Abstract
Background
Venous thromboembolism (VTE) is a frequent complication in critically ill patients with coronavirus disease 2019 (COVID-19) and is associated with mortality. Early diagnosis and treatment of VTE is warranted.
Objective
To develop a prediction model for VTE in critically ill COVID-19 patients.
Patients and methods
In this retrospective cohort study, 127 adult patients with confirmed COVID-19 infection admitted to the intensive care unit of two teaching hospitals were included. VTE was diagnosed with either ultrasound or computed tomography scan.
Univariate receiver operating characteristic (ROC) curves were constructed for Positive End Expiratory Pressure, PaO2/FiO2 ratio, platelet count, international normalized ratio, activated partial thromboplastin time as well as levels of fibrinogen, antithrombin, D-dimer and C-reactive protein (CRP). Multivariate analysis was done using binary linear regression.
Results
Variables associated with VTE in both univariate and multivariate analysis were D-dimer and CRP with an area under the curve (AUC) of 0.64, P = 0.023 and 0.75, P = 0.045, respectively. Variables indicating hypoxemia were not predictive. The ROC curve of D-dimer and CRP combined had an AUC of 0.83, P < 0.05. Categorized values of D-dimer and CRP were used to compute a mean absolute risk for the combination of these variables with a high positive predictive value. The predicted probability of VTE with a D-dimer > 15 in combination with a CRP > 280 was 98%. The negative predictive value of D-dimer was low.
Conclusion
Elevated CRP and D-dimer have a high positive predictive value for VTE in critically ill COVID-19 patients. We developed a prediction table with these biomarkers that can aid clinicians in the timing of imaging in patients with suspected VTE.
Abbreviations: aPTT, activated partial thromboplastin time; ARDS, acute respiratory distress syndrome; AUC, area under the curve; COVID-19, coronavirus disease 2019; CRP, c-reactive protein; CT, computed tomography; DVT, deep venous thrombosis; ICU, intensive care unit; INR, international normalized ratio; Kg, kilogram; LMWH, low-molecular-weight heparin; NPV, negative predicting value; PCR, polymerase chain reaction; PE, pulmonary embolism; PEEP, positive end expiratory pressure; PPV, positive predicting value; PT, prothrombin time; ROC, receiver operating characteristics; SARS-CoV-2, severe Acute Respiratory Syndrome Coronavirus-2; VTE, venous thromboembolism
Keywords: COVID-19, C-reactive protein, Critical illness, D-dimer, Venous thromboembolism
Highlights
-
•
Venous thromboembolisms are a frequently observed complication of COVID-19.
-
•
Markers of oxygenation are not predictive of venous thromboembolism.
-
•
Elevated C-reactive protein and D-dimer have the potential to predict venous thromboembolism.
-
•
We created a prediction tool based on elevations in both CRP and D-dimer to optimize time of imaging.
1. Introduction
Venous thromboembolism (VTE) is a frequent complication in critically ill patients with coronavirus disease 2019 (COVID-19). Estimated incidences of thrombotic complications, including deep venous thrombosis (DVT) and pulmonary embolism (PE) have been reported at 30–38%, despite the use of prophylactic anticoagulation [1,2]. Furthermore, the development of thrombosis is associated with increased mortality [2], whereas the use of heparin is associated with a decrease in mortality in observational studies in critically ill COVID-19 patients [3]. This indicates that early diagnosis and treatment of thrombotic complications is of paramount importance.
The diagnosis of VTE can be challenging in critically ill patients with COVID-19. DVT can be difficult to recognise in sedated and often obese patients. Moreover, PE is also challenging to diagnose as worsening hypoxemia can be attributed to concomitant pneumonia or acute respiratory distress syndrome (ARDS), leading to underdiagnoses. Also, optimal timing of imaging is a challenge, as it was shown that when computed tomography (CT) scanning for a suspected PE is performed in COVID-19 patients, 75% of the scans are negative [4]. Transportation of a patient to perform a CT scan while on mechanical ventilation with high pressures is a logistic challenge with risks for the patient as well as risks of contamination. Thereby, the use of a prediction model with the ability to differentiate between patients with high and low risk for VTE is likely to improve diagnostic efficiency and early commencement of anticoagulant therapy.
The use of biomarkers in the prognosis of COVID-19 has already been described. A markedly elevated D-dimer was shown to be a predictor of mortality [5,6]. Other deranged coagulation tests in patients with COVID-19 are slightly decreased platelet counts and prolongation of the prothrombin time (PT) [7]. Monitoring of these biomarkers in patients with COVID-19 is recommended by the International Society of Thrombosis and Haemostasis, as an increase may justify more aggressive critical care support [8]. In addition, although pathophysiology of COVID-19 induced coagulopathy remains unclear, it is suggested that thrombosis is inflammatory driven [7,9]. Thereby, acute phase reactant proteins could also be a useful tool in the diagnosis of VTE.
The aim of this study was to develop a prediction model for the occurrence of VTE in critically ill COVID-19 patients. Markers of inflammation and haemostasis as well as markers of hypoxemia were analysed for their diagnostic ability to detect VTE in critically ill COVID-19 patients.
2. Methods
2.1. Study design and participants
This retrospective cohort study was conducted in all adult patients (≥18 years old) admitted to the intensive care unit (ICU) of a university and large teaching hospital in Amsterdam between March 13th and April 9th. Patients with a polymerase chain reaction (PCR) confirmed diagnosis of COVID-19 from a nose or throat swab or tracheal aspirate positive for Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) were included. There were no exclusion criteria. Thrombosis prophylaxis was part of standard of care. The observations of a high incidence of thrombosis led us to intensify the dose of prophylactic low-molecular-weight heparin (LMWH). From April 3rd onwards in the OLVG Hospital and from April 9th onwards in the Amsterdam UMC, location AMC, patients received a double dose of nadroparin, which was nadroparin 2850 IU twice daily (bid) for patients with a body weight <100 kg and 3800 or 5700 IU bid for those ≥100 kg.
Clinical, laboratory and treatment data during the first 20 days of ICU admission (which comprises the time period in which most VTE develop) or until discharge or decease were collected from electronic medical records using a standardised data collection form. In our prediction tool we included the laboratory and ventilation values that were measured most recently before the diagnosis of VTE was confirmed with either ultrasound or CT-scan imaging.
Formal approval from the Medical Ethics Review Committee was not required as the Medical Research Involving Human Subjects Act (WMO) does not apply due to the retrospective nature of this observational study involving no potential risk to patients.
2.2. Venous thromboembolism
VTE was classified as deep venous thrombosis (DVT), pulmonary embolism (PE) or other thrombosis. Diagnosis of DVT or other thrombosis was based on ultrasound, performed as part of weekly screening in all admitted patients. The diagnosis of PE was based on CT imaging, which was performed on clinical suspicion, primarily based on deterioration of gas exchange. Patients with both DVT and PE were scored for PE. Patients with other thrombosis, consisting of superficial vein or venous catheter thrombosis, and DVT were scored for DVT.
2.3. Laboratory and ventilation values
Daily blood examinations consisted of complete blood count, platelets, international normalized ratio (INR), activated Partial Thromboplastin Time (aPTT), serum biochemical tests for liver and kidney function, electrolytes and C-reactive protein (CRP).
D-dimer, fibrinogen and antithrombin were measured twice a week. Oxygenation parameters included the level of PEEP, which is the positive pressure at the end of expiration given to prevent the alveoli form collapsing, and PaO2/FiO2 ratio, which is a clinical indicator of hypoxaemia. The laboratory and ventilation values measured most recently prior to imaging (ultrasound or CT-scan) that confirmed VTE were used for the prediction model.
2.4. Statistical analysis
Based on normality, data is either presented as mean with standard deviation or median with interquartile ranges and differences between groups was analysed with either the Student t-test or Mann Whitney U test as appropriate. Categorical data is presented as percentage and significance was calculated with the Fisher's exact test. Two tailed P values <0.05 were considered statistically significant.
Univariate statistical analysis included Receiver Operating Characteristics (ROC) curve analysis and computation of diagnostic performance parameters for the most optimal cut-off values. All variables with a significance of P < 0.20 in univariate analysis were selected for multivariate analysis. Multivariate analysis was done using binary linear regression with backward stepwise procedure to exclude statistically significant variables from the model. The 95% confidence interval was reported if appropriate.
Based on the results of the logistic regression model, diagnostic performance (sensitivity, specificity, positive predictive value and negative predictive value) for D-dimer and CRP was calculated. Furthermore, odd's ratios for D-dimer and CRP were computed, with a reference value for D-dimer of 1 μg/ml and for CRP a reference value of <20 mg/dl. Intervals to compute a mean absolute risk for the combination of D-dimer and CRP were based on the univariate analysis of ROC curves.
A sub analysis was performed to identify risk factors for PE following the same procedure. Variables were selected for multivariate logistic regression if the AUC had a significance of P < 0.20.
Statistical analysis was performed with SPSS© version 26.0. (IBM©, New York, New York, the United States). Graphs were made with Prism Graphpad version 8.0.2 (San Diego, California, The United States).
3. Results
One-hundred and twenty-seven patients were enrolled in the study. Table 1 shows the baseline characteristics of all patients as well as for the VTE and no VTE subgroups separately at the time of ICU admission. Of 127 included patients, 53 (41.7%) developed VTE. Of patients who developed VTE during ICU stay 16.5% had PE, 20.5% DVT and 4.7% other thromboses, consisting of venous catheter or superficial vein thrombosis. The median time to develop a VTE was eight (IQR 4–12) days after ICU admission.
Table 1.
Baseline characteristics of critically ill patients with COVID-19. Data are reported as median (IQR) unless otherwise specified. BMI, body mass index; CRP, C-reactive protein; VTE, venous thromboembolism; PE, pulmonary embolism. Laboratory values measured on day of admission. Mechanical ventilation at any point during ICU stay.
| Variable | All patients N = 127 |
VTE group N = 53 |
No VTE group N = 74 |
P value |
|---|---|---|---|---|
| Age (years) | 62 (55–70) | 62 (55–71) | 62 (55–70) | 0.73 |
| Male sex, % | 77.0% | 75.5% | 78.1% | 0.73 |
| BMI (kg/m2) | 27.0 (24.7–30.2) | 27.0 (24.2–30.5) | 26.2 (25.0–30.1) | 0.83 |
| Use of vasopressors at admission, % | 53.5% | 69.2% | 43.1% | 0.006 |
| Mechanical ventilation, % | 90.6% | 100% | 83.8% | 0.001 |
| PEEP on admission (cmH2O) | 12 (10–15) | 14 (10–15) | 12 (10–15) | 0.21 |
| P/F ratio on admission | 151 (110–190) | 151 (99–185) | 155 (119–200) | 0.98 |
| INR | 0.93 (0.89–0.99) | 0.93 (0.89–1.00) | 0.93 (0.88–0.98) | 0.77 |
| PT (s) | 11.3 (10.8–12.0) | 11.3 (10.8–11.9) | 11.3 (10.7–12.0) | 0.96 |
| aPTT (s) | 27.4 (25.0–31.0) | 27.7 (25.0–30.7) | 27.4 (25.0–31.0) | 0.66 |
| Platelets (×10.9/l) | 237 (172–307) | 240 (167–354) | 223 (176–298) | 0.81 |
| Fibrinogen (mg/dl) | 7.7 (5.7–8.4) | 7.5 (5.6–8.6) | 7.7 (5.6–8.3) | 0.92 |
| D-dimer (μg/ml) | 1.68 (0.79–5.51) | 2.31 (0.82–29.2) | 1.25 (0.73–3.00) | 0.10 |
| CRP (mg/dl) | 176 (114–247) | 214 (128–270) | 153 (97–230) | 0.035 |
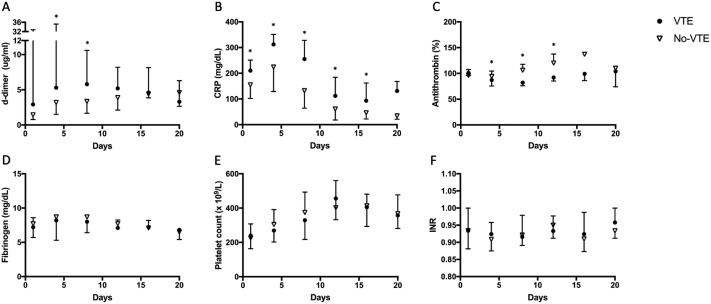
Fig. 1 shows coagulation tests plotted over time during ICU stay in patients who developed VTE and in those who did not. Median levels of D-dimer, CRP and fibrinogen were high in COVID-19. Antithrombin activity gradually increased over time in ICU. Median D-dimer level was significantly increased on day 4 and 8 in the VTE group compared to the non-VTE group. Median levels of CRP were also significantly higher in the VTE group compared to the non-VTE group at day of admission up to day 16. In contrast, fibrinogen levels did not differ between the two groups at any point in time. The median level of antithrombin was significantly lower in the VTE group when compared with the non-VTE group on day 4, 8 and 12. However, median levels were within normal range for both groups.
Fig. 1.
Median (A) D-dimer n, (B) CRP (C) antithrombin (D) fibrinogen, (E) platelet count and (F) INR, plotted against days since ICU admission for patients who were diagnosed with a venous thromboembolism (VTE) and patients who had no proven VTE (no-VTE). Error bars show the upper limit of the IQR for the group with the highest median and lower limit of the IQR for the group with the lowest median.
3.1. Analysis of risk factors for VTE
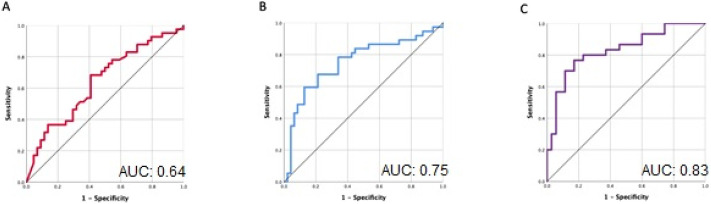
In the univariate analysis, D-dimer and CRP showed statistically significant AUCs (0.64 and 0.75 respectively, P < 0.05, Table 2 and Fig. 2 ) and were included in binary logistic regression. The final model to predict the risk of VTE from both biomarkers had the following relationship:
| (1) |
where x 1 is D-dimer level and x 2 is CRP level. p is the probability of developing VTE and is the odds of developing VTE. D-dimer and CRP remained significantly associated with VTE in the binary logistic regression model with an AUC of 0.83 (0.73–0.93), Fig. 2. The sensitivity, specificity, positive predictive value and negative predictive value of these markers are shown in Table 3 and Table 4 . Other variables were not predictive of VTE. Odd's ratios are shown in supplemental Table C. The categorized variables were used to compute a mean absolute risk for the combination of D-dimer and CRP and shown in Table 5 .
Table 2.
AUC values of ROC analysis of all variables for VTE. AUC, area under the curve. P-value of AUC differing from 0.5.
| Test | AUC | P value |
|---|---|---|
| D-dimer | 0.640 | 0.023 |
| INR | 0.426 | 0.555 |
| Antithrombin | 0.625 | 0.241 |
| Platelet count | 0.562 | 0.622 |
| CRP | 0.752 | 0.045 |
| aPTT | 0.479 | 0.870 |
| Fibrinogen | 0.380 | 0.341 |
| P/F ratio | 0.393 | 0.393 |
| PEEP | 0.483 | 0.896 |
Fig. 2.
Receiver operating characteristics curves for (A) D-dimer (B) CRP and (C) D-dimer and CRP combined computed through logistic regression.
Table 3.
Diagnostic performance of D-dimer in the diagnosis of VTE. Values in parentheses indicate the 95% confidence interval. PPV, positive predictive value; NPV, negative predictive value.
| D-dimer (μg/ml) cut-off | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| >2.0 | 80 (61–92) | 29 (15–49) | 53 (46–61) | 60 (38–79) |
| >3 | 71 (51–85) | 50 (34–69) | 55 (45–65) | 67 (52–79) |
| >4.5 | 70 (51–85) | 40 (24–58) | 50 (41–59) | 61 (44–76) |
| >11 | 37 (20–56) | 94 (81–99) | 84 (57–96) | 63 (57–70) |
Table 4.
Diagnostic performance of CRP in the diagnosis of VTE. Values in parentheses indicate the 95% confidence interval. PPV, positive predictive value; NPV, negative predictive value.
| CRP (mg/dl) cut off | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| >70 | 87 (69–96) | 49 (31–66) | 59 (50–67) | 81 (62–92) |
| >130 | 77 (58–90) | 63 (46–79) | 64 (45–79) | 76 (61–86) |
| >160 | 67 (47–83) | 77 (60–90) | 71 (56–83) | 73 (61–82) |
| >200 | 60 (41–77) | 89 (73–97) | 82 (63–92) | 72 (62–80) |
| >245 | 43 (26–63) | 97 (85–99) | 93 (64–99) | 67 (59–73) |
Table 5.
Predicted probability of VTE for combinations of biomarkers. CRP, C-reactive protein. Values indicate the probability (in percentage) of VTE given ranges of CRP and D-dimer.
| CRP (mg/dl) |
|||||||
|---|---|---|---|---|---|---|---|
| <20 | 21–80 | 81–160 | 161–220 | 221–280 | >280 | ||
| D-dimer (μg/ml) | <1 | 9% | 12% | 20% | 32% | 45% | 73% |
| 1–4 | 11% | 15% | 25% | 38% | 51% | 77% | |
| 5–9 | 16% | 21% | 33% | 48% | 61% | 84% | |
| 10–15 | 29% | 36% | 52% | 67% | 77% | 92% | |
| >15 | 64% | 72% | 83% | 90% | 94% | 98% | |
3.2. Analysis of risk factors for PE
As we found that variables indicating hypoxemia were not predictive for the development of VTE, we repeated the analysis in the subgroup of patients with a PE (n = 21). In the univariate analysis, PEEP and CRP were significantly associated with PE development. However, in the multivariate model, significance of PEEP was lost, and only CRP remained as a predictor for pulmonary embolism (supplemental Table B).
4. Discussion
In this study, increased D-dimer and CRP levels were predictors of VTE with a high sensitivity. Of note, markers of oxygenation were not predictive of VTE or the presence of a pulmonary embolism. Risk stratification in patients is important to ensure VTEs are not missed and adequate anticoagulation is administered [10]. We constructed a prediction table with various cut off values of the commonly used biomarkers D-dimer and CRP that can aid physicians in the timing of imaging in patients suspected for DVT and PE.
In our study, 41.7% of included patients developed VTE. This high number is similar to VTE incidences in critically ill COVID-19 patients reported elsewhere [1,4]. In the present study, both D-dimer and CRP were able to predict the development of VTE in COVID-19 patients, with the combination of D-dimer and CRP showing the highest positive predictive value. For instance, our results indicate a predicted probability for VTE of 92% when D-dimer is >9 μg/ml and CRP > 280 mg/ml.
Elevated D-dimer levels indicate coagulation activation and subsequent hyper fibrinolysis. Therefore, D-dimer is generally used to detect an active thrombus with a high sensitivity but low specificity [11]. In clinical practice, a D-dimer of less than 0.5 μg/ml is used to rule out VTE [12,13]. A recently published study on D-dimer in Asian COVID-19 patients reported a negative predicting value (NPV) for VTE of 92.5% using a D-dimer cut off value of 3.0 μg/ml [14]. In contrast, the ability of a D-dimer level of <3.0 μg/ml to rule out VTE in this study was only 67%. An explanation may be the larger sample size in this study. Alternatively, the different patient population characteristics can be an explanation. There are genetic differences between Asians and Caucasians with regard to the coagulation system [15], which may have led to different predictive values of D-dimer. However, a high D-dimer level has a high positive predictive value for VTE. Thereby, in contrast to non-COVID-19 patients, D-dimer levels in COVID-19 appear not useful to rule out VTE, but could be useful in timing of imaging in COVID-19 patients suspected of VTE.
High CRP levels had the best performance to predict VTE. It has already been suggested that rises in CRP reflect physiologic complications of COVID-19 [16]. Furthermore, this underlines the current hypothesis that inflammation is the driving force behind the development of thrombosis in COVID-19 patients [7,9]. The implication of our finding is that an increase in CRP should not only raise suspicion of ongoing viremia or a nosocomial infection, but also of the occurrence of VTE. Of note, we did not find fibrinogen levels to be predictive for VTE in COVID-19 patients. This is remarkable, as fibrinogen is also an acute phase reactant and high levels of fibrinogen have been reported to be a prominent feature of COVID-19 induced coagulopathy [17]. Apparently, not all inflammatory proteins contribute to the pathogenesis of thrombosis in COVID-19. This remains an important area of further research. Furthermore, the observation that in both the VTE and the no VTE group antithrombin levels fall within reference values and fibrinogen levels are not decreased suggest that disseminated intravascular coagulation is not the underlying cause of coagulopathy.
In clinical practice, suspicion of PE usually arises with worsening gas exchange or hypoxemia. In our study, however, variables indicating hypoxemia were not predictive for VTE. In a sub analysis in COVID-19 patients with PE, high PEEP was a risk factor for VTE in univariate analysis, but this association was lost in the multivariate model. This suggests that hypoxemia is not predictive for the development of PE in COVID-19 patients, but rather that high PEEP may be an indicator of the severity of inflammation. Of note, in this study PE diagnosis was based on CT imaging. However, it is suggested that COVID-19 coagulopathy may be caused by local thrombotic microangiopathy [7], which may not be visible on CT imaging. This could also be an explanation for loss of significance. Alternatively, the sub analysis was done in a small sample size, which could also have accounted for loss of significance.
A limitation of this study is the fact that CT imaging was performed upon clinical suspicion and not as standard of care, possibly causing underdiagnosis of PE. Another limitation is the retrospective design and that, as a result, some parameters used in our model were collected three days prior to imaging. This may have caused a decrease in sensitivity. Also, data collection commenced at ICU admission and not from onset of symptoms.
In conclusion, we constructed a prediction model based on D-dimer and CRP, which are widely available biomarkers that can help to differentiate between patients with a high and a low risk for VTE. It can be used to increase awareness of the possibility of VTE as well as optimize timing of imaging for critically ill patients with COVID-19 suspected for VTE.
Disclosures
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
There was no writing assistance.
CRediT authorship contribution statement
Romein W.G. Dujardin: Methodology, Data curation, Formal analysis, Writing - original draft, Writing - review & editing, Validation. Bashar N. Hilderink: Methodology, Data curation, Investigation, Formal analysis, Writing - original draft, Writing - review & editing, Validation. Wolmet E. Haksteen: Methodology, Data curation, Investigation, Writing - review & editing, Validation. S. Middeldorp: Methodology, Data curation, Writing - review & editing, Validation. Alexander P.J. Vlaar: Methodology, Data curation, Writing - review & editing, Validation. J. Thachil: Methodology, Data curation, Writing - review & editing, Validation. Marcella C.A. Müller: Methodology, Data curation, Writing - review & editing, Validation. Nicole P. Juffermans: Methodology, Data curation, Writing - original draft, Writing - review & editing, Validation.
Declaration of competing interest
All authors declare no competing interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.thromres.2020.09.017.
Appendix A. Supplementary data
Supplementary tables
References
- 1.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb. Res. 2020 doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Middeldorp S., Coppens M., van Haaps T.F., Foppen M., Vlaar A.P., Muller M.C.A. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020 doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020 doi: 10.1016/s2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thachil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J. Thromb. Haemost. 2020;18(5):1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGonagle D., O’Donnell J.S., Sharif K., Emery P., Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020 doi: 10.1016/s2665-9913(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan I.H., Savarimuthu S., Leung M.S.T., Harky A. The need to manage the risk of thromboembolism in COVID-19 patients. J. Vasc. Surg. 2020 doi: 10.1016/j.jvs.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koch V., Biener M., Muller-Hennessen M., Vafaie M., Staudacher I., Katus H.A. Diagnostic performance of D-dimer in predicting venous thromboembolism and acute aortic dissection. Eur. Heart J. Acute Cardiovasc. Care. 2020 doi: 10.1177/2048872620907322. 2048872620907322. [DOI] [PubMed] [Google Scholar]
- 12.Wells P.S., Anderson D.R., Rodger M., Forgie M., Kearon C., Dreyer J. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N. Engl. J. Med. 2003;349(13):1227–1235. doi: 10.1056/NEJMoa023153. [DOI] [PubMed] [Google Scholar]
- 13.Crawford F., Andras A., Welch K., Sheares K., Keeling D., Chappell F.M. D-dimer test for excluding the diagnosis of pulmonary embolism. Cochrane Database Syst. Rev. 2016;8:CD010864. doi: 10.1002/14651858.CD010864.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zakai N.A., McClure L.A. Racial differences in venous thromboembolism. J. Thromb. Haemost. 2011;9(10):1877–1882. doi: 10.1111/j.1538-7836.2011.04443.x. [DOI] [PubMed] [Google Scholar]
- 16.Kermali M., Khalsa R.K., Pillai K., Ismail Z., Harky A. The role of biomarkers in diagnosis of COVID-19 — a systematic review. Life Sci. 2020;254 doi: 10.1016/j.lfs.2020.117788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020 doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables