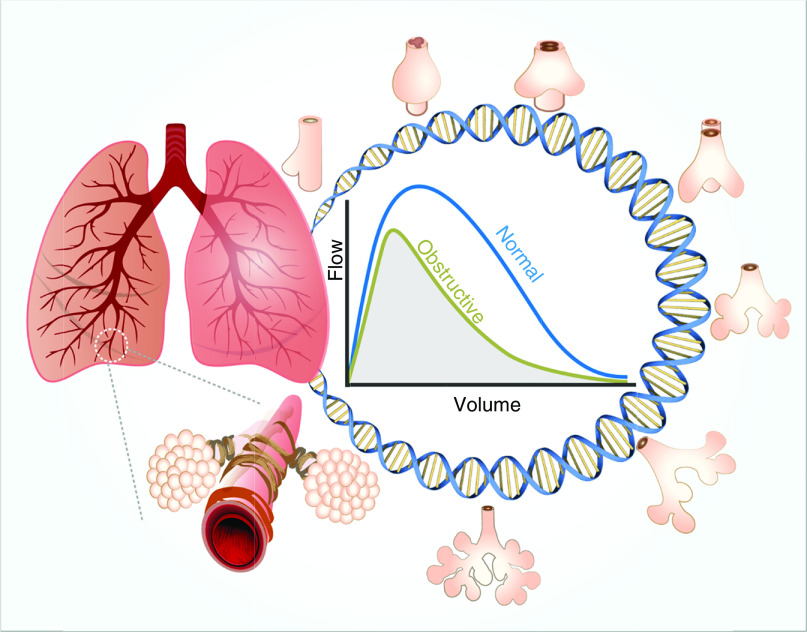
Figure 1.
A schematic figure depicting the study design by Portas and colleagues. Genes known to be involved in lung development were selected as candidate genes to explore associations with lung function in adults (flow–volume indices FVC and FEV1/FVC). Illustration by Fuad Bahram, FB Scientific Art Design.
In the study by Portas and colleagues, almost 350,000 subjects with mean age 56 years (range, 39–70 yr) contributed cross-sectional lung function data from the well-powered U.K. Biobank (6, 7). The list of genes related to lung development was prepared by two authors, summarizing both human and experimental data in a variety of model organisms. In addition, this list was further extended to include relevant genes based on pathway information from four databases. In total, 391 genes (represented by 106,384 variants) believed to influence lung development were tested for association with prebronchodilator FVC and FEV1/FVC. Using a two-stage and “best SNP per gene” approach, novel independent signals from 36 genes were identified and replicated internally; 16 were uniquely associated with FVC, 19 were uniquely with FEV1/FVC, and only one signal was associated with both traits. Next, the authors used meta-analysis data from previous GWASs in the CHARGE (Cohorts for Heart and Aging Research in Genomic Epidemiology) and SpiroMeta consortia (n > 100,000 in both datasets) and replicated 16 variants. Pathway analyses revealed that identified genes belong primarily to the following pathways: growth factors, transcriptional regulators, cell–cell adhesion/cytoskeletal, and extracellular matrix, which was not surprising given the fact that genes were preselected based on involvement in lung development in the first place. Finally, a majority of the key SNPs were found to influence expression in the blood and/or lung tissue.
The results emerging from this methodologically sound sequence of analyses have important implications. If the missing heritability of complex traits resides at least partly in genetic variants that are missed by traditional genome-wide significance thresholds, using a priori knowledge to reduce the search space may be an effective approach to retrieve these missing genomic components. Using this hypothesis-driven approach, which is reminiscent of the classical candidate gene or pathway study, this study identified 16 novel variants associated with lung function that were sufficiently robust to survive both internal and external replication. Of note, although all these variants were significant after Bonferroni correction, only a few of them reached genome-wide significance in the U.K. Biobank, and none did in the external replication. Therefore, this approach identified successfully multiple novel robust genetic variants for lung function that could have been missed in a traditional GWAS. Naturally, any approach that is based on a priori knowledge is as good as the knowledge on which it is based. Although the authors did try to formalize their selection process of genes, it should be noted that this process eventually boils down to expert opinion and the integration of data from animal and human studies, which could be perceived as subjective. Future approaches guided by single cell–specific transcriptomic signatures obtained during different stages of lung development may represent another way to select genes and limit the search space of a GWAS (8).
Complex traits are complex not only because of their multifactorial nature but also because of their phenotypic heterogeneity. Lung function impairment is no exception, as it is associated with different profiles of risk factors and morbidities (and genetic determinants) depending on whether the “impairment” refers to FEV1, FVC, or their ratio. Not surprisingly, in the study by Portas and colleagues, the vast majority (97%) of the identified susceptibility genes affected either FVC or FEV1/FVC uniquely, and only one variant was associated with both indices. Although deficits in FEV1/FVC identify the obstructive pattern and are the hallmark of chronic obstructive pulmonary disease (COPD), low levels of FVC in the presence of a conserved ratio could be indicative of a spirometric restrictive pattern (albeit not diagnostic), which has been shown to carry a substantial and frequently overlooked morbidity and mortality burden in the general population (9, 10).
It is now clear that substantial heterogeneity exists also in the trajectories by which lung function patterns develop (11, 12). It has been conclusively shown that adults may develop irreversible airflow limitation, the functional hallmark of COPD, by either having an accelerated decline of FEV1 in adult years, by reaching suboptimal maximal FEV1 levels by young adulthood, or by any combination of the two (13). To what extent these trajectories are influenced by different molecular pathways and genetic determinants is largely unknown. By focusing on genes involved in lung development, this study captured genetic contributions that are likely relevant to a persistently low lung function trajectory into adult life. Interestingly, previous studies that tested genetic variants known to be associated with levels of adult lung function failed to find those variants to be associated with the decline of lung function (14). This suggests that the effects of genetic variants identified to date are possibly mediated more through development and growth of lung function than susceptibility to accelerated decline. The differential expression of lung function genes during fetal lung development in previous studies lends support for this observation (6, 15).
Because of the cross-sectional nature and the age range of participants in the U.K. Biobank, the study by Portas and colleagues could not address genetic contributions to lung function trajectories. We recommend that the newly identified genetic variants should be studied in the context of longitudinal lung function from cohorts that transition from childhood into adult life. This will enable the research community to fully exploit the opportunities that the fetal origins hypothesis offers to advance risk stratification and preserve lung health across the life span.
Supplementary Material
Footnotes
E.M. is supported by grants from the European Research Council (TRIBAL grant agreement 757919), the Swedish Research Council, the Swedish Heart-Lung Foundation, and Region Stockholm (ALF project).
Originally Published in Press as DOI: 10.1164/rccm.202006-2123ED on July 7, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Hanson M, Gluckman P. Developmental origins of noncommunicable disease: population and public health implications. Am J Clin Nutr. 2011;94(Suppl):1754S–1758S. doi: 10.3945/ajcn.110.001206. [DOI] [PubMed] [Google Scholar]
- 2. Martinez FD. Early-life origins of chronic obstructive pulmonary disease. N Engl J Med. 2016;375:871–878. doi: 10.1056/NEJMra1603287. [DOI] [PubMed] [Google Scholar]
- 3. Melén E, Guerra S, Hallberg J, Jarvis D, Stanojevic S. Linking COPD epidemiology with pediatric asthma care: implications for the patient and the physician. Pediatr Allergy Immunol. 2019;30:589–597. doi: 10.1111/pai.13054. [DOI] [PubMed] [Google Scholar]
- 4. Allinson JP, Hardy R, Donaldson GC, Shaheen SO, Kuh D, Wedzicha JA. Combined impact of smoking and early-life exposures on adult lung function trajectories. Am J Respir Crit Care Med. 2017;196:1021–1030. doi: 10.1164/rccm.201703-0506OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Portas L, Pereira M, Shaheen SO, Wyss AB, London SJ, Burney PGJ, et al. Lung development genes and adult lung function. Am J Respir Crit Care Med. 2020;202:853–865. doi: 10.1164/rccm.201912-2338OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wain LV, Shrine N, Miller S, Jackson VE, Ntalla I, Soler Artigas M, et al. UK Brain Expression Consortium (UKBEC); OxGSK Consortium. Novel insights into the genetics of smoking behaviour, lung function, and chronic obstructive pulmonary disease (UK BiLEVE): a genetic association study in UK Biobank. Lancet Respir Med. 2015;3:769–781. doi: 10.1016/S2213-2600(15)00283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shrine N, Guyatt AL, Erzurumluoglu AM, Jackson VE, Hobbs BD, Melbourne CA, et al. Understanding Society Scientific Group. New genetic signals for lung function highlight pathways and chronic obstructive pulmonary disease associations across multiple ancestries. Nat Genet. 2019;51:481–493. doi: 10.1038/s41588-018-0321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schiller HB, Montoro DT, Simon LM, Rawlins EL, Meyer KB, Strunz M, et al. The human lung cell atlas: a high-resolution reference map of the human lung in health and disease. Am J Respir Cell Mol Biol. 2019;61:31–41. doi: 10.1165/rcmb.2018-0416TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Godfrey MS, Jankowich MD. The vital capacity is vital: epidemiology and clinical significance of the restrictive spirometry pattern. Chest. 2016;149:238–251. doi: 10.1378/chest.15-1045. [DOI] [PubMed] [Google Scholar]
- 10. Guerra S, Sherrill DL, Venker C, Ceccato CM, Halonen M, Martinez FD. Morbidity and mortality associated with the restrictive spirometric pattern: a longitudinal study. Thorax. 2010;65:499–504. doi: 10.1136/thx.2009.126052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Agusti A, Faner R. Lung function trajectories in health and disease. Lancet Respir Med. 2019;7:358–364. doi: 10.1016/S2213-2600(18)30529-0. [DOI] [PubMed] [Google Scholar]
- 12. Bui DS, Lodge CJ, Burgess JA, Lowe AJ, Perret J, Bui MQ, et al. Childhood predictors of lung function trajectories and future COPD risk: a prospective cohort study from the first to the sixth decade of life. Lancet Respir Med. 2018;6:535–544. doi: 10.1016/S2213-2600(18)30100-0. [DOI] [PubMed] [Google Scholar]
- 13. Lange P, Celli B, Agustí A, Boje Jensen G, Divo M, Faner R, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373:111–122. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]
- 14. John C, Soler Artigas M, Hui J, Nielsen SF, Rafaels N, Paré PD, et al. Genetic variants affecting cross-sectional lung function in adults show little or no effect on longitudinal lung function decline. Thorax. 2017;72:400–408. doi: 10.1136/thoraxjnl-2016-208448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Loth DW, Soler Artigas M, Gharib SA, Wain LV, Franceschini N, Koch B, et al. Genome-wide association analysis identifies six new loci associated with forced vital capacity. Nat Genet. 2014;46:669–677. doi: 10.1038/ng.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.