Abstract
Background:
Methamphetamine (METH) use is a public health crisis that disproportionately affects men who have sex with men (MSM). There are currently no FDA-approved pharmacological interventions to treat methamphetamine use disorder (MUD). MUD is associated with social impairments and extremely high treatment attrition rates. Administration of oxytocin, a neuropeptide involved in social attachment, may be a novel approach to addressing these issues. Moreover, oxytocin administration has shown promise for reducing METH-related addictive behavior in animal models, but has not yet been investigated in clinical trials for MUD. Last, oxytocin is known to modulate stress responsivity via regulation of the autonomic nervous system, which is dysregulated in METH users. We hypothesize that oxytocin, in combination with group psychotherapy, will increase treatment engagement, reduce addiction behavior, and mitigate stress hyperreactivity.
Methods:
This is a randomized, double blind trial of oxytocin 40-IU (n = 24) or placebo (n = 24) administered intranasally prior to each of six weekly motivational interviewing group therapy (MIGT) sessions for MUD in MSM. Primary outcome: (a) session attendance. Secondary outcomes: (b) group cohesion, (c) anxiety, (d) METH craving, (e) METH use, and (f) in-session cardiac physiology.
Results:
Participants receiving oxytocin had significantly higher group therapy attendance than those receiving placebo, OR 3.26, 95% CI [1.27–8.41], p = .014. There was a small effect of oxytocin on group cohesion, but not anxiety or craving. METH use did not change over the six-week MIGT course in either treatment arm. Participants receiving oxytocin had lower average heart rates during MIGT sessions and higher heart rate variability. There were positive main effects of MIGT over Time regardless of study drug.
Conclusions:
This evidence, and the lack of any serious adverse events, suggests that oxytocin may safely increase treatment attendance. One possible mechanism by which it may do so is its modulation of the autonomic nervous system. Further investigation is warranted.
Keywords: Psychotherapy, group; Oxytocin; Methamphetamine; Sexual and gender minorities; Motivational interviewing; Retention in care
1. Introduction
Methamphetamine (METH) is a potent psychostimulant with high addiction potential (Insel, 2003; Taylor, Lewis, & Olive, 2013). METH use is a widespread global health concern (UNODC, August, 2018). SAMHSA estimated prevalence of past year use in 2018 in the United States to be 0.7% (SAMHSA, 2019). However, among lesbian, gay, and bisexual individuals in the United States, estimated prevalence was 2.4% (SAMHSA, 2019), and prevalence of METH use over the past six months for men who have sex with men (MSM) in San Francisco was an estimated 8% (SFDPH, 2019). High concentration of METH use among MSM is a public health crisis and is associated with significant psychosocial and biomedical harm (Knight et al., 2019). METH-related fatalities have been on the rise (SFDPH, 2019), primarily due to cardiovascular failure (Hassan, Wearne, Cornish, & Goodchild, 2016; SFDPH, 2019).
Treatment options for methamphetamine use disorder (MUD) are limited and poorly utilized. Behavioral interventions are the gold standard treatment for MUD (Baker & Lee, 2003; Shearer, 2007). However, METH use is associated with drastically high treatment drop-out rates. Almost 25% of patients drop out of addiction treatment within 30 days (Stevens, Radcliffe, Sanders, & Hunt, 2008) and >75% within 180 days (Bhatt et al., 2016; Cook, Quinn, Heinzerling, & Shoptaw, 2017; Hermanstyne et al., 2014; Maglione, Chao, & Anglin, 2000; Stevens et al., 2008). Moreover, in clinical trials investigating treatments for MUD, >50% of participants drop out prior to study completion (Bhatt et al., 2016; Cook et al., 2017), and ongoing METH use is associated with poor adherence to experimental pharmacological interventions (Hermanstyne et al., 2014). Poor retention in clinical trials reduces internal validity, making results difficult to interpret and limiting the development of much-needed innovative treatment strategies for MUD. Despite significant effort, there are currently no pharmacological treatments that are FDA-approved for any stimulant use disorder (Knight et al., 2019; Morley, Cornish, Faingold, Wood, & Haber, 2017).
METH use has been linked to maladaptive social impairments, such as social withdrawal and misperceiving neutral social cues as threatening (Homer et al., 2008; Payer et al., 2008), which may help to explain difficulties engaging with treatment. Conversely, lack of social support has been linked to treatment drop-out in stimulant users (McMahon, Kouzekanani, & Malow, 1999). It is important to note that there is a dose-response relationship between duration of retention in addiction treatment and positive outcomes (Brecht & Herbeck, 2014; Moos, Finney, Federman, & Suchinsky, 2000; Siqueland et al., 2002). Given the notoriously high drop-out rates associated with METH use juxtaposed against the significant positive impact of longer treatment duration, there is a critical need for novel approaches that combat METH-related social impairments and enhance treatment engagement and efficacy (Volkow, 2020).
Oxytocin is a hypothalamic neuropeptide known to increase social salience (Shamay-Tsoory & Abu-Akel, 2016), has demonstrated anti-addiction properties (Lee, Rohn, Tanda, & Leggio, 2016; Sarnyai & Kovacs, 2014), and modulates stress reactivity (Flanagan, Baker, McRae-Clark, Brady, & Moran-Santa Maria, 2015; Sippel et al., 2017). In the context of safety, oxytocin, via its central role in mammalian attachment, facilitates selective social engagement with trusted individuals and supports emotion regulation (Carter, 2017). Intranasal administration of exogenous oxytocin together with social support from a friend have been found to synergistically ameliorate reactivity to a laboratory stress test (Heinrichs, Baumgartner, Kirschbaum, & Ehlert, 2003; Riem, Kunst, Bekker, Fallon, & Kupper, 2020). Conversely, oxytocin's effects on addiction and psychophysiology have been linked to increased motivation and capacity for social engagement (Kemp et al., 2012; Tops, Koole, Ijzerman, & Buisman-Pijlman, 2014). Thus, oxytocin administration may be ideal for increasing treatment retention and engagement among METH users, which would improve clinical treatment outcomes and boost internal validity of clinical trials aimed at investigating new treatments for MUD.
Our research group previously conducted a randomized, controlled, pilot study of intranasal oxytocin administered twice daily for two weeks to cocaine users in a methadone clinic (Stauffer et al., 2016). An exploratory analysis revealed improved clinic attendance for participants receiving oxytocin versus placebo. Though we provided a supportive environment, which has been shown to bolster oxytocin's therapeutic effects (Heinrichs et al., 2003; Riem et al., 2020), the study did not involve any manualized psychotherapeutic intervention. Other researchers have begun to explore the effects of oxytocin administration to augment psychotherapy for post-traumatic stress disorder (Flanagan, Sippel, Wahlquist, Moran-Santa Maria, & Back, 2018) and major depressive disorder (Ellenbogen, Cardoso, Serravalle, & Virginia, 2018). Research has found motivational interviewing (MI), a supportive, client-centered, therapeutic approach designed to help individuals overcome ambivalence and change problematic behavior (Miller & Rollnick, 1991), to be effective for the treatment of MUD among MSM (Parsons, Lelutiu-Weinberger, Botsko, & Golub, 2014; Zule et al., 2012). Moreover, although a recent Cochrane review (Klimas et al., 2018) found no difference in treatment retention between MI and other standard psychosocial interventions for addiction, MI implemented during intake has been shown to improve retention in subsequent addiction treatment (Carroll et al., 2006). Therefore, the combination of oxytocin administration and MI, conducted in groups geared toward fostering a supportive context among peers, may help to boost treatment engagement as well as address other symptoms of MUD.
In addition to its social effects, oxytocin appears to demonstrate anti-addiction effects for a wide variety of substances (Lee et al., 2016; Sarnyai & Kovacs, 2014). For example, oxytocin has been found to diminish several aspects of METH-related reward in animals (Baracz & Cornish, 2016). Specifically, oxytocin administered to animal models of addiction has demonstrated reduction in METH self-administration and conditioned place preference (Baracz et al., 2012; Carson, Cornish, Guastella, Hunt, & McGregor, 2010; Cox et al., 2017; Hicks, Cornish, Baracz, Suraev, & McGregor, 2016; Qi et al., 2009); METH-induced locomotor hyperactivity (Carson et al., 2010; Qi et al., 2008); cue-, drug-, and stress-induced reinstatement of METH use (Baracz, Everett, & Cornish, 2015; Baracz, Everett, McGregor, & Cornish, 2016; Carson, Cornish, et al., 2010; Cox et al., 2017; Everett, Baracz, & Cornish, 2019; Everett, Baracz, & Cornish, 2020; Hicks et al., 2016; Qi et al., 2009); and METH-induced cFos expression in the nucleus accumbens (Everett et al., 2019). While early clinical trials have investigated oxytocin's effects on alcohol, cannabis, opioid, cocaine, and tobacco use disorders (Lee & Weerts, 2016; Stauffer et al., 2016), no clinical trial has yet investigated the effects of oxytocin administration on METH use in humans.
Oxytocin generally increases vagal tone (i.e., parasympathetic nervous system activity) and plays a cardioprotective role (Gutkowska, Jankowski, & Antunes-Rodrigues, 2014). Moreover, intranasal oxytocin administration acutely modulates heart rate variability (HRV) in response to laboratory stressors in humans (Kemp et al., 2012; Kubzansky, Mendes, Appleton, Block, & Adler, 2012; Norman et al., 2011; Riem et al., 2020; Romney, Hahn-Holbrook, Norman, Moore, & Holt-Lunstad, 2019; Tracy, Gibson, Labuschagne, Georgiou-Karistianis, & Giummarra, 2018). HRV refers to variation in the interval between successive heart beats, a marker of autonomic flexibility and ability to regulate emotional responses (Appelhans & Luecken, 2006). Interestingly, the direction of oxytocin's effects on HRV depends on social context, emphasizing the intimate link between oxytocin's social and physiological properties. In samples of healthy individuals, administration of oxytocin, compared to placebo, in a challenging context seems to decrease HRV, essentially reducing vagal tone and allowing the sympathetic nervous system to increase arousal and orchestrate a fight-or-flight response; whereas, oxytocin administration in a non-threatening context (e.g., at rest) seems to increase HRV, allowing vagal regulation of emotional processes underlying social approach behavior (Appelhans & Luecken, 2006; Kemp & Guastella, 2010). The presence of a supportive friend (Kemp et al., 2012; Kubzansky et al., 2012; Norman, Cacioppo, Morris, Malarkey, et al., 2011; Riem et al., 2020; Romney et al., 2019; Tracy et al., 2018) and loneliness ratings (Norman, Cacioppo, Morris, Malarkey, et al., 2011) further moderate the effects of oxytocin on HRV. On the other hand, METH use is associated with acute sympathomimetic effects (Gutkowska et al., 2014; Henry, Minassian, & Perry, 2012; Schwarzbach, Lenk, & Laufs, 2020) as well as chronic reductions in HRV (Henry et al., 2012). Recent studies have focused on interventions to increase HRV as a correlate for symptom improvement in substance use disorders (Dolezal et al., 2014; Eddie, Kim, Lehrer, Deneke, & Bates, 2014). Despite the evidence above outlining oxytocin's effects on cardiac physiology in healthy individuals, along with the negative cardiac effects of METH, to our knowledge this is the first investigation of the effects of oxytocin administration on HRV in individuals with a substance use disorder.
The purpose of the current study was to test the hypothesis that intranasal oxytocin, compared to matched intranasal placebo, administered immediately before each of six sessions of motivational interviewing group therapy (MIGT) will improve clinical treatment outcomes for MUD in a sample of MSM. We will refer to this psychopharmacology-psychotherapy combination treatment as oxytocin-enhanced motivational interviewing group therapy (OE-MIGT). In addition to being well tolerated (e.g., no serious adverse events), we specifically hypothesized that OE-MIGT will (a) increase group therapy session attendance. Secondarily, we hypothesized that OE-MIGT will (b) increase self-reported group cohesion, (c) decrease self-reported anxiety, (d) reduce METH craving and (e) METH use, and (f) reduce heart rate and increase HRV during group therapy sessions (i.e., psychophysiology reflecting social approach-related motivation) (Kemp et al., 2012).
2. Methods
2.1. Trial design
This was a randomized, double-blind, placebo-controlled, clinical trial (NCT02881177) examining the effects of OE-MIGT for MUD among MSM. The rationale, study design, and analytic plan have been previously published in greater detail (Stauffer et al., 2019), but we outline them here.
We compensated participants $50 for each study visit (i.e., a possible total of $350 for attending the baseline screening assessment and all six MIGT sessions). For the MIGT sessions, participants received $25 immediately after the session and an additional $25 was placed into a “bonus pot”, which they would receive only if they attended the final session (i.e., they would receive a total of $175 at session 6 if they had attended all sessions prior). The University of California, San Francisco (UCSF) Institutional Review Board provided ethics review and safety monitoring for this study.
2.2. Participants
2.2.1. Inclusion/exclusion criteria
To be eligible for participation, individuals had to be between 18 and 65 years old, male-identified (or genderqueer and assigned male at birth), have a history of sexual contact with men, and meet criteria for severe MUD as defined by the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5). At the time of screening, we required participants to be treatment-seeking for MUD and have used METH at least once in the past 30 days.
Exclusion criteria included: (a) diagnosis of bipolar I disorder; (b) severe alcohol use disorder with withdrawal symptoms; (c) suicidal ideation with intent or plan, a suicide attempt in the past six months, and/or homicidal ideation within the past ninety days; (d) evidence of opioid use in the past month; (e) cognitive impairment or behavioral issues precluding participation in group therapy; (f) diseases likely to influence hormonal or neuroendocrine status; (g) conditions preventing nasal spray administration (e.g., nasal obstruction, frequent nose bleeds); and (h) known allergic reaction to the preservatives within the nasal spray.
2.2.2. Recruitment and screening
We recruited participants from March 2017 through July 2018 in San Francisco, CA, USA, from various community health centers specializing in the treatment of substance use disorders among MSM populations. We also posted study flyers throughout the community. Interested participants contacted study staff and underwent a brief screening interview prior to being invited to attend a more thorough in-person screening assessment. We obtained written informed consent before we performed any study procedures. A trained clinical interviewer with at least master's level training in clinical psychology conducted screening assessments, which included pertinent sections of the Mini International Neuropsychiatric Interview (MINI) 7.0.0 (Sheehan et al., 2015) and the suicide subsection from the Structured Clinical Interview for DSM-5 (SCID-5) (First, Williams, Karg, & Spitzer, 2016). Last, a study physician conducted a brief medical history and physical examination, including assessment of the nasal parenchyma.
2.2.3. Randomization and blinding
We randomly assigned eligible individuals to an MIGT cohort, with a 1:1 chance of being placed in an OE-MIGT cohort or a control MIGT cohort receiving intranasal placebo prior to each session. Each member of a cohort received the same study drug as the other members of their cohort, and each cohort received the same study drug for all six sessions. Wellspring Compounding Pharmacy (Berkeley, CA, USA) implemented and maintained blinding procedures.
2.3. Interventions
2.3.1. Study drug
Study drug was oxytocin 40 International Units (IU) or matched placebo. Wellspring Compounding Pharmacy prepared the drug. Oxytocin concentration was 40-IU per milliliter (mL). Prior work has shown the onset of oxytocin's effects to occur within 30 min and last at least 90 min (Norman et al., 2011). The study psychiatrist administered the study drug intranasally, with all participants present in the group therapy space, 30 min prior to the beginning of each MIGT session. The study psychiatrist separated each study drug dose into two 1-mL Luer lock syringes and administered, one per nostril, in a standardized fashion (Guastella et al., 2013) through an attached mucosal atomization device (MAD300; Teleflex technologies, Mooresville, NC).
2.3.2. Motivational interviewing group therapy (MIGT)
MI is a conversational technique designed to address ambivalence and strengthen an individual's own motivation to change a behavior. Participants generate their own goals for change, along with personalized plans for attaining stated goals. Reducing or discontinuing METH use or increasing engagement in treatment are examples of goals for healthy behavioral change within the context of our study. In MIGT, therapists normalize the fact that participants in the group may be at different stages of change and have different goals and plans for treatment (Wagner & Ingersoll, 2013).
Two therapists, a licensed psychiatrist and a mental health trainee (either a clinical psychology Ph.D./Psy.D. candidate or a psychiatry resident in their final year of training), co-facilitated each of the MIGT sessions in this study. Each closed-admission MIGT cohort consisted of four to six research participants. We adapted a treatment manual from Wagner and Ingersoll (2013) for OE-MIGT for MUD in MSM (see Supplemental Material). We gave special attention to social context in our study design, as oxytocin administration can enhance “in-group/out-group” dynamics (De Dreu, 2012; De Dreu, Shalvi, Greer, Van Kleef, & Handgraaf, 2012; Ten Velden, Daughters, & De Dreu, 2017; Zhang, Gross, De Dreu, & Ma, 2019). In other words, we specifically designed our harm reduction intervention to provide a generally supportive and nonjudgmental social context in an attempt to maximize the potential benefits of intranasal oxytocin related to social approach behavior while minimizing risks, such as scapegoating.
Participants arrived 45 min prior to the beginning of each 90-min MIGT session for study drug administration, to complete questionnaires, and to provide a urine sample. Participants also completed questionnaires for approximately 15 min after each MIGT session. The sixth and final MIGT session lasted only 60 min to provide time for completion of additional post-session measures. We recorded all MIGT sessions (n = 60) for ongoing review and clinical supervision.
We randomly selected audio recordings from one third of the MIGT sessions (n = 20), half from oxytocin cohorts and half from placebo cohorts, for two expert raters to assess treatment integrity using the Motivational Interviewing Treatment Integrity Coding Manual (MITI) 4.2 (Moyers et al., 2014). The MITI 4.2 includes global ratings and behavior counts. Global rating categories include “relational”, which describes the extent to which therapists exhibited partnership and empathy, as well as “technical”, which describes the extent to which therapists engaged in techniques designed to strengthen change talk and soften sustain talk in relation to participants' behavioral goals. Global ratings range from 1 to 5. Behavior counts describe how often therapists engaged in Mi-adherent interventions (e.g., affirmations, seeking collaboration, and emphasizing autonomy) and MI-non-adherent behavior (e.g., persuade, confront). We double-coded a random sample of five sessions to establish inter-rater reliability. We determined inter-rater reliability using a two-way absolute single-measures intra-class correlation (ICC), which provides a conservative estimate of reliability that is generalizable to single- and double-coded sessions (Hallgren, 2012).
2.4. Outcome measures
See Table 1 for timing of the outcome measurements described here. We collected self-report data using Research Electronic Data Capture hosted by UCSF on Google Nexus 7 tablets.
Table 1.
Eligibility and assessment timetable
| BL | 0b | MIGT Sessions 1–6a |
|||
|---|---|---|---|---|---|
| Pre | During | Post | |||
| Enrollment | |||||
| Informed Consent | X | ||||
| Medical Screen | X | ||||
| Allocation | X | ||||
| Assessments | |||||
| Sociodemographic | X | ||||
| MINI 7.0.0 | X | ||||
| SCID-5, Suicide Assessment | X | ||||
| ACE | X | ||||
| ECR-S | X | ||||
| STAI (full) | X | ||||
| OTSE | X | ||||
| Session Attendance | X | ||||
| GQ | X | ||||
| STAI-6 | X | X | |||
| MCQ-Br | X | X | |||
| Urine Toxicology | X | X | |||
| TLFB | X | ||||
| Physiology Recording | X | ||||
ACE = Adverse Childhood Experience Questionnaire, BL = Baseline, ECR-S = Experiences in Close Relationships – Short Form, GQ = Group Questionnaire, MCQ-Br = Methamphetamine Craving Questionnaire-Brief, MINI = Mini International Neuropsychiatric Interview, OTSE = Oxytocin Side Effect Checklist, SCID-5 = Structured Clinical Interview for DSM-5, STAI=State-Trait Anxiety Inventory, STAI-6 = State-Trait Anxiety Inventory Short Form, TLFB = Timeline Follow Back.
Intranasal study drug was administered 30 min prior to each MIGT session. “Pre”, “During", and “Post” refer to timing around MIGT session.
Enrollment.
2.4.1. Baseline measures
At the screening assessment, the clinical interviewer collected baseline sociodemographic factors, including sexual orientation using the Kinsey scale (Weinrich, 1990). We assessed relative frequency of use for various substances: (1) number of days used over the past 30 days and (2) lifetime use = (number of years on average participant used ≥3 days per week)/(age in years). We asked participants their preferred term for METH, and all subsequent references to METH in self-assessment questionnaires used the individual respondent's preferred term. Baseline self-assessment measures included: the Adverse Childhood Experience Questionnaire (Felitti et al., 1998), the State-Trait Anxiety Inventory (Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983), the Experiences in Close Relationships – Short Form (Wei, Russell, Mallinckrodt, & Vogel, 2007), and the Readiness to Change Questionnaire (Heather & Hönekopp, 2008).
2.4.2. Tolerability
After each MIGT session, participants responded to a list of the most commonly reported side effects from previous oxytocin studies, adapted from MacDonald et al. (2011). Each participant rated each side effect reported was subjectively rated by the participant as mild, moderate, or severe. We asked participants to report any moderate-severe or unexpected (i.e., not on the list) side effects directly to the study physician for further assessment.
2.4.3. Primary clinical outcome
MIGT session attendance was the primary clinical outcome. At the screening assessment, participants provided a primary contact number as well as contact information for a secondary individual who would know how to get ahold of them (e.g., family member, friend, case manager, etc.). If study staff were unsuccessful in reaching the participant via their primary contact information, they would try the secondary contact. Study staff called each participant the day prior to each weekly MIGT session as a reminder and left a discreet voicemail if the participant did not answer. If a participant was not present by 40 min prior to starting group therapy (i.e., 5 min late), and study staff had not yet heard from them, one of the therapists attempted to contact them by telephone at this time. If they arrived with <15 min before the start of the MIGT session, they were unable to participate that day. Participants were aware that missing more than two MIGT sessions was grounds for termination. We encouraged participants to discuss with study staff as soon as possible any unexpected circumstances leading to an inability to attend sessions.
2.4.4. Secondary outcome measures
2.4.4.1. Group cohesion.
Study staff administered the 30-item Group Questionnaire (GQ; (Krogel et al., 2013) after each MIGT session to assess the quality of the therapeutic relationships within the group. The three GQ constructs, Positive Bonding (cohesion, engagement, and emotional bond), Positive Working (agreement on therapeutic goals and tasks), and Negative Relationship (conflict and empathic failure), are subscales of the GQ.
2.4.4.2. Anxiety.
We used the Six-Item State-Trait Anxiety Inventory Short Form (STAI-6; [Spielberger et al., 1983]), using the three highest anxiety-present and three highest anxiety-absent items from the full State-Trait Anxiety Inventory (Spielberger et al., 1983), to measure self-reported state anxiety before and after each MIGT session.
2.4.4.3. METH craving.
We used the Methamphetamine Craving Questionnaire-Brief (MCQ-Br), which we adapted from the 10-item Stimulant Craving Questionnaire-Brief (Northrup, Green, Walker, Greer, & Trivedi, 2015), to measure self-reported METH craving before and after each MIGT session.
2.4.4.4. METH use.
Study staff collected a urine sample at each MIGT session, and we analyzed it using a point-of-care, CLIA-waived, 10-panel, Toxicology iCup Dx (Alere Inc., Waltham, MA) to screen for METH and other common substances of abuse. We also measured METH use through a self-administered (Sobell, Brown, Leo, & Sobell, 1996) Timeline Follow Back assessment of quantity used each day over the past week (Hoeppner, Stout, Jackson, & Barnett, 2010).
2.4.4.5. Psychophysiology.
We equipped each participant with a Zephyr™ BioHarness v3.0 (Zephyr Technology, Auckland, New Zealand), wearable, wireless, psychophysiology data collection equipment, prior to each MIGT session. The Zephyr™ TEAM System OmniSense Software (Version 4.2.4) then recorded continuous ECG data for each 90-min MIGT session, sampled at 250 Hz.
2.5. Analysis plan
2.5.1. Sample size calculation
The general purpose of this early phase clinical trial was to investigate preliminary outcomes, collect participant and clinician feedback, and to evaluate feasibility and acceptability for the purpose of refining our intervention. Therefore, we set the sample size of N = 50 (25 in each of the two conditions) primarily for practical and clinical reasons; it was not driven by hypothesis testing. We did, however, plan to conduct a preliminary evaluation of the outcomes and conduct statistical tests (see NCT02881177 as well as our previous publication of the protocol design and analysis plan; [Stauffer et al., 2019]). A sample size of 25 per treatment condition provided us with >99% power to detect an effect size comparable to the large between-condition effect size (d = 1.44) observed for attendance from our preliminary 3-week trial in cocaine users (Stauffer et al., 2016). We used a two-tailed alpha of 0.05 for all analyses.
2.5.2. Statistical methods
Our modified intention-to-treat sample comprised all participants who were randomized to treatment and received at least one dose of study drug (i.e., attended at least the first MIGT session). Participants who did not attend session 1 did not receive any study intervention, and we excluded them from further participation.
We performed a chi-square test of independence to assess for significant differences in frequencies of each reported side effect between those receiving oxytocin and those receiving placebo.
We compared session attendance (present or absent) and urine toxicology (METH-positive versus METH-negative), dichotomous variables, between the oxytocin and placebo treatment conditions using generalized estimating equations (GEE) to estimate odds ratios. GEE accounted for correlations among the observations originating from the same participant. For attendance GEE, attendance for session 1 was 100% in both treatment groups. Therefore, we omitted session 1 data from analysis. We also omitted attendance data from session 6 from analysis given the increased financial incentive to attend this final session compared to other sessions. For urine toxicology GEE, we omitted screening visit and session 1 toxicology data as dependent variables, but we included session 1 results in the model as a baseline, because they reflect METH use prior to receiving any study intervention. At session 1, almost twice as many participants assigned to the placebo arm had METH-positive urine (70.83%) compared to those assigned to the oxytocin arm (37.5%). This is despite more comparable METH use between the two treatment arms at screening when randomization took place (41.67% versus 34.78%, respectively, see Fig. 3f). Because we did not equally distribute active METH users were not equally distributed between the two treatment arms at session 1, and because active METH use versus abstinence may be confounding, we controlled for METH use in our analysis of session attendance. When assessing for the influence of METH on attendance, we used the previous session's toxicology result in our GEE analysis as a predictor (i.e., urine toxicology from sessions 1–4 to correspond to attendance at sessions 2–5, without imputing missing data). In analysis of urine toxicology, we imputed missing data (i.e., absences) to be METH-positive.
Fig. 3.
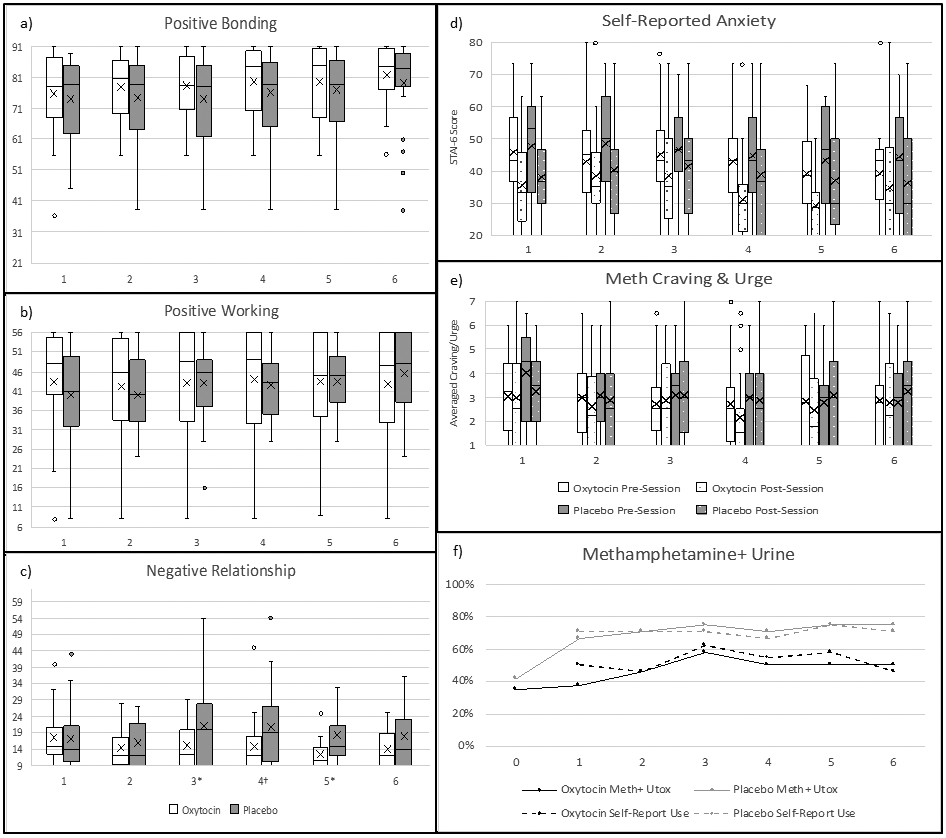
Self-report outcomes and METH use. METH use not controlled for. a-c) Group Questionnaire (GQ) subscale summary scores (subscale score ranges: Positive Bonding 13–91, Positive Working 8–56, Negative Relationship 9–63), d) STAI-6 (score range: 20–80), e) MCQ-Br items #2 (urge) & #5 (craving) averaged (score range: 1–7), f) percent with Methamphetamine-Positive Urine Toxicology (solid lines) and any self-reported METH use over the 3 days prior to each urine sample (dotted lines). x-axes = group therapy session number. a-e = box plots. Missing data imputed by carrying value from previous session forward. †p < .10. *p < .05, for significant differences between oxytocin and placebo treatment conditions (i.e., Negative Relationship Sessions 3–5).
We used linear mixed effect models (LMEM) to assess continuous secondary outcome variables for group cohesion, anxiety, METH craving, and psychophysiology, because LMEM can accommodate missing data and multiple time points. LMEM assessed for the effects of OE-MIGT (versus placebo + MIGT), METH use (versus METH-negative urine samples), and Time across the six MIGT sessions. For METH use, we used toxicology results from all sessions in our LMEM (with missing data imputed as METH-positive). When the main effects of OE-MIGT and METH were both significant, we then controlled for METH in a post-hoc analysis of OE-MIGT treatment effect. We calculated Cohen's d effect size proxy by taking the estimated fixed effect, B, for each secondary outcome divided by an estimated standard deviation using standard error, d = B/[sqrt(n)*standard error]. We refer to conventional effect size benchmarks proposed by (Cohen, 1988): small (d = 0.2), medium (d = 0.5), and large (d = 0.8).
We assessed GQ subscales (positive bonding, positive working, and negative relationship) separately. Some participants were confused by the phrasing of the reverse-scored items from the MCQ-Br (i.e., rating them in the same range as the regularly scored items); therefore, we used only data for the questions “I have an urge for [METH]” and “I crave [METH] right now” for analysis rather than the MCQ-Br summary score. In our LMEM model, we used pre-session anxiety and METH urge/craving as a marker of sustained effects of OE-MIGT and pre- to postsession change in these variables as a marker of the acute effects of OE-MIGT.
We divided all continuous raw ECG data from MIGT sessions into one-minute segments and pre-processed them using MindWare HRV software (v3.1.2, MindWare Technologies, Ltd., Gahanna, OH, USA). MindWare is a program that automatically identifies the R peaks of successive ECG waveforms to calculate the average heart rate and markers of HRV, including respiratory sinus arrhythmia (RSA) and root mean square of successive R-R differences (RMSSD), per 60-second interval. RSA, derived from frequency-domain methods, is considered a sensitive operational marker of cardiac vagal control (Berntson, Cacioppo, & Quigley, 1993) and emotion regulation (Tonhajzerova, Mestanik, Mestanikova, & Jurko, 2016). RMSSD, calculated using the beat-to-beat variance in heart rate, is the primary time-domain measure used to estimate vagally-mediated changes reflected in HRV (Shaffer & Ginsberg, 2017). We identified artifacts within each segment via MindWare's dual MAD/MED and IBI Min/Max artifact detection algorithms derived from established strategy (Berntson, Quigley, Jang, & Boysen, 1990). We then manually reviewed all software-identified R peaks and artifacts and edited them for accuracy. Lastly, we averaged one-minute segments for heart rate, RSA, and RMSSD across the entire MIGT session, and we used session averages for subsequent analyses.
We conducted GEE analyses for dichotomous variables using IBM SPSS Statistics for Macintosh, version 25.0 (IBM Corp., Armonk, NY, USA). We conducted LMEM analyses using R, version 3.6.1 (R Core Team, Vienna, Austria) and the ImerTest package for statistical analysis (Kuznetsova, Brockhoff, & Christensen, 2017).
3. Results
3.1. Participants
See Fig. 1 for participant flow diagram (Moher, Schulz, & Altman, 2001). Forty-eight participants attended the first MIGT session and were included in analysis for the primary outcome. There were a total of 10 MIGT cohorts, 5 cohorts received oxytocin (n = 24) and 5 cohorts received placebo (n = 24). See Table 2 for demographics and Table 3 for baseline characteristics. One participant from the placebo arm was removed from analyses of self-report measures due to unreliable data (answered the maximum rating for every question at each collection point).
Fig. 1.
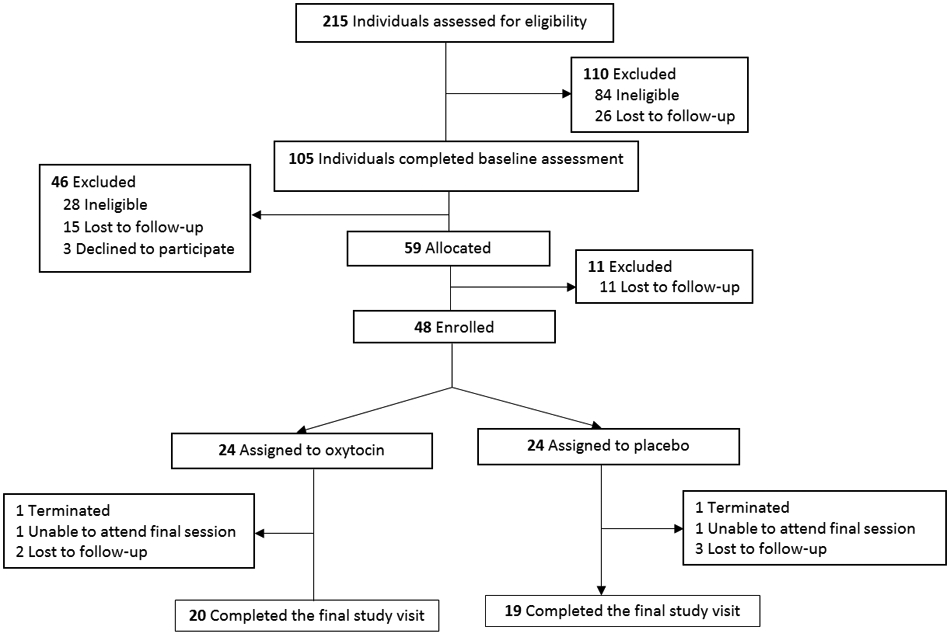
Participant flow diagram.
Table 2.
Demographics.
| Oxytocin (n = 24) | Placebo (n = 24) | Overall (n = 48) | ||
|---|---|---|---|---|
| Age; Mean(SD) | 43.96(11.04) | 42.75(11.00) | 43.35(10.91) | |
| Gender Identity; n (%) | ||||
| Male | 22 (91.67%) | 23 (95.83%) | 45 (93.75%) | |
| Transgender male | 0 (0.00%) | 1 (4.17%) | 1 (2.08%) | |
| Genderqueera | 2 (8.33%) | 0 (0.00%) | 2 (4.17%) | |
| Kinsey Score; Mean(SD) | 5.17(1.18) | 4.96(1.27) | 5.06(1.24) | |
| Race; n (%) | ||||
| African American/Black | 6 (25.00%) | 7 (29.17%) | 13(27.08%) | |
| Multiracial | 1 (4.17%) | 6 (25.00%) | 7 (14.58%) | |
| Native American/Pacific Islander | 3 (12.5%) | 3 (12.5%) | 6 (12.5%) | |
| White | 14 (58.33%) | 8 (33.33%) | 22(45.83%) | |
| Ethnicity; n (%) | Hispanic/Latino | 2 (8.33%) | 6 (25.00%) | 8 (16.67%) |
| Education; n (%) | ||||
| ≤High school graduate | 3 (13.04%) | 8 (33.33%) | 11 (23.40%) | |
| Some college | 14 (60.87%) | 9 (37.50%) | 23 (48.93%) | |
| College graduate | 5 (21.74%) | 3 (12.50%) | 8 (16.67%) | |
| Graduate degree | 2 (8.70%) | 4 (16.67%) | 6 (12.50%) | |
| Annual Income; n (%) | ≤$11,880b | 7 (29.17%) | 8 (33.33%) | 15 (31.25%) |
| Employed; n (%) | 4 (16.67%) | 4 (16.67%) | 8 (16.67%) | |
| Disability; n (%) | 11 (45.83%) | 8 (33.33%) | 19 (39.58%) | |
| Housing Status; n (%) | RTF/SLE | 3 (12.50%) | 6 (25.00%) | 9 (18.75%) |
| Homeless, past year | 5 (20.83%) | 11 (47.83%) | 16 (33.33%) | |
| Relationship Status; n (%) | Primary relationshipc | 5 (20.83%) | 3 (12.50%) | 8 (16.67%) |
| Hx of Incarceration; n (%) | 13 (54.17%) | 9 (37.50%) | 22 (45.83%) | |
| HIV Positive; n (%) | 16 (66.67%) | 22 (91.67%) | 37 (77.08%) | |
HIV=Human Immunodeficiency Virus, Hx = History, RTF = Residential Treatment Facility, SD = standard deviation, SLE = Sober Living Environment.
Assigned male at birth.
2016 United States Department of Health and Human Services poverty guideline.
Someone who you are currently in love with or feel a commitment to.
Table 3.
Baseline characteristics.
| Psychiatric Meds; n (%) | Oxytocin (n=24) |
Placebo (n=24) |
Overall (n=48) |
|||
|---|---|---|---|---|---|---|
| Antidepressant | 8 (33.33%) | 11 (45.83%) | 19 (39.58%) | |||
| Anxiolytic | 4 (16.67%) | 3 (12.50%) | 7 (14.58%) | |||
| Antipsychotic | 6 (25.00%) | 4 (16.67%) | 10 (20.83%) | |||
| Mood stabilizer | 3 (12.50%) | 1 (4.17%) | 4 (8.33%) | |||
| None | 13 (54.17%) | 8 (33.33%) | 21 (43.75%) | |||
| Substance Use; Mean(SD) | 30-day* | Lifetime† | 30-day* | Lifetime† | 30-day* | Lifetime† |
| Methamphetamine | .38(.35) | .23(.16) | .43(.33) | .24(.16) | .40(.35) | .24(.16) |
| Primary IV; n (%) | 8 (33.33%) | 5 (20.83%) | 13 (27.08%) | |||
| Alcohol | .19(.29) | .25(.22) | .10(.15) | .21(.16) | .14(.24) | .23(.19) |
| Cannabis | .25(.42) | .14(.19) | .31(.42) | .20(.17) | .28(.43) | .17(.18) |
| Cocaine | .03(.10) | .10(.18) | .01(.02) | .10(.11) | .02(.07) | .10(.15) |
| Poppers (amyl nitrate) | .10(.22) | .04(.12) | .05(.10) | .01(.03) | .07(.17) | .03(.09) |
| Oxytocin (n=24) |
Placebo (n=23) |
Overall (n=47) |
||
|---|---|---|---|---|
| ACE; Mean(SD) | 5.33(2.67) | 5.65(2.14) | 5.49(2.46) | |
| Trait Anxiety‡; Mean(SD) | 48.26(13.93) | 52.56(10.14) | 50.41(12.51) | |
| Attachment Anxiety§; Mean(SD) | 27.87(7.43) | 27.09(8.12) | 27.48(7.88) | |
| RTCQ∣; Mean(SD) | Pre-Contemplation | −3.96(2.84) | −2.74(2.88) | −3.36(2.89) |
| Contemplation | 4.29(2.97) | 4.13(2.16) | 4.21(2.58) | |
| Action | 3.75(3.81) | 3.39(3.09) | 3.57(3.44) |
ACE=Adverse Childhood Experience Questionnaire, IV=intravenous, SD=standard deviation
Number of days used in past 30 days/30
Number of years used ≥3 times per week/age in years
State-Trait Anxiety Inventory, trait items
Experiences in Close Relationships Scale – Short Form
Readiness to Change Questionnaire
3.2. OE-MIGT fidelity rating
All fidelity rating codes demonstrated acceptable inter-rater reliability. According to guidelines set forth by Cicchetti (1994), inter-rater reliabilities were good to excellent for relational (ICC = 0.73), technical (ICC = 0.94), Mi-adherent (ICC = 1.0), and Mi-nonadherent (ICC = 1.0) behaviors (these perfect ICCs were a result of a low frequency of Mi-adherent and Mi-nonadherent behaviors in the five double-coded sessions). Overall, therapists demonstrated upper-range global ratings on the relational, M(SD) = 4.1(0.37), and technical M(SD) = 3.3(0.48) components of MI. In addition, therapists did engage in more MI-adherent behaviors M(SD) = 1.5(1.9) than MI-nonadherent behaviors M(SD) = 0.7(1.5). Using a paired-sample t-test, the difference between MI-adherent and MI-nonadherent counts was statistically significant, t( 18) = 2.14, p = .05. There were no significant differences in any MITI outcome between treatment groups.
3.3. Outcomes and estimates
3.3.1. Tolerability
There were no serious adverse events related to the study intervention. The five most commonly reported items from the list of common side effects were “calm/relaxed” (placebo 28.8%, oxytocin 17.9%), “runny nose” (placebo 14.4%, oxytocin 23.6%), “drowsiness/sleepy” (placebo 15.4%, oxytocin 15.4%), “nasal irritation” (placebo 5.8%, oxytocin 21.1%), and “anxious/worried” (placebo 14.4%, oxytocin 11.4%). Those receiving placebo reported feeling calm/relaxed significantly more often than those receiving oxytocin, X2(1, N = 48) = 3.83, p = .05. Participants more commonly reported nasal irritation, X2(1, N = 48) = 10.99, p < .001, and runny nose, X2(1, N = 48) = 3.02, p = .08, after receiving oxytocin versus placebo. Subjective severity (mild, moderate, or severe) of nasal irritation was primarily rated as mild (78.1%) and moderate (15.6%), and runny nose was primarily rated as mild (86.4%) and moderate (11.4%). The prevalence of remaining side effects demonstrated no difference between oxytocin and placebo.
3.3.2. Primary outcome
There was a significant effect of OE-MIGT on session attendance (OR 3.26, 95% CI [1.27–8.41], p = .014). This was after controlling for METH use (i.e., urine sample from the previous MIGT session), which had a significant main effect on attendance (p = .037). Overall, there were 35 absences in the placebo arm and 19 absences in the oxytocin arm (see Fig. 2). Furthermore, 47.4% of the 19 absences in the oxytocin arm-while only 34.3% of the 35 absences in the placebo arm-involved contact with providers (i.e., were either planned absences, the participant otherwise made telephone contact with providers prior to the MIGT session, or the participant arrived too late to participate for that session). The other absences involved no contact with providers prior to group therapy, and the participants either subsequently returned or were lost to follow-up.
Fig. 2.
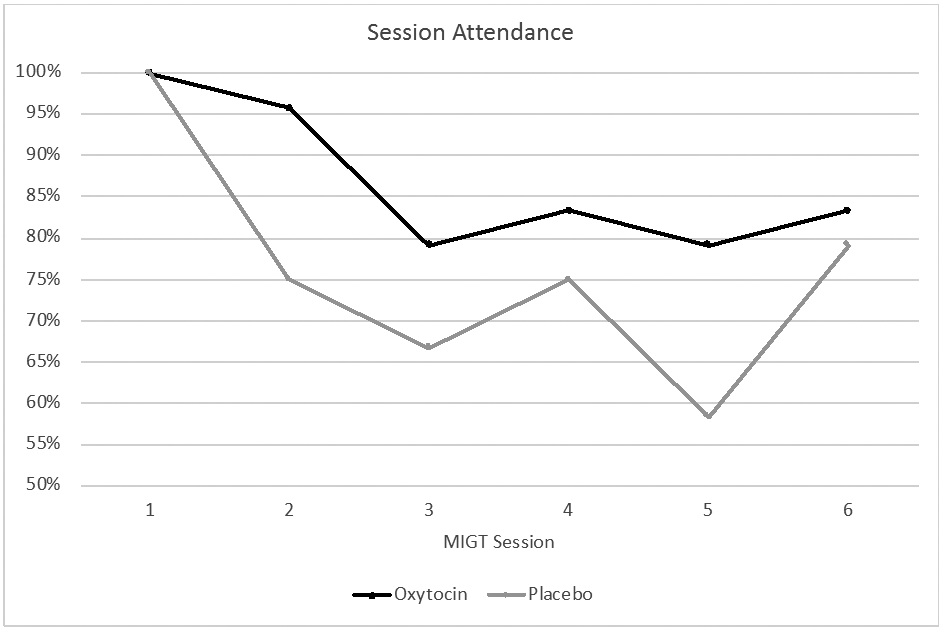
Attendance chart. MIGT = Motivational Interviewing Group Therapy.
Five participants in the placebo arm and four participants in the oxytocin arm did not attend the final MIGT session; thus, they were considered noncompleters (see Fig. 1). This included two participants terminated by the study therapists, one from the placebo arm for making a threat toward another group member during session 4, and one from the oxytocin arm who was hospitalized with pneumonia during sessions 3–5 (although he maintained contact with providers and expressed a desire to attend if he were not in the hospital).
3.3.3. Secondary outcomes
See Figs. 3 and 4 for visualization of secondary outcomes. See Table 4 for LMEM main effects of OE-MIGT, METH, and Time on secondary outcomes.
Fig. 4.
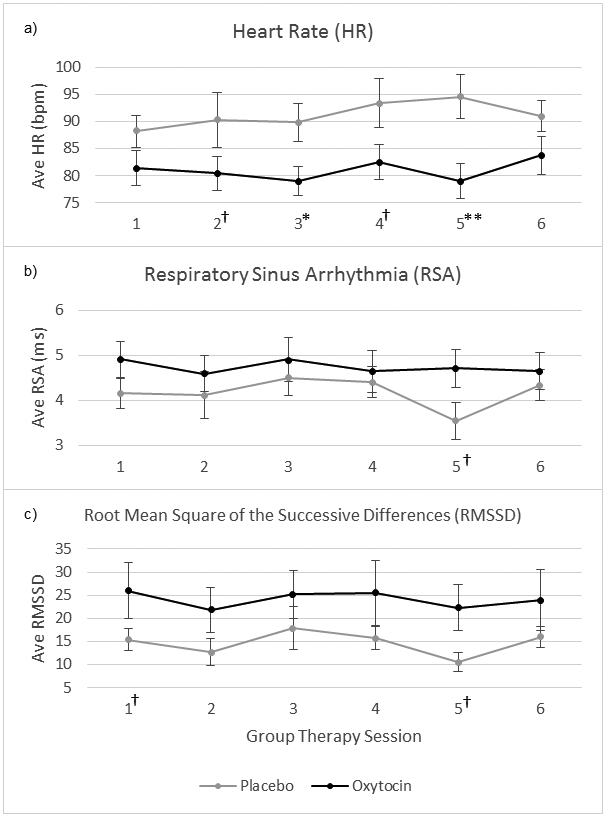
Psychophysiology outcomes. 60-second blocks averaged across entire session (90 min for Sessions 1–5 and 60 min for Session 6). METH use not controlled for. bpm = beats per minute, ms = millisecond. †p < .10, *p < .05, **p < .01, for significant differences between oxytocin and placebo treatment conditions for individual session.
Table 4.
LMEM outcomes.
| # | Drug (Oxytocin, Placebo) | METH+ (Urine Toxicology) | Time (across 6 sessions) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ob | n | B(SE) | 95% CI | d | p | B(SE) | 95% CI | d | p | B(SE) | 95% CI | d | p | ||
| GQ | PB | 228 | 47 | 1.59(2.86) | [−4.02–7.20] | 0.08 | .581 | −1.26(1.99) | [−5.17–2.64] | −0.09 | .526 | 1.27(0.27) | [0.73–1.79] | .69 | <.001 |
| PW | 228 | 47 | 0.69(3.10) | [−5.39–6.77] | 0.03 | .825 | −0.354(1.86) | [−3.99–3.29] | −0.03 | .849 | 0.52(0.23) | [0.06–0.97] | 0.33 | .027 | |
| NR | 228 | 47 | −2.91(1.61) | [−6.06–0.25] | −0.26 | .078 | 3.48(1.37) | [0.79–6.17] | 0.37 | .013 | −0.46(0.25) | [−0.94–0.02] | −0.27 | .064 | |
| Pre-Session | Anxiety | 229 | 47 | −2.58(3.43) | [−9.31–4.15] | −0.11 | .456 | 6.19(2.59) | [1.11–11.28] | 0.35 | .018 | −1.22(0.39) | [−1.99 - −0.45] | −0.46 | .002 |
| Craving | 229 | 47 | −0.47(0.40) | [−1.26–0.32] | −0.17 | .249 | 0.56(0.32) | [−0.07–1.19] | 0.26 | .082 | −0.11(0.05) | [−0.21–0.004] | −0.32 | .043 | |
| Urge | 229 | 47 | −0.14(0.39) | [−0.91–0.63] | −0.05 | .719 | 0.57(0.31) | [−0.04–1.18] | 0.27 | .072 | −0.18(0.05) | [−0.28 - −0.09] | −0.53 | <.001 | |
| Pre- to Post-Session Change | Anxiety | 230 | 47 | −0.30(1.74) | [−3.71–3.10] | −0.03 | .863 | −1.82(1.65) | [−5.06–1.42] | −0.16 | .276 | 0.26(0.42) | [−0.56–1.09] | 0.09 | .533 |
| Craving | 229 | 47 | 0.24(0.21) | [−0.17–0.65] | 0.17 | .256 | −0.11(0.21) | [−0.52–0.31] | −0.08 | .618 | 0.05(0.06) | [−0.07–0.16] | 0.12 | .437 | |
| Urge | 229 | 47 | −0.15(0.23) | [−0.59–0.30] | −0.10 | .527 | −0.10(0.22) | [−0.52–0.32] | −0.07 | .639 | 0.11(0.06) | [−0.01–0.23] | 0.27 | .065 | |
| Psycho-physiology | HR | 181 | 46 | −9.14(3.49) | [−15.98 - −2.30] | −0.39 | .013 | 9.14(2.75) | [3.76–14.52] | 0.49 | .001 | 0.27(0.41) | [−0.53–1.07] | 0.10 | .510 |
| RSA | 181 | 46 | 0.66(0.43) | [−0.18–1.50] | 0.23 | .132 | −1.27(0.28) | [−1.81 - −0.72] | −0.67 | <.001 | −0.01(0.04) | [−0.08–0.06] | −0.04 | .774 | |
| RMSSD | 180 | 46 | 10.09(5.07) | [−0.16–20.03] | 0.29 | .052 | −13.60(4.08) | [−21.58 - −5.61] | −0.49 | .001 | −0.24(0.64) | [−1.50–1.02] | −0.06 | .710 | |
Fixed effect estimates (B), standard error (SE), 95% Confidence Interval (CI), effect sizes (d), and p-values for secondary outcomes, looking at main effect of Drug (oxytocin versus placebo), METH use, and Time (across six sessions).
GQ = Group Questionnaire, HR = heart rate, LMEM = linear mixed effects model, METH = methamphetamine, NR = Negative Relationship, Ob = Observations, PB=Positive Bonding, PW=Positive Working, RMSSD = root mean square of the successive differences, RSA = respiratory sinus arrhythmia.
3.3.3.1. Group cohesion.
Main effects of OE-MIGT and METH on positive bonding and positive working were negligible. For negative relationship, there was a significant main effect of OE-MIGT, such that OE-MIGT was associated with lower negative relationship ratings, and of METH, such that METH-positive urine was associated with higher negative relationship ratings. Positive bonding and positive working increased across the six sessions for the overall sample, with medium and small effect sizes, respectively, and negative relationship decreased overall across the six sessions with a small effect size. See Fig. 3a-c and Table 4.
When we controlled for METH, OE-MIGT had a small effect on reducing negative relationship, B(SE) = −2.21(1.57), 95% CI [−5.28–0.86], d = −0.21.
3.3.3.2. Self-reported anxiety.
The main effect of OE-MIGT on pre-session anxiety and change in anxiety pre- to post-session was negligible. METH had a small effect on pre-session anxiety, such that METH-positive urine was associated with higher anxiety, and a negligible effect on pre- to post-session change in anxiety. Pre-session anxiety decreased across the six sessions, with a small effect size, and pre- to post-session change in anxiety had no notable change over the course of treatment. See Fig. 3d and Table 4.
3.3.3.3. METH urge/craving.
The main effect of OE-MIGT on pre-session urge and craving for METH was negligible. METH had a small effect on pre-session METH urge and craving, such that METH-positive urine was associated with higher METH urge/craving. There were no notable effects of OE-MIGT and METH on pre- to post-session change in METH urge/craving. METH craving/urge tended to decrease across the six sessions for the overall sample, with small to medium effect sizes. See Fig. 3e and Table 4.
3.3.3.4. METH use.
See Fig. 3f. To control for baseline differences in METH use between groups, we factored session 1 urine toxicology results into our GEE model as a baseline predictor. When doing so there was no significant main effect of OE-MIGT on METH use (p = .866).
3.3.3.5. Psychophysiology.
OE-MIGT had small effects on heart rate, RSA, and RMSSD, such that heart rate was lower and RSA/RMSSD were higher compared to the control group. METH had small-medium effects in the opposite direction. There were no notable changes in these psychophysiological measures across the six sessions. See Fig. 4 and Table 4.
After controlling for the effect of METH, a small effect of OE-MIGT remained for heart rate, B(SE) = −6.93(3.27), 95% CI [−13.35 - −0.51], d = −0.31, and RMSSD, B(SE) = 7.26(4.68), 95% CI [−1.92–16.44], d = 0.23, counter to the direction of METH's effects. There was no notable effect of OE-MIGT, after controlling for METH use, on RSA, B(SE) = 0.35(0.37), 95% CI [−0.38–1.07], d = 0.14.
4. Discussion
Consistent with our hypotheses, participants who received intranasal oxytocin (compared to placebo) prior to each of six weekly group therapy sessions demonstrated significantly higher session attendance, lower in-session heart rate, and higher RMSSD. Aside from a small effect on negative relationship, we did not detect any notable differences between oxytocin and placebo administration on self-report measures of group cohesion, anxiety, METH craving/urge, or on objective measures of METH use. This randomized, controlled, clinical trial of OE-MIGT for MUD among MSM is the first published study of the tolerability and preliminary effectiveness of intranasal oxytocin on METH-related clinical outcomes. Our findings suggest that intranasal oxytocin might be used to enhance engagement in treatment interventions for individuals with MUD, possibly related to changes in psychophysiology and negative interpersonal perceptions. Given our previous findings of improved clinic attendance in individuals with co-occurring cocaine and opioid use disorders (Stauffer et al., 2016), oxytocin's positive effects on treatment engagement may also generalize to other substance use disorders or other clinical populations experiencing high attrition rates. If true, this would represent an entirely new target for pharmacological intervention with profound impact on the effectiveness of psychosocial treatments.
The precise mechanism by which oxytocin increased attendance in our sample remains unclear. One possibility reflects oxytocin's key role in mammalian attachment (Carter, 2017), which, at its core, involves proximity maintenance to trusted individuals and modulation of physiological arousal (Bolwby, 1969). Porges (2011) describes this as “immobility without fear”, representing a “neural choice to stay in one place” in the context of safety (Carter, 2017). Attachment behavior is thought to be a largely automatic process driven by implicit memory and the autonomic nervous system (Knox, 1999; Ogden, Minton, & Pain, 2006). This may help to explain why oxytocin administration did not notably alter self-report measures but did significantly affect objective measures of social approach and cardiac physiology. Moreover, the link between attachment insecurity, or difficulty utilizing close relationships to modulate stress, and substance use disorders is well-established (Schindler, 2019). Stimulant use is thought to co-opt social reward circuitry (Insel, 2003; Taylor et al., 2013) and may reflect a maladaptive effort to regulate autonomic hyperreactivity in response to stressors when close relationships are lacking or are themselves associated with threat (Corrigan, Fisher, & Nutt, 2011). Interestingly, as measured by the GQ, oxytocin did not seem to affect positive aspects of group cohesion but did temper perceptions of interpersonal conflict (i.e., negative relationship). Thus, our findings may reflect an oxytocin-induced shift in salience (Shamay-Tsoory & Abu-Akel, 2016), allowing consideration of building trust and utilizing positive social reward, rather than depending solely on drug-related coping, to modulate stress (Tops et al., 2014). Anecdotally, childhood abuse and rejection by families and communities of origin were common topics brought forth by participants during MIGT sessions in the current study. From this perspective, oxytocin-induced reductions in physiological arousal may reflect a widened “window of tolerance”, an optimal arousal state during which emotions can be tolerated and difficult experiences can be therapeutically integrated (Corrigan et al., 2011). This is supported by the fact that those receiving oxytocin reported feeling calm and relaxed significantly less often than those receiving placebo, despite showing reductions in physiological arousal. Thus, an oxytocin-induced widening of the window of tolerance may be linked to our findings of increased attendance and reduced physiological arousal in a clinical population with high levels of attachment insecurity and known difficulty with treatment adherence. Such interpretations of our findings are merely theoretical until future trials can further elucidate the complex interrelationship among oxytocin, addiction, the autonomic nervous system, and treatment retention; however, attachment theory is a useful lens moving forward.
Our sample consisted of a particularly high-risk group of methamphetamine-using MSM, which limits generalizability. Participants enrolled in this trial, on average, spent almost a quarter of their lives using METH ≥ 3 times per week (see Table 3). The chronicity of heavy use in our sample may indicate treatment resistance, which is why we may not have seen any change in METH use over the six-week course of treatment. We did, however, see improvements in many of our self-report measures in both treatment arms across the six-week intervention (see Table 4, time column). Nonetheless, for the most part, there were no notable differences between those receiving OE-MIGT and those receiving MIGT with placebo. Due to limitations in our budget, we were unable to assess for longer term effects of our treatment intervention (e.g., effects of OE-MIGT on future relapse compared to those who received placebo along with MIGT). Future studies may investigate potential benefits of a longer treatment course of OE-MIGT as well as incorporate post-treatment follow-up.
Not surprisingly, METH use affected several of our outcome measures, including all cardiac physiology measures, heightened negative perspectives on group cohesion, increased self-reported anxiety, and worsened METH craving/urge. After controlling for METH use, we still saw a small positive effect of OE-MIGT on session attendance, ratings of negative relationship, heart rate, and RMSSD (see Table 4). Encouragingly, even when the effects of oxytocin were negligible, they consistently trended in the opposite direction from the effects of METH—a signal that suggests further investigation into the potential benefits of OE-MIGT for the treatment of MUD.
Our cardiac physiology findings are consistent with literature demonstrating that oxytocin's effects on heart rate are generally the inverse of the acute sympathomimetic effects of METH (Gutkowska et al., 2014; Henry et al., 2012; Schwarzbach et al., 2020). Moreover, findings were consistent with previous literature on oxytocin's HRV effects (Kemp et al., 2012; Kubzansky et al., 2012; Norman, Cacioppo, Morris, Malarkey, et al., 2011; Riem et al., 2020; Romney et al., 2019; Tracy et al., 2018), although oxytocin's effects on RSA were negligible after controlling for METH (Kemp et al., 2012; Kubzansky et al., 2012; Norman, Cacioppo, Morris, Malarkey, et al., 2011; Riem et al., 2020; Romney et al., 2019; Tracy et al., 2018). While the direction of acute effects of oxytocin administration on HRV are highly dependent on social context, chronic METH use is associated with persistent and significant reductions in HRV (Henry et al., 2012). Persistently reduced HRV indicates impaired vagal function and is linked to increased arousal in response to stress, compromised implementation of adaptive coping strategies, cardiovascular disease, and all-cause mortality (Appelhans & Luecken, 2006; Kemp, Koenig, & Thayer, 2017). The chronicity of MUD in our sample may indicate more chronically reduced HRV and, thus, resistance to significant oxytocin-induced changes in HRV. Overall, the effects of oxytocin administration on HRV in METH-using populations is likely to be more complicated than in previously studied healthy participants (Kemp et al., 2012; Kubzansky et al., 2012; Norman, Cacioppo, Morris, Malarkey, et al., 2011; Riem et al., 2020; Romney et al., 2019; Tracy et al., 2018). Further investigation is warranted and current models of the psychophysiology of stress (Hughes, Steffen, & Thayer, 2018; Porges, 2011) should guide this investigation.
Research on the clinical benefits of intranasal oxytocin is known to suffer from methodological concerns, such as replication issues (Lane et al., 2015) and a lack of consensus on optimal strategies for delivery and course of treatment (Erdozain & Penagarikano, 2019; Leng & Ludwig, 2016). We chose a standard oxytocin dosage for the field, but future research would benefit from dose-finding studies, particularly in METH users, given the robust effects of METH in the opposite direction from the effects of oxytocin. Furthermore, the question of central penetration of oxytocin via the intranasal route remains controversial. However, peripheral oxytocin levels are known to increase to supra-physiologic levels after intranasal delivery (Leng & Ludwig, 2016). It remains unclear, then, whether our findings were the result of diffuse peripheral effects of oxytocin alone or if they were, at least partially, due to more targeted and centrally mediated oxytocin effects. Additionally, previous evidence suggests that oxytocin administration may lead to different outcomes based on the gender (Hoge et al., 2014) and sexual orientation (Thienel et al., 2014) of recipients; thus, our results may not generalize beyond MSM. Finally, participants receiving oxytocin experienced significantly more nasal irritation, which may have impacted blinding. Despite these challenges, our understanding of how best to utilize oxytocin in a clinical setting continues to evolve.
The current study had several additional limitations worth mentioning. First, heart rate is a nonspecific marker of arousal, and the sample rate (250 Hz) of our cardiac physiology measurement was relatively low for HRV analysis (Appelhans & Luecken, 2006), making our psychophysiological findings difficult to interpret. Second, METH use, versus abstinence, was unequally distributed between the treatment arms; although, we did control for this in our analyses. Third, participants received most of their compensation at the end of session 6, which was a design element meant to enhance retention; consequently, this may have influenced attendance at session 6 and rates of non-completion, which were similar for those receiving oxytocin compared to placebo. Last, due to our limited sample size, we did not take interdependence resulting from MIGT cohort membership into account in our analyses.
In conclusion, this is the first trial investigating oxytocin administration in human participants using METH. Oxytocin administration improved treatment engagement while also countering the effects of METH on cardiac physiology during group therapy sessions. While our time-limited, six-week intervention did not change METH use in this relatively small sample, oxytocin administration may improve retention in future MUD research, introducing the potential to boost internal validity and facilitate the development of new treatments. Future studies might also investigate the effects of oxytocin-psychotherapy combination on treatment retention in additional clinical populations and settings where attrition is known to be problematically high. Further analyses might assess individual factors (e.g., childhood trauma, attachment insecurity) that have been shown to moderate the effects of intranasal oxytocin (Olff et al., 2013; Riem et al., 2020) and analyze the effects of oxytocin on objective measures of group cohesiveness and synchrony (Gonzales, Hancock, & Pennebaker, 2010; McCraty, 2017). Based on promising preexisting animal data and results from the current clinical trial, larger trials of the effects of OE-MIGT on METH use in humans are certainly warranted.
Supplementary Material
Acknowledgements
We acknowledge the support of additional study therapists: Jennifer Hagstrom, MD; Nicole Amaro, PsyD; and Gabrielle Agin-Liebes, PhD; and additional research assistants: Salem Samson and Harleen Mangat. We would like to express our gratitude for the research participants, who opened their hearts and shared their stories to advance scientific investigation into new treatments for addiction.
Funding
This study was primarily funded by the UCSF Academic Senate via the UCSF Resource Allocation Program, Pilot Award Program in HIV/ AIDS. Additional salary support for CSS was provided by the Department of Veterans Affairs, Clinical Science Research and Development, Federal Award Identification Number IK2CX001495. JN was supported by the VISN 21 Sierra-Pacific Mental Illness Education and Clinical Center. BB's contribution is the result of work supported with resources and the use of facilities at the San Francisco Veterans Affairs Medical Center and the Northern California Institute for Research and Education. None of the funding bodies had any influence on the trial. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Veterans Affairs or the United States Government.
Abbreviations:
- ECG
electrocardiogram
- GEE
generalized estimating equations
- HRV
heart rate variability
- ICC
intra-class correlation
- LMEM
linear mixed effects models
- MI
motivational interviewing
- MIGT
motivational interviewing group therapy
- MINI
Mini International Neuropsychiatric Interview
- MITI
Motivational Interviewing Treatment Integrity Coding Manual
- METH
methamphetamine
- MSM
men who have sex with men
- MUD
methamphetamine use disorder
- OE-MIGT
oxytocin-enhanced motivational interviewing group therapy
- RMSSD
root mean square of successive differences
- RSA
respiratory sinus arrhythmia
- SCID-5
Structured Clinical Interview for DSM-5
- STAI-6
State-Trait Anxiety Inventory Short Form
- UCSF
University of California, San Francisco
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Appelhans BM, & Luecken LJ (2006). Heart rate variability as an index of regulated emotional responding. Review of General Psychology, 10(3), 229–240. 10.1037/1089-2680.10.3.229. [DOI] [Google Scholar]
- Baker A, & Lee NK (2003). A review of psychosocial interventions for amphetamine use. Drug and Alcohol Review, 22(3), 323–335. 10.1080/0959523031000154472. [DOI] [PubMed] [Google Scholar]
- Baracz SJ, & Cornish JL (2016). The neurocircuitry involved in oxytocin modulation of methamphetamine addiction. Frontiers in Neuroendocrinology, 43, 1–18. 10.1016/j.yfrne.2016.08.001. [DOI] [PubMed] [Google Scholar]
- Baracz SJ, Everett NA, & Cornish JL (2015). The involvement of oxytocin in the subthalamic nucleus on relapse to methamphetamine-seeking behaviour. PLoS One, 10(8), Article e0136132 10.1371/journal.pone.0136132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baracz SJ, Everett NA, McGregor IS, & Cornish JL (2016). Oxytocin in the nucleus accumbens core reduces reinstatement of methamphetamine-seeking behaviour in rats. Addiction Biology, 21(2), 316–325. 10.1111/adb.12198. [DOI] [PubMed] [Google Scholar]
- Baracz SJ, Rourke PI, Pardey MC, Hunt GE, McGregor IS, & Cornish JL (2012). Oxytocin directly administered into the nucleus accumbens core or subthalamic nucleus attenuates methamphetamine-induced conditioned place preference. Behavioural Brain Research, 228(1), 185–193. 10.1016/j.bbr.2011.11.038. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, & Quigley KS (1993). Respiratory sinus arrhythmia: Autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology, 30(2), 183–196. 10.1111/j.1469-8986.1993.tb01731.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Quigley KS, Jang JF, & Boysen ST (1990). An approach to artifact identification: Application to heart period data. Psychophysiology, 27(5), 586–598. 10.1111/j.1469-8986.1990.tb01982.x. [DOI] [PubMed] [Google Scholar]
- Bhatt M, Zielinski L, Baker-Beal L, Bhatnagar N, Mouravska N, Laplante P, … Samaan Z (2016). Efficacy and safety of psychostimulants for amphetamine and methamphetamine use disorders: A systematic review and meta-analysis. Systematic Reviews, 5(1), 189 10.1186/s13643-016-0370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwby J (1969). Attachment and loss Vol I: Attachment. New York: Basic. [Google Scholar]
- Brecht ML, & Herbeck D (2014). Time to relapse following treatment for methamphetamine use: A long-term perspective on patterns and predictors. Drug Alcohol Dependence, 139, 18–25. 10.1016/j.drugalcdep.2014.02.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Ball SA, Nich C, Martino S, Frankforter TL, Farentinos C, … Network, N. I. o. D. A. C. T (2006). Motivational interviewing to improve treatment engagement and outcome in individuals seeking treatment for substance abuse: A multisite effectiveness study. Drug Alcohol Dependence, 81(3), 301–312. 10.1016/j.drugalcdep.2005.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson DS, Cornish JL, Guastella AJ, Hunt GE, & McGregor IS (2010). Oxytocin decreases methamphetamine self-administration, methamphetamine hyperactivity, and relapse to methamphetamine-seeking behaviour in rats. Neuropharmacology, 58(1), 38–43. 10.1016/j.neuropharm.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Carson DS, Hunt GE, Guastella AJ, Barber L, Cornish JL, Arnold JC, … McGregor IS (2010). Systemically administered oxytocin decreases methamphetamine activation of the subthalamic nucleus and accumbens core and stimulates oxytocinergic neurons in the hypothalamus. Addiction Biology, 15(4), 448–463. 10.1111/j.1369-1600.2010.00247.x. [DOI] [PubMed] [Google Scholar]
- Carter CS (2017). The role of oxytocin and vasopressin in attachment. Psychodyn Psychiatry, 45(4), 499–517. 10.1521/pdps.2017.45.4.499. [DOI] [PubMed] [Google Scholar]
- Cicchetti DV (1994). Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychological Assessment, 6(4), 284. [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences. Hillsdale, N.J: L. Erlbaum Associates. [Google Scholar]
- Cook R, Quinn B, Heinzerling K, & Shoptaw S (2017). Dropout in clinical trials of pharmacological treatment for methamphetamine dependence: The role of initial abstinence. Addiction, 112(6), 1077–1085. 10.1111/add.13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan FM, Fisher JJ, & Nutt DJ (2011). Autonomic dysregulation and the Window of Tolerance model of the effects of complex emotional trauma. Journal of Psychopharmacology, 25(1), 17–25. 10.1177/0269881109354930. [DOI] [PubMed] [Google Scholar]
- Cox BM, Bentzley BS, Regen-Tuero H, See RE, Reiehel CM, & Aston-Jones G (2017). Oxytocin acts in nucleus accumbens to attenuate methamphetamine seeking and demand. Biological Psychiatry, 81(11), 949–958. 10.1016/j.biopsych.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Dreu CK (2012). Oxytocin modulates cooperation within and competition between groups: An integrative review and research agenda. Hormones and Behavior, 61(3), 419–428. 10.1016/j.yhbeh.2011.12.009. [DOI] [PubMed] [Google Scholar]
- De Dreu CK, Shalvi S, Greer LL, Van Kleef GA, & Handgraaf MJ (2012). Oxytocin motivates non-cooperation in intergroup conflict to protect vulnerable in-group members. PLoS One, 7(11), Article e46751 10.1371/journal.pone.0046751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolezal BA, Chudzynski J, Dickerson D, Mooney L, Rawson RA, Garfinkel A, & Cooper CB (2014). Exercise training improves heart rate variability after methamphetamine dependency. Medicine and Science in Sports and Exercise, 46(6), 1057–1066. 10.1249/mss.0000000000000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddie D, Kim C, Lehrer P, Deneke E, & Bates ME (2014). A pilot study of brief heart rate variability biofeedback to reduce craving in young adult men receiving inpatient treatment for substance use disorders. Applied Psychophysiology and Biofeedback, 39(3–4), 181–192. 10.1007/s10484-014-9251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbogen M, Cardoso C, Serravalle L, & Virginia T (2018). T142. Intranasal oxytocin augments the efficacy of psychotherapy for major depressive disorder. Biological Psychiatry, 83(9), Article S183 10.1016/j.biopsych.2018.02.479. [DOI] [Google Scholar]
- Erdozain AM, & Penagarikano O (2019). Oxytocin as treatment for social cognition, not there yet. Frontiers in Psychiatry, 10, 930 10.3389/fpsyt.2019.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett N, Baracz S, & Cornish J (2019). Oxytocin treatment in the prelimbic cortex reduces relapse to methamphetamine-seeking and is associated with reduced activity in the rostral nucleus accumbens core. Pharmacology, Biochemistry, and Behavior, 183, 64–71. 10.1016/j.pbb.2019.06.002. [DOI] [PubMed] [Google Scholar]
- Everett NA, Baracz SJ, & Cornish JL (2020). The effect of chronic oxytocin treatment during abstinence from methamphetamine self-administration on incubation of craving, reinstatement, and anxiety. Neuropsychopharmacology, 45(4), 597–605. 10.1038/s41386-019-0566-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, … Marks JS (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medicine, 14(4), 245–258. 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- First MB, Williams JB, Karg RS, & Spitzer RL (2016). Structured clinical interview for DSM-5 disorders: SCID-5-CV clinician version. American Psychiatric Association Publishing. [Google Scholar]
- Flanagan JC, Baker NL, McRae-Clark AL, Brady KT, & Moran-Santa Maria MM (2015). Effects of adverse childhood experiences on the association between intranasal oxytocin and social stress reactivity among individuals with cocaine dependence. Psychiatry Research, 229(1–2), 94–100. 10.1016/j.psychres.2015.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan JC, Sippel LM, Wahlquist A, Moran-Santa Maria MM, & Back SE (2018). Augmenting Prolonged Exposure therapy for PTSD with intranasal oxytocin: A randomized, placebo-controlled pilot trial. Journal of Psychiatric Research, 98, 64–69. 10.1016/j.jpsychires.2017.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales AL, Hancock JT, & Pennebaker JW (2010). Language style matching as a predictor of social dynamics in small groups. Communication Research, 37(1), 3–19. 10.1177/0093650209351468. [DOI] [Google Scholar]
- Guastella AJ, Hickie IB, McGuinness MM, Otis M, Woods EA, Disinger HM, … Banati RB (2013). Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology, 38(5), 612–625. https://doi.Org/10.1016/j.psyneuen.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Gutkowska J, Jankowski M, & Antunes-Rodrigues J (2014). The role of oxytocin in cardiovascular regulation. Brazilian Journal of Medical and Biological Research, 47, 206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren KA (2012). Computing inter-rater reliability for observational data: An overview and tutorial. Tutor Quant Methods Psychol, 8(1), 23–34. 10.20982/tqmp.08.1.p023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan SF, Wearne TA, Cornish JL, & Goodchild AK (2016). Effects of acute and chronic systemic methamphetamine on respiratory, cardiovascular and metabolic function, and cardiorespiratory reflexes. The Journal of Physiology, 594(3), 763–780. 10.1113/jp271257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heather N, & Hönekopp J (2008). A revised edition of the Readiness to Change Questionnaire [treatment version]. Addiction Research and Theory, 16(5), 421–433. 10.1080/16066350801900321. [DOI] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, & Ehlert U (2003). Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry, 54(12), 1389–1398. 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Henry BL, Minassian A, & Perry W (2012). Effect of methamphetamine dependence on heart rate variability. Addiction Biology, 17(3), 648–658. 10.1111/j.1369-1600.2010.00270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermanstyne KA, Santos GM, Vittinghoff E, Santos D, Colfax G, & Coffin P (2014). Event-level relationship between methamphetamine use significantly associated with non-adherence to pharmacologic trial medications in event-level analyses. Drug and Alcohol Dependence, 143, 277–280. 10.1016/j.drugalcdep.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks C, Cornish JL, Baracz SJ, Suraev A, & McGregor IS (2016). Adolescent pre-treatment with oxytocin protects against adult methamphetamine-seeking behavior in female rats. Addiction Biology, 21(2), 304–315. 10.1111/adb.12197. [DOI] [PubMed] [Google Scholar]
- Hoeppner BB, Stout RL, Jackson KM, & Barnett NP (2010). How good is finegrained Timeline Follow-back data? Comparing 30-day TLFB and repeated 7-day TLFB alcohol consumption reports on the person and daily level. Addictive Behaviors, 35(12), 1138–1143. 10.1016/j.addbeh.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge EA, Anderson E, Lawson EA, Bui E, Fischer LE, Khadge SD, et al. (2014). Gender moderates the effect of oxytocin on social judgments. Human Psychopharmacology, 29(3), 299–304. 10.1002/hup.2402. [DOI] [PubMed] [Google Scholar]
- Homer BD, Solomon TM, Moeller RW, Mascia A, DeRaleau L, & Halkitis PN (2008). Methamphetamine abuse and impairment of social functioning: A review of the underlying neurophysiological causes and behavioral implications. Psychological Bulletin, 134(2), 301–310. 10.1037/0033-2909.134.2.301. [DOI] [PubMed] [Google Scholar]
- Hughes BM, Steffen PR, & Thayer JF (2018). The psychophysiology of stress and adaptation: Models, pathways, and implications. International Journal of Psychophysiology, 131, 1–3. 10.1016/j.ijpsycho.2018.06.003. [DOI] [PubMed] [Google Scholar]
- Insel TR (2003). Is social attachment an addictive disorder? Physiology & Behavior, 79(3), 351–357. 10.1016/s0031-9384(03)00148-3. [DOI] [PubMed] [Google Scholar]
- Kemp AH, & Guastella AJ (2010). Oxytocin: Prosocial behavior, social salience, or approach-related behavior? Biol Psychiatry, 67(6), 10.1016/j.biopsych.2009.11.019 (e33–34; author reply e35). [DOI] [PubMed] [Google Scholar]
- Kemp AH, Koenig J, & Thayer JF (2017). From psychological moments to mortality: A multidisciplinary synthesis on heart rate variability spanning the continuum of time. Neuroscience and Biobehavioral Reviews, 83, 547–567. 10.1016/j.neubiorev.2017.09.006. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Kuhnert RL, Griffiths K, Hickie IB, & Guastella AJ (2012). Oxytocin increases heart rate variability in humans at rest: Implications for social approach-related motivation and capacity for social engagement. PLoS One, 7(8), Article e44014 10.1371/journal.pone.0044014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimas J, Fairgrieve C, Tobin H, Field CA, O’Gorman CS, Glynn LG, … Cullen W (2018). Psychosocial interventions to reduce alcohol consumption in concurrent problem alcohol and illicit drug users. Cochrane Database Syst Rev, 12, Article Cd009269 10.1002/14651858.CD009269.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight R, Karamouzian M, Carson A, Edward J, Carrieri P, Shoveller J, … Fast D (2019). Interventions to address substance use and sexual risk among gay, bisexual and other men who have sex with men who use methamphetamine: A systematic review. Drug and Alcohol Dependence, 194, 410–429. 10.1016/j.drugalcdep.2018.09.023. [DOI] [PubMed] [Google Scholar]
- Knox J (1999). The relevance of attachment theory to a contemporary Jungian view of the internal world: Internal working models, implicit memory and internal objects. The Journal of Analytical Psychology, 44(4), 511–530. 10.1111/1465-5922.00117. [DOI] [PubMed] [Google Scholar]
- Krogel J, Burlingame G, Chapman C, Renshaw T, Gleave R, Beecher M, & Macnair-Semands R (2013). The Group Questionnaire: A clinical and empirically derived measure of group relationship. Psychotherapy Research, 23(3), 344–354. 10.1080/10503307.2012.729868. [DOI] [PubMed] [Google Scholar]
- Kubzansky LD, Mendes WB, Appleton AA, Block J, & Adler GK (2012). A heartfelt response: Oxytocin effects on response to social stress in men and women. Biological Psychology, 90(1), 1–9. 10.1016/j.biopsycho.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, & Christensen RHB (2017). ImerTest package: Tests in linear mixed effects models. Journal of Statistical Software, 82(13), 1–26. 10.18637/jss.v082.i13. [DOI] [Google Scholar]
- Lane A, Mikolajczak M, Treinen E, Samson D, Corneille O, de Timary P, & Luminet O (2015). Failed replication of oxytocin effects on trust: The envelope task case. PLoS One, 10(9), Article e0137000 10.1371/journal.pone.0137000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Rohn MC, Tanda G, & Leggio L (2016). Targeting the oxytocin system to treat addictive disorders: Rationale and Progress to date. CNS Drugs, 30(2), 109–123. 10.1007/s40263-016-0313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, & Weerts EM (2016). Oxytocin for the treatment of drug and alcohol use disorders. Behavioural Pharmacology, 27(8), 640–648. 10.1097/fbp.0000000000000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G, & Ludwig M (2016). Intranasal oxytocin: Myths and delusions. Biological Psychiatry, 79(3), 243–250. 10.1016/j.biopsych.2015.05.003. [DOI] [PubMed] [Google Scholar]
- MacDonald E, Dadds MR, Brennan JL, Williams K, Levy F, & Cauchi AJ (2011). A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research. Psychoneuroendocrinology, 36(8), 1114–1126. 10.1016/j.psyneuen.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Maglione M, Chao B, & Anglin MD (2000). Correlates of outpatient drug treatment drop-out among methamphetamine users. Journal of Psychoactive Drugs, 32(2), 221–228. 10.1080/02791072.2000.10400232. [DOI] [PubMed] [Google Scholar]
- McCraty R (2017). New Frontiers in heart rate variability and social coherence research: Techniques, technologies, and implications for improving group dynamics and outcomes. Frontiers in Public Health, 5, 267 10.3389/fpubh.2017.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon RC, Kouzekanani K, & Malow RM (1999). A comparative study of cocaine-treatment completers and dropouts. Journal of Substance Abuse Treatment, 16(1), 17–22. https://doi.org/10.1016A0740-5472(97)00317-6. [DOI] [PubMed] [Google Scholar]
- Miller WR, & Rollnick S (1991). Motivational interviewing: Preparing people to change addictive behavior. New York, NY, US: The Guilford Press. [Google Scholar]
- Moher D, Schulz KF, & Altman DG (2001). The CONSORT statement: Revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet, 357(9263), 1191–1194. [PubMed] [Google Scholar]
- Moos RH, Finney JW, Federman EB, & Suchinsky R (2000). Specialty mental health care improves patients’ outcomes: Findings from a nationwide program to monitor the quality of care for patients with substance use disorders. J Stud Alcohol, 61(5), 704–713. 10.15288/jsa.2000.61.704. [DOI] [PubMed] [Google Scholar]
- Morley KC, Cornish JL, Faingold A, Wood K, & Haber PS (2017). Pharmacotherapeutic agents in the treatment of methamphetamine dependence. Expert Opinion on Investigational Drugs, 26(5), 563–578. 10.1080/13543784.2017.1313229. [DOI] [PubMed] [Google Scholar]
- Moyers T, Manuel J, Ernst D, Moyers T, Manuel J, Ernst D, … Fortini C (2014). Motivational interviewing treatment integrity coding manual 4.1 (MITI 4.1). Unpublished manual.
- Norman GJ, Cacioppo JT, Morris JS, Karelina K, Malarkey WB, Devries AC, & Berntson GG (2011). Selective influences of oxytocin on the evaluative processing of social stimuli. Journal of Psychopharmacology, 25(10), 1313–1319. 10.1177/0269881110367452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman GJ, Cacioppo JT, Morris JS, Malarkey WB, Berntson GG, & Devries AC (2011). Oxytocin increases autonomic cardiac control: Moderation by loneliness. Biological Psychology, 86(3), 174–180. 10.1016/j.biopsycho.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Northrup TF, Green C, Walker R, Greer TL, & Trivedi MH (2015). On the invariance of the Stimulant Craving Questionnaire (STCQ) across cocaine and methamphetamine users. Addictive Behaviors, 42, 144–147. 10.1016/j.addbeh.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden P, Minton K, & Pain C (2006). Trauma and the body: A sensorimotor approach to psychotherapy. New York, NY, US: W W Norton & Co. [Google Scholar]
- Olff M, Frijling JL, Kubzansky LD, Bradley B, Ellenbogen MA, Cardoso C, … van Zuiden M (2013). The role of oxytocin in social bonding, stress regulation and mental health: An update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology, 38(9), 1883–1894. 10.1016/j.psyneuen.2013.06.019. [DOI] [PubMed] [Google Scholar]
- Parsons JT, Lelutiu-Weinberger C, Botsko M, & Golub SA (2014). A randomized controlled trial utilizing motivational interviewing to reduce HIV risk and drug use in young gay and bisexual men. Journal of Consulting and Clinical Psychology, 82(1), 9–18. 10.1037/a0035311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payer DE, Lieberman MD, Monterosso JR, Xu J, Fong TW, & London ED (2008). Differences in cortical activity between methamphetamine-dependent and healthy individuals performing a facial affect matching task. Drug and Alcohol Dependence, 93(1–2), 93–102. 10.1016/j.drugalcdep.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges S (2011). The polyvagal theory: Neurophysiological foundations of emotions, attachment, communication and self-regulation. New York: Norton. [Google Scholar]
- Qi J, Yang JY, Song M, Li Y, Wang F, & Wu CF (2008). Inhibition by oxytocin of methamphetamine-induced hyperactivity related to dopamine turnover in the mesolimbic region in mice. Naunyn-Schmiedeberg’s Archives of Pharmacology, 376(6), 441–448. 10.1007/s00210-007-0245-8. [DOI] [PubMed] [Google Scholar]
- Qi J, Yang JY, Wang F, Zhao YN, Song M, & Wu CF (2009). Effects of oxytocin on methamphetamine-induced conditioned place preference and the possible role of glutamatergic neurotransmission in the medial prefrontal cortex of mice in reinstatement. Neuropharmacology, 56(5), 856–865. 10.1016/j.neuropharm.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Riem MME, Kunst LE, Bekker MHJ, Fallon M, & Kupper N (2020). Intranasal oxytocin enhances stress-protective effects of social support in women with negative childhood experiences during a virtual Trier Social Stress Test. Psychoneuroendocrinology, 111, 104482 10.1016/j.psyneuen.2019.104482. [DOI] [PubMed] [Google Scholar]
- Romney C, Hahn-Holbrook J, Norman GJ, Moore A, & Holt-Lunstad J (2019). Where is the love? A double-blind, randomized study of the effects of intranasal oxytocin on stress regulation and aggression. International Journal of Psychophysiology, 136, 15–21. 10.1016/j.ijpsycho.2018.08.010. [DOI] [PubMed] [Google Scholar]
- SAMHSA (2019). 2018 National Survey on Drug Use and Health: Methodological summary and definitions. MD: Retrieved from Rockville, https://www.samhsa.gov/data/. [Google Scholar]
- Sarnyai Z, & Kovacs GL (2014). Oxytocin in learning and addiction: From early discoveries to the present. Pharmacology, Biochemistry, and Behavior, 119, 3–9. 10.1016/j.pbb.2013.11.019. [DOI] [PubMed] [Google Scholar]
- Schindler A (2019). Attachment and substance use disorders-theoretical models, empirical evidence, and implications for treatment. Frontiers in Psychiatry, 10, 727 10.3389/fpsyt.2019.00727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbach V, Lenk K, & Laufs U (2020). Methamphetamine-related cardiovascular diseases. ESC Heart Fail, 10.1002/ehf2.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SFDPH (2019). San Francisco Methamphetamine Task Force: Final report. Retrieved from https://www.sfdph.org/dph/comupg/knowlcol/MethTaskForce/default.asp.
- Shaffer F, & Ginsberg JP (2017). An overview of heart rate variability metrics and norms. Frontiers in Public Health, 5, 258 10.3389/fpubh.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, & Abu-Akel A (2016). The social salience hypothesis of oxytocin. Biological Psychiatry, 79(3), 194–202. 10.1016/j.biopsych.2015.07.020. [DOI] [PubMed] [Google Scholar]
- Shearer J (2007). Psychosocial approaches to psychostimulant dependence: A systematic review. Journal of Substance Abuse Treatment, 32(1), 41–52. 10.1016/j.jsat.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Sheehan D, Janavs J, Baker R, Sheehan K, Knapp E, & Sheehan M (2015). Mini international neuropsychiatric interview-version 7.0. 0 DSM-5. 2014. In. [Google Scholar]
- Sippel LM, Allington CE, Pietrzak RH, Harpaz-Rotem I, Mayes LC, & Olff M (2017). Oxytocin and stress-related disorders: Neurobiological mechanisms and treatment opportunities. Chronic Stress (Thousand Oaks), 1 10.1177/2470547016687996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueland L, Crits-Christoph P, Gallop R, Barber JP, Griffin ML, Thase ME, … Gladis M (2002). Retention in psychosocial treatment of cocaine dependence: Predictors and impact on outcome. The American Journal on Addictions, 11(1), 24–40. 10.1080/10550490252801611. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Brown J, Leo GI, & Sobell MB (1996). The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug and Alcohol Dependence, 42(1), 49–54. 10.1016/0376-8716(96)01263-x. [DOI] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch R, Lushene R, Vagg P, & Jacobs G (1983). Consulting Psychologists Press; Palo Alto, CA: 1983. (Manual for the state-trait anxiety inventor). [Google Scholar]
- Stauffer CS, Moschetto JM, McKernan SM, Hsiang E, Borsari B, & Woolley JD (2019). Oxytocin-enhanced motivational interviewing group therapy for methamphetamine use disorder in men who have sex with men: Study protocol for a randomized controlled trial. Trials, 20(1), 145 10.1186/s13063-019-3225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer CS, Musinipally V, Suen A, Lynch KL, Shapiro B, & Woolley JD (2016). A two-week pilot study of intranasal oxytocin for cocaine-dependent individuals receiving methadone maintenance treatment for opioid use disorder. Addiction Research and Theory, 24(6), 490–498. 10.3109/16066359.2016.1173682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens A, Radcliffe P, Sanders M, & Hunt N (2008). Early exit: Estimating and explaining early exit from drug treatment. Harm Reduction Journal, 5, 13 10.1186/1477-7517-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SB, Lewis CR, & Olive MF (2013). The neurocircuitry of illicit psychostimulant addiction: Acute and chronic effects in humans. Substance Abuse and Rehabilitation, 4, 29–43. 10.2147/sar.S39684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Velden FS, Daughters K, & De Dreu CKW (2017). Oxytocin promotes intuitive rather than deliberated cooperation with the in-group. Hormones and Behavior, 92, 164–171. 10.1016/j.yhbeh.2016.06.005. [DOI] [PubMed] [Google Scholar]
- Thienel M, Heinrichs M, Fischer S, Ott V, Born J, & Hallschmid M (2014). Oxytocin’s impact on social face processing is stronger in homosexual than heterosexual men. Psychoneuroendocrinology, 39, 194–203. 10.1016/j.psyneuen.2013.09.013. [DOI] [PubMed] [Google Scholar]
- Tonhajzerova I, Mestanik M, Mestanikova A, & Jurko A (2016). Respiratory sinus arrhythmia as a non-invasive index of “brain-heart” interaction in stress. The Indian Journal of Medical Research, 144(6), 815–822. 10.4103/ijmr.IJMR_1447_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tops M, Koole SL, Ijzerman H, & Buisman-Pijlman FTA (2014). Why social attachment and oxytocin protect against addiction and stress: Insights from the dynamics between ventral and dorsal corticostriatal systems. Pharmacology, Biochemistry, and Behavior, 119, 39–48. 10.1016/j.pbb.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Tracy LM, Gibson SJ, Labuschagne I, Georgiou-Karistianis N, & Giummarra MJ (2018). Intranasal oxytocin reduces heart rate variability during a mental arithmetic task: A randomised, double-blind, placebo-controlled cross-over study. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 81, 408–415. 10.1016/j.pnpbp.2017.08.016. [DOI] [PubMed] [Google Scholar]
- UNODC (August 2018). Methamphetamine continues to dominate synthetic drug markets.
- Volkow ND (2020). Personalizing the treatment of substance use disorders. The American Journal of Psychiatry, 177(2), 113–116. 10.1176/appi.ajp.2019.19121284. [DOI] [PubMed] [Google Scholar]
- Wagner CC, & Ingersoll KS (2013). Motivational interviewing in groups. Guilford Press. [Google Scholar]
- Wei M, Russell DW, Mallinckrodt B, & Vogel DL (2007). The Experiences in Close Relationship scale (ECR)-short form: Reliability, validity, and factor structure. Journal of Personality Assessment, 88(2), 187204 10.1080/00223890701268041. [DOI] [PubMed] [Google Scholar]
- Weinrich JD (1990). The Kinsey Scale in biology, with a note on Kinsey as a biologist McWhirter DP, Sander- SA, and Reinisch JM (eds) homosexuality: Concepts of sexual orientation. Oxford University: Press. [Google Scholar]
- Zhang H, Gross J, De Dreu C, & Ma Y (2019). Oxytocin promotes coordinated outgroup attack during intergroup conflict in humans. Elife, 8 10.7554/eLife.40698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zule WA, Poulton WE, Coomes CM, Mansergh G, Charania M, Wechsberg WM, & Jones HE (2012). Results of a pilot study to reduce methamphetamine use and sexual risk behaviors among methamphetamine-using men who have sex with men (MSM) not currently in treatment. Journal of Psychoactive Drugs, 44(5), 351–358. 10.1080/02791072.2012.736794. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


