Abstract
Human eccrine sweat contains numerous biomarkers which can provide information on health, performance, and aging. Non-invasive collection and measurement of biomarkers has become especially important in recent times given viral outbreaks like SARS-CoV-2. In the current study we describe a method of sweat collection from palmar surfaces in participants via surface capture using glass beads and the resulting analysis of biomarkers from very low volumes of sweat using liquid chromatography mass spectrometry with selected ion monitoring. Study participants underwent a cognitive and physical stress task with easy and hard conditions with sweat being collected after each task. Resulting analysis found a signal for 22 steroid biomarkers and we report detailed information on selected biomarkers, given their applicability to timely real-world exemplars, including cortisol, dehydroepiandrosterone, allopregnanolone, estrone, aldosterone, and 20α/β-dihydrocortisone.
Keywords: eccrine sweat, sweat biomarkers, non-invasive measurement, surface capture, OIF/OEF/OND, precision medicine
1.0. Introduction
Sweat is a biofluid rich in biomarkers that can provide information about human cognitive performance, health, disease state(s), nutrition and environmental impacts. Steroids and other immune biomarkers have been detected in eccrine sweat (1–12). Non-invasive measurement tools of biomarkers are needed and have become especially salient given recent viral outbreaks like SARS-CoV-2, where in-person blood collection is not feasible or unadvisable. The monitoring of some biomarkers may help to monitor human performance in industrial and occupational settings, may help to predict and track acquired disease processes like obesity, and may help to determine how psychiatric sequalae are experienced and expressed.
Steroids of interest for the monitoring and enhancement of human performance and health are many and include cortisol, dehydroepiandrosterone (DHEA), allopregnanolone, estrone and aldosterone. Cortisol is secreted in response to stress, suppressing the hypothalamic-pituitary-adrenocortical (HPA) axis and negatively effecting health and cognition (13). DHEA is implicated in many physiological processes with impacts on disease resistance and immune function (14). DHEA and cortisol are the most common products of the stress response from the endocrine system, mediating short and long-term stress responses via the HPA-axis; DHEA can be converted into dehydroepiandrosterone sulfate (DHEA-S) and has been shown to antagonize the effects of cortisol (15). In humans, basal DHEA levels can be altered by exposure to traumatic events like military combat and lower cortisol/DHEA ratios have been found in patients with post-traumatic stress disorder (PTSD) (16). Positive correlations have also been found between current PTSD symptoms and the ratio of DHEA to allopregnanolone (17). Allopregnanolone is a metabolite of progesterone and plays a role in neuronal excitability at the synaptic and extrasynaptic ƴ-aminobutyric acid (GABAA)receptor (18); GABAergic neurotransmission has been shown to be impaired in subjects with PTSD (19). Furthermore, allopregnanolone is a key therapeutic target for research and development of neurodegenerative and age-related diseases (20). Estrone is one of the three most common estrogens found in humans. In men, estrone has been shown to increase as BMI rises (21) and may be a sensitive marker of acquired Type-II diabetes risk (22). Aldosterone, through the Renin-Angiotensin-Aldosterone System (RAAS) has health implications for the vascular, renal and cardiovascular systems, which is of heightened interest due to the SARS-CoV-2 virus and resulting COVID-19 disease (23).
Steroid compounds of interest in human health and performance have been measured in plasma, serum, urine, saliva, tissue and sweat (4,11–12,23). Liquid chromatography/mass spectrometry (LCMS) has been used to investigate the production and clearance of free cortisol (via downstream inactive metabolites) in human eccrine sweat during heat and/or exercise induced stress with sweat cortisol concentrations being similar to those measured in saliva (4,12).
Analysis of target biomarkers in sweat has typically required a defined volume of sweat necessary to apply benchtop assays for measurement or have utilized wearable devices for collection via exercise stress (5,12,25). Using pouches, wrist worn tubes (e.g. Macroduct), skin surface scraping, vacuuming of sweat droplets, skin swabbing with absorbent materials, glass rollers and collecting sweat from a whole body rinse have all been previously employed to collect volumes of human eccrine sweat needed for analysis (26,27). 2-D molecular mapping of latent finger prints using LCMS with electrospray ionization (LCMS-ESI) has been successful in identifying gender, age, ethnicity and disease markers of human subjects with over 80% accuracy showing that monitoring of biomarkers in very low volumes of secretions via surface capture is possible(28).
In the current study, we explore the possibility of utilizing glass beads to collect very small volumes of eccrine sweat via surface capture from the palms of the hands for targeted steroid biomarker analysis in response to stress. Furthermore, we utilize a novel washing method to extract the target biomarkers from the glass beads which simplifies sample preparation and clean up in comparison to standard approaches such as solid phase extraction that is often used for plasma and urine biofluid samples. Finally, the developed approach inclusive of sample collection, sample preparation and LCMS methodology allows for robust analysis of non-derivatized steroids thereby simplifying the analysis. Our discussion of results focuses predominately on cortisol, DHEA, allopregnanolone, aldosterone and estrone given interest in enhancement of human performance as mentioned earlier. We hypothesized that collection of palmar sweat via surface capture would be a non-invasive, rapid, easily scalable method which would yield sweat samples rich in molecular information correlating with physiological systems, and provide a sensitive method for measuring biomarker changes on a time scale of minutes in response to stress.
2.0. Materials and Methods
2.1. Participants
All participants gave informed consent before taking part in the experiment. The experiment was approved by the University of Arizona Institutional Review Board. Participants were university students recruited through a program enabling them to receive educational credit for participation. 5 participants took part in the study (1 male, 4 female; ages 18–22).
2.2. Stress tasks and sample collection
Study participants were asked to complete a series of cognitive and physical tasks on a computer over the course of 45 minutes during which palmar sweat samples were collected concurrently with real-time, high-resolution thermographic imaging of sweat pore activation from the left lateral malleolus. Of note, this current manuscript focuses on the collection and characterization of biomarkers collected from palmar sweat rather than thermographic imaging. A concurrent report details the thermal imaging of sweat pore activation (29).
In this study, participants followed prompts on the screen from a program designed in MATLAB. A neurocognitive task (N-back 1&2 digits) was presented with two difficulty conditions (easy/hard). Participants then engaged with a physical task (finger/keyboard tapping) with two difficulty conditions (easy/hard) which were randomized within task for each participant.
A batch of 4mm glass beads made from borosilicate were baked at 475 °C for 4 hours to remove organic contaminates, allowed to return to room temperature, divided into 35 bead aliquots and stored at room temperature in falcon tubes until use. Participants had these glass beads poured from the tubes into their hands and then instructed to roll them between the palms of their hands for 60 seconds. After collection of these samples, the beads were returned to the containers, and then were stored at 4 °C until biomarker analysis. Sample collection was performed at the beginning of the study session, after completion of N-back cognitive tasks, and after completion of physical tasks.
2.2. LCMS-ESI-SIM of steroid biomarkers
LCMS with electrospray ionization and selected ion monitoring (LCMS-ESI-SIM; ThermoScientific TSQ Quantiva) was used to analyze the extract of steroid biomarkers from the glass beads. Four 4mm glass beads from each participant were washed with 50 μL of 70/30 LCMS water/ACN (1% formic acid) in glass LCMS vials and then transferred to a second LCMS vial with a clean glass insert. 2 μL of glass bead extract was injected into the instrument for analysis by LCMS-ESI-SIM. Chromatography of the extract was accomplished using a Restek Raptor Biphenyl column (200 mm × 2.1 mm; 2.7 um bead) held at 30C and a water/ACN (0.1% formic acid) binary solvent system. The solvent flow rate was set to 0.5 mL/min with a gradient of ACN from 10% - 100% over 3 minutes followed by a column washing step for 1-minute at 100% ACN and a 3-minute column equilibrium step at 10% ACN before the next sample injection. Mass spectrometer settings used for detection of steroid biomarkers were as follows: 3kV in positive ion mode, 0.4 resolution, 20usec dwell time, 300 °C vaporizer temperature and ion transfer tube temperature, 50abs N2 sheath gas, 25Arb N2 aux gas. Table 1 shows the steroid biomarkers tracked in the extract samples.
Table 1.
Steroid Biomarker Surveyed in Palmar Sweat
| Steroid target biomarker | Mass [m/z +1; +H] | Steroid classification |
|---|---|---|
| Estrone | 269 | Estrogen |
| Dihydroepiandrosterone (DHEA) | 271 | Androgen |
| Etiocholanolone | 273 | |
| Androsterone | 273 | |
| Androstenedione | 287 | |
| Testosterone | 289 | |
| Dihydrotestosterone | 291 | |
| Progesterone | 315 | Progestogen |
| 17a-hydroxypregnenolone | 315 | |
| Pregnenolone | 317 | |
| Pregnanolone | 319 | |
| Allopregnanolone | 319 | |
| 17a-hydroxyprogesterone | 331 | |
| 11-deoxycorticosterone | 331 | Corticosteroids |
| 11-deoxycortisol | 347 | |
| Corticosterone | 347 | |
| Aldosterone | 359 | |
| Cortisone | 361 | |
| 20α/β-dihydrocortisone (20α/β-DHCN) | 363 | |
| Cortisol | 363 | |
| 20α/β-dihydrocortisol (20α/β-DHCL) | 365 | |
| Cholesterol | 387 |
3.0. Results
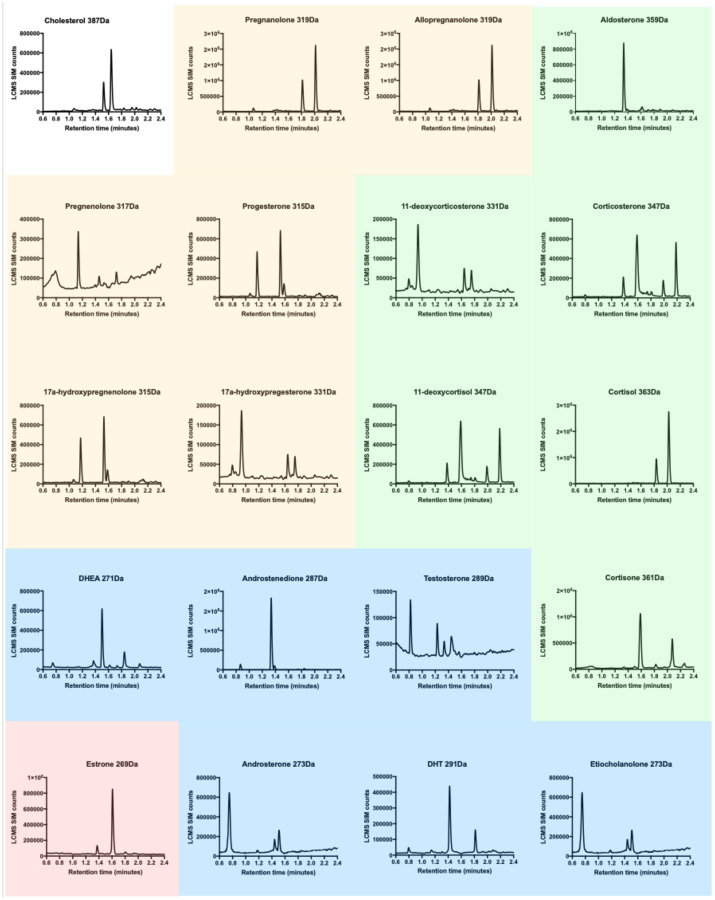
The results shown below in Figure 1 summarize the different steroid molecules that were found in palmar sweat utilizing an adapted LCMS methodology from (11).
Figure 1.
LCMS-ESI-SIM chromatograms for steroid biomarkers found in palmar sweat. Progestogens (yellow), corticosteroids (green), androgens (blue), estrogens (pink), cholesterol (white).
3.1. Stress induced changes in steroid biomarkers
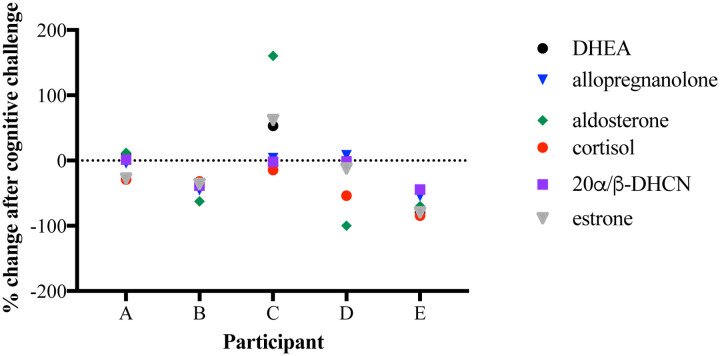
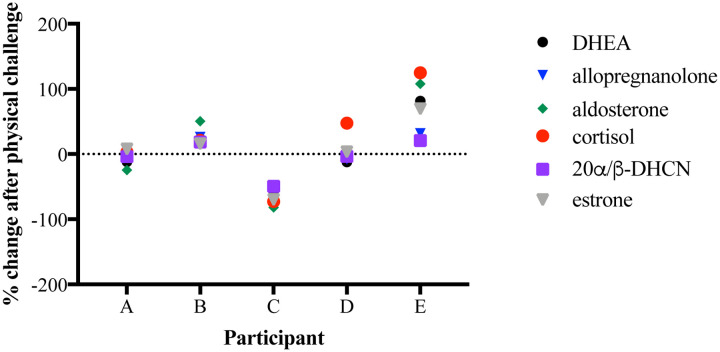
Healthy human volunteers were subjected to both cognitive and physical stress challenges on a computer. Figures 2 & 3 present aggregated data for cortisol, 20α/β-dihydrocortisone (a metabolite of cortisol), DHEA, estrone, allopregnanolone, and aldosterone after completion of each challenge. Figure 2 shows the biomarker response to a cognitive challenge as a percent change in measured signal. Generally, a decrease in signal was observed for most biomarkers across participants. Figure 3 shows the biomarker response to a physical challenge as a percent change in measured signal as well. Generally, an increase was observed for most biomarkers across participants. For both types of challenges, significant biomarker responses were observed within minutes of the challenge.
Figure 2.
Percent change in palmar sweat steroid biomarker signal after cognitive challenge.
Figure 3.
Percent change in palmar sweat steroid biomarker signal after physical challenge.
3.2. LCMS-ESI-SIM reproducibility
Eight different steroid biomarkers were used to determine the reproducibility and robustness of the novel LCMS-ESI-SIM method to measure the target steroid biomarkers. The overall relative standard deviation of the LCMS-ESI-SIM measurement, inclusive of sample collection, sample preparation, and instrument variability, was 7%. Figure 4 shows the linear response of these eight steroids when palmar sweat was extracted using the same method but with different numbers of glass beads. The correlation of each least-squares-fit (R2) ranged from 0.977 – 0.997 providing confidence in the extraction method. The slope of the lines ranged from 49468 – 355476 (SIM counts/4mm glass bead) highlighting the different sensitivity of LCMS detection for each molecule which may be, in part, influenced by the differential solubility of each steroid in the 70/30 water/ACN extraction solvent and the differential ionization potential for each molecule in the LCMS instrument.
Figure 4.
LCMS-ESI-SIM linear response to increasing glass beads used for extraction of target steroid biomarkers from palmar sweat.
4.0. Discussion
In this study, we hypothesized that collection of palmar sweat would yield sweat samples rich in molecular information and provide a sensitive method for measuring biomarker changes on a time scale of minutes in response to stress challenges. The data presented supports this hypothesis and shows that the steroid response is both individualistic and dynamic in real time. The change of measured biomarkers in response to short cognitive or physical challenges is significant and occurs within the time frame of minutes which agrees with the known time frame response of salivary and sweat cortisol (12). The presentation of the data in Figures 2 & 3 as a percent change in biomarker levels allows for comparison between individuals despite the strong individual variability of raw data observed between study participants.
Given multiple factors that may influence sweat production, contents, volume, and activation of individual pores (i.e., hydration, health status, medications, diet, gender, topical treatments, etc.), the concentrations of steroid biomarkers in palmar sweat can vary from person to person, sometimes by orders of magnitude. For example, the LCMS-ESI-SIM response for DHEA spanned from 105 to 107 counts for the participants. The general observed trend in the data was an increase in biomarker concentrations after completion of the physical task, with the exception of a single participant (Participant C) as seen in Figure 3. By comparison, the general trend observed was a decrease in biomarker concentration after completion of the cognitive task as shown in Figure 2. Hyde and colleagues (29) (data not shown) also demonstrated a 4-fold increase in the number of sweat pores activated after completion of the finger tapping physical challenge compared with the N-back cognitive task using thermographic imaging, suggesting that an increase in sweat pore activation contributes in part to the increased biomarker signal measured by LCMS-ESI-SIM. Further studies are needed to deconvolute the individual contributions of increased sweat volume and increased biomarker concentration to the measured LCMS signal for each biomarker.
5.0. Conclusion
Non-invasive methods for detecting biomarkers are needed in order to enhance the ability to monitor and track human performance, health, and disease process. The selected biomarkers presented are of particular interest for tracking stress responsiveness and changes in aging populations. U.S. military veterans from Operation Iraqi Freedom (OIF), Operation Enduring Freedom (OEF) and Operation New Dawn (OND) are a timely example of a population with multiple acquired physical and psychiatric conditions where biomarker tracking may be beneficial. OIF/OEF/OND servicemembers and veterans have high rates of PTSD at 23% (30) and high rates of obesity (BMI >30kg/m2) at 44% (31). While still relatively young, these complex conditions are unlikely to resolve as this cohort continues to age, further complicating typical age-related physical issues and normal age-related cognitive decline. As previously noted, biomarkers like cortisol, DHEA, allopregnanolone, estrone and aldosterone are all implicated and play a role in the moderation of these conditions. Further, monitoring the status of steroids and immune biomarkers of servicemembers during their active-duty service may provide useable and actionable information necessary to enhance overall performance and potentially decrease the likelihood of negative outcomes after service. In veterans and the general population, biomarker monitoring may help to predict and determine the onset of disease, the course of disease processes, and may influence precision medicine strategies.
We have now shown that using non-invasive methods of eccrine sweat collection in very low volumes of sweat is possible via surface capture using glass beads. Multiple biomarkers can be found and measured and may hold promise in monitoring human performance and health. Future studies should focus on applying this methodology to large and diverse cohorts with broad demographics across age ranges, ethnicities, gender, etc., and validating the results against established values in other biofluids such as saliva, serum or urine.
6.0. Acknowledgements
The authors would like to thank UArizona Research Innovation and Impact and NIH Grant #NIHT35 HL07479 (Short-Term Institutional Research Training Grant), the Andrew Weil Center for Integrative Medicine for administrative support, and the Arizona Arthritis Center for facilities support.
7.0 References
- 1.Hu Y, Converse C, Lyons MC, Hsu WH. Neural control of sweat secretion: a review. Br J Dermatol. 2018;178(6):1246–56. [DOI] [PubMed] [Google Scholar]
- 2.Baker LB. Physiology of sweat gland function: The roles of sweating and sweat composition in human health. Temperature [Internet]. 2019. July 3;6(3):211–59. Available from: 10.1080/23328940.2019.1632145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jia M, Belyavskaya E, Deuster P, Sternberg EM. Development of a sensitive microarray immunoassay for the quantitative analysis of neuropeptide y. Anal Chem. 2012;84(15):6508–14. [DOI] [PubMed] [Google Scholar]
- 4.Jia M, Chew W, Feinstein Y, Skeath P, Sternberg E. Quantification of Cortisol in Human Eccrine Sweat by Liquid Chromatography - Tandem Mass Spectrometry. Analyst [Internet]. 2016; Available from: http://pubs.rsc.org/en/Content/ArticleLanding/2016/AN/C5AN02387D [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sonner Z, Wilder E, Heikenfeld J, Kasting G, Beyette F, Swaile D, et al. The microfluidics of the eccrine sweat gland, including biomarker partitioning, transport, and biosensing implications. Biomicrofluidics. 2015;9(3):031301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouslimani A, Porto C, Rath CM, Wang M, Guo Y, Gonzalez A, et al. Molecular cartography of the human skin surface in 3D. Proc Natl Acad Sci. 2015;112(17):E2120–E2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor NAS, Machado-Moreira CA. Regional variations in transepidermal water loss, eccrine sweat gland density, sweat secretion rates and electrolyte composition in resting and exercising humans. Extrem Physiol Med. 2013;2(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marques-Deak A, Cizza G, Eskandari F, Torvik S, Christie IC, Sternberg EM, et al. Measurement of cytokines in sweat patches and plasma in healthy women: Validation in a controlled study. J Immunol Methods. 2006;315(1–2):99–109. [DOI] [PubMed] [Google Scholar]
- 9.Cizza G, Marques AH, Eskandari F, Christie IC, Torvik S, Silverman MN, et al. Elevated Neuroimmune Biomarkers in Sweat Patches and Plasma of Premenopausal Women with Major Depressive Disorder in Remission: The POWER Study. Biol Psychiatry. 2008;64(10):907–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marques AH, Silverman MN, Sternberg EM. Evaluation of stress systems by applying noninvasive methodologies: Measurements of neuroimmune biomarkers in the sweat, heart rate variability and salivary cortisol. Neuroimmunomodulation. 2010;17(3):205–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Kock N, Acharya SR, Ubhayasekera SJKA, Bergquist J. A Novel Targeted Analysis of Peripheral Steroids by Ultra-Performance Supercritical Fluid Chromatography Hyphenated to Tandem Mass Spectrometry. Sci Rep [Internet]. 2018;8(1):16993 Available from: 10.1038/s41598-018-35007-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Runyon JR, Jia M, Goldstein MR, Skeath P, Abrell L, Chorover J, et al. Dynamic behavior of cortisol and cortisol metabolites in human eccrine sweat. Int J Progn Heal Manag. 2019; [Google Scholar]
- 13.Lee DY, Kim E, Choi MH. Technical and clinical aspects of cortisol as a biochemical marker of chronic stress. BMB Rep [Internet]. 2015. April 30;48(4):209–16. Available from: http://koreascience.or.kr/journal/view.jsp?kj=E1MBB7&py=2015&vnc=v48n4&sp=209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prall SP, Muehlenbein MP. DHEA Modulates Immune Function: A Review of Evidence. Vitam Horm [Internet]. 2018;108:125–44. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0083672918300347 [DOI] [PubMed] [Google Scholar]
- 15.Kamin HS, Kertes DA. Cortisol and DHEA in development and psychopathology. Horm Behav [Internet]. 2017. March;89:69–85. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0018506X1630215X [DOI] [PubMed] [Google Scholar]
- 16.van Zuiden M, Haverkort SQ, Tan Z, Daams J, Lok A, Olff M. DHEA and DHEA-S levels in posttraumatic stress disorder: A meta-analytic review. Psychoneuroendocrinology [Internet]. 2017. October;84:76–82. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0306453017303281 [DOI] [PubMed] [Google Scholar]
- 17.Rasmusson AM, Pinna G, Paliwal P, Weisman D, Gottschalk C, Charney D, et al. Decreased Cerebrospinal Fluid Allopregnanolone Levels in Women with Posttraumatic Stress Disorder. Biol Psychiatry [Internet]. 2006;60(7):704–13. Available from: http://www.sciencedirect.com/science/article/pii/S0006322306004021 [DOI] [PubMed] [Google Scholar]
- 18.Paul SM, Pinna G, Guidotti A. Allopregnanolone: From molecular pathophysiology to therapeutics. A historical perspective. Neurobiol Stress [Internet]. 2020. May;12:100215 Available from: https://linkinghub.elsevier.com/retrieve/pii/S2352289520300059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cruz DA, Glantz LA, McGaughey KD, Parke G, Shampine LJ, Kilts JD, et al. Neurosteroid Levels in the Orbital Frontal Cortex of Subjects With PTSD and Controls: A Preliminary Report. Chronic Stress [Internet]. 2019. January 1;3:2470547019838570 Available from: 10.1177/2470547019838570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernandez GD, Brinton RD. Allopregnanolone as a Therapeutic to Regenerate the Degenerated Brain In: Brinton RD, Genazzani AR, Simoncini T, Stevenson JC, editors. Sex Steroids’ Effects on Brain, Heart and Vessels: Volume 6: Frontiers in Gynecological Endocrinology [Internet]. Cham: Springer International Publishing; 2019. p. 111–23. Available from: 10.1007/978-3-030-11355-1_7 [DOI] [Google Scholar]
- 21.Mezzullo M, Di Dalmazi G, Fazzini A, Baccini M, Repaci A, Gambineri A, et al. Impact of age, body weight and metabolic risk factors on steroid reference intervals in men. Eur J Endocrinol [Internet]. 2020. May;182(5):459–71. Available from: https://eje.bioscientifica.com/view/journals/eje/182/5/EJE-19-0928.xml [DOI] [PubMed] [Google Scholar]
- 22.Jasuja GK, Travison TG, Davda M, Rose AJ, Zhang A, Kushnir MM, et al. Circulating Estrone Levels Are Associated Prospectively With Diabetes Risk in Men of the Framingham Heart Study. Diabetes Care [Internet]. 2013. September;36(9):2591–6. Available from: http://care.diabetesjournals.org/lookup/doi/10.2337/dc12-2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ingraham NE, Barakat AG, Reilkoff R, Bezdicek T, Schacker T, Chipman JG, et al. Understanding the Renin-Angiotensin-Aldosterone-SARS-CoV-Axis: A Comprehensive Review. Eur Respir J [Internet]. 2020. January 1;2000912 Available from: http://erj.ersjournals.com/content/early/2020/04/20/13993003.00912-2020.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaikwad NW. Ultra Performance Liquid Chromatography-Tandem Mass Spectrometry Method for Profiling of Steroid Metabolome in Human Tissue. Anal Chem [Internet]. 2013. May 21;85(10):4951–60. Available from: 10.1021/ac400016e [DOI] [PubMed] [Google Scholar]
- 25.Heikenfeld J, Jajack A, Feldman B, Granger SW, Gaitonde S, Begtrup G, et al. Accessing analytes in biofluids for peripheral biochemical monitoring. Nat Biotechnol [Internet]. 2019; Available from: 10.1038/s41587-019-0040-3 [DOI] [PubMed] [Google Scholar]
- 26.Hussain JN, Mantri N, Cohen MM. Working Up a Good Sweat - The Challenges of Standardising Sweat Collection for Metabolomics Analysis. Clin Biochem Rev [Internet]. 2017. February;38(1):13–34. Available from: https://pubmed.ncbi.nlm.nih.gov/28798503 [PMC free article] [PubMed] [Google Scholar]
- 27.Kutyshenko VP, Molchanov M, Beskaravayny P, Uversky VN, Timchenko MA. Analyzing and Mapping Sweat Metabolomics by High-Resolution NMR Spectroscopy. PLoS One [Internet]. 2011. December 14;6(12):e28824 Available from: 10.1371/journal.pone.0028824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Z, Zare RN. Personal Information from Latent Fingerprints Using Desorption Electrospray Ionization Mass Spectrometry and Machine Learning. Anal Chem [Internet]. 2017. January 17;89(2):1369–72. Available from: 10.1021/acs.analchem.6b04498 [DOI] [PubMed] [Google Scholar]
- 29.Hyde JN, Majdi MS, Kromenacker B, Wilson RC, Staroschack C, Rodriguez JJ, et al. Thermographic imaging for detection of changes in autonomic nervous system activity and response to varying stress levels through changes in sweat pore activation. PLoS One. 2020;Under review. [Google Scholar]
- 30.Fulton JJ, Calhoun PS, Wagner HR, Schry AR, Hair LP, Feeling N, et al. The prevalence of posttraumatic stress disorder in Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) Veterans: a meta-analysis. J Anxiety Disord. 2015. April;31:98–107. [DOI] [PubMed] [Google Scholar]
- 31.Wischik DL, Magny-Normilus C, Whittemore R. Risk Factors of Obesity in Veterans of Recent Conflicts: Need for Diabetes Prevention. Curr Diab Rep [Internet]. 2019. September;19(9):70 Available from: http://link.springer.com/10.1007/s11892-019-1191-9 [DOI] [PMC free article] [PubMed] [Google Scholar]