Abstract
Cardiovascular health in midlife is an established risk factor for cognitive function later in life. Knowing mechanisms of this association may allow preventative steps to be taken to preserve brain health and cognitive performance in older age. In this study, we investigated the association of the Framingham stroke-risk score, a validated multifactorial predictor of 10-year risk of stroke, with brain measures and cognitive performance in stroke-free individuals. We used a large (N = 800) longitudinal cohort of community-dwelling adults of the Whitehall II imaging sub-study with no obvious structural brain abnormalities, who had Framingham stroke risk measured five times between 1991 and 2013 and MRI measures of structural integrity, and cognitive function performed between 2012 and 2016 [baseline mean age 47.9 (5.2) years, range 39.7–62.7 years; MRI mean age 69.81 (5.2) years, range 60.3–84.6 years; 80.6% men]. Unadjusted linear associations were assessed between the Framingham stroke-risk score in each wave and voxelwise grey matter density, fractional anisotropy and mean diffusivity at follow-up. These analyses were repeated including socio-demographic confounders as well as stroke risk in previous waves to examine the effect of residual risk acquired between waves. Finally, we used structural equation modelling to assess whether stroke risk negatively affects cognitive performance via specific brain measures. Higher unadjusted stroke risk measured at each of the five waves over 20 years prior to the MRI scan was associated with lower voxelwise grey and white matter measures. After adjusting for socio-demographic variables, higher stroke risk from 1991 to 2009 was associated with lower grey matter volume in the medial temporal lobe. Higher stroke risk from 1997 to 2013 was associated with lower fractional anisotropy along the corpus callosum. In addition, higher stroke risk from 2012 to 2013, sequentially adjusted for risk measured in 1991–94, 1997–98 and 2002–04 (i.e. ‘residual risks’ acquired from the time of these examinations onwards), was associated with widespread lower fractional anisotropy, and lower grey matter volume in sub-neocortical structures. Structural equation modelling suggested that such reductions in brain integrity were associated with cognitive impairment. These findings highlight the importance of considering cerebrovascular health in midlife as important for brain integrity and cognitive function later in life (ClinicalTrials.gov Identifier: NCT03335696).
Keywords: Framingham stroke risk, cardiovascular health, brain health, structural brain integrity, cognition
Higher stroke-risk score over 20 years before the magnetic resonance imaging scan was associated with whole-brain grey matter volume and white matter microstructure in community-dwelling older adults. Structural equation models suggest that brain integrity was associated with cognitive function. Results highlight the need for targeting modifiable risk factors in midlife.
Graphical Abstract
Graphical Abstract.
Introduction
The Framingham stroke-risk score (FSRS) is a multifactorial predictor of 10-year risk of stroke (D’Agostino et al., 1994). It includes cardiovascular, metabolic and health measures and is routinely used in clinical practice to predict the risk of stroke and the need for prophylaxis. Higher FSRS is also linked with lower grey and white matter (WM) integrity in older age (Pase et al., 2018; Zsoldos et al., 2018), lower fractional anisotropy (FA) in late-onset depression (Allan et al., 2012), cerebral small-vessel disease progression and cognitive decline in middle-aged hypertensive patients (Uiterwijk et al., 2018), cognitive decline in the over 50s (Dregan et al., 2013) and poor cognition in older individuals even in the absence of Alzheimer’s biomarkers or neuropathology (Hohman et al., 2015). While individual vascular risk factors of stroke, such as hypertension, atrial fibrillation, diabetes, smoking and obesity in midlife, have been linked with an increased rate of progression of vascular brain injury (Allan et al., 2015), various brain measures (Debette et al., 2011) and poor cognitive performance (Dregan et al., 2013; Kaffashian et al., 2013b; Nishtala et al., 2018; Moran et al., 2019), the combined effects of particular vascular risk factors may expedite the process of cognitive decline (Dregan et al., 2013; Kaffashian et al., 2013a; Cox et al., 2019). For example, findings from Dregan et al. imply that, while cigarette smoking is categorically associated with global cognition, memory and executive function, age and duration of high systolic blood pressure levels have a cumulative, detrimental long-term effect on cognition over time. It has been recommended that interventions to limit cognitive decline should target multiple vascular risk factors rather than manage individual risk (Dregan et al., 2013). It is therefore imperative to understand if cerebrovascular health in midlife is important for brain integrity and cognitive function later in life.
The aim of this study was to assess whether Framingham stroke risk over a 20-year period before the magnetic resonance imaging (MRI) scan was associated with structural brain integrity measures, even after accounting for the effects of confounding variables, such as age (Uiterwijk et al., 2018). We investigated which grey and WM structures were affected by new stroke risk acquired between the study waves. We also examined whether FSRS-predicted cognitive performance was mediated by structural brain measures. Our hypothesis was that higher FSRS will be associated with lower cognitive performance and this association is mediated by lower structural brain measures.
Materials and methods
Participant characteristics
A total of 800 Whitehall II participants were randomly recruited from the 2012–13 wave of the study to take part in the Whitehall II imaging sub-study between April 2012 and December 2016 where they underwent multi-modal MRI scanning and neuropsychological testing (Filippini et al., 2014). At study inception (1985–88), the Whitehall II study included 10 308 British civil service workers aged 35–55 years (born between 1932 and 1955), of whom 6895 were men. Follow-up health examinations were conducted over the following 30 years, approximately every 5 years. The present analysis uses data from 1991 to 1994, 1997 to 1999, 2002 to 2004, 2007 to 2009 and 2012 to 2013. A total of 74% of participants who took part at the 2012–13 wave had reached or passed the statutory retirement age of 65 years. Ethical approval was obtained from the University of Oxford Medical Sciences Interdivisional Research Ethics Committee (Reference: MS IDREC-C1-2011-71) and the University College London Committee on the Ethics of Human Research (Reference: 85/0938). All participants provided informed written consent.
Inclusion/exclusion criteria
Detailed information is provided in the Supplementary Material. In brief, participants were excluded from analysis if they did not have an MRI scan, had obvious structural abnormalities, for example suggesting a stroke, or poor image quality that pre-processing and artefact correction could not fix, or had a missing FSRS at any wave.
MRI acquisition and analysis
T1-weighted, fluid-attenuated inversion recovery (a modified T2-weighted sequence) and diffusion-weighted MRI images were acquired at the Oxford Centre for Functional MRI of the Brain, Wellcome Centre for Integrative Neuroimaging. The first 550 participants were scanned on a 3-T Siemens MAGNETOM Verio (Erlangen, Germany) scanner with a 32-channel receive head coil (between April 2012 and December 2014), and due to a scanner upgrade, the last 250 participants were scanned on a 3-T Siemens MAGNETOM Prisma scanner with 64-channel receive head–neck coil (between July 2015 and December 2016) (for sequence parameters, see Supplementary Table 1). All images were processed and analysed using FMRIB Software Library v.6.0 tools (Smith et al., 2004) or FreeSurfer version 5.3. Full technical details are given in the Supplementary Material.
In brief, cortical atrophy was estimated by scaling the grey matter (GM) values for the total intracranial volume (GM + WM + CSF) resulting in percentage total GM volume. Hippocampal volume was also estimated. WM lesions appear brighter (hyperintense) on T2-weighted images and are attributed to degenerative changes in small, deep penetrating arteries (Wardlaw et al., 2013), cardiovascular risk factors and age (de Leeuw et al., 2001; Li et al., 2013). WM hyperintensities (WMHs) were automatically segmented on fluid -attenuated inversion recovery images (Griffanti et al., 2016). Voxelwise analysis of GM density was performed using FMRIB Software Library-VBM (Douaud et al., 2007), an optimized voxel-based morphometry protocol (Good et al., 2001). Diffusion tensor imaging quantifies the directionality and rate of diffusion of water molecules within different tissues and allows inferences about the structural integrity of WM tracts. When movement is anisotropic, such as in healthy myelinated fibres, diffusion is restricted perpendicular to the longitudinal axis of the fibre. We carried out voxelwise analysis of diffusion tensor data [FA and mean diffusivity (MD)] with tract-based spatial statistics.
Framingham stroke-risk score
The FSRS is a stroke-risk appraisal function that empirically relates cardiovascular risk factors to the probability of a stroke within 10 years (D’Agostino et al., 1994). The probability of stroke depends on an individual’s presence and level of risk factors and is expressed as a percentage score. Risk factors include cardiovascular health (systolic blood pressure, prior cardiovascular disease, atrial fibrillation, left ventricular hypertrophy and antihypertensive medication), diabetes mellitus, smoking habits, sex and age. The percentage risk score was computed using beta coefficients based on the Cox proportional hazards regression model in the Framingham study at each data wave (further details in the Supplementary Material).
Assessment of cognition and premorbid functioning
The Hopkins Verbal Learning Test-Revised (Brandt, 1991) and Test of Premorbid Functioning (Wechsler, 2011) were administered by a trained psychology graduate on the day of the MRI scan, prior to the scan (details in the Supplementary Material).
Assessment of confounding variables
Age (linear and quadratic term) at the time of scan, sex, ethnicity, education, employment grade and scanner type were used as confounding variables. Ethnicity was limited to white versus non-white. Education years were calculated as the difference between the age at which the participant commenced primary school and the age at which they first left full-time education. Socio-economic status was classified according to the occupation grade between 1985 and 1988: senior managers and administrators (highest grade), professionals and executives (middle grade) and clerical and support staff (lowest grade). Scanner model was defined as Siemens 3-T Verio versus Prisma.
Statistical analysis
Voxelwise general linear models were generated for the analysis of GM density, FA and MD data using ‘Randomise’ (Winkler et al., 2014), a permutation-based non-parametric statistical programme, running 5000 permutations and correcting for multiple comparisons across space with P < 0.05, using threshold-free cluster enhancement (Smith and Nichols, 2009). We used the Harvard–Oxford cortical and sub-cortical structural atlases for VBM and the John Hopkins University diffusion tensor imaging-based WM atlases for tract-based spatial statistics.
Model I: linear associations were assessed between FSRS in each of the five waves and voxelwise GM, and diffusion tensor imaging data, including MRI scanner type as a confounding variable.
Model II: Model I analyses were repeated including all confounding variables.
Model III: results for associations between FSRS at 2012–13 and voxelwise GM, FA and MD were repeated using scanner type and FSRS at 1991–93, 1997–99, 2002–03 or 2007–09 as confounders, to remove between-subject variability including age and sex and examine the effect of residual risk acquired over a 5-, 10-, 15- and 20-year period before the scan.
Model IV: scanner type, percentage GM and WMH volumes were entered as confounding variables to test whether the relationship between FSRS between 2012 and 2013 and lower FA was mediated by percentage GM (i.e. an estimate of cortical atrophy and presumed Wallerian degeneration) or WMH volume (i.e. presumed vascular lesion, as opposed to WM rarefication originating from GM loss).
Structural equation modelling
IBM SPSS Amos version 25 structural equation modelling software was employed to build additional models to test the hypothesis that stroke risk negatively affects cognitive performance via specific brain measures. Models were optimized by backwards removal of non-significant effects until all effects were significant. With the removal of each variable, the measures of goodness of fit improved. All available data in the covariance matrix were used.
Data availability
The study follows Medical Research Council data-sharing policies (https://www.mrc.ac.uk/research/policies-and-guidance-for-researchers/data-sharing/, 20 March 2020, date last accessed). Data will be accessible from the authors after 2019.
Results
Descriptive statistics
Participant exclusion/inclusion
VBM analysis was based on a final available sample of N = 566, tract-based spatial statistics on N = 548 and structural equation modelling on N = 775 (for details of exclusions, see Supplementary Material).
Socio-demographic variables
The mean participant age at the time of scan and during the previous study waves is listed in Table 1; 80.6% of participants were male, with on average 14 years of education, reflecting the demographics of the British Civil Service at recruitment to the Whitehall II study in 1985 (Table 2). Both the mean and range of FSRSs increased with time, on average by 2.5% every 5 years. Participants scored M = 27 (4.7 SD) out of 36 on total Hopkins Verbal Learning Test memory recall and M = 9 (2.7 SD) out of 12 on delayed memory recall (Table 3).
Table 1.
Mean follow-up time between study waves and participant age at each wave
| 1991–93 | 1997–99 | 2003–04 | 2007–09 | 2012–13 | MRI scan: 2012–16 | |
|---|---|---|---|---|---|---|
| Time to scan (years): Mean (SD), Range | 22 (1.4) 18–25 | 16 (1.4) 13–19 | 10 (1.4) 7–13 | 5 (1.4) 3–8 | 1 (1.3) 0–4 | NA |
| Age (years): Mean (SD), Range | 47.9 (5.2) 39.7–62.7 | 53.6 (5.2) 45.3–67.5 | 59.1 (5.2) 50.5–72.6 | 64.0 (5.2) 55.6–77.8 | 68.1 (5.2) 59.8–81.8 | 69.8 (5.2) 60.3–84.6 |
| Age (years): Median | 46.7 | 52.4 | 58.0 | 62.9 | 66.9 | 68.8 |
NA = not applicable; SD = standard deviation.
Table 2.
Sociodemographic characteristics
| N | Verio and Prisma | N | Verio sample | N | Prisma sample | N | VBM analysis | N | TBSS analysis | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age (years): Mean (SD), Range | 775 | 69.8 (5.2), 60.3–84.6 | 552 | 69.5 (5.3), 60.3–83.0 | 223 | 70.6 (4.8), 63.2–84.6 | 566 | 69.9 (5.2), 60.3–84.6 | 548 | 69.9 (5.2), 60.3–84.6 |
| Sex: N (%), male | 775 | 625 (80.6) | 552 | 444 (80.4) | 223 | 181 (81.2) | 566 | 450 (79.5) | 548 | 435 (79.4) |
| Ethnicity: | ||||||||||
| White N (%) | 775 | 733 (94.6) | 552 | 516 (93.5) | 223 | 217 (97.3) | 566 | 534 (94.3) | 548 | 517 (94.3) |
| Occupation: N (%) | ||||||||||
| Administrative (highest) | 775 | 320 (42.4) | 552 | 234 (42.4) | 223 | 86 (38.6) | 566 | 230 (40.6) | 548 | 220 (40.1) |
| Professional/executive | 775 | 399 (52.9) | 552 | 273 (49.5) | 223 | 126 (56.5) | 566 | 292 (51.6) | 548 | 286 (52.2) |
| Clerical/support (lowest) | 775 | 56 (7.4) | 552 | 45 (8.2) | 223 | 11 (4.9) | 566 | 44 (7.7) | 548 | 42 (7.6) |
| Education (years): Mean (SD), Range | 775 | 14.05 (3.1), 6–23 | 552 | 14.00 (3.1), 6–23 | 223 | 14.20 (3.05), 6–22 | 566 | 13.97 (3.01), 6–23 | 548 | 14.01 (3.0), 6–23 |
| Framingham stroke risk (%): Mean (SD), Range | ||||||||||
| 1991–94 | 709 | 3.32 (1.4), 1–13 | 502 | 3.32 (1.4), 1–13 | 207 | 3.29 (1.4), 1–13 | 566 | 3.27 (1.4), 1–13 | 548 | 3.26 (1.4), 1–13 |
| 1997–99 | 684 | 3.87 (2.3), 1–20 | 486 | 3.88 (2.3), 1–20 | 198 | 3.83 (2.2), 1–17 | 566 | 3.87 (2.3), 1–20 | 548 | 3.84 (2.3), 1–20 |
| 2002–04 | 718 | 5.35 (4.3), 1–52 | 508 | 5.50 (4.4), 1–52 | 210 | 5.00 (4.2), 1–37 | 566 | 5.29 (4.0), 1–29 | 548 | 5.18 (3.8), 1–29 |
| 2007–09 | 735 | 6.50 (4.9), 1–52 | 522 | 6.75 (5.2), 1–52 | 213 | 5.9 (3.92), 1–26 | 566 | 6.55 (5.0), 1–52 | 548 | 6.39 (4.5), 1–37 |
| 2012–13 | 743 | 8.50 (6.2), 1–64 | 524 | 8.76 (6.6), 1–52 | 219 | 7.89 (5.2), 1–29 | 566 | 8.59 (6.5), 1–64 | 548 | 8.50 (6.4), 1–64 |
SD = standard deviation; TBSS = tract-based spatial statistics.
Table 3.
Memory performance and segmented brain values of the Verio and Prisma samples
| Verio and Prisma samples |
Verio sample |
Prisma sample |
||||
|---|---|---|---|---|---|---|
| N | Mean (SD), range | N | Mean (SD), range | N | Mean (SD), range | |
| HVLT-R (total recall) | 775 | 27.4 (4.7), 10–36 | 552 | 27.5 (4.8), 10–36 | 223 | 27.2 (4.4), 15–35 |
| HVLT-R (delayed recall) | 775 | 9.2 (2.7), 0–12 | 552 | 9.2 (2.8), 0–12 | 223 | 9.1 (2.6), 0–12 |
| Right hippocampusa (mm3) | 773 | 3700 (525), 1370–5412 | 550 | 3580 (493), 1370–5064 | 223 | 3998 (484), 2782–5412 |
| Left hippocampusa (mm3) | 773 | 3651 (485), 1996–5305 | 550 | 3580 (468), 1996–5150 | 223 | 3826 (483), 2292–5305 |
| Total intracranial volumea (mm3) | 773 | 1 589 563 (205 927), 860 447—2 242 966 | 550 | 1 656 169 (172 573), 860 447–2 242 966 | 223 | 1 425 286 (188 990), 914 417–1 926 628 |
| Cerebrospinal fluidb (mm3) | 771 | 349 903 (64 992), 172 836–610 075 | 550 | 330 289 (56 983), 172 836–610 075 | 221 | 398 717 (57 667), 280 711–580 161 |
| Grey matterb (mm3) | 771 | 557 938 (48 097), 415 276–707 896 | 550 | 552 412 (46 067), 415 276–707 896 | 221 | 571 690 (50 343), 452 639–703 112 |
| White matterb (mm3) | 771 | 551 874 (59 735), 376 172–776 406 | 550 | 558 713 (60 122), 377 965–776 406 | 221 | 534 855 (55 325), 376 172–681 453 |
| Intracranial volumeb (mm3) | 771 | 1 459 718 (135 542), 1 023 552–1 927 776 | 550 | 1 441 418 (130 870), 1 023 552–197 776 | 221 | 1 505 262 (136 529), 1 118 270–1 798 853 |
| Cortical atrophy (%)b (mm3) | 771 | 38.3 (2.0), 28.9–44.5 | 550 | 38.4 (2.0), 28.9–44.5 | 221 | 38.0 (2.0), 30.4–42.6 |
| White matter hyperintensity volumec (mm3) | 770 | 0.46 (0.3), 0.08–2.47 | 549 | 0.42 (0.3), 0.08–2.5 | 221 | 0.55 (0.3), 0.27–2.34 |
FreeSurfer.
FAST.
BIANCA.
HVLT-R = Hopkins Verbal Learning Test-Revise; SD = standard deviation.
Voxel-based morphometry
Model I: the FSRS in each of the five waves, even as early as 20 years preceding the MRI scan, was associated with widespread lower GM density at follow-up, across the cortex bilaterally, including higher cortical areas, the operculum and insular cortex, occipital cortex and medial temporal lobes (MTLs) (Fig. 1). Overall, FSRS measured in the earlier waves (at younger mean age) was associated with lower GM in as large a number of voxels, as risk measured at mean age 68 (5.2 SD) years. Peak cluster locations were in the right MTL and the amygdala [1991–94: t (563) = 3.27, P < 0.001; 2002–04: t (563) = 3.24, P < 0.001; 2007–09: t (563) = 3.38, P < 0.001], right caudate [1997–99: t (563) = 3.36, P < 0.001] and left central opercular cortex [2012–13: t (563) = 3.29, P < 0.001, see Supplementary Table 2].
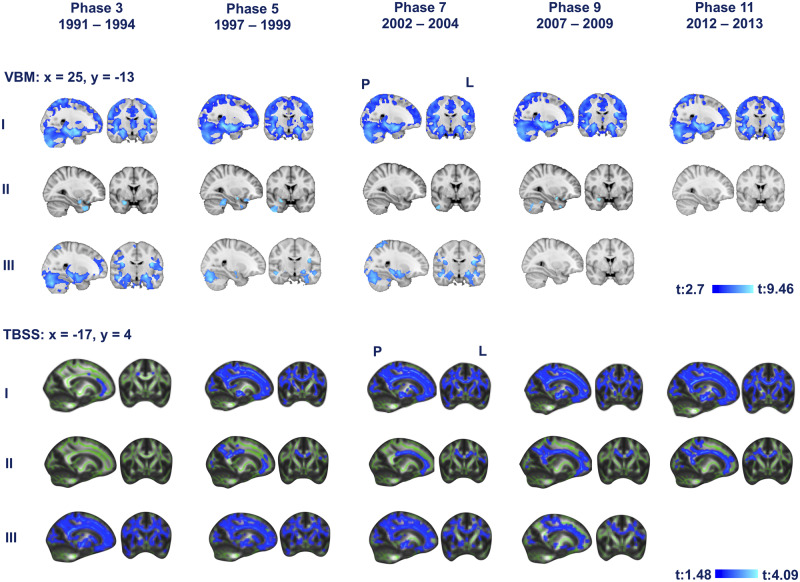
Figure 1.
The association of midlife Framingham stroke risk and lower grey matter density (top) and FA at older ages. Rows I correspond to Model I (baseline model, uncorrected Framingham association with grey and white matter integrity). Rows II correspond to Model II (corrected model, Framingham stroke risk and grey and white matter integrity corrected for confounders). Rows III correspond to Model III (longitudinal model, analyses with significant results for associations between FSRS between 2012 and 2013, and voxelwise GM, and FA were repeated using scanner type and FSRS between 1991 and 1994, 1997 and 1999, 2002 and 2004 and 2007 and 2009 as a confounder). Blue represents regions significant at P < 0.05, threshold-free cluster enhancement, corrected for multiple comparisons. Coordinates are in MNI space. L = left; M = mean; P = posterior.
Model II: after adjusting for the confounders, FSRS measured at mean ages 47.9, 53.6, 59.1 and 64.0 (5.2 SD) years remained significantly associated with lower GM values, although the number of significant voxels was markedly reduced (Fig. 1, Supplementary Table 2). Results were localized in the cerebellar hemisphere, right MTL, middle temporal gyrus and frontal orbital cortex. A higher number of statistically significant voxels than in other waves were found with stroke risk measured on average 6 years before the scan. Maximum t-values were located in the temporal pole [1991–94: t (557) = 5.07, P = 0.008; 1997–99: t (557) = 6.05, P = 0.011; 2002–04: t (557) = 5.47, P = 0.018; 2007–09: t (557) = 5.13, P = 0.011, see Supplementary Table 2].
Model III: after removing the contribution of FSRS at mean ages 47.9, 53.6 and 59.1 (5.2 SD) years, FSRS between 2012 and 2013 [at mean age 68.1 (5.2 SD) years] predicted lower GM density in the MTLs. New FSRS risk acquired between the youngest and oldest study waves was associated with most widespread lower GM [t (563) = 2.96, P < 0.001]. After removing the contribution of FSRS during the penultimate study wave at mean age 64 (5.2 SD) years, FSRS between 2012 and 2013 [at mean age 68.1 (5.2 SD) years] did ‘not’ predict lower GM density.
Tract-based spatial statistics
Model I: the FSRS at each of the five waves, at mean ages 47.9, 53.6, 59.1, 64.0 and 68.1 (5.2 SD) years and as early as 20 years preceding the brain scan, was associated with widespread lower FA at follow-up. FSRS measured closest to the time of scan, at mean age 68.1 (5.3 SD) years, was associated with the most widespread effect, including anterior thalamic radiation, cingulum, anterior and superior corona radiata, corpus callosum, corticospinal tract, external capsule, forceps major, forceps minor, internal capsule and superior longitudinal fasciculus. Peak cluster location was in the left corona radiata and forceps minor [t (547) = 2.5, P < 0.001]. The association was similar for higher MD, peak cluster location in left corona radiata [t (547) = 3.34, P < 0.001].
Model II: after controlling the association for the confounders, FSRS measured at four time points between 1997 and 2013 [at mean ages 53.6, 59.1, 64.0 and 68.1 (5.2 SD) years] remained significantly associated with lower FA and higher MD values, although the number of significant voxels was markedly reduced. Associations were localized to the corpus callosum, longitudinal fasciculus, cingulate gyrus and anterior thalamic radiation (Fig. 1, Supplementary Table 3). FSRS closest to the time of scan (2012–13) remained the best predictor of lower FA and higher MD. Maximum t-values were located in the left cingulum [FA: t (541) = 1.8, P = 0.015, MD: t (541) = 2.94, P = 0.045].
Model III: after removing the contribution of FSRS at mean ages 47.9, 53.6, 59.1, 64.0 and 68.1 (5.2 SD) years, FSRS between 2012 and 2013 [at mean age 68.1 (5.2 SD) years] predicted lower FA and higher MD in the cingulum, anterior corona radiata, corpus callosum, longitudinal fasciculus and posterior thalamic radiation. The most widespread association between new FSRS risk and lower WM integrity was acquired between the youngest and oldest ages [FA: t (546) = 2.42, P < 0.001, and MD: t (546) = 3.28, P < 0.001].
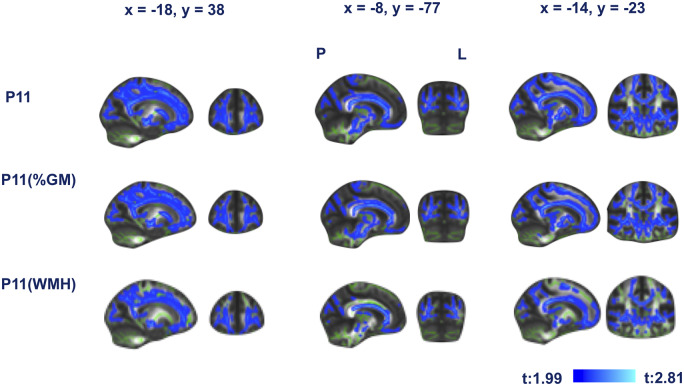
Model IV: the association of FSRS measured at mean age 68.1 (5.2 SD) years and lower FA remained significant after controlling for GM [t (546) = 2.1, P < 0.001] and after controlling for WMH volume both as percentages of whole-brain volume [t (546) = 2.03, P < 0.001] (Fig. 2).
Figure 2.
Framingham stroke-risk predicted changes in white matter microstructure (FA) are primary to white matter lesions and secondary to Wallerian degeneration. First row shows lower FA associated with Framingham stroke risk. Second row shows first row controlled for percentage grey matter (an estimate of Wallerian degeneration). Third row shows first row controlled for white matter hyperintensity volume. Blue represents regions significant at P < 0.05, threshold-free cluster enhancement, corrected for multiple comparisons. Coordinates are in MNI space. L = left; P = posterior.
Scanner differences
Voxelwise analyses were also performed separately on data acquired with the Verio and Prisma scanners, yielding results in the same locations, as previously found in a sample of Verio participants (Zsoldos, 2017). Sample characteristics, segmented GM and WMHs of both samples are presented in Tables 2 and 3.
Structural equation modelling
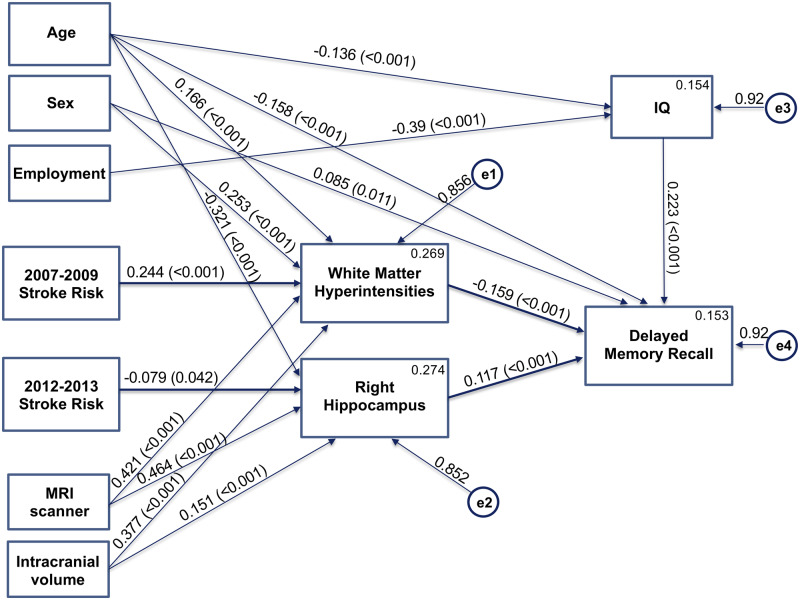
The structural equation modelling used 18 variables. Log-transformed FSRS between 1991 and 1994, 1997 and 1999, 2002 and 2004, 2007 and 2009 and 2012 and 2013, employment grade, age at scan, sex, scanner type and FreeSurfer-estimated total intracranial volume were entered as observed, exogenous variables (independent variables). WMH volume, right hippocampus, full-scale IQ (FSIQ; estimated from the Test of Premorbid Functioning) and delayed memory recall were entered as observed, endogenous variables (dependent variables). Each endogenous variable had an error term (unobserved, exogenous variable) with mean set to 0, and variance to 1. Covariances between exogenous variables and direct effects between each exogenous and endogenous variables were also modelled. Non-significant direct effects were removed until a suitable model fit of CMIN/DF <3 was achieved. The final model was significant with χ2 = 74.062 (df = 36, P < 0.001, χ2/df = 2.06). Higher 2007–09 FSRS predicted higher WMH volume, and higher 2012–13 FSRS predicted lower right hippocampal volume—both of which in turn predicted poorer delayed memory recall. Other significant direct effects were as follows: (i) higher age with higher WMH, lower hippocampal volume, FSIQ and delayed recall, (ii) women had higher WMH and delayed memory recall, (iii) participants scanned with the 3-T Prisma had higher WMH and right hippocampal volume, (iv) higher intracranial volume (ICV) with higher WMH and right hippocampal volume, (v) higher employment grade with higher FSIQ and (vi) higher FSIQ with higher delayed recall (Fig. 3, Supplementary Tables 4 and 5).
Figure 3.
Structural equation modelling results. Framingham stroke risk in later waves was best associated with white matter hyperintensity and hippocampal volume, which, in turn, was associated with memory performance in older life. Covariances are not shown.
As we were interested in direct and indirect (via anatomical measures) effects of FSRS on delayed memory recall, we also evaluated a number of models including the direct effects of FSRS on delayed memory. Presumably, due to the collinearity of the FSRS measured at different time points (Pearson’s correlation between FSRS between 2007 and 2009 and between 2012 and 2013 = 0.826, P < 0.001) and the consequent different signs of effects, the sum of absolute direct and indirect effects did not add up to the absolute total effect, making interpretations difficult (Hayes, 2018). After removing the values for times not significantly associated with anatomical measures (leaving 2012–13 for hippocampal size and 2007–09 for WMH volumes), direct and indirect effects added up to total effects. In particular, the model retaining 2012–13 FSRS only achieved a χ2 = 74.100 (df = 18, P < 0.001, χ2/df = 4.12) and estimated direct effects of FSRS on Hopkins Verbal Learning Test as β = −0.226 and indirect effects via hippocampal size as β = −0.036, i.e. as 86% and 14%, respectively. The model retaining 2007–09 FSRS values only achieved a χ2 = 60.804 (df = 18, P < 0.001, χ2/df = 3.38) and estimated direct effects of FSRS on Hopkins Verbal Learning Test as β = −0.002 and indirect effects via volume of WMHs as β = −0.180, i.e. as 2% and 98%, respectively.
Discussion
In this large group of community-dwelling older adults, unadjusted higher Framingham stroke risk measured across a 20-year period prior to the MRI scan was associated with lower whole-brain GM density and WM integrity. Higher stroke risk in younger ages, as early as 20 years before the MRI scan predicted lower GM density across the cortex and sub-cortical areas, and FA in corpus callosum. After removing the effect of confounding variables, including linear and squared age effects, higher stroke risk during earlier, but not at the most recent study wave, was associated with lower GM in the MTL. In addition, residual stroke risk over the 20-year period was associated with lower FA in widespread tracts, and lower GM density, confined mainly to sub-neocortical structures. Higher stroke risk at mean ages of 64 and 68.1 (5.2 SD) years statistically predicted lower Hopkins Verbal Learning Test-measured delayed memory performance. WMH volumes largely (90%) mediated this effect, while hippocampal volume did so only marginally.
Grey matter
Our findings suggest that the unique effects of 10-year stroke risk impact GM density in (right) medial temporal structures up to old age. This means that FSRS does not only predict established clinical stroke but also ‘subclinical’ GM atrophy, particularly in MTL structures that may be of relevance in the development of cognitive impairment (Bastos-Leite et al., 2007), depressive disorder (Santos et al., 2018), Alzheimer’s disease (Burton et al., 2009; den Heijer et al., 2010) and dementia of vascular type (Laakso et al., 1996). The effects of FSRS are independent of chronological age, which by itself predicts GM atrophy (Fotenos et al., 2008) and the related cognitive decline in the general population (Jack et al., 2005; Craik and Bialystok, 2006) and in the Whitehall II cohort (Singh-Manoux et al., 2012). We added a quadratic age term because the effects of FSRS were still present after removing the effects of chronological age. This did not remove all the effects of FSRS, so FSRS makes a unique contribution to lower GM density, presumably based on vascular pathology beyond a simple function of time (Uiterwijk et al., 2018). In a recent study of a subset (N = 116) of the Whitehall II imaging sub-study, we found that cardiovascular risk in midlife was significantly associated with lower GM perfusion at older ages, whereas this association was not significant for cardiovascular risk in later life, which lends further support to these results (Suri et al., 2019). FSRS is a composite score and is incremental with age, which makes it difficult to estimate when it starts to affect GM, and when its effects become evident. FSRS at mean age 68.1 (5.2 SD) years, after removing the contribution of FSRS at mean ages of 47.9, 53.6 and 59.1 (5.2 SD) years, predicted lower GM density in the MTLs. This may mean that, while cardio-metabolic risk in the late 40s already predicts lower GM density at the age of 70 years in ‘neocortical areas’ located in middle cerebral artery vascular territories, which is the largest branch of the internal carotid and most often occluded by embolism, ‘sub-neocortical areas’ remain sensitive to residual risk over the following 20-year period. This is consistent with our knowledge that hippocampi remain plastic in adulthood and can regenerate (Spalding et al., 2013; Bergmann et al., 2015).
White matter
FSRS in most data waves was associated with widespread lower microstructural WM integrity. FSRS measured 20 years prior to scan and, after the removal of confounders, was only associated with changes in the body of corpus callosum, superior corona radiata and their associated tracts in the right hemisphere. Change in stroke risk over a 20-year period was associated with lower FA in all major tracts. The findings that the associations were significantly reduced after removing confounding effects suggest that a large amount of variance is shared between WM microstructure and demographic factors. WM changes that are visible in fluid-attenuated inversion recovery images are generally interpreted clinically as microvascular changes (Debette and Markus, 2010). FSRS was predictive of WMHs in the present study as in previous ones (Jeerakathil et al., 2004; Uiterwijk et al., 2018). In our study, the effect of FSRS on WM microstructure was primarily mediated by widespread WM changes that are visible in fluid-attenuated inversion recovery images but was to some extent also secondary to Wallerian degeneration, suggesting that GM changes might not be of primary importance in generating the loss of WM integrity. WMHs affect cognitive abilities in healthy individuals (Debette et al., 2010) and may also be related to the late-onset depression and the maintenance of impaired cognitive function in late-life depression (Kohler et al., 2010).
Cognition
Stroke and dementia have common risk factors, and there is strong evidence for the link between vascular risk factors and cognitive impairment (Elkins et al., 2004; van Oijen et al., 2007). Contrary to expectations (Pase et al., 2018), FSRS in later but not in earlier waves was directly associated with higher WMH volume and lower hippocampal volume, which, in turn, was associated with worse memory performance in later life. Moreover, the effects of FSRS on verbal memory mediated by hippocampal size were relatively small, while the effect mediated via WMHs was accounting for almost all of the effect of FSRS on memory. Previous studies have found that vascular risk was a useful predictor of higher WMH burden and lower overall cognitive performance (Uiterwijk et al., 2018) and have concluded that lowering vascular risk in midlife can potentially prevent dementias (Gorelick et al., 2011; Hachinski and World Stroke Organization, 2015; Pase et al., 2017). Furthermore, it is possible that Alzheimer’s biomarkers and cardiovascular risk predict cognitive performance through independent pathways (Buckner, 2004; Hohman et al., 2015). The hippocampus is highly sensitive to cardiovascular and age-related damage or impaired regeneration (Nagy et al., 1996; Molendijk et al., 2012). However, it is likely that the stroke-risk effects are not only vascular in nature. Our voxelwise results support this finding, as neither an index of Wallerian degeneration nor WMHs could fully explain the association between FSRS and WM microstructure.
Strengths of this study are the 22-year repeated prospective data on Framingham stroke risk, the availability of a large amount of MRI data and the advanced methods of imaging analysis. The main limitation is that longitudinal MRI data are not available in parallel with the repeated measures of FSRS. However, the prospective association between FSRS assessed at mean age 47.9 (5.2 SD), two decades prior to the scan, with imaging measures highlighting the potential for targeting modifiable risk factors in midlife.
Findings of this study show that the Framingham 10-year probability stroke-risk score may relevant for primary prevention not only for stroke risk itself but also for subclinical GM atrophy in younger ages, and as early as 20 years in advance, and subsequent memory changes in later life. Brain areas such as the MTL are often implicated in cognitive impairment and dementias; thus, preserving them into older age is important. In the future, we may be able to use imaging results to track and target the modification of risk factors throughout adulthood.
Supplementary Material
Acknowledgements
We thank all Whitehall II participants, the Whitehall II staff at the University College London, Mandy Pipkin and Barbora Krausova for assisting with recruitment and data collection, Mark Jenkinson for providing invaluable advice on voxelwise analysis methods and the core staff at Oxford Centre for Functional MRI of the Brain in the Wellcome Centre for Integrative Neuroimaging for their helpful collaboration.
Funding
Work on the Whitehall imaging sub-study was funded by the UK Medical Research Council (G1001354) and the HDH Wills 1965 Charitable Trust (1117747). Work on this research study, E.Zs. and S.S. were funded by the EU Horizon 2020 (732592). N.F. and C.E.M. are supported by the National Institute for Health Research (NIHR) Oxford Health Biomedical Research Centre (BRC). L.G. is supported by the Oxford Parkinson’s Disease Centre (Parkinson’s UK Monument Discovery Award) and the Medical Research Council Dementias Platform UK. A.S.-M. receives research support from the US National Institutes of Health (R01AG056477). M.K. is supported by the UK Medical Research Council (K013351), the US National Institute on Aging (R01AG056477), NordForsk and the Academy of Finland (311492).
Competing interests
All authors declare grant support for the submitted work as described above; C.M. declares personal and financial interest in Exprodo Software; no other relationships or activities that could appear to have influenced the submitted work.
Glossary
- FA =
fractional anisotropy
- FSRS =
Framingham stroke-risk score
- GM =
grey matter
- MD =
mean diffusivity
- MRI =
magnetic resonance imaging
- MTL =
medial temporal lobe
- WM =
white matter
- WMH =
white matter hyperintensity
References
- Allan CL, Sexton CE, Kalu UG, McDermott LM, Kivimaki M, Singh-Manoux A, et al. Does the Framingham Stroke Risk Profile predict white-matter changes in late-life depression? Int Psychogeriatr 2012; 24: 524–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan CL, Zsoldos E, Filippini N, Sexton CE, Topiwala A, Valkanova V, et al. Lifetime hypertension as a predictor of brain structure in older adults: cohort study with a 28-year follow-up. Br J Psychiatry 2015; 206: 308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos-Leite AJ, van der Flier WM, van Straaten EC, Staekenborg SS, Scheltens P, Barkhof F.. The contribution of medial temporal lobe atrophy and vascular pathology to cognitive impairment in vascular dementia. Stroke 2007; 38: 3182–5. [DOI] [PubMed] [Google Scholar]
- Bergmann O, Spalding KL, Frisen J.. Adult neurogenesis in humans. Cold Spring Harb Perspect Biol 2015; 7: a018994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J. The Hopkins Verbal Learning Test: development of a new memory test with six equivalent forms. Clin Neuropsychol 1991; 5: 125–42. [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron 2004; 44: 195–208. [DOI] [PubMed] [Google Scholar]
- Burton EJ, Barber R, Mukaetova-Ladinska EB, Robson J, Perry RH, Jaros E, et al. Medial temporal lobe atrophy on MRI differentiates Alzheimer’s disease from dementia with Lewy bodies and vascular cognitive impairment: a prospective study with pathological verification of diagnosis. Brain 2009; 132: 195–203. [DOI] [PubMed] [Google Scholar]
- Cox SR, Lyall DM, Ritchie SJ, Bastin ME, Harris MA, Buchanan CR, et al. Associations between vascular risk factors and brain MRI indices in UK Biobank. Eur Heart J 2019; 40: 2290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik FIM, Bialystok E.. Cognition through the lifespan: mechanisms of change. Trends Cogn Sci 2006; 10: 131–8. [DOI] [PubMed] [Google Scholar]
- D’Agostino RB, Wolf PA, Belanger AJ, Kannel WB.. Stroke risk profile: adjustment for antihypertensive medication. The Framingham Study. Stroke 1994; 25: 40–3. [DOI] [PubMed] [Google Scholar]
- de Leeuw FE, de Groot JC, Achten E, Oudkerk M, Ramos LMP, Heijboer R, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry 2001; 70: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debette S, Beiser A, DeCarli C, Au R, Himali JJ, Kelly-Hayes M, et al. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: the Framingham Offspring Study. Stroke 2010; 41: 600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debette S, Markus HS.. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 2010; 341: c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology 2011; 77: 461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Heijer T, van der Lijn F, Koudstaal PJ, Hofman A, van der Lugt A, Krestin GP, et al. A 10-year follow-up of hippocampal volume on magnetic resonance imaging in early dementia and cognitive decline. Brain 2010; 133: 1163–72. [DOI] [PubMed] [Google Scholar]
- Douaud G, Smith S, Jenkinson M, Behrens T, Johansen-Berg H, Vickers J, et al. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain 2007; 130: 2375–86. [DOI] [PubMed] [Google Scholar]
- Dregan A, Stewart R, Gulliford MC.. Cardiovascular risk factors and cognitive decline in adults aged 50 and over: a population-based cohort study. Age Ageing 2013; 42: 338–45. [DOI] [PubMed] [Google Scholar]
- Elkins JS, O'Meara ES, Longstreth WT, Carlson MC, Manolio TA, Johnston SC.. Stroke risk factors and loss of high cognitive function. Neurology 2004; 63: 793–9. [DOI] [PubMed] [Google Scholar]
- Filippini N, Zsoldos E, Haapakoski R, Sexton CE, Mahmood A, Allan CL, et al. Study protocol: The Whitehall II imaging sub-study. BMC Psychiatry 2014; 14: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotenos AF, Mintun MA, Snyder AZ, Morris JC, Buckner RL.. Brain volume decline in aging: evidence for a relation between socioeconomic status, preclinical Alzheimer disease, and reserve. Arch Neurol 2008; 65: 113–20. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS.. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 2001; 14: 21–36. [DOI] [PubMed] [Google Scholar]
- Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42: 2672–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffanti L, Zamboni G, Khan A, Li L, Bonifacio G, Sundaresan V, et al. BIANCA (Brain Intensity AbNormality Classification Algorithm): a new tool for automated segmentation of white matter hyperintensities. Neuroimage 2016; 141: 191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachinski V; World Stroke Organization. Stroke and potentially preventable dementias proclamation: updated world stroke day proclamation. Stroke 2015; 46: 3039–40. [DOI] [PubMed] [Google Scholar]
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis, 2nd edn New York: The Guilford Press; 2018. [Google Scholar]
- Hohman TJ, Samuels LR, Liu D, Gifford KA, Mukherjee S, Benson EM, et al. Stroke risk interacts with Alzheimer’s disease biomarkers on brain aging outcomes. Neurobiol Aging 2015; 36: 2501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Shiung MM, Weigand SD, O'Brien PC, Gunter JL, Boeve BF, et al. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology 2005; 65: 1227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeerakathil T, Wolf PA, Beiser A, Massaro J, Seshadri S, D’Agostino RB, et al. Stroke risk profile predicts white matter hyperintensity volume: the Framingham Study. Stroke 2004; 35: 1857–61. [DOI] [PubMed] [Google Scholar]
- Kaffashian S, Dugravot A, Brunner EJ, Sabia S, Ankri J, Kivimaki M, et al. Midlife stroke risk and cognitive decline: a 10-year follow-up of the Whitehall II cohort study. Alzheimers Dement 2013a; 9: 572–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaffashian S, Dugravot A, Elbaz A, Shipley MJ, Sabia S, Kivimaki M, et al. Predicting cognitive decline: a dementia risk score vs. the Framingham vascular risk scores. Neurology 2013b; 80: 1300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler S, Thomas AJ, Lloyd A, Barber R, Almeida OP, O’Brien JT.. White matter hyperintensities, cortisol levels, brain atrophy and continuing cognitive deficits in late-life depression. Br J Psychiatry 2010; 196: 143–9. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Partanen K, Riekkinen P, Lehtovirta M, Helkala EL, Hallikainen M, et al. Hippocampal volumes in Alzheimer’s disease, Parkinson’s disease with and without dementia, and in vascular dementia: an MRI study. Neurology 1996; 46: 678–81. [DOI] [PubMed] [Google Scholar]
- Li L, Simoni M, Kuker W, Schulz UG, Christie S, Wilcock GK, et al. Population-based case-control study of white matter changes on brain imaging in transient ischemic attack and ischemic stroke. Stroke 2013; 44: 3063–70. [DOI] [PubMed] [Google Scholar]
- Molendijk ML, van Tol MJ, Penninx BW, van der Wee NJ, Aleman A, Veltman DJ, et al. BDNF val66met affects hippocampal volume and emotion-related hippocampal memory activity. Transl Psychiatry 2012; 2: e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran C, Beare R, Wang W, Callisaya M, Srikanth V; Alzheimer's Disease Neuroimaging Initiative (ADNI). Type 2 diabetes mellitus, brain atrophy, and cognitive decline. Neurology 2019; 92: e823–30.30674592 [Google Scholar]
- Nagy Z, Jobst KA, Esiri MM, Morris JH, King EM, MacDonald B, et al. Hippocampal pathology reflects memory deficit and brain imaging measurements in Alzheimer’s disease: clinicopathologic correlations using three sets of pathologic diagnostic criteria. Dementia 1996; 7: 76–81. [DOI] [PubMed] [Google Scholar]
- Nishtala A, Piers RJ, Himali JJ, Beiser AS, Davis-Plourde KL, Saczynski JS, et al. Atrial fibrillation and cognitive decline in the Framingham Heart Study. Heart Rhythm 2018; 15: 166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pase MP, Davis-Plourde K, Himali JJ, Satizabal CL, Aparicio H, Seshadri S, et al. Vascular risk at younger ages most strongly associates with current and future brain volume. Neurology 2018; 91: e1479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pase MP, Satizabal CL, Seshadri S.. Role of improved vascular health in the declining incidence of dementia. Stroke 2017; 48: 2013–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos MAO, Bezerra LS, Carvalho A, Brainer-Lima AM.. Global hippocampal atrophy in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Trends Psychiatry Psychother 2018; 40: 369–78. [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A, Kivimaki M, Glymour MM, Elbaz A, Berr C, Ebmeier KP, et al. Timing of onset of cognitive decline: results from Whitehall II prospective cohort study. BMJ 2012; 344: d7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004; 23 (Suppl 1): S208–19. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE.. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 2009; 44: 83–98. [DOI] [PubMed] [Google Scholar]
- Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell 2013; 153: 1219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri S, Topiwala A, Chappell MA, Okell TW, Zsoldos E, Singh-Manoux A, et al. Association of midlife cardiovascular risk profiles with cerebral perfusion at older ages. JAMA Netw Open 2019; 2: e195776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uiterwijk R, Staals J, Huijts M, de Leeuw PW, Kroon AA, van Oostenbrugge RJ.. Framingham Stroke Risk Profile is related to cerebral small vessel disease progression and lower cognitive performance in patients with hypertension. J Clin Hypertens 2018; 20: 240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oijen M, de Jong FJ, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM.. Atherosclerosis and risk for dementia. Ann Neurol 2007; 61: 403–10. [DOI] [PubMed] [Google Scholar]
- Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013; 12: 822–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Test of premorbid functioning. UK version (TOPF UK). Bloomington, MN: Pearson Inc; 2011. [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE.. Permutation inference for the general linear model. Neuroimage 2014; 92: 381–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsoldos E, Brain markers of cumulative stress response and allostatic load in the ageing whitehall II cohort. Oxford: University of Oxford; 2017. [Google Scholar]
- Zsoldos E, Filippini N, Mahmood A, Mackay CE, Singh-Manoux A, Kivimaki M, et al. Allostatic load as a predictor of grey matter volume and white matter integrity in old age: the Whitehall II MRI study. Sci Rep 2018; 8: 6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study follows Medical Research Council data-sharing policies (https://www.mrc.ac.uk/research/policies-and-guidance-for-researchers/data-sharing/, 20 March 2020, date last accessed). Data will be accessible from the authors after 2019.