Abstract
Objective
To reveal the distribution signature of cancer susceptibility genes in patients with gastric adenocarcinoma, offering a diagnostic and prognostic surrogate for disease risk management and therapeutic decisions.
Methods
A total of 282 patients with gastric adenocarcinoma (182 males and 100 females) were enrolled in this study, with peripheral blood genomic DNA extracted. Mutations of 69 canonical cancer susceptibility genes or presumably tumor-related genes were analyzed by targeted capture-based high-throughput sequencing. Candidate mutations were particularly selected for discussion on tumor pathogenesis according to the American College of Medical Genetics and Genomics (ACMG) guidelines.
Results
In this study, 7.1% (20/282) of patients with gastric adenocarcinoma were found to harbor mutations of canonical or presumable cancer susceptibility genes. The detection rate in male patients (3.8%, 7/182) was significantly lower than that in female patients (13%, 13/100) (P=0.004). The most recurrent mutations were in A-T mutated (ATM) (1.1%, 3/282), followed by BRCA1, BRIP1 and RAD51D, all showed a detection rate of 0.7% (2/282). Mutations in three genes associated with hereditary gastric cancer syndromes were detected, namely, PMS2 and EPCAM associated with Lynch syndrome and CDH1 associated with hereditary diffuse gastric cancer. The detection frequencies were all 0.4% (1/282). Notwithstanding no significant difference observed, the age of patients with pathogenic mutations or likely pathogenic mutations is slightly younger than that of non-carriers (median age: 58.5 vs. 60.5 years old), while the age of patients with ATM mutations was the youngest overall (median age: 49.3 years old).
Conclusions
Our study shed more light on the distribution signature and pathogenesis of mutations in gastric cancer susceptibility genes, and found the detection rate of pathogenic and likely pathogenic mutations in male patients was significantly lower than that in female patients. Some known and unidentified mutations were found in gastric cancer, which allowed us to gain more insight into the hereditary gastric cancer syndromes from the molecular perspective.
Keywords: Gastric adenocarcinoma, cancer-susceptibility-associated genes, pathogenic mutations
Introduction
Gastric cancer (GC) is the fifth most common cancer and the third leading cause of cancer mortality worldwide (1,2). Although the substantive molecular underpinnings of gastric cancer remain largely elusive, germline genotypes, risky behaviors, alcohol consumption, smoking and unhealthy diet, and infectious pathogens such as Helicobacter pylori (H. pylori) all showed close associations with gastric cancer pathogenesis (3-6).
Characterized by multifocal signet-ring cells pathology, hereditary diffuse gastric cancer (HDGC) is the most typical form of hereditary gastric cancer, in which germline mutations in E-cadherin (CDH1) gene were frequently reported. CDH1 germline mutations were reported in 30%−50% of HDGC cases, with more than 100 pathogenic mutations in this gene identified (7-9). Other hereditary gastrointestinal cancer syndromes include Lynch syndrome caused by mutations in DNA mismatch repair genes (10), Peutz-Jeghers syndrome mostly associated with mutations in the Serine/Threonine Kinase (STK11) and Li-Fraumeni syndrome associated with germline Tumor Protein P53 (TP53) mutations (11,12). Furthermore, germline oncogenic mutations in A-T mutated (ATM), breast cancer susceptibility gene 2 (BRCA2), and Partner and localizer of BRCA2 (PALB2) which regulate DNA mismatch repair were also found in some families with HDGC (13).
In the past decades, researchers have analyzed 114 cancer related genes with the data of The Cancer Genome Atlas (TCGA), and suggested that 11% of patients with gastric adenocarcinoma harbored pathogenic or likely pathogenic mutations, among which mutations in genes triggering Fanconi anemia signaling pathways by itself or indirectly were the most dominant (14,15). It was shown that mutations in ATM and PALB2 were significantly more prevalent in patients with gastric adenocarcinoma compared with other cancer types, indicating these mutations might essentially increase carriers’ risks of developing gastric adenocarcinoma (16-18).
Materials and methods
Patients
A total of 282 patients with gastric adenocarcinoma were enrolled in this study, including 182 males and 100 females. Ages of diagnosis were reported in 92% (259/282) of patients, with a median age of 58.8 years old. Written informed consent were obtained from all patients in data collection, processing and publication. This study was approved by the Institutional Review Board of Peking University Cancer Hospital & Institute and all procedures were conducted in accordance with the 1964 Helsinki Declaration and its later amendments.
Targeted capture-based genomic sequencing
Genomic DNA was extracted from peripheral blood samples using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany), and further sheared by Bioruptor (Bioruptor, Diagenode, Liege, Belgium) according to manufacturer’s instructions. Indexed NGS libraries were constructed utilizing the NEBNext UltraII DNA Library Preparation Kit (New England Biolabs, Inc., Ipswich, USA). All libraries were hybridized to custom-designed oligonucleotide probes (IDT, Integrated DNA Technologies, Inc., Coralville, USA) spanning the whole exome of 288 cancer susceptibility genes. DNA sequencing was then performed with PE75 sequencing strategy on the Illumina Sequencing System.
Sequencing data were further subjected to processing as follows. The original FASTQ data underwent quality control to remove the low-quality reads. The sequencing reads remained were then mapped to the reference human genome (hg19) using the Burrows-Wheel Aligner (BWA) (19). Single-nucleotide variations (SNVs) and insertions/deletions (indels) were obtained with The Gene Analysis Toolkit (GATK). Finally, National Center of Biotechnology Information (NCBI) annotation release 104, frequency database dbSNP135, 1000human, ESP6500, Exome Aggregation Consortium (ExAC) were used for annotation. Human Genome Variation Society (HGVS) was used for standardized naming of variations, while Online Mendelian Inheritance in Man (OMIM), The Human Gene Mutation Database (HGMD) disease databases and clinical genome database (CGD) were used for mutations and disease annotation.
In total, 69 germline mutations in cancer susceptibility genes were selected for analysis and further discussed as follows: APC, ATM, AXIN2, BAP1, BARD1, BLM, BMPR1A, BRCA1, BRCA2, BRIP1, CDC73, CDH1, CDK4, CDKN1B, CDKN2A, CHEK2, DICER1, EPCAM, FANCC, FH, FLCN, GALNT12, HOXB13, KIT, MAX, MEN1, MET, MLH1, MLH3, MRE11A, MSH2, MSH3, MSH6, MUTYH, NBN, NF1, NF2, NTRK1, PALB2, PDGFRA, PIK3CA, PMS1, PMS2, POLD1, POLE, PTCH1, PTCH2, PTEN, RAD50, RAD51C, RAD51D, RB1, RET, SDHA, SDHAF2, SDHB, SDHC, SDHD, SMAD4, SMARCA4, SMARCB1, STK11, TMEM127, TP53, TSC1, TSC2, VHL, WT1, XRCC2.
Statistical analysis
Chi-square test was performed to determine difference in detection rates of pathogenic and likely pathogenic mutations between males and females. Mann-Whitney test was carried out when appropriate for comparing ages between germline mutations carriers and non-carriers. For comparisons in variant allele frequency, Fisher’s exact test was used. Statistical analyses were carried out using IBM SPSS Statistics (Version 20.0; IBM Corp., New York, USA). Odds ratios (ORs) are presented with 95% confidence interval (95% CI). Two-sided P<0.05 was considered statistically significant.
Results
Germline pathogenic variants (GPVs) in male patients were lower than in female patients
Overall, we identified 35,243 germline variants within the whole exome and respective flanks (±10 bp) in 282 patients. After being filtered by qualities, frequencies and biological functions (Figure 1A ) (20), 1,151 candidate variants were screened out eventually. The clinical significance of candidate variants was analyzed according to American College of Medical Genetics and Genomics (ACMG) guideline (21), with pathogenic and likely pathogenic mutations (Table 1 ) identified in 7.1% (20/282) patients. The detection rate in male patients (3.8%, 7/182) was significantly lower than that in female patients (13%, 13/100) (P=0.004) when smoking, H. pylori infection, chronic gastritis and tumor stage were consistent in the two groups (Table 2 ).
1.
Landscape of pathogenic and likely pathogenic mutations in gastric adenocarcinoma. (A) Analysis flow of pathogenic and likely pathogenic mutations in 282 patients; (B) Landscape of pathogenic and likely pathogenic mutations in 282 patients. EAS, East Asians; ExAC, Exome Aggregation Consortium; ACMG, American College of Medical Genetics and Genomics; VUS, variants of uncertain significance.
1. Pathogenic mutations and likely pathogenic mutations.
| Sample ID | Mutation name | Function | Mutation frequency | Depth | Clinical significance |
| GC001 | NM_000051.3(ATM): c.8473C>T (p.Q2825*) | Nonsense | 0.50 | 254 | Pathogenic |
| GC002 | NM_000051.3(ATM): c.8435_8436delCT (p.S2812Ffs*2) | Frameshift | 0.45 | 220 | Pathogenic |
| GC003 | NM_000051.3(ATM): c.6100C>T (p.R2034*) | Nonsense | 0.50 | 206 | Pathogenic |
| GC004 | NM_007294.3(BRCA1): c.2138C>G (p.S713*) | Nonsense | 0.50 | 424 | Pathogenic |
| GC005 | NM_007294.3(BRCA1): c.3359_3363delTTAAT (p.V1120Dfs*11) | Frameshift | 0.42 | 329 | Likely Pathogenic |
| GC006 | NM_032043.2(BRIP1): c.3185_3186delCA (p.T1062Ifs*18) | Frameshift | 0.48 | 465 | Likely Pathogenic |
| GC007 | NM_032043.2(BRIP1): c.2990_2993delCAAA (p.T997Rfs*61) | Frameshift | 0.49 | 176 | Pathogenic |
| GC008 | NM_004360.3(CDH1): c.603delT (p.V202Lfs*13) | Frameshift | 0.43 | 291 | Pathogenic |
| GC009 | NM_002354.2(EPCAM): c.753T>G (p.Y251*) | Nonsense | 0.45 | 182 | Likely Pathogenic |
| GC010 | NM_024642.4(GALNT12): c.5G>A (p.W2*) | Nonsense | 0.41 | 117 | Likely Pathogenic |
| GC011 | NM_001040108.1(MLH3): c.429dupG (p.T144Dfs*7) | Frameshift | 0.47 | 311 | Likely Pathogenic |
| GC012 | NM_002439.4(MSH3): c.1764-2A>G | Splice-3 | 0.43 | 230 | Likely Pathogenic |
| GC013 | NM_001128425.1(MUTYH): c.55C>T (p.R19*) | Nonsense | 0.49 | 267 | Pathogenic |
| GC014 | NM_002485.4(NBN): c.2206G>T (p.E736*) | Nonsense | 0.46 | 154 | Likely Pathogenic |
| GC015 | NM_024675.3(PALB2): c.3114-2A>G | Splice-3 | 0.50 | 114 | Likely Pathogenic |
| GC016 | NM_000535.5(PMS2): c.24-1G>C | Splice-3 | 0.45 | 211 | Likely Pathogenic |
| GC017 | NM_005732.3(RAD50): c.2165_2166insT (p.K722Nfs*6) | Frameshift | 0.49 | 333 | Pathogenic |
| GC018 | NM_002878.3(RAD51D): c.270_271dupTA (p.K91Ifs*13) | Frameshift | 0.45 | 164 | Pathogenic |
| GC019 | NM_002878.3(RAD51D): c.270_271dupTA (p.K91Ifs*13) | Frameshift | 0.41 | 259 | Pathogenic |
| GC020 | NM_003000.2(SDHB): c.137G>A (p.R46Q) | Missense | 0.53 | 209 | Pathogenic |
2. Clinical characteristics of patients.
| Variables | Samples without GPVs (n) | Samples with GPVs (n) | P |
| GPV, germline pathogenic variants; H. pylori, Helicobacter pylori. | |||
| Sex | |||
| Male | 175 | 7 | |
| Female | 87 | 13 | 0.004 |
| Median age (year) | 60.5 | 58.5 | 0.718 |
| Smoking | |||
| Yes | 80 | 6 | |
| No | 182 | 14 | 0.960 |
| H. pylori infection | |||
| Yes | 115 | 10 | |
| No | 97 | 7 | |
| Unknown | 50 | 3 | 0.843 |
| Chronic gastritis | |||
| Yes | 262 | 20 | |
| No | 0 | 0 | 0.148 |
| Stage | |||
| I | 6 | 1 | |
| II | 4 | 1 | |
| III | 33 | 2 | |
| IV | 219 | 16 | 0.584 |
Known and unidentified mutations were both found in GC
The known GC-related syndromes genes were identified in three cases, namely, PMS2 and EPCAM associated with Lynch syndrome, and HDGC associated CDH1 gene (Figure 1B ).
Among mutations with unidentified significance, the c.1172_1173delCT of PTCH2 gene is of particular interest, given that previous studies indicated this mutation might be related to the nevoid basal cell carcinoma syndrome (NBCCS), but the variant allele frequency (VAF) reported in ExAC (East Asians) and our study were 29/8,644 (0.34%) and 3/564 (0.53%), accordingly, both showing no significant difference (P=0.444) (22).
Patients with the most recurrent mutations were slightly younger than non-carriers
The most recurrent mutations were observed in ATM gene (1.1%, 3/282), followed by BRCA1, BRIP1 and RAD51D (0.7%, 2/282) (Figure 1B , Table 3 ). Of note, ATM, BRCA1 and RAD51D might be associated with gastric adenocarcinoma, in spite of the modest sample size which leads to insufficient statistical significance.
3. Candidate genes associated with gastric adenocarcinoma.
| Genes | Cases, mutated samples (n/N) | ExAC_EAS, mutated samples (n/N) | OR (95% CI) | P |
| OR, odds ratio; 95% CI, 95% confidence interval. | ||||
| ATM | 3/282 | 16/3,931 | 2.63 (0.76−9.09) | 0.130 |
| BRCA1 | 2/282 | 6/3,933 | 4.68 (0.94−23.28) | 0.096 |
| BRIP1 | 2/282 | 17/3,933 | 1.65 (0.38−7.16) | 0.366 |
| RAD51D | 2/282 | 9/4,327 | 3.43 (0.74−15.94) | 0.143 |
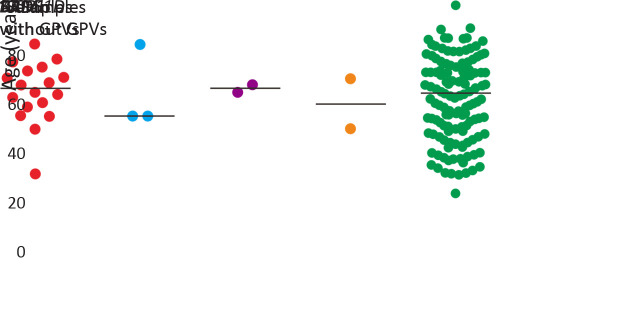
In this study, patients with GPVs were slightly younger than non-carriers (median age: 58.5 vs. 60.5 years old, P=0.718). Patients with ATM mutations were the youngest (median age: 49.3 years old), whereas no significant difference could be observed as well (Figure 2 ).
2.
Age distribution of patients with pathogenic mutations or likely pathogenic mutations. GPV, germline pathogenic variants.
Discussion
In this study, 282 patients with gastric adenocarcinoma were sequenced by targeted capture NGS, and the mutational characteristics of 69 cancer susceptibility genes were analyzed. We found that 7.1% of the patients had pathogenic or likely pathogenic mutations. To our knowledge, this novel study demonstrates that the detection rate of pathogenic and likely pathogenic mutations in male patients (3.8%, 7/182) was significantly lower than that in female patients (13%, 13/100).
Most of these patients harbored mutations in genes like ATM, RAD51D, and so on. Next-generation sequencing efforts have revealed that ATM is among the most commonly aberrant genes in sporadic cancers and reports point mutations in 1%−5% of GC (17), which was the same as our results about GC. Though loss of ATM protein expression was associated with worse prognosis in colorectal cancer (17), it still remains unknown for gastric adenocarcinoma.
The c.270_271dupTA mutation of RAD51D gene was determined as a founder mutation in Asian population, increasing the risk of developing and decreasing progression-free survival of ovarian cancer (20,23). While it was 3.0% in ovarian cancer, the incidence of this mutation in GC is approximately 0.2% and 0.7% (2/282) in our study (P>0.05) (24). Nevertheless, in this regard whether the mutation imposes a higher risk of GC pathogenesis or poor prognosis to carriers requires further study.
HDGC is an autosomal dominant cancer syndrome, characterized by poorly differentiated adenocarcinoma. With infiltration into the gastric wall, HDGC results in thickening of the gastric wall without ostensible bulk formation. Diffuse GC is also referred to as signet-ring carcinoma or isolated cell-type carcinoma. The average age of onset of HDGC is around 38 years old and the disease mostly occurs before the age of 40 years (25). CDH1 gene is the most essential and well-characterized cancer susceptibility gene in HDGC (26,27), with its mutations identified in 30%−50% of patients. From the epidemiological perspective, the incidence of GC varies across geographic regions, with the prevalence of germlineCDH1 mutations among GC patients ranging from 1% to 3% (28). The cumulative incidence of GC by the age of 80 years in patients with this mutation were 70 % in males and 56 % in females, and 42% of women also had a risk of breast cancer (29). In our study, CDH1 mutation (c.603delT) was identified in a female patient who was diagnosed with poorly differentiated adenocarcinoma on the gastric angle at the age of 71 years. While the majority of variants were found by Lo et al, included the truncating variants c.1003C>T, c.1212delC, c.1792C>T and c.2398delC and the splice site variants c.1008G>T, c.1137G>A and c.1679C>G (27). It was believed that different CDH1 genotypes were associated with different biological and clinical manifestations in this cancer predisposition syndrome. The observation showed that somatic structural CDH1 alterations conferred a poorer outcome when compared with epigenetic silencing or differences in the second hit of the CDH1 allele in primary vs. metastatic lesions.
The c.1172_1173delCT mutation in PTCH2 gene was defined in ClinVar database as a likely pathogenic mutation. In 2013, researchers reported a case where a 13-year-old girl was diagnosed with NBCCS, due to detection of multiple keratocystic odontogenic tumors, rib abnormalities, and c.1172_1173delCT mutation in PTCH2 gene (22). The disease is an autosomal dominant genetic disorder characterized by a considerably high likelihood of developmental defects and oncogenesis presence. It was indicated in the discussion that NBCCS individuals with PTCH2 mutations might exhibit a less aggressive phenotype. However, in 2019, researchers also found a healthy person harboring homozygous PTCH2 mutants. Moreover, in vivo animal model experiments delineated PTCH2 knock-out mice showed no NBCCS pathology as well. Considering that previously reported PTCH2 mutants harboring patients were not diagnosed according to the recommended diagnostic protocols, the pathogenicity of these mutations remains disputable (30). In our study, the detecting rate of corresponding mutations was similar to that from the ExAC database, therefore they were presumably determined as mutations with unknown significance. Further analysis may be performed in a cohort study to determine the casual genes.
In this study, the overall age of patients carrying pathogenic mutations and likely pathogenic mutations was younger than that of non-carriers, yet there was no significant difference observed probably due to a modest sample size. Also, it is worthy to be noted that most of these genes show relatively low penetrance.
Conclusions
This study analyzed the detection rates and mutational characteristics of 69 cancer susceptibility genes in 282 Han Chinese patients with gastric adenocarcinoma, including pathogenesis and prognosis. These results showed the detection rate of pathogenic and likely pathogenic mutations in male patients was significantly lower than that in female patients. Besides, some known and unidentified mutations were found in gastric cancer so that we gained more insight into the hereditary gastric cancer syndromes from the molecular perspective. A diagnostic and prognostic surrogate for disease risk management and treatment decisions were also offered, but further investigations and discussion on this topic were needed in the future.
Acknowledgements
This study was supported by Shenzhen Sanming Project (No. SZSM201612051) and Shenzhen Science and Technology Innovation Commission Project (No. ZDSYS20190902092855097, No. GJHZ20180420180754917).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Contributor Information
Li Feng, Email: 66552483@qq.com.
Guoqing Lyu, Email: lgqqq_3528@qq.com.
References
- 1.Chen W, Sun K, Zheng R, et al Cancer incidence and mortality in China, 2014. Chin J Cancer Res. 2018;30:1–12. doi: 10.21147/j.issn.1000-9604.2018.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, et al Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Park SA, Ko A, Lee NG Stimulation of growth of the human gastric pathogen Helicobacter pylori by atmospheric level of oxygen under high carbon dioxide tension. BMC Microbiol. 2011;11:96. doi: 10.1186/1471-2180-11-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang L, Zheng R, Wang N, et al Incidence and mortality of stomach cancer in China, 2014. Chin J Cancer Res. 2018;30:291–8. doi: 10.21147/j.issn.1000-9604.2018.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee YC, Chiang TH, Chou CK, et al Association between helicobacter pylori eradication and gastric cancer incidence: A systematic review and meta-analysis. Gastroenterology. 2016;150:1113–24.e5. doi: 10.1053/j.gastro.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 6.Amieva M, Peek RM Jr Pathobiology of helicobacter pylori-induced gastric cancer. Gastroenterology. 2016;150:64–78. doi: 10.1053/j.gastro.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xicola RM, Li S, Rodriguez N, et al Clinical features and cancer risk in families with pathogenic CDH1 variants irrespective of clinical criteria. J Med Genet. 2019;56:838–43. doi: 10.1136/jmedgenet-2019-105991. [DOI] [PubMed] [Google Scholar]
- 8.Kaurah P, MacMillan A, Boyd N, et al Founder and recurrent CDH1 mutations in families with hereditary diffuse gastric cancer. JAMA. 2007;297:2360–72. doi: 10.1001/jama.297.21.2360. [DOI] [PubMed] [Google Scholar]
- 9.Oliveira C, Seruca R, Carneiro F Genetics, pathology, and clinics of familial gastric cancer. Int J Surg Pathol. 2006;14:21–33. doi: 10.1177/106689690601400105. [DOI] [PubMed] [Google Scholar]
- 10.Dray BK, Raveendran M, Harris RA, et al Mismatch repair gene mutations lead to lynch syndrome colorectal cancer in rhesus macaques. Genes Cancer. 2018;9:142–52. doi: 10.18632/genesandcancer.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Lier MG, Westerman AM, Wagner A, et al High cancer risk and increased mortality in patients with Peutz-Jeghers syndrome. Gut. 2011;60:141–7. doi: 10.1136/gut.2010.223750. [DOI] [PubMed] [Google Scholar]
- 12.Masciari S, Dewanwala A, Stoffel EM, et al Gastric cancer in individuals with Li-Fraumeni syndrome. Genet Med. 2011;13:651–7. doi: 10.1097/GIM.0b013e31821628b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sahasrabudhe R, Lott P, Bohorquez M, et al Germline mutations in PALB2, BRCA1, and RAD51C, which regulate DNA recombination repair, in patients with gastric cancer. Gastroenterology. 2017;152:983–6.e6. doi: 10.1053/j.gastro.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu C, Xie M, Wendl MC, et al Patterns and functional implications of rare germline variants across 12 cancer types. Nat Commun. 2015;6:10086. doi: 10.1038/ncomms10086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang A, Li Z, Wang Q, et al Diagnostic value of negative enrichment and immune fluorescence in situ hybridization for intraperitoneal free cancer cells of gastric cancer. Chin J Cancer Res. 2019;31:945–54. doi: 10.21147/j.issn.1000-9604.2019.06.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang KL, Mashl RJ, Wu Y, et al Pathogenic germline variants in 10, 389 adult cancers. Cell. 2018;173:355–70.e14. doi: 10.1016/j.cell.2018.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi M, Kipps T, Kurzrock R ATM mutations in cancer: Therapeutic implications. Mol Cancer Ther. 2016;15:1781–91. doi: 10.1158/1535-7163.MCT-15-0945. [DOI] [PubMed] [Google Scholar]
- 18.Fewings E, Larionov A, Redman J, et al Germline pathogenic variants in PALB2 and other cancer-predisposing genes in families with hereditary diffuse gastric cancer without CDH1 mutation: a whole-exome sequencing study. Lancet Gastroenterol Hepatol. 2018;3:489–98. doi: 10.1016/S2468-1253(18)30079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Durbin R Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–60. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schrader KA, Cheng DT, Joseph V, et al Germline variants in targeted tumor sequencing using matched normal DNA. JAMA Oncol. 2016;2:104–11. doi: 10.1001/jamaoncol.2015.5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richards S, Aziz N, Bale S, et al Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujii K, Ohashi H, Suzuki M, et al Frameshift mutation in the PTCH2 gene can cause nevoid basal cell carcinoma syndrome. Fam Cancer. 2013;12:611–4. doi: 10.1007/s10689-013-9623-1. [DOI] [PubMed] [Google Scholar]
- 23.Song H, Dicks E, Ramus SJ, et al Contribution of germline mutations in the RAD51B, RAD51C, and RAD51D genes to ovarian cancer in the population. J Clin Oncol. 2015;33:2901–7. doi: 10.1200/JCO.2015.61.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson ER, Rowley SM, Sawyer S, et al Analysis of RAD51D in ovarian cancer patients and families with a history of ovarian or breast cancer. PLoS One. 2013;8:e54772. doi: 10.1371/journal.pone.0054772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H, Feng M, Feng Y, et al Germline mutations in hereditary diffuse gastric cancer. Chin J Cancer Res. 2018;3:122–30. doi: 10.21147/j.issn.1000-9604.2018.01.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bustos-Carpinteyro AR, Oliveira C, Sousa A, et al CDH1 somatic alterations in Mexican patients with diffuse and mixed sporadic gastric cancer. BMC Cancer. 2019;19:69. doi: 10.1186/s12885-019-5294-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lo W, Zhu B, Sabesan A, et al Associations of CDH1 germline variant location and cancer phenotype in families with hereditary diffuse gastric cancer (HDGC) J Med Genet. 2019;56:370–9. doi: 10.1136/jmedgenet-2018-105361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corso G, Marrelli D, Roviello F Familial gastric cancer and germline mutations of E-cadherin. Ann Ital Chir. 2012;83:177–82. [PubMed] [Google Scholar]
- 29.Hansford S, Kaurah P, Li-Chang H, et al Hereditary diffuse gastric cancer syndrome: CDH1 mutations and beyond. JAMA Oncol. 2015;1:23–32. doi: 10.1001/jamaoncol.2014.168. [DOI] [PubMed] [Google Scholar]
- 30.Altaraihi M, Wadt K, Ek J, et al A healthy individual with a homozygous PTCH2 frameshift variant: Are variants of PTCH2 associated with nevoid basal cell carcinoma syndrome? Hum Genome Var. 2019;6:10. doi: 10.1038/s41439-019-0041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]