Abstract
Rationale: Coronavirus disease (COVID-19) is a global threat to health. Its inflammatory characteristics are incompletely understood.
Objectives: To define the cytokine profile of COVID-19 and to identify evidence of immunometabolic alterations in those with severe illness.
Methods: Levels of IL-1β, IL-6, IL-8, IL-10, and sTNFR1 (soluble tumor necrosis factor receptor 1) were assessed in plasma from healthy volunteers, hospitalized but stable patients with COVID-19 (COVIDstable patients), patients with COVID-19 requiring ICU admission (COVIDICU patients), and patients with severe community-acquired pneumonia requiring ICU support (CAPICU patients). Immunometabolic markers were measured in circulating neutrophils from patients with severe COVID-19. The acute phase response of AAT (alpha-1 antitrypsin) to COVID-19 was also evaluated.
Measurements and Main Results: IL-1β, IL-6, IL-8, and sTNFR1 were all increased in patients with COVID-19. COVIDICU patients could be clearly differentiated from COVIDstable patients, and demonstrated higher levels of IL-1β, IL-6, and sTNFR1 but lower IL-10 than CAPICU patients. COVID-19 neutrophils displayed altered immunometabolism, with increased cytosolic PKM2 (pyruvate kinase M2), phosphorylated PKM2, HIF-1α (hypoxia-inducible factor-1α), and lactate. The production and sialylation of AAT increased in COVID-19, but this antiinflammatory response was overwhelmed in severe illness, with the IL-6:AAT ratio markedly higher in patients requiring ICU admission (P < 0.0001). In critically unwell patients with COVID-19, increases in IL-6:AAT predicted prolonged ICU stay and mortality, whereas improvement in IL-6:AAT was associated with clinical resolution (P < 0.0001).
Conclusions: The COVID-19 cytokinemia is distinct from that of other types of pneumonia, leading to organ failure and ICU need. Neutrophils undergo immunometabolic reprogramming in severe COVID-19 illness. Cytokine ratios may predict outcomes in this population.
Keywords: COVID-19, cytokines, neutrophils, alpha-1 antitrypsin, immunometabolism
At a Glance Commentary
Scientific Knowledge on the Subject
The characteristics of the cytokinemia associated with coronavirus disease (COVID-19) are incompletely understood. Data on immunometabolic alterations in patients with severe illness are scarce.
What This Study Adds to the Field
Here, we demonstrate that the COVID-19 cytokinemia is distinct from that observed in other critical care presentations, with marked differences in the balance between proinflammatory and antiinflammatory cytokines and a blunted alpha-1 antitrypsin acute-phase response. Neutrophils display altered immunometabolism in severe COVID-19.
In late 2019, multiple pneumonia cases of unknown origin were identified in Wuhan, China (1). The inciting pathogen was subsequently identified as a novel enveloped RNA betacoronavirus, now known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), giving rise to coronavirus disease (COVID-19). The World Health Organization has since declared COVID-19 a public health emergency of international concern. As of mid-June 2020, more than 8 million laboratory-confirmed cases have been documented globally, with over 450,000 deaths (2).
Little is known about the prehospital course of the disease. However, in-hospital studies have described a proinflammatory syndrome with a disproportionately high rate of progression to acute respiratory distress syndrome (ARDS), acute renal failure, shock, and arrhythmia (3, 4). Profound hypoxemia at initial presentation is common. Current management remains supportive, focusing on supplemental oxygen, vasopressors to maintain perfusion pressure, and mechanical support in the event of end-organ failure. Despite the implications for global health, the inflammatory characteristics of patients with COVID-19 are incompletely understood, as are the inflammatory mediators and attendant molecular mechanisms underlying them. Indeed, whether a distinct COVID-19 inflammatory profile exists, as opposed to the inflammation seen in these patients being merely the product of a generic response to severe illness, remains unclear, as does the question of whether subphenotypes exist within the COVID-19 cohort. Similarly, the degree of the endogenous antiinflammatory response to SARS-CoV-2 infection, and the role of this response in delineating those who recover from those who experience severe morbidity and mortality, has not been fully elucidated.
Critical illness is notable for markedly increased energy demands. ATP serves as the building block of this energy and is produced via two linked metabolic pathways, glycolysis and the tricarboxylic acid cycle, also known as Krebs’ cycle. Although quiescent human neutrophils demonstrate tricarboxylic acid cycle activity, their metabolism is predominantly glycolytic. Certain circumstances, such as hypoxemia, infection, and inflammation, stand to shift the metabolism of circulating neutrophils further toward glycolysis.
The key step in glycolysis is the conversion of phosphoenolpyruvate to pyruvate, a nonequilibrium reaction catalyzed by PK (pyruvate kinase). Two major PK isoenzymes exist. PKM1 (pyruvate kinase M1) is considered to be the more enzymatically active form and promotes entry of pyruvate into Krebs’ cycle via pyruvate dehydrogenase. PKM2, on the other hand, displays lower enzymatic activity than PKM1, and instead promotes increased glycolysis with resultant cytosolic accumulation of lactate and other metabolic intermediates (5). PKM2 can assume different conformations, with dimers existing in equilibrium with tetramers. Critically, PKM2 dimers are capable of nuclear translocation, where they directly interact with the transcription factor HIF-1α (hypoxia-inducible factor-1α) (6, 7). At the nucleus, in addition to driving the adaptor response to hypoxia (8), HIF-1α transcribes glycolytic machinery and proinflammatory cytokines (9), particularly the master proinflammatory cytokine IL-1β, a pivotal cause of chronic and acute inflammation (10) and generation of the febrile response in humans (11), and IL-6. This proinflammatory shift is compounded by concomitant downregulation of antiinflammatory and proresolution cytokines such as IL-10 (6). In addition to their well-defined role in systemic inflammation, IL-1β, IL-6, and IL-10 have been shown to drive airway inflammation in ARDS, a syndrome characterized by acute hypoxemic respiratory failure, disruption of the alveolar-capillary barrier, and excessive neutrophil-predominant inflammation (12, 13).
In this study, we evaluated the in-hospital cytokine profile of the patient with COVID-19 to determine whether the clinical status of these individuals could be identified using such a profile and whether the inflammation seen in COVID-19 could be distinguished from that seen in patients with severe non–COVID-19 pneumonia requiring ICU support. Given the role of immunometabolism in inflammatory cytokine regulation, we also searched for evidence of metabolic reprogramming of circulating immune cells during severe COVID-19 illness. Finally, we assessed the acute phase antiinflammatory response of AAT (alpha-1 antitrypsin) to COVID-19 and whether shortcomings in this response were associated with worse outcome in those with severe illness.
Methods
Ethical approval was received from the Beaumont Hospital Ethics Committee (REC #18/52, #17/06, with both projects amended specifically for inclusion of subjects with COVID-19). Healthy control (HC) subjects (n = 15) and patients with a laboratory-confirmed diagnosis of COVID-19 infection who were hospitalized and symptomatic but clinically stable 7 days after onset of symptoms (COVIDstable patients, n = 20) were compared with patients positive for COVID-19 and symptomatic for 7 days who required admission to the ICU at that time for intubation and mechanical ventilation in the context of hypoxemic respiratory failure (COVIDICU patients, n = 20). Patients with severe community-acquired pneumonia (CAP) receiving intubation and mechanical ventilation (CAPICU patients, n = 15) were also studied for the purpose of further comparison. Initial blood samples for cytokine measurements and hospital-based blood testing in the ICU cohorts were obtained from the same blood draw at the time of intubation, with follow-up sampling at 2-day intervals while in ICU. A confirmed case of COVID-19 was defined by a positive result on an RT-PCR assay of a specimen collected on a nasopharyngeal swab. Prolonged ICU stay was defined as an ICU length of stay of greater than 12 consecutive days. Patients were excluded if they were immunosuppressed; receiving long-term oral corticosteroids, anti–IL-1, anti–IL-6, or anti-TNF (tumor necrosis factor) therapy; known to be pregnant; had active neoplasia; or had a history of vasculitis or connective tissue disease.
Sample Preparation
Human blood neutrophils were isolated as previously described (14) using dextran sedimentation followed by lymphoprep (Axis-Shield Poc AS) centrifugation. Neutrophil cytosolic and nuclear fractions were obtained at 4°C in the presence of protease and phosphatase inhibitors (Table E1 in the online supplement) as previously described (15). For each neutrophil cytosol preparation, 1 × 107 neutrophils were used. For nuclear extracts, 8.8 × 106 neutrophils were required. Plasma was isolated by centrifugation of whole blood in lithium heparin tubes at 240 × g for 5 minutes at room temperature. AAT protein phenotypes in plasma were determined by immunofixation of serum glycoforms via isoelectric focusing gel electrophoresis prior to analysis (16).
Plasma Cytokines
Cytokine levels were measured in plasma from the whole blood of HC subjects (n = 15), COVIDstable patients (n = 20), COVIDICU patients (n = 20), and CAPICU patients (n = 15). IL-1β, IL-6, IL-8, IL-10, and sTNFR1 (soluble TNF receptor 1) were measured by ELISA (all R&D Systems) in accordance with the manufacturer’s instructions. The lower limits of detection for each assay are provided in Table E2. Where cytokine levels were undetectable, a value of 0 was assigned.
Immunometabolic Markers
PKM2, phosphorylated PKM2, and HIF-1α were detected by sodium dodecyl sulfate polyacrylamide gel electrophoresis and Western blotting. Cytosolic lactate and pyruvate were measured by colorimetric assay (both Abcam). For experiments involving immunometabolic markers, samples from COVIDICU patients (n = 8) were compared with samples from HC subjects (n = 8) matched for age, sex, and body mass index. A complete list of product details for key resources used is available in Table E2.
Statistical Analysis
Results are reported as absolute numbers, medians, or means and SDs, as appropriate. Categorical variables are summarized as counts and percentages. No imputation was made for missing data. Changes in the levels of biomarkers between groups were analyzed using GraphPad Prism 8.0 software for Windows, with an unpaired t test used for comparisons between two groups in the event of normally distributed data and nonparametric Mann-Whitney testing used in the event of data failing the test for normality. Comparisons across three or more groups were by ANOVA or Kruskal-Wallis test. Changes in biomarkers over time between patient groups were analyzed using patient group and direction of change in biomarker (slope) as factors. P values were derived by Tukey’s post hoc multiple comparison test to control for familywise error rate. Where significant interaction between factors was observed, groupwise P values are used to refer to simple effects within factors. P < 0.05 was considered statistically significant.
Results
Characteristics of the COVID-19 Cohorts
The demographic and clinical characteristics of the patients with COVID-19 are shown in Table 1, with further details regarding the COVIDICU group as well as the HC and CAPICU groups provided in Tables E3–E5. The mean (±SD) age of the overall COVID-19 cohort was 55.5 ± 17.7 years; 62.5% were men. The most common symptoms on admission to the hospital were fever, dyspnea, and cough. The mean duration of symptoms prior to blood sampling was 7 days. Fifteen percent had a history of travel to a country where COVID-19 was declared endemic within the previous month (China, South Korea, Italy, Spain, or Iran). Forty-five percent reported being in close contact with a confirmed case, though this may be underrepresentative because of the testing restrictions in effect in Ireland at the time of the study. Thirty percent were healthcare workers. Community-acquired infection, defined for the purpose of this study as a positive diagnosis in the absence of either known close contact with any other confirmed case or recent travel to an area officially designated as high risk, accounted for 40% of the positive cases described. Comorbidities were common in the study population. Over one-quarter of the total cohort had preexisting lung disease, 40% had hypertension, 20% had diabetes mellitus, 18% had documented coronary artery disease, and 23% had chronic kidney disease. Twenty patients (50%) were current or former smokers and five were active vapers. COVIDICU patients also had elevated white cell counts and higher levels of circulating neutrophils, CRP (C-reactive protein), fibrinogen, and lactate when compared with COVIDstable patients (Table 2). When compared with patients with severe CAP requiring intubation and mechanical ventilation, patients with COVID-19 exhibited markedly lower eosinophil counts and higher levels of ferritin and LDH (lactate dehydrogenase). Total white cell count, neutrophil count, and CRP were higher in the CAPICU cohort than in either COVID-19 group.
Table 1.
Clinical Characteristics of the Two COVID-19 Cohorts
| COVIDstable (n = 20) | COVIDICU (n = 20) | Total (n = 40) | |
|---|---|---|---|
| Age, yr | 56.6 ± 17.3 | 54.3 ± 18.2 | 55.5 ± 17.7 |
| Sex, M/F | 12/8 | 13/7 | 25/15 |
| Days since onset of symptoms | 7.00 ± 0.58 | 7.05 ± 0.81 | 7.03 ± 0.74 |
| Symptoms at admission | |||
| Fever | 17 (85) | 18 (90) | 35 (88) |
| Dyspnea | 11 (55) | 15 (75) | 26 (65) |
| Cough | 11 (55) | 14 (70) | 25 (60) |
| Sputum production | 5 (25) | 5 (25) | 10 (25) |
| Myalgia | 8 (40) | 7 (35) | 15 (38) |
| Sore throat | 5 (25) | 4 (20) | 9 (23) |
| Nasal congestion | 1 (5) | 0 (0) | 1 (3) |
| Headache | 6 (30) | 5 (25) | 11 (28) |
| Fatigue | 13 (65) | 14 (70) | 13 (68) |
| Anorexia | 5 (25) | 6 (30) | 11 (28) |
| Nausea | 5 (25) | 5 (25) | 10 (25) |
| Vomiting | 0 (0) | 1 (5) | 1 (3) |
| Diarrhea | 4 (20) | 3 (15) | 7 (18) |
| Chest pain | 6 (30) | 6 (30) | 6 (30) |
| Anosmia | 4 (20) | 3 (15) | 7 (18) |
| Circumstances surrounding infection | |||
| Recent travel to high-risk area | 4 (20) | 2 (10) | 6 (15) |
| Close contact with infected person | 8 (40) | 10 (50) | 18 (45) |
| Community acquired | 8 (40) | 8 (40) | 16 (40) |
| Comorbidities | |||
| Hypertension | 8 (40) | 8 (40) | 16 (40) |
| Coronary artery disease | 4 (20) | 3 (15) | 7 (18) |
| Diabetes mellitus | 4 (20) | 4 (20) | 8 (20) |
| Obesity | 13 (65) | 14 (70) | 27 (68) |
| Chronic lung disease | 6 (30) | 5 (25) | 11 (28) |
| Chronic kidney disease | 5 (25) | 4 (20) | 9 (23) |
| Smoking history | |||
| Current | 6 (30) | 6 (30) | 12 (30) |
| Former | 4 (20) | 4 (20) | 8 (20) |
| Never | 10 (50) | 10 (50) | 20 (50) |
| Vaping history | |||
| Current | 3 (15) | 2 (10) | 5 (13) |
| Former | 0 (0) | 0 (0) | 0 (0) |
| Never | 17 (85) | 18 (90) | 35 (88) |
Definition of abbreviations: COVID-19 = coronavirus disease; COVIDICU = patients with COVID-19 requiring ICU admission; COVIDstable = hospitalized but stable patients with COVID-19.
Data are presented as mean ± SD or absolute number (percentage of group total).
Table 2.
Laboratory Findings at Study Entry
| COVIDstable
(n = 20) |
COVIDICU
(n = 20) |
CAPICU
(n = 15) |
||||
|---|---|---|---|---|---|---|
| Mean (SD) | Median | Mean (SD) | Median | Mean (SD) | Median | |
| White cell count (4.0–11.0) | 8.29 (2.27) | 7.99 | 13.12 (5.36) | 12.22 | 16.31 (5.77) | 16.00 |
| Neutrophils (2.0–7.5) | 7.85 (3.18) | 6.95 | 11.17 (5.17) | 10.29 | 14.11 (5.12) | 12.41 |
| Lymphocytes (1.0–4.0) | 1.55 (0.66) | 1.46 | 1.09 (0.34) | 1.03 | 1.88 (0.89) | 1.80 |
| Monocytes (0.2–1.0) | 0.65 (0.25) | 0.58 | 0.41 (0.25) | 0.38 | 0.84 (0.43) | (0.66) |
| Eosinophils (0.04–0.4) | 0.04 (0.02) | 0.02 | 0.01 (0.01) | 0.000 | 0.1 (0.07) | 0.07 |
| Platelets (140–400) | 266 (56.4) | 253 | 228 (54.2) | 236 | 226 (52.2) | 230 |
| Hb (13.0–17.5 g/dl) | 13.7 (1.08) | 13.1 | 12.9 (1.44) | 12.8 | 13.0 (1.39) | 12.9 |
| C-reactive protein (0–5 mg/L) | 62.1 (61.7) | 47 | 232 (104.5) | 192 | 253 (101.4) | 272 |
| Aspartate aminotransferase (0–40 U/L) | 51 (24.0) | 38 | 63 (21.4) | 66 | 59 (23.2) | 53 |
| Alanine aminotransferase (0–41 IU/L) | 59 (40.3) | 43 | 47 (16.1) | 49 | 49 (15.8) | 46 |
| γ-Glutamyltransferase (0–59 IU/L) | 74 (57.6) | 57 | 94 (52.8) | 90 | 62 (38.9) | 60 |
| Bilirubin (0–21 μmol/L) | 8 (2.8) | 9 | 10 (4.3) | 9 | 8 (3.1) | 8 |
| Albumin (35–52 g/L) | 37 (4.82) | 37 | 33 (5.76) | 34 | 34 (4.32) | 34 |
| Fibrinogen (1.90–3.50 g/L) | 3.98 (0.72) | 4.00 | 5.14 (1.13) | 4.70 | 4.22 (0.36) | 4.10 |
| Ferritin* (30–400 ng/ml) | 1,710 (863) | 1,473 | 1,812 (1,276) | 1,599 | 773 (421) | 796 |
| Lactate (0.5–1.0 mmol/L) | 0.9 (0.3) | 0.9 | 2.9 (1.3) | 2.9 | 2.8 (1.2) | 2.8 |
| Lactate dehydrogenase† (135–225 U/L) | 304 (105.8) | 267 | 806 (102.2) | 787 | 332 (98.4) | 320 |
| Alpha-1 antitrypsin (0.90–1.80 g/L) | 2.10 (0.51) | 2.16 | 2.89 (0.49) | 2.85 | 2.85 (0.39) | 2.85 |
Definition of abbreviations: CAPICU = patients with severe community-acquired pneumonia requiring ICU support; COVID-19 = coronavirus disease; COVIDICU = patients with COVID-19 requiring ICU admission; COVIDstable = hospitalized but stable patients with COVID-19.
Reference ranges for each blood measurement are included in parentheses.
Data available for 39 patients with COVID-19 and 13 patients with community-acquired pneumonia.
Data available for 37 patients with COVID-19 and 13 patients with community-acquired pneumonia. All cell counts are ×109/L.
Characterization of the COVID-19–associated Cytokinemia
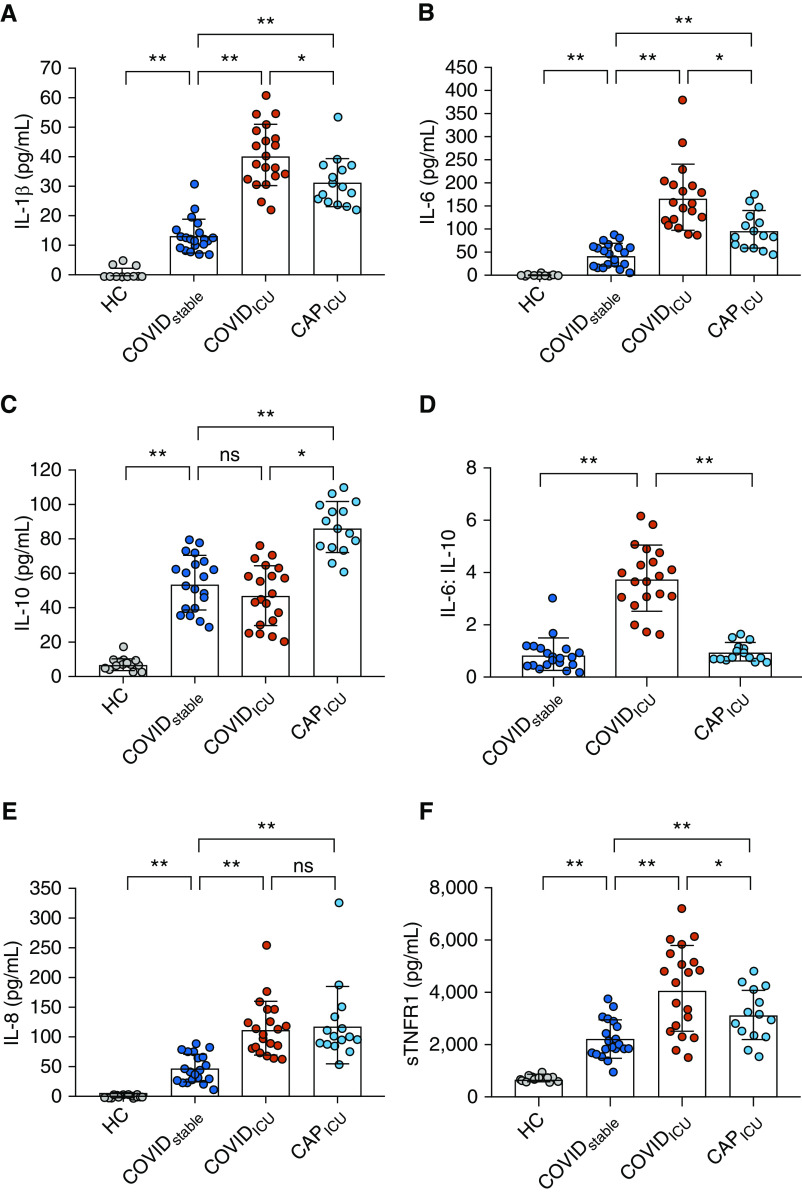
Circulating levels of IL-1β were elevated in patients with a diagnosis of COVID-19 compared with HC subjects (P < 0.0001, Figure 1A), with a significant difference in plasma IL-1β between the COVIDstable and COVIDICU groups (13.7 ± 5.8 pg/ml and 40.8 ± 10.4 pg/ml, respectively; P < 0.0001; Figure 1A). A significant difference was also observed between patients in the COVIDICU group and those in the CAPICU group (31.8 ± 8.2 pg/ml; P = 0.009; Figure 1A).
Figure 1.
The coronavirus disease (COVID-19) cytokinemia. (A) Plasma was obtained from healthy control (HC) subjects (n = 15), patients with COVID-19 infection who required hospitalization but were stable at ward level (COVIDstable patients; n = 20), severely unwell patients with COVID-19 requiring intubation and mechanical ventilation (COVIDICU patients; n = 20), and patients with severe community-acquired pneumonia in ICU (CAPICU patients; n = 15). IL-1β levels were elevated in COVIDstable patients compared with HC subjects, with an increase observed between the COVIDstable and COVIDICU groups. IL-1β levels were higher in COVIDICU than in CAPICU patients. (B) IL-6 levels were elevated in COVIDstable patients compared with HC subjects, with an additional increase in COVIDICU patients. IL-6 levels were higher in CAPICU than in COVIDstable patients but significantly lower than in COVIDICU patients. (C) IL-10 was higher in COVIDstable patients than in HC subjects. IL-10 in CAPICU patients was higher than in both COVID-19 groups. (D) IL-6:IL-10 was higher in COVIDICU patients than in COVIDstable and CAPICU patients. (E) IL-8 was increased in COVIDstable plasma, with a further rise in COVIDICU patients. No difference between COVIDICU and CAPICU patients was observed. (F) Levels of sTNFR1 were higher in COVIDstable than in HC subjects, with a further increase in COVIDICU patients. Levels were higher in COVIDICU than in CAPICU patients. *P < 0.05 and **P < 0.001. ns = not significant; sTNFR1 = soluble tumor necrosis factor receptor 1.
A similar pattern was observed for IL-6, with plasma levels in patients with COVID-19 increasing with severity of illness (HC: 0.8 ± 1.6 pg/ml; COVIDstable: 45.9 ± 24.8 pg/ml; COVIDICU: 169.4 ± 70.7 pg/ml; P < 0.0001; Figure 1B). IL-6 levels in the CAPICU group (99.4 ± 40.5 pg/ml) were higher than in COVIDstable patients (P = 0.0001) but significantly lower than in COVIDICU patients (P = 0.0005, Figure 1B). Though circulating levels of the antiinflammatory cytokine IL-10 were higher in COVIDstable patients than in HC subjects (54.7 ± 15.7 pg/ml and 7.5 ± 3.6 pg/ml, respectively; P < 0.0001, Figure 1C), a further increase between COVIDstable and COVIDICU patients was not observed (COVIDICU: 47.5 ± 17.4; P = 0.17; Figure 1C). In contrast, patients in the CAPICU group exhibited markedly increased IL-10 levels in response to inflammation when compared with COVIDICU patients (86.8 ± 14.9 pg/ml; P < 0.0001; Figure 1C), indicating that in addition to the rise in proinflammatory mediators, concomitant loss of antiinflammatory protection may also be clinically relevant. In support of this concept, the ratios of IL-6:IL-10 and IL-1β:IL-10 were significantly increased in the COVIDICU cohort (both P < 0.0001; Figures 1D and E1). Levels of the potent neutrophil chemoattractant IL-8 were also higher in patients positive for COVID-19, with a further increase observed between the COVIDstable and COVIDICU groups (HC: 1.9 ± 2.1 pg/ml; COVIDstable: 48.2 ± 24.0 pg/ml; COVIDICU: 115.5 ± 46.4 pg/ml; P < 0.0001; Figure 1E). Unlike IL-6 and IL-1β, the IL-8 response to infection was not more pronounced in severe COVID-19 than in severe CAP (121.4 ± 65.5 pg/ml; P = 0.98; Figure 1E). Additionally, sTNFR1, a surrogate for circulating TNF-α, was markedly increased in both COVID groups, with a significant difference observed between COVIDICU patients (4.2 ± 1.7 ng/ml) and both COVIDstable and CAPICU patients (2.22 ± 0.7 ng/ml, P = 0.0001 and 3.2 ± 0.98 ng/ml, P = 0.04, respectively; Figure 1F).
Neutrophils Undergo Metabolic Reprogramming in Severe COVID-19 Illness
Given that the relationship observed between IL-1β, IL-6, and IL-10 in inflamed COVIDICU patients was consistent with increased HIF-mediated transcription, we searched for evidence of altered immunometabolism in these individuals. Neutrophils were chosen for this purpose, as they produce all of the aforementioned cytokines (17, 18), were increased in the peripheral blood of COVIDICU patients, and are heavily implicated in the pathogenesis of ARDS (12), where HIF-mediated signaling is upregulated (19). PKM2 was significantly increased in COVIDICU neutrophil cytosols compared with HC subjects (P < 0.0001; Figure 2A). Assumption of a tetramer conformation prevents nuclear translocation, thereby impairing interaction with HIF-1α (9). Phosphorylation of PKM2 on tyrosine 105 prevents PKM2 from attaining a tetramer conformation and is thus considered indicative of dimer formation. Cytosolic levels of phosphorylated PKM2 were also higher in COVIDICU neutrophil cytosols compared with HC subjects (P < 0.0001; Figure 2B).
Figure 2.
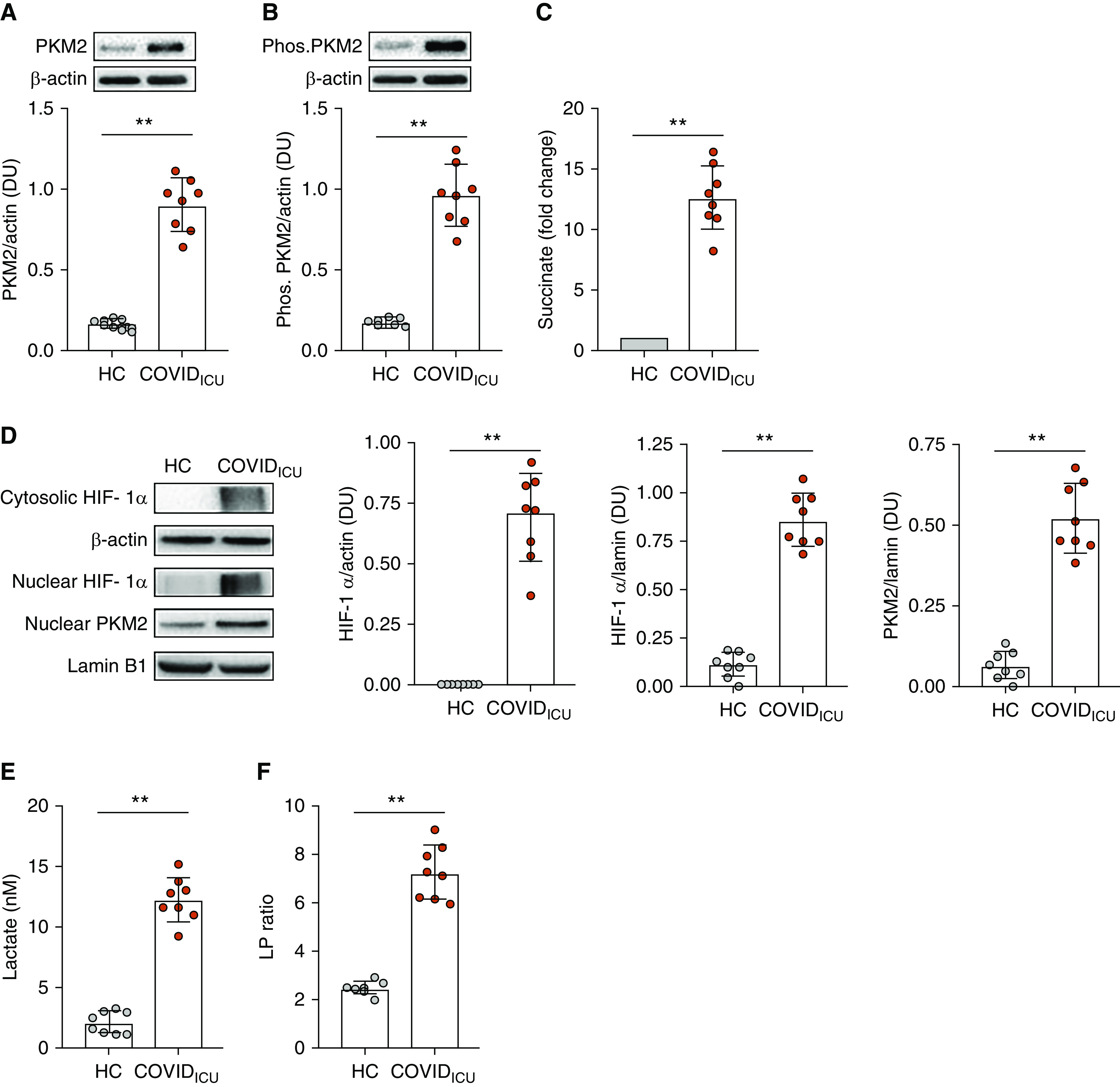
Neutrophil immunometabolism is altered in severe coronavirus disease (COVID-19) illness. (A) Neutrophils were isolated from the peripheral blood of healthy control (HC) subjects (n = 8) and patients with severe COVID-19 illness requiring intubation and mechanical ventilation (COVIDICU patients; n = 8), and cytosolic fractions were obtained. PKM2 (pyruvate kinase M2) was significantly increased in COVIDICU neutrophil cytosols compared with HC neutrophil cytosols (P < 0.0001). (B) Cytosolic levels of phosphorylated PKM2, indicative of PKM2 dimer formation, were also significantly increased in COVIDICU patients compared with HC subjects (P < 0.0001). (C) Cytosolic succinate levels were higher in COVIDICU than in HC neutrophils (fold increase 10.41 ± 1.97; P < 0.0001). (D) Cytosolic HIF-1α (hypoxia-inducible factor-1α) was higher in COVIDICU neutrophils than in HC neutrophil cytosols (P < 0.0001). Nuclear levels of HIF-1α and PKM2 were also increased in neutrophils from the same infected patients (both P < 0.0001). (E) Cytosolic lactate was higher in circulating COVIDICU neutrophils than in HC neutrophils (P < 0.0001). (F) Cytosolic lactate:pyruvate ratio was similarly increased (P < 0.0001). **P < 0.001. DU = densitometric units; LP = lactate:pyruvate; Phos. PKM2 = phosphorylated PKM2.
In addition to being an important control point in glycolysis, PKM2 has been shown to function as a coactivator of HIF-1α (7), a key mediator of acute inflammation that also exerts a positive feedback effect by inducing expression of proglycolytic enzymes such as LDH, PDKs (pyruvate dehydrogenase kinases), and the glucose transporter GLUT-1. In a healthy resting cell, HIF-1α is hydroxylated at conserved proline residues by PHDs (prolyl hydroxylases), marking it for ubiquitination and rapid proteasomal degradation. PHDs are oxygen dependent. Thus, in a state of normoxia, HIF-1α typically displays a short cytosolic half-life, high turnover, and low basal levels, but in a state of relative hypoxemia, it is conserved (8). HIF-1α breakdown is also prevented by cytosolic accumulation of succinate (9), a PHD inhibitor and inflammatory danger signal. Cytosolic succinate was elevated in COVIDICU neutrophils compared with HC neutrophils (P < 0.0001; Figure 2C). Consistent with this, cytosolic levels of HIF-1α were also markedly increased in COVIDICU neutrophil cytosols (P < 0.0001; Figure 2D). The preservation of HIF-1α in the cytosol was reflected at the nucleus, where it was also increased, an effect similarly observed for phosphorylated PKM2 (P = 0.0002 and P < 0.0001, respectively; Figure 2D). Cytosolic lactate levels were significantly higher in COVIDICU neutrophils than in HC neutrophils (12.23 ± 1.82 nM vs. 2.07 ± 0.88 nM; P < 0.0001; Figure 1E). Cytosolic lactate:pyruvate ratio was also increased in COVIDICU neutrophils (COVIDICU: 7.24 ± 1.16; HC: 2.49 ± 0.25; P < 0.0001; Figure 1F), confirming that the increase in lactate was due to a fundamental metabolic shift rather than merely an increase in overall metabolism.
The Acute Phase Response of AAT Is Insufficient in Severe COVID-19 Illness
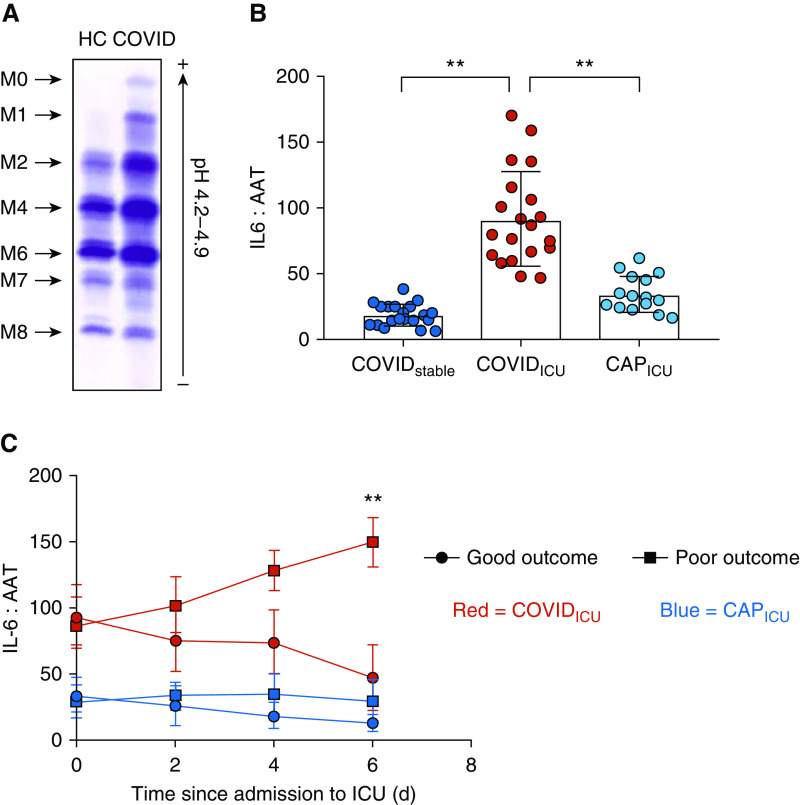
Having shown increased levels of IL-6 and IL-1β in the presence of an inadequate IL-10 response, we investigated whether other endogenous acute phase antiinflammatories were similarly outstripped in patients with severe COVID-19. AAT is a 52 kD glycoprotein synthesized primarily in the liver. In addition to its role as a serine protease inhibitor, AAT is a potent antiinflammatory and a key modulator of the acute phase immune response in humans (14, 20–23). Stimulation of HepG2 cells with human IL-6 triggered increased production of AAT mRNA and increased secretion of AAT protein (Figures E2A and E2B), and circulating AAT levels were significantly elevated in both COVID-19 and severe CAP (Table 2). Glycosylation of AAT is also altered in response to infection, with increased sialylation of AAT previously shown to enhance the protein’s antiinflammatory effects (24). Immunofixation of plasma from patients with COVID-19 by isoelectric focusing gel electrophoresis revealed the presence of the highly sialylated M0 and M1 AAT glycoforms (Figure 3A). These data indicated that affected individuals were mounting an acute AAT response to increased inflammation. However, this response failed to keep pace with IL-6 in patients with COVID-19 who were critically unwell. Despite COVIDICU patients having significantly higher IL-6 levels than patients with severe CAP, no difference in circulating AAT levels was observed between the two groups, leading to a clear difference in the plasma IL-6:AAT ratio between the two groups (P < 0.0001; Figure 3B). Similarly, the IL-6:AAT ratio in COVIDICU patients was substantially higher than in COVIDstable patients (P < 0.0001; Figure 3B). Further analysis of the COVIDICU cohort demonstrated that, in patients sampled prospectively at 2-day intervals for 6 days after ICU admission, an increase in IL-6:AAT between Day 0 and Day 6 was associated with poor outcome, defined as death or prolonged ICU stay, whereas a reduction in IL-6:AAT was associated with clinical improvement and discharge to the ward (Figures 3C and E4). Specifically, logistic regression analysis demonstrated that an IL-6:AAT ratio ≤85.02 on Day 6 predicted a good outcome perfectly, and a decrease from Day 0 to Day 6 of ≥25.68 predicted a good outcome perfectly. Furthermore, a decrease from Day 0 to Day 4 of ≥3.76 predicted a good outcome perfectly, whereas a one-unit increase in the change in IL-6:AAT from Day 0 to Day 2 increased the odds of a poor outcome by 17% (odds ratio, 1.17; 95% confidence interval, 1.01–1.35; P = 0.039). In patients with severe CAP sampled in the same manner, the trends in IL-6:AAT observed were different, with a decrease in IL-6:AAT observed earlier in those who resolved clinically and a flatter IL-6:AAT trajectory in those who went on to poor outcome (Figure 3C). Therefore, though multiple endogenous antiinflammatory responses are present in patients with severe COVID-19, they are overwhelmed by an excessive IL-6 burden, a feature of inflammatory dysregulation that distinguishes this condition from others in the ICU setting.
Figure 3.
The acute phase response of AAT (alpha-1 antitrypsin) is overwhelmed in severe coronavirus disease (COVID-19) illness. AAT is a 52 kD glycosylated protein synthesized primarily in the liver. (A) Immunofixation of plasma from patients with COVID-19 after isoelectric focusing gel electrophoresis demonstrated the presence of the highly sialylated M0 and M1 AAT glycoforms indicative of an attempt to mount a response to inflammation. (B) Plasma IL-6:AAT ratios were significantly higher in patients who required ICU support (COVIDstable: 19.00 ± 8.41; COVIDICU: 92.05 ± 35.61; CAPICU: 35.26 ± 13.77; P = 0.0002). (C) Sequential plasma samples were obtained from 16 COVIDICU patients (indicated in red), 8 of whom resolved sufficiently within 10 days of entering ICU to be discharged to the ward, and 8 of whom had a poor outcome (death or prolonged ICU stay). A progressive increase in IL-6:AAT was observed in COVIDICU patients who had a poor outcome, whereas a decrease in IL-6:AAT was seen in COVIDICU patients who recovered. By comparison, CAPICU patients (indicated in blue; good outcome: n = 8, poor outcome: n = 7) did not exhibit the same trend. **P < 0.001. CAPICU = patients with severe community-acquired pneumonia requiring ICU support; COVIDICU = patients with COVID-19 requiring ICU admission; COVIDstable = hospitalized but stable patients with COVID-19; HC = healthy control subjects.
Discussion
Here, we define the COVID-19 cytokinemia and the inflammatory phenotype of the critically unwell patient with COVID-19 and show for the first time the presence of immunometabolic reprogramming in patients with severe COVID-19.
Differences in laboratory values between COVID-19 patient groups were mirrored by a series of proinflammatory cytokines. IL-1β, IL-6, IL-8, and sTNFR1 were all increased in infected patients compared with HC subjects. Furthermore, the COVIDICU cohort could be clearly differentiated from ICU patients with severe CAP and those who were positive for COVID but stable. The most unanticipated differentiating factor between patients with severe COVID-19 and severe CAP, however, was not the degree of increase in proinflammatory cytokines but rather the relatively blunted antiinflammatory responses of IL-10 and AAT. Indeed, the profound increases observed in the ratios of IL-6:IL-10, IL-1β:IL-10, and IL-6:AAT in severe COVID-19 highlights a distinct inflammatory phenotype, one that may be associated with altered immunometabolism.
Neutrophils from patients with severe COVID-19 displayed increased cytosolic levels of the proinflammatory metabolic regulator and glycolytic marker PKM2, with its phosphorylation at tyrosine 105 also increased. Nuclear translocation of the phosphorylated PKM2 was evident in the COVIDICU group, as was an increase in both cytosolic and nuclear levels of HIF-1α.
Though there are several well-described circumstances in which the latter phenomenon may arise in other cell types, such as in neoplastic cells or in muscle cells during intense exertion, there are two in particular that apply to circulating neutrophils. The first, hypoxemia, is intuitive and is pertinent here. Patients with COVID-19 requiring ICU admission displayed profound hypoxemia when sampled immediately prior to intubation. The second involves build-up of HIF-1α after its stabilization by succinate in the cytosol (9). Succinate was significantly elevated in neutrophil cytosols from COVIDICU patients. Cytosolic accumulation of succinate is not exclusive to hypoxia; it can also be triggered by infection and severe inflammation (9, 15, 25), both of which are observed in the COVIDICU cohort.
Though the metabolic rewiring of neutrophils described in these patients supports the concept that the cytokinemia observed is driven by circulating immune cells, it is certainly possible that cytokine leakage from a permeabilized lung also plays a role. In this regard, we are informed by prior experiments delineating the mechanism of IL-6 release into the serum after administration of adenovirus to the airway (26), in which a loss of the integrity of the respiratory epithelial barrier, coupled with de novo synthesis by airway neutrophils, led to passage of IL-6 into the local tissue and, subsequently, the systemic circulation. It should also be noted that other immune cell types besides neutrophils, such as macrophages and monocytes, are likely to contribute to the cytokine burden observed. Similarly, the elevations seen in circulating lactate and LDH (Table 2) in COVIDICU patients are likely to be driven by increased glycolytic activity in multiple cell types, although cell breakdown in the context of critical illness may also play a contributing role. Furthermore, the predilection for increased glycolytic activity varies across different tissues and cells. Tumor cells preferentially employ glycolysis and HIF-1α to drive angiogenesis, for example, whereas skeletal muscle typically only reverts to glycolysis as its predominant source of ATP after an anaerobic threshold is reached.
Physiologically, it has been suggested that the ARDS observed in patients with severe COVID-19 differs from “typical” ARDS, with relatively preserved compliance (27, 28), though this is not universally accepted (29). We observed that our patients had abnormal compliance, with a median dynamic compliance of 33.7 ml/cm H2O (interquartile range 30.1–43.0). From an inflammatory perspective, comparing the COVID-19 cytokine profile described here to data from previous studies in ARDS and sepsis is made more difficult by the heterogeneity of these conditions and differences in the cohorts studied. This applies to several factors, including severity and duration of illness, timing of sampling, socioeconomic and demographic factors, diagnostic criteria used, treatments available at the time of study commencement, and underlying etiology. Methodological variation also exists between studies, from the assay type used and the sample type assayed to the processing, handling, and storage of samples (30–34). This is of particular relevance when interpreting biomarker data from large clinical trials or biobanks. Indeed, a recent study by Stapleton and colleagues compared baseline plasma cytokine levels from patients in a current ARDS randomized control trial with baseline values from similar historical control subjects from previous ARDS randomized control trials and found that modern-day values were generally substantially lower, most notably in the level of IL-6 (35). Though the cytokine levels described in our manuscript are closer to the numbers described by Stapleton and colleagues than to many of these historical studies, the balance of these cytokines in COVID-19 is different, particularly for the IL-10 response to infection relative to both IL-1β and IL-6.
The data provide some insight regarding potential therapeutic options, some of which have already been licensed for use in humans. However, it is apparent that limitations to each approach exist. Drugs such as tocilizumab, anakinra, and infliximab inhibit the action of specific cytokines but do not offer broad-spectrum antiinflammatory cover. Steroid therapy induces an indiscriminate pancytokinaemia, with suppression of antiinflammatory and proresolution cytokines such as IL-10 in addition to those that are proinflammatory. This may explain why recent attempts to use steroids in this population have proven unsuccessful (36).
Targeting metabolism represents another means of modulating inflammation. Most of the pyruvate generated by glycolysis is converted to acetyl-CoA via pyruvate dehydrogenase or to lactate via LDH. As the transcription factor driving both LDH and PDK, HIF-1α dictates the fate of pyruvate and holds the key to cellular metabolic balance (37). Dichloroacetate, a PDK inhibitor, has been shown to redirect the conversion of pyruvate away from lactate and back toward acetyl-CoA and oxidative phosphorylation by catalyzing pyruvate decarboxylation and has been successfully administered to humans (38).
Other potential strategies, such as the maintenance of PKM2 in a tetramer conformation by molecules such as TEPP-46 (5) or the use of itaconate as an antiinflammatory and antioxidant (39) hold promise but have yet to progress to the clinical arena.
The IL-6:AAT ratio in COVIDICU patients was more than twice that seen in CAPICU patients, and the progression of IL-6 relative to AAT over time matched clinical trajectory in patients with severe COVID-19. In this regard, supplementation of the acute AAT response with exogenous AAT may merit consideration, as it has been shown to modulate the production and activity of the key proinflammatory cytokines described here (14, 20, 24, 32, 40) while preserving the production of IL-10 (41). Indeed, we have recently shown that abrupt cessation of AAT augmentation therapy for patients with hereditary AAT deficiency results in marked increases in levels of these specific proinflammatory cytokines, loss of IL-10, and subsequent progression to respiratory failure (42).
Although one or more of the abovementioned therapies may yet prove to be beneficial in severe COVID-19 illness, it is important that the urgency surrounding the current pandemic does not prompt hasty engagement in treatment strategies that may, though well intentioned, do more harm than good. It is our shared responsibility to emphasize the importance of applying therapies to vulnerable patients only when there is sufficient preclinical data to support their advancement to expedited, properly conducted clinical trials. Identifying potential targets, as we have done here, stands to reduce the number of inappropriate off-label therapies being administered and inform the selection of candidate therapies for robust evaluation against defined outcomes in the clinical trial setting.
Supplementary Material
Footnotes
O.J.M. received support from the Elaine Galwey Memorial Research Bursary and the American Thoracic Society (ATS) in the form of an ATS International Trainee Scholarship award and an ATS abstract scholarship award.
Author Contributions: O.J.M., G.F.C., and N.G.M. conceptualized the study. O.J.M., T.P.C., G.F.C., and N.G.M. designed experiments. O.J.M., N.L.M., O.F.M., J.C., E.O’C., S.W., and B.M. recruited patients and collected samples. O.J.M., O.F.M., T.P.C., and D.M.D. performed experiments. O.J.M., O.N.C., J.C., D.R., I.S., C.G., P.B., M.E.O’B., R.K.M., R.W.C., K.H., S.W., E.d.B., C.M., S.M., S.G., F.K., J.O’R., R.D., M.P., P.G., C.L., R.A.O’L., J.F., A.G., B.M., G.F.C., and N.G.M. attended patients. O.J.M., N.L.M., O.F.M., O.N.C., J.C., E.O’C., and S.W. collected clinical data. O.J.M., T.P.C., M.P.M., G.H., F.B., G.F.C., and N.G.M. analyzed data. O.J.M., T.P.C., M.P.M., G.F.C., and N.G.M. cowrote the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202005-1583OC on June 25, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. Coronavirus disease (COVID-19): situation report–150. 2020 Jun 18 [accessed 2020 Jun 18]. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200618-covid-19-sitrep-150.pdf?sfvrsn=aa9fe9cf_2.
- 3.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. [online ahead of print] 13 Mar 2020; DOI: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed]
- 4. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. doi: 10.1001/jama.2020.2648. [online ahead of print] 24 Feb 2020; DOI: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5. Palsson-McDermott EM, Curtis AM, Goel G, Lauterbach MA, Sheedy FJ, Gleeson LE, et al. Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab. 2015;21:65–80. doi: 10.1016/j.cmet.2014.12.005. [Published erratum appears in Cell Metab 21:347.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Corcoran SE, O’Neill LA. HIF1α and metabolic reprogramming in inflammation. J Clin Invest. 2016;126:3699–3707. doi: 10.1172/JCI84431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luo W, Hu H, Chang R, Zhong J, Knabel M, O’Meally R, et al. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145:732–744. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. West JB. Physiological effects of chronic hypoxia. N Engl J Med. 2017;376:1965–1971. doi: 10.1056/NEJMra1612008. [DOI] [PubMed] [Google Scholar]
- 9. Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dinarello CA. Interleukin-1 beta, interleukin-18, and the interleukin-1 beta converting enzyme. Ann N Y Acad Sci. 1998;856:1–11. doi: 10.1111/j.1749-6632.1998.tb08307.x. [DOI] [PubMed] [Google Scholar]
- 11. Dinarello CA, Gatti S, Bartfai T. Fever: links with an ancient receptor. Curr Biol. 1999;9:R147–R150. doi: 10.1016/s0960-9822(99)80085-2. [DOI] [PubMed] [Google Scholar]
- 12. Goodman RB, Strieter RM, Martin DP, Steinberg KP, Milberg JA, Maunder RJ, et al. Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996;154:602–611. doi: 10.1164/ajrccm.154.3.8810593. [DOI] [PubMed] [Google Scholar]
- 13. Donnelly SC, Strieter RM, Reid PT, Kunkel SL, Burdick MD, Armstrong I, et al. The association between mortality rates and decreased concentrations of interleukin-10 and interleukin-1 receptor antagonist in the lung fluids of patients with the adult respiratory distress syndrome. Ann Intern Med. 1996;125:191–196. doi: 10.7326/0003-4819-125-3-199608010-00005. [DOI] [PubMed] [Google Scholar]
- 14. Bergin DA, Reeves EP, Meleady P, Henry M, McElvaney OJ, Carroll TP, et al. α-1 Antitrypsin regulates human neutrophil chemotaxis induced by soluble immune complexes and IL-8. J Clin Invest. 2010;120:4236–4250. doi: 10.1172/JCI41196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McElvaney OJ, Zaslona Z, Becker-Flegler K, Palsson-McDermott EM, Boland F, Gunaratnam C, et al. Specific inhibition of the NLRP3 inflammasome as an antiinflammatory strategy in cystic fibrosis. Am J Respir Crit Care Med. 2019;200:1381–1391. doi: 10.1164/rccm.201905-1013OC. [DOI] [PubMed] [Google Scholar]
- 16. Zerimech F, Hennache G, Bellon F, Barouh G, Jacques Lafitte J, Porchet N, et al. Evaluation of a new Sebia isoelectrofocusing kit for alpha 1-antitrypsin phenotyping with the Hydrasys System. Clin Chem Lab Med. 2008;46:260–263. doi: 10.1515/CCLM.2008.036. [DOI] [PubMed] [Google Scholar]
- 17. Melani C, Mattia GF, Silvani A, Carè A, Rivoltini L, Parmiani G, et al. Interleukin-6 expression in human neutrophil and eosinophil peripheral blood granulocytes. Blood. 1993;81:2744–2749. [PubMed] [Google Scholar]
- 18. Tecchio C, Micheletti A, Cassatella MA. Neutrophil-derived cytokines: facts beyond expression. Front Immunol. 2014;5:508. doi: 10.3389/fimmu.2014.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Juss JK, House D, Amour A, Begg M, Herre J, Storisteanu DM, et al. Acute respiratory distress syndrome neutrophils have a distinct phenotype and are resistant to phosphoinositide 3-kinase inhibition. Am J Respir Crit Care Med. 2016;194:961–973. doi: 10.1164/rccm.201509-1818OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bergin DA, Reeves EP, Hurley K, Wolfe R, Jameel R, Fitzgerald S, et al. The circulating proteinase inhibitor α-1 antitrypsin regulates neutrophil degranulation and autoimmunity. Sci Transl Med. 2014;6:217ra1. doi: 10.1126/scitranslmed.3007116. [DOI] [PubMed] [Google Scholar]
- 21. Hurley K, Lacey N, O’Dwyer CA, Bergin DA, McElvaney OJ, O’Brien ME, et al. Alpha-1 antitrypsin augmentation therapy corrects accelerated neutrophil apoptosis in deficient individuals. J Immunol. 2014;193:3978–3991. doi: 10.4049/jimmunol.1400132. [DOI] [PubMed] [Google Scholar]
- 22. O’Dwyer CA, O’Brien ME, Wormald MR, White MM, Banville N, Hurley K, et al. The BLT1 inhibitory function of α-1 antitrypsin augmentation therapy disrupts leukotriene B4 neutrophil signaling. J Immunol. 2015;195:3628–3641. doi: 10.4049/jimmunol.1500038. [DOI] [PubMed] [Google Scholar]
- 23. Jonigk D, Al-Omari M, Maegel L, Müller M, Izykowski N, Hong J, et al. Anti-inflammatory and immunomodulatory properties of α1-antitrypsin without inhibition of elastase. Proc Natl Acad Sci USA. 2013;110:15007–15012. doi: 10.1073/pnas.1309648110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McCarthy C, Dunlea DM, Saldova R, Henry M, Meleady P, McElvaney OJ, et al. Glycosylation repurposes alpha-1 antitrypsin for resolution of community-acquired pneumonia. Am J Respir Crit Care Med. 2018;197:1346–1349. doi: 10.1164/rccm.201709-1954LE. [DOI] [PubMed] [Google Scholar]
- 25. Mills E, O’Neill LA. Succinate: a metabolic signal in inflammation. Trends Cell Biol. 2014;24:313–320. doi: 10.1016/j.tcb.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 26. McElvaney NG, Crystal RG. IL-6 release and airway administration of human CFR cDNA adenovirus vector. Nat Med. 1995;1:182–184. doi: 10.1038/nm0395-182b. [DOI] [PubMed] [Google Scholar]
- 27. Gattinoni L, Chiumello D, Rossi S. COVID-19 pneumonia: ARDS or not? Crit Care. 2020;24:154. doi: 10.1186/s13054-020-02880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201:1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ziehr DR, Alladina J, Petri CR, Maley JH, Moskowitz A, Medoff BD, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med. 2020;201:1560–1564. doi: 10.1164/rccm.202004-1163LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Keustermans GC, Hoeks SB, Meerding JM, Prakken BJ, de Jager W. Cytokine assays: an assessment of the preparation and treatment of blood and tissue samples. Methods. 2013;61:10–17. doi: 10.1016/j.ymeth.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 31. O’Brien ME, Fee L, Browne N, Carroll TP, Meleady P, Henry M, et al. Activation of complement component 3 is associated with airways disease and pulmonary emphysema in alpha-1 antitrypsin deficiency. Thorax. 2020;75:321–330. doi: 10.1136/thoraxjnl-2019-214076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pott GB, Chan ED, Dinarello CA, Shapiro L. Alpha-1-antitrypsin is an endogenous inhibitor of proinflammatory cytokine production in whole blood. J Leukoc Biol. 2009;85:886–895. doi: 10.1189/jlb.0208145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Riches P, Gooding R, Millar BC, Rowbottom AW. Influence of collection and separation of blood samples on plasma IL-1, IL-6 and TNF-alpha concentrations. J Immunol Methods. 1992;153:125–131. doi: 10.1016/0022-1759(92)90314-j. [DOI] [PubMed] [Google Scholar]
- 34. Waage A, Brandtzaeg P, Halstensen A, Kierulf P, Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock: association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med. 1989;169:333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stapleton RD, Suratt BT, Neff MJ, Wurfel MM, Ware LB, Ruzinski JT, et al. Bronchoalveolar fluid and plasma inflammatory biomarkers in contemporary ARDS patients. Biomarkers. 2019;24:352–359. doi: 10.1080/1354750X.2019.1581840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Du Y, Tu L, Zhu P, Mu M, Wang R, Yang P, et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. Am J Respir Crit Care Med. 2020;201:1372–1379. doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 38. Michelakis ED, Gurtu V, Webster L, Barnes G, Watson G, Howard L, et al. Inhibition of pyruvate dehydrogenase kinase improves pulmonary arterial hypertension in genetically susceptible patients. Sci Transl Med. 2017;9:eaao4583. doi: 10.1126/scitranslmed.aao4583. [DOI] [PubMed] [Google Scholar]
- 39. Mills EL, Ryan DG, Prag HA, Dikovskaya D, Menon D, Zaslona Z, et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature. 2018;556:113–117. doi: 10.1038/nature25986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Joosten LA, Crişan TO, Azam T, Cleophas MC, Koenders MI, van de Veerdonk FL, et al. Alpha-1-anti-trypsin-Fc fusion protein ameliorates gouty arthritis by reducing release and extracellular processing of IL-1β and by the induction of endogenous IL-1Ra. Ann Rheum Dis. 2016;75:1219–1227. doi: 10.1136/annrheumdis-2014-206966. [DOI] [PubMed] [Google Scholar]
- 41. Janciauskiene SM, Nita IM, Stevens T. Alpha1-antitrypsin, old dog, new tricks. Alpha1-antitrypsin exerts in vitro anti-inflammatory activity in human monocytes by elevating cAMP. J Biol Chem. 2007;282:8573–8582. doi: 10.1074/jbc.M607976200. [DOI] [PubMed] [Google Scholar]
- 42. McElvaney OJ, Carroll TP, Franciosi AN, Sweeney J, Hobbs BD, Kowlessar V, et al. Consequences of abrupt cessation of alpha1-antitrypsin replacement therapy. N Engl J Med. 2020;382:1478–1480. doi: 10.1056/NEJMc1915484. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.