Abstract
Objective
Family caregivers (FCs) of breast cancer patients play a vital role throughout the treatment process. Psychological distress of FCs is common and often ignored. A simple and effective instrument for screening psychological distress would help in selecting those FCs requiring special attention and intervention. Here, the validity of distress thermometer (DT) in FCs of Chinese breast cancer patients receiving postoperative chemotherapy was assessed, and the prevalence of anxiety and depression was evaluated.
Methods
We recruited 200 FCs of hospitalized breast cancer patients in this cross-sectional descriptive study. Before the first cycle of adjuvant chemotherapy, the levels of anxiety and depression among FCs were assessed using DT and Hospital Anxiety and Depression Scale (HADS). In total, 191 valid cases were analyzed. HADS was used as the diagnostic standard to assess the effectiveness of DT as a screening tool for anxiety and depression as well as to analyze the diagnostic efficiency of DT at various cutoff points.
Results
The definitive prevalence of both anxiety and depression was 8.90%. The mean level of anxiety and depression among FCs was 5.64±3.69 and 5.09±3.85, respectively, both of which were significantly higher than corresponding Chinese norms (P<0.01). The areas under receiver operating characteristic curves of DT for the diagnoses of FCs’ anxiety and depression were 0.904 and 0.885, respectively. A cutoff value of 5 produced the best diagnostic effects of DT for anxiety and depression.
Conclusions
The levels of both anxiety and depression were higher in the FCs of Chinese breast cancer patients receiving postoperative chemotherapy than the national norm. DT might be an effective tool to initially screen psychological distress among FCs. This process could be integrated into the palliative care of breast cancer patients and warrant further research.
Keywords: Breast cancer, distress thermometer, anxiety, depression
Introduction
According to the 2018 Global Cancer Epidemiological Statistics, over 2 million new cases of female breast cancer are diagnosed annually worldwide, and breast cancer accounts for around one-quarter of the world’s confirmed female cancer cases, making it the most common malignant tumor among women (1). Although globally China is at a relatively lower risk of breast cancer, the burden of this disease is increasing in the country (2-4).
Surgery is the primary treatment regimen for breast cancer; however, standard adjuvant chemotherapy has greatly improved the prognosis of early breast cancer patients (5-8). Nevertheless, breast cancer patients preparing for postoperative adjuvant chemotherapy face many stressful events, including diagnosis of a malignant tumor, breast loss and multiple worries, such as vomiting, hair loss and other chemotherapy-related physical symptoms. Mental disorders, such as anxiety, depression, fear and decreased self-efficacy, are also very common among these patients (9-11).
Similar mental disorders also arise in the caregivers of breast cancer patients, who are typically informal family caregivers (FCs); 50% of their caregivers are spouses who play a vital role throughout the treatment process (12-14). Indeed, these FCs have been reported to spend more than 2,000 h caring for breast cancer patients in the first 2 years after diagnosis (15). The FCs of breast cancer patients not only face emotional distress due to their loved one’s condition but also bear the economic and social pressure of the situation. Consequently, psychological distress, including the incidence of anxiety and depression, occurs frequently among these caregivers (16-18). Previous studies found that 12.3% of the FCs of Malaysian breast cancer patients suffered from major depression, whereas 11.5% of them receiving oncologic treatment were diagnosed with anxiety disorders (16,19). Meanwhile, the psychological distress of FCs can in turn have an adverse effect on the breast cancer patients’ quality of life, treatment compliance and prognosis (20-27).
Previous cancer research has mainly focused on prolonging the survival time of patients with the disease. Recently, an increasing amount of evidence has suggested that early integration of palliative care into the cancer treatment process would benefit patients, their families, hospitals and society to various degrees (28-30). An important form of palliative care is the psychological care of the FCs of cancer patients. This is beneficial not only for FCs but also for patients themselves, as it alleviates their low mood and improves their prognosis (13,25,31,32). However, owing to limited clinical resources and a lack of professional knowledge among medical staff, the FCs of patients receiving chemotherapy and other anticancer treatments are often ignored in the literature. Therefore, studies conducted in this area are important.
Thus, effective instruments for screening psychological distress in FCs are required. The distress thermometer (DT), proposed by Roth et al. in 1998 (33), has been recommended by the National Comprehensive Cancer Network (NCCN) for screening the psychological distress of cancer patients (34). Multiple versions of the DT in different languages have been shown suitable for cancer patients (35-38). Previously, Tang et al. (38) verified the validity of Chinese DT in cancer patients. Although some studies outside China have reported that DT is an effective screening tool for psychological distress among FCs of cancer patients, similar studies are scare in China (39-42). In addition, studies regarding the psychological distress of FCs of breast cancer patients receiving adjuvant chemotherapy remains to be conducted.
Therefore, in this study, we tested the validity of DT as a screening tool for anxiety and depression in FCs of Chinese breast cancer patients at the beginning of postoperative adjuvant chemotherapy. We adopted the classical Hospital Anxiety and Depression Scale (HADS) as the diagnostic standard to detect the validity of DT, explore the main reason for psychological distress among FCs, and confirm the optimal cutoff value for the application of DT. Our study will help practitioners meet the increasing need for the psychological screening of FCs of breast cancer patients, lay the foundations for further related research, and push the shift from patient-disease to patient-caregiver dyadic coping.
Materials and methods
Study design
This cross-sectional descriptive study was based on data from a prospective clinical trial. The trial was designed to analyze the prevalence and associated factors of psychological distress among Chinese breast cancer patients and conducted at the Cancer Hospital of the Chinese Academy of Medical Sciences (Beijing, China) between January 2018 and September 2019. The study protocol was approved by the Institutional Review Board of the Ethical Committee of Cancer Hospital, Chinese Academic of Medical Science (No. 19-013/1798), with an identifying NCT number of NCC1867. All participants provided informed consent. Psychological scale data and general information were collected within 24 h of the start of the first cycle of adjuvant chemotherapy. The scale was invalid when any data related to the DT, HADS-anxiety (HADS-A) or HADS-depression (HADS-D) were missing. Finally, available data from 191 FCs were analyzed, with an effective recovery rate of 95.5%.
Participants
This study originally included 200 FCs of breast cancer patients receiving postoperative adjuvant chemotherapy at the Cancer Hospital of the Chinese Academy of Medical Sciences.
The inclusion criteria were as follows: 1) patients aged ≥18 years and ≤75 years; 2) FC was the patient’s family member and provided primary care; 3) the breast cancer patient was admitted to the hospital for the first cycle of adjuvant chemotherapy after breast cancer surgery; 4) those who were able to independently complete the psychological scales without communication or language barriers; and 5) those who voluntarily provided informed consent.
The exclusion criteria were as follows: 1) those who were unable to clearly express their feelings; 2) those with a history of suicide or mental illness; 3) those who were taking antianxiety or antidepressant medicines; 4) those with other potential factors, such as psychological, familial, social, or geographic factors, that might hinder the research progress; or 5) a participant that was unable to complete the study, as judged by the researchers.
Measurements
The residents had been trained in a standard manner to guide the FCs to fill out the paper questionnaires one by one, including the HADS and DT. Residents presented the precautions before the assessment and provided specific guidance for items that might be misunderstood but did not provide any hints for possible answers. After the questionnaire was completed, two researchers simultaneously but separately entered and checked the data to ensure its accuracy.
HADS was compiled by Zigmond and Snaith in 1983 (43). It is a 14-item scale that is categorized into two 7-item subscales: the HADS-A and HADS-D subscales. Every item is scored on a four-point Likert scale rated from 0 (no problem) to 3 (high level of problems), and the total score of each subscale is 0−21. In FCs of cancer patients, the Chinese version of HADS has good internal consistency and acceptable concurrent validity (44), with Cronbanch’ s alpha coefficients of 0.92, 0.86, and 0.85 for the total scale, HADS-A, and HADS-D, respectively. In each of the two subscales, a score of ≤7 indicates non-anxiety/depression, 8−10 indicates borderline anxiety/depression, and 11−21 indicates definite anxiety/depression (45). Thus, based on this previous research, we adopted a threshold subscale score of 11 for anxiety and depression in this study.
DT is a self-reporting instrument with two parts: a visual image scale ranging from 0 (no distress) to 10 (extreme distress), which is rated according to the level of distress over the past week, and a problem listed with 36 questions, which are categorized into five problem categories: practical, communication, emotional, physical and spiritual or religious. The Chinese version of DT has been shown to be easily understood and accepted by patients and has high retest reliability in cancer patients (r=0.800, P<0.01) (38).
Statistical analysis
The SAS software (Version 9.4; SAS Institute Inc., Cary, USA) was used to analyze the data. The distribution of HADS-A and HADS-D score was analyzed using normality test. Additionally, the scores of HADS-A and HADS-D were compared with the corresponding Chinese norms using Student’s t-test based on normal distribution. The Chinese norms for HADS were derived from research relating to 11,766 medical outpatients in Shanghai. The diagnostic efficacy of DT for anxiety and depression was analyzed using receiver operating characteristic (ROC) curves. From these ROC curves, an area under the curve (AUC) value of 0.5−0.7 represented low accuracy, 0.7−0.9 represented moderate accuracy, and 0.9−1.0 represented high accuracy. Sensitivity, specificity, positive predictive value and negative predictive value were used to analyze and determine the best cutoff value for the DT. P<0.05 was considered to statistically significant in all analyses.
Results
Demographic characteristics
The 191 FCs analyzed included 152 (79.58%) males and 39 (20.42%) females. All demographic characteristics of these FCs are summarized in Table 1. Their median age (range) was 46 (19−72) years. Among them, 130 (68.06%) were the spouses of breast cancer patients and 171 (89.53%) were married.
1. Demographic characteristics of family caregivers of breast cancer patients from China (N=191).
| Variables | n | % |
| Age (year) | ||
| <40 | 56 | 29.32 |
| 40−50 | 59 | 30.89 |
| 50−60 | 58 | 30.37 |
| >60 | 18 | 9.42 |
| Gender | ||
| Male | 152 | 79.58 |
| Female | 39 | 20.42 |
| Marital status | ||
| Married | 171 | 89.53 |
| Others | 11 | 5.76 |
| Missing data | 9 | 4.71 |
| Relationship with patients | ||
| Spouse | 130 | 68.06 |
| Child | 37 | 19.37 |
| Others | 24 | 12.57 |
| Educational level | ||
| University or above | 108 | 56.54 |
| High school or less | 82 | 42.93 |
| Missing data | 1 | 0.52 |
| Chronic diseases | ||
| Yes | 44 | 23.04 |
| No | 138 | 72.25 |
| Missing data | 9 | 4.71 |
| Hospital charges paid by | ||
| National Healthcare | 183 | 95.81 |
| Others | 6 | 3.14 |
| Missing data | 2 | 1.05 |
| Living with patients | ||
| Yes | 144 | 75.39 |
| No | 38 | 19.90 |
| Missing data | 9 | 4.71 |
Prevalence of anxiety and depression among FCs
The prevalence of definite (HADS-A: 11−21) and borderline (HADS-A: 8−10) cases of anxiety were 8.90% and 20.42%, respectively, whereas those for depression were 8.90% (HADS-D: 11−21) and 18.85% (HADS-D: 8−10), respectively. The lack of anxiety and depression among FCs was observed in 70.68% and 72.25% of cases, respectively. Both of the HADS-A and HADS-D scores were following the normal distribution. The mean level anxiety and depression in FCs were 5.64±3.69 and 5.09±3.85, respectively. The levels of both anxiety and depression were significantly higher among male and female FCs than the Chinese norms (P<0.01) (Table 2).
2. Comparison of levels of anxiety and depression in family caregivers of Chinese breast cancer patients with the Chinese norms for these conditions.
| Variables | Female | Male | |||||||
| Our study | Chinese norms | T | P | Our study | Chinese norms | T | P | ||
| Anxiety | 5.76±3.62 | 3.37±2.81 | 5.25 | <0.01 | 5.77±3.71 | 3.14±2.62 | 12.13 | <0.01 | |
| Depression | 5.68±4.39 | 3.83±3.14 | 3.64 | <0.01 | 5.07±3.73 | 3.68±3.14 | 5.32 | <0.01 | |
Level and distribution of DT scores
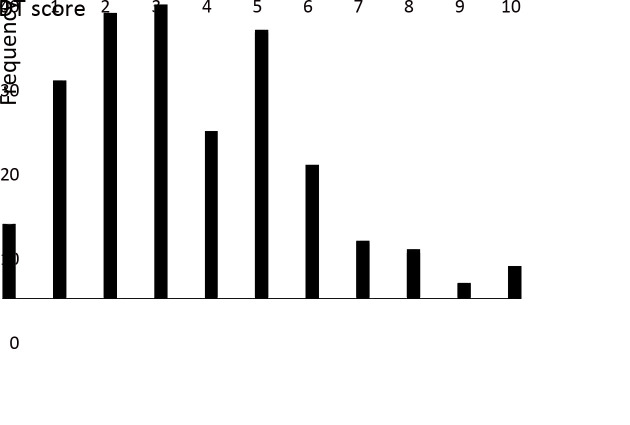
The mean DT score of FCs was 3.61±2.26, and 87 FCs (45.55%) had a score of >4. The frequency distribution of these scores is shown in Figure 1. Among the problem list, items with an incidence of >20% included worry (51.83%), taking care of children or parents (39.27%), insurance/financial problems (36.65%), memory loss or inattention (26.18%), nervousness (24.61%) and fatigue (20.42%).
1.
Frequency distribution of distress thermometer (DT) scores of family caregivers of Chinese breast cancer patients.
Diagnostic efficacy of DT for anxiety and depression
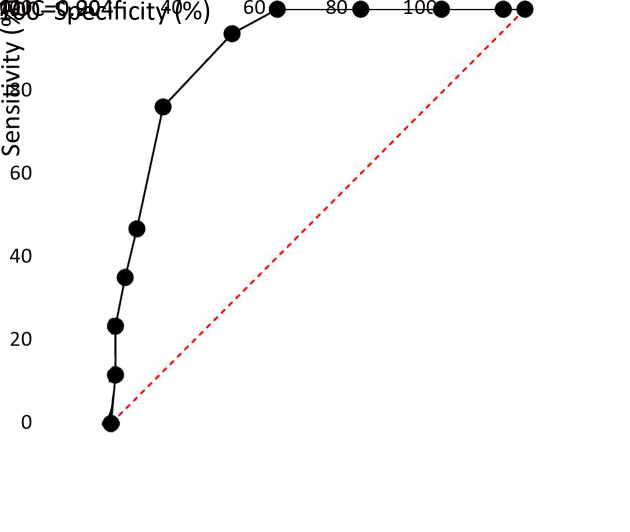
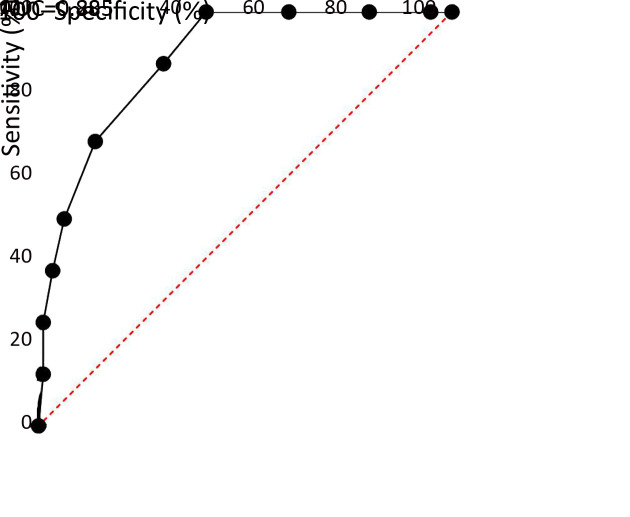
The AUC of DT for the diagnosis of anxiety and depression among FCs was 0.904 (according to HADS-A; Figure 2) and 0.885 (according to HADS-D; Figure 3), respectively. With DT, the best diagnostic efficacy for anxiety and depression was obtained with a cutoff value of 5 (anxiety: sensitivity=94.12%, specificity=70.69%; depression: sensitivity=87.50%, specificity=69.71%; Table 3).
2.
Receiver operating characteristic curve of distress thermometer for diagnosing anxiety (HADS-A ≥11). AUC, area under the curve; HADS, Hospital Anxiety and Depression Scale.
3.
Receiver operating characteristic curve of distress thermometer for diagnosing depression (HADS-D ≥11). AUC, area under the curve; HADS, Hospital Anxiety and Depression Scale.
3. Diagnostic efficacy of distress thermometer at different cutoff values.
| Cutoff | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
| Diagnostic criterion for anxiety: HADS-A ≥11; diagnostic criterion for depression: HADS-D ≥11. | ||||
| Anxiety | ||||
| 1 | 100 | 5.17 | 9.34 | 100 |
| 2 | 100 | 20.11 | 10.90 | 100 |
| 3 | 100 | 39.66 | 13.93 | 100 |
| 4 | 100 | 67.10 | 25.00 | 100 |
| 5 | 94.12 | 70.69 | 23.88 | 99.19 |
| 6 | 76.47 | 87.36 | 37.14 | 97.44 |
| 7 | 47.06 | 93.68 | 42.11 | 94.77 |
| 8 | 35.29 | 96.55 | 50.00 | 93.85 |
| 9 | 23.53 | 98.85 | 66.67 | 92.97 |
| Depression | ||||
| 1 | 100 | 5.14 | 8.79 | 100 |
| 2 | 100 | 20.00 | 10.26 | 100 |
| 3 | 100 | 39.43 | 13.11 | 100 |
| 4 | 100 | 59.43 | 18.39 | 100 |
| 5 | 87.50 | 69.71 | 20.90 | 98.39 |
| 6 | 68.75 | 86.29 | 31.43 | 96.79 |
| 7 | 50.00 | 93.71 | 42.11 | 95.35 |
| 8 | 37.50 | 96.57 | 50.00 | 94.41 |
| 9 | 25.00 | 98.86 | 66.67 | 93.51 |
Discussion
The provision of supportive care to breast cancer patients and the pressure it creates can have negative effects on the mental health of FCs at various levels, and the psychological state of FCs in turn affects the psychology and prognosis of cancer patients. Therefore, providing psychological support to FCs is an important part of palliative care. In our study, we found that the percentage of definite cases of both anxiety and depression among FCs of breast cancer patients at the beginning of postoperative adjuvant chemotherapy was 8.90%, while those of borderline cases of anxiety (HADS-A: 8−10) and depression (HADS-D: 8−10) were 20.42% and 18.85%, respectively. This morbidity is consistent with the results reported by Jaafaret al. (19) in Malaysia and higher than that in the general Chinese population. In contrast, the incidence of anxiety and depression among 6,172 outpatients of internal medicine in Shanghai was only 6.93% and 3.66%, respectively (46). In our study, the mean levels of anxiety and depression were 5.64±3.69 and 5.09±3.85, which were significantly higher than the Chinese norms. Geng et al. (47) found that the risk factors for these conditions included the time of care, educational level of FCs, age of FCs, general condition of the patient, and cancer staging. Therefore, anxiety and depression among FCs of Chinese breast cancer patients at the beginning of postoperative adjuvant chemotherapy are apparently relatively common and severe, warranting the screening of psychological distress.
A simple and effective screening tool can help improve the completion rate and accuracy of psychological screening and can enable (without any extra burden on medical staff) the rapid selection of FCs requiring additional attention and further intervention. With its simple design, DT can be easily operated by most participants and can be generally completed within 3 min. Very few studies have investigated the application of DT to FCs of cancer patients, and most studies have focused on terminal-stage cancer patients (21,39,41,42,48). Therefore, our study was the first to explore the value of DT for screening anxiety and depression in FCs of cancer patients at the beginning of postoperative adjuvant chemotherapy in China. The mean DT score of these Chinese FCs was 3.61, and ROC analysis showed that DT was an effective screening tool for anxiety and depression in this cohort. Zwahlen et al. (42) reported a similar mean DT score of 3.7 in FCs of cancer patients. However, Halkett et al. (41) reported a higher mean DT score of 5.1 in FCs of high-grade glioma patients undergoing chemoradiotherapy, while Oechsle et al. (39) reported an even higher mean score of up to 7.9 in FCs of advanced cancer patients. The main possible reasons for the relatively low DT scores in our study are as follows: 1) the overall survival and prognosis of breast cancer is longer and better than those of most other malignant tumors; and 2) breast cancer patients receiving postoperative adjuvant chemotherapy are generally at an early TNM stage. In the present study, breast cancer patients at TNM stage I and II accounted for 80.11% of all patients according to the 8th edition TNM classification.
In our study, the optimal cutoff value of DT for diagnosis of anxiety and depression was 5. The NCCN guidelines recommends that the cutoff value of DT for cancer patients should be 4 and that patients with a score ≥4 require further professional evaluation and treatment. A meta-analysis covering more than 14,000 cases of cancer patients showed that the pooled sensitivity and specificity of DT was 81% and 72%, respectively, when the recommended cutoff value was 4 (49). At present, the cutoff value of 4 is also recommended for screening among Chinese cancer patients. However, some studies have presented a different conclusion about the best cutoff value of DT (36,50,51). Moreover, there are few recommendations for an appropriate cutoff value of DT for FCs of cancer patients. Zwahlen et al. (42) showed that the cutoff value for FCs of cancer patients should be 4 or 5 using the HADS as a diagnostic criterion. However, Oechsle et al. (39) recommended that the cutoff value of DT for FCs of advanced cancer patients with severe distress should be 8. The reasons for differences in optimal cutoff values are related to the type of cancer, sample heterogeneity, and different diagnostic tools or criteria.
The PL of DT questionnaire covers the possible causes of distress. Our study showed that emotional and practical problems were common in FCs of Chinese breast cancer patients receiving postoperative adjuvant chemotherapy. The top three problems were worry, taking care of children or parents, and insurance or financial problems. FCs worry about the prognosis, recurrence, and treatment of the disease and take additional responsibility for caring for other family members during the anticancer period. Sometimes, FCs must also reduce their employment time to take care of the patient and their family, which may result in a loss of family income. In our study, none of the FCs reported spiritual and religious problems, which is likely related to the Chinese traditional culture and religious beliefs.
Our study has some limitations. First, it was a cross-sectional study and did not detect the dynamic changes of HADS and DT for FCs during adjuvant chemotherapy and the follow-up period. Second, due to the limited sample size in our study, we were unable to analyze risk factors. Third, all the enrolled subjects in our study were inpatients; therefore, we effectively excluded outpatients, and the evaluation time was limited to the beginning of adjuvant chemotherapy. Thus, further research is required before our results could be robustly applied to all the FCs of breast cancer patients. In addition, we suggest that the mini-Suda professional psychological test scale should be included in future studies to further confirm diagnoses.
Conclusions
Our study was the first to investigate psychological distress in FCs of Chinese breast cancer patients at the beginning of postoperative adjuvant chemotherapy. We found that anxiety and depression were common among our cohort and that the diagnostic efficacy of DT for anxiety and depression was good. The DT could therefore be an effective tool for initially screening the psychological state of FCs of breast cancer patients and is worthy of further study.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.National Health Commission of the People’s Republic of China Chinese guidelines for diagnosis and treatment of breast cancer 2018 (English version) Chin J Cancer Res. 2019;31:259–77. doi: 10.21147/j.issn.1000-9604.2019.02.02><PMID:31156298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo R, Si J, Xue J, et al Changing patterns and survival improvements of young breast cancer in China and SEER database, 1999-2017. Chin J Cancer Res. 2019;31:653–62. doi: 10.21147/j.issn.1000-9604.2019.04.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonadonna G, Moliterni A, Zambetti M, et al 30 years’ follow up of randomised studies of adjuvant CMF in operable breast cancer: cohort study. BMJ. 2005;330:217. doi: 10.1136/bmj.38314.622095.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100, 000 women in 123 randomised trials. Lancet. 2012;379:432–44. doi: 10.1016/s0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldvaser H, Ribnikar D, Majeed H, et al Absolute benefit from adjuvant chemotherapy in contemporary clinical trials: A systemic review and meta-analysis. Cancer Treat Rev. 2018;71:68–75. doi: 10.1016/j.ctrv.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 8.de Almeida FK, Rosa DD Adjuvant dose-dense chemotherapy for breast cancer: Available evidence and recent updates. Breast Care (Basel) 2018;13:447–52. doi: 10.1159/000488026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gold M, Dunn LB, Phoenix B, et al Co-occurrence of anxiety and depressive symptoms following breast cancer surgery and its impact on quality of life. Eur J Oncol Nurs. 2016;20:97–105. doi: 10.1016/j.ejon.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.So WK, Marsh G, Ling WM, et al Anxiety, depression and quality of life among Chinese breast cancer patients during adjuvant therapy. Eur J Oncol Nurs. 2010;14:17–22. doi: 10.1016/j.ejon.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Antoni MH, Jacobs JM, Bouchard LC, et al Post-surgical depressive symptoms and long-term survival in non-metastatic breast cancer patients at 11-year follow-up. Gen Hosp Psychiatry. 2017;44:16–21. doi: 10.1016/j.genhosppsych.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grunfeld E, Coyle D, Whelan T, et al Family caregiver burden: results of a longitudinal study of breast cancer patients and their principal caregivers. CMAJ. 2004;170:1795–801. doi: 10.1503/cmaj.1031205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borstelmann NA, Rosenberg SM, Ruddy KJ, et al Partner support and anxiety in young women with breast cancer. Psychooncology. 2015;24:1679–85. doi: 10.1002/pon.3780. [DOI] [PubMed] [Google Scholar]
- 14.Zhu P, Fu JF, Wang B, et al Quality of life of male spouse caregivers for breast cancer patients in China. Asian Pac J Cancer Prev. 2014;15:4181–5. doi: 10.7314/apjcp.2014.15.10.4181. [DOI] [PubMed] [Google Scholar]
- 15.Yabroff KR, Kim Y Time costs associated with informal caregiving for cancer survivors. Cancer. 2009;115:4362–73. doi: 10.1002/cncr.24588. [DOI] [PubMed] [Google Scholar]
- 16.Selamat Din SH, Nik Jaafar NR, Zakaria H, et al Anxiety disorders in family caregivers of breast cancer patients receiving oncologic treatment in malaysia. Asian Pac J Cancer Prev. 2017;18:465–71. doi: 10.22034/apjcp.2017.18.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoellen F, Wagner JF, Lüdders DW, et al Anxiety in caregiving partners of breast cancer patients. Arch Gynecol Obstet. 2019;300:993–1005. doi: 10.1007/s00404-019-05253-2. [DOI] [PubMed] [Google Scholar]
- 18.Segrin C, Badger TA, Sikorskii A, et al Longitudinal dyadic interdependence in psychological distress among Latinas with breast cancer and their caregivers. Support Care Cancer. 2020;28:2735–43. doi: 10.1007/s00520-019-05121-4. [DOI] [PubMed] [Google Scholar]
- 19.Nik Jaafar NR, Selamat Din SH, Mohamed Saini S, et al Clinical depression while caring for loved ones with breast cancer. Compr Psychiatry. 2014;55(Suppl 1):S52–9. doi: 10.1016/j.comppsych.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Hodges LJ, Humphris GM, Macfarlane GA A meta-analytic investigation of the relationship between the psychological distress of cancer patients and their carers. Soc Sci Med. 2005;60:1–12. doi: 10.1016/j.socscimed.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 21.Rosenberger C, Höcker A, Cartus M, et al Outpatient psycho-oncological care for family members and patients: access, psychological distress and supportive care needs. Psychother Psychosom Med Psychol (in German) 2012;62:185–94. doi: 10.1055/s-0032-1304994. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs JM, Shaffer KM, Nipp RD, et al Distress is interdependent in patients and caregivers with newly diagnosed incurable cancers. Ann Behav Med. 2017;51:519–31. doi: 10.1007/s12160-017-9875-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Q, Lin Y, Xu Y, et al The impact of depression and anxiety on quality of life in Chinese cancer patient-family caregiver dyads, a cross-sectional study. Health Qual Life Outcomes. 2018;16:230. doi: 10.1186/s12955-018-1051-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Q, Xu Y, Zhou H, et al Factors influencing the health-related quality of life of Chinese advanced cancer patients and their spousal caregivers: a cross-sectional study. BMC Palliat Care. 2016;15:72. doi: 10.1186/s12904-016-0142-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alam S, Hannon B, Zimmermann C Palliative care for family caregivers. J Clin Oncol. 2020;38:926–36. doi: 10.1200/jco.19.00018. [DOI] [PubMed] [Google Scholar]
- 26.Parmelee Streck B, LoBiondo-Wood G A systematic review of dyadic studies examining depression in couples facing breast cancer. J Psychosoc Oncol. 2020;38:463–80. doi: 10.1080/07347332.2020.1734894. [DOI] [PubMed] [Google Scholar]
- 27.Segrin C, Badger TA, Sikorskii A, et al A dyadic analysis of stress processes in Latinas with breast cancer and their family caregivers. Psychooncology. 2018;27:838–46. doi: 10.1002/pon.4580. [DOI] [PubMed] [Google Scholar]
- 28.Temel JS, Greer JA, Muzikansky A, et al Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–42. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 29.Temel JS, Greer JA, El-Jawahri A, et al Effects of early integrated palliative care in patients with lung and GI cancer: A randomized clinical trial. J Clin Oncol. 2017;35:834–41. doi: 10.1200/jco.2016.70.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrell BR, Temel JS, Temin S, et al Integration of palliative care into standard oncology care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017;35:96–112. doi: 10.1200/jco.2016.70.1474. [DOI] [PubMed] [Google Scholar]
- 31.Badger TA, Segrin C, Sikorskii A, et al Randomized controlled trial of supportive care interventions to manage psychological distress and symptoms in Latinas with breast cancer and their informal caregivers. Psychol Health. 2020;35:87–106. doi: 10.1080/08870446.2019.1626395. [DOI] [PubMed] [Google Scholar]
- 32.Fu F, Zhao H, Tong F, et al A systematic review of psychosocial interventions to cancer caregivers. Front Psychol. 2017;8:834. doi: 10.3389/fpsyg.2017.00834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roth AJ, Kornblith AB, Batel-Copel L, et al Rapid screening for psychologic distress in men with prostate carcinoma: a pilot study. Cancer. 1998;82:1904–8. doi: 10.1002/(sici)1097-0142(19980515)82:10<1904::aid-cncr13>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 34.Riba MB, Donovan KA, Andersen B, et al Distress Management, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17:1229–49. doi: 10.6004/jnccn.2019.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alosaimi FD, Abdel-Aziz N, Alsaleh K, et al Validity and feasibility of the Arabic version of distress thermometer for Saudi cancer patients. PLoS One. 2018;13:e0207364. doi: 10.1371/journal.pone.0207364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iskandarsyah A, de Klerk C, Suardi DR, et al The Distress Thermometer and its validity: a first psychometric study in Indonesian women with breast cancer. PLoS One. 2013;8:e56353. doi: 10.1371/journal.pone.0056353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shim EJ, Shin YW, Jeon HJ, et al Distress and its correlates in Korean cancer patients: pilot use of the distress thermometer and the problem list. Psychooncology. 2008;17:548–55. doi: 10.1002/pon.1275. [DOI] [PubMed] [Google Scholar]
- 38.Tang LL, Zhang YN, Pang Y, et al Validation and reliability of distress thermometer in chinese cancer patients. Chin J Cancer Res. 2011;23:54–8. doi: 10.1007/s11670-011-0054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oechsle K, Ullrich A, Marx G, et al Psychological burden in family caregivers of patients with advanced cancer at initiation of specialist inpatient palliative care. BMC Palliat Care. 2019;18:102. doi: 10.1186/s12904-019-0469-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujinami R, Sun V, Zachariah F, et al Family caregivers’ distress levels related to quality of life, burden, and preparedness. Psychooncology. 2015;24:54–62. doi: 10.1002/pon.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Halkett GKB, Lobb EA, Shaw T, et al Distress and psychological morbidity do not reduce over time in carers of patients with high-grade glioma. Supportive Care in Cancer. 2017;25:887–93. doi: 10.1007/s00520-016-3478-6. [DOI] [PubMed] [Google Scholar]
- 42.Zwahlen D, Hagenbuch N, Carley MI, et al Screening cancer patients’ families with the distress thermometer (DT): a validation study. Psychooncology. 2008;17:959–66. doi: 10.1002/pon.1320. [DOI] [PubMed] [Google Scholar]
- 43.Zigmond AS, Snaith RP The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 44.Li Q, Lin Y, Hu C, et al The Chinese version of hospital anxiety and depression scale: Psychometric properties in Chinese cancer patients and their family caregivers. Eur J Oncol Nurs. 2016;25:16–23. doi: 10.1016/j.ejon.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 45.Arving C, Glimelius B, Brandberg Y Four weeks of daily assessments of anxiety, depression and activity compared to a point assessment with the Hospital Anxiety and Depression Scale. Qual Life Res. 2008;17:95–104. doi: 10.1007/s11136-007-9275-4. [DOI] [PubMed] [Google Scholar]
- 46.Ling Z, Sha L, Ji JL, et al Use of hospital anxiety and depression scale (Chinese version) in Chinese outpatient in department of internal medicine. Shanghai Jing Shen Yi Xue. 2010;22:204–6. doi: 10.3969/j.issn.1002-0829.2010.04.003. [DOI] [Google Scholar]
- 47.Geng HM, Chuang DM, Yang F, et al Prevalence and determinants of depression in caregivers of cancer patients: A systematic review and meta-analysis. Medicine (Baltimore) 2018;97:e11863. doi: 10.1097/md.0000000000011863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martínez P, Galdón MJ, Andreu Y, et al The Distress Thermometer in Spanish cancer patients: convergent validity and diagnostic accuracy. Support Care Cancer. 2013;21:3095–102. doi: 10.1007/s00520-013-1883-7. [DOI] [PubMed] [Google Scholar]
- 49.Ma X, Zhang J, Zhong W, et al The diagnostic role of a short screening tool--the distress thermometer: a meta-analysis. Support Care Cancer. 2014;22:1741–55. doi: 10.1007/s00520-014-2143-1. [DOI] [PubMed] [Google Scholar]
- 50.Vitek L, Rosenzweig MQ, Stollings S Distress in patients with cancer: definition, assessment, and suggested interventions. Clin J Oncol Nurs. 2007;11:413–8. doi: 10.1188/07.Cjon.413-418. [DOI] [PubMed] [Google Scholar]
- 51.Ploos van Amstel FK, Tol J, Sessink KH, et al A specific distress cutoff score shortly after breast cancer diagnosis. Cancer Nurs. 2017;40:E35–e40. doi: 10.1097/ncc.0000000000000380. [DOI] [PubMed] [Google Scholar]