Abstract
Objective
National Health Commission of the People’s Republic of China collaborated with many ministries and commissions government and initiated a population-based cancer screening program in high-risk area of rural China, targeting three types of cancer that are most prevalent in these areas, including esophageal, stomach and liver cancer. This study protocol was reported to show the design and evaluate the effectiveness of cancer screening and appropriate screening strategies of three cancers in rural China.
Methods and analysis
A two-step design with cancer risk assessment based on questionnaire interview, Hepatitis B surface antigen (HBsAg) test strip and subsequent clinical intervention for high-risk populations was adopted free of charge at the local hospitals designated in the program.
Ethic and dissemination
This study was approved by the Institutional Review Board of Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. The results will evaluate the effectiveness of cancer screening and appropriate screening strategies in rural China.
Keywords: Esophageal cancer screening, stomach cancer screening, liver cancer screening, study protocol
Introduction
With the development of global aging and socio-demographic changes, cancer is a chronic disease and the leading cause of death. In 2018, GLOBOCAN reported 14.1 million new cancer cases and 8.2 million deaths from cancer worldwide (1). In 2015, a total of 4,292,000 new cancer cases and 2,814,000 cancer deaths were reported in China, with esophageal, stomach, and liver cancers accounting for 45% of all newly diagnosed cancers. And esophageal, stomach and liver cancers were reported to be 40.7% in rural China (2,3). How to effectively control the growing burden of cancer is a major challenge for China.
Previous evidence and practices have revealed that screening is effective in reducing the mortality and burden of cancers (4). Given the potential benefit of screening, many countries have implemented nationwide organized cancer screening programs. For instance, national health departments in Russia, South Korea and Japan carried out nationwide organized stomach cancer screening (5-7). Comprehensive evaluation of the effectiveness and cost-effectiveness of cancer screening in rural populations in China is highly demanded (8).
In 2007, the National Health Commission of the People’s Republic of China collaborated with many ministries and commissions government and initiated a population-based cancer screening program in high-risk area of rural China, targeting three types of cancer that are most prevalent in these areas, including esophageal, stomach and liver cancer. Screening strategy has been adopted to high-risk population who have cancer risk assessment based on epidemiological surveys in the program. Detailed follow-ups have been collected and documented in a central database. The aims of the program were: 1) to establish a cancer screening cohort with long-term follow-ups in high-risk area of selected rural China; 2) to improve the rate of early detection and early treatment, increase survival rate, and reduce the mortality of esophageal, stomach and liver cancers in these areas; and 3) to evaluate the effectiveness of cancer screening and appropriate screening strategies in rural China. In the protocol, the design of the national program of the esophageal, stomach and liver cancer screening program was given in detail.
Materials and methods
Ethical approval and informed consent
This study was approved by the Institutional Review Board of the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. Staffs in each screening site have explained and obtained written informed consent from participants regarding the following items: objectives, reasons for enrollment, the methods and time period, potential benefits and risks, need of personal information and means for information storage.
Screening sites selection and population recruitment
The program was conducted in rural regions with relative higher rates of esophageal, stomach and liver cancers from four provinces in China (Henan, Shandong, Anhui and Jiangsu), which were appointed by the government. Thirty-two counties (there were 6 counties covered by the program in the first year and gradually increased to 32 counties in 2019) in these rural regions were selected by cluster sampling among candidate screening sites according to the sampling principle including: 1) with solid foundation of cancer registry and death registry in order to carry out follow-up and quality control; 2) counties with higher incidence rate and mortality rate on esophageal, stomach and liver cancers compared with native average level of morbidity according to the cancer registry report; and 3) with experiences of carrying out scientific researches or population programs related to prevention and control of chronic non-communicable diseases. Each county for the three types of cancer was selected differently. For esophageal cancer, 11 counties were selected by the above criteria. The incidence rate of 2 counties in Jiangsu province and 1 county in Shandong province were 2 times higher than that in the whole country. The incidence rate has doubled among 1 county in Jiangsu province and 4 counties in Shandong province. The incidence rate of other 3 counties in Henan province and Anhui province were almost similar to that of the whole country. For stomach cancer, 7 counties were selected. The incidence rate of 2 counties in Jiangsu and Shandong were 2 times higher than that in the whole country. The incidence rate has doubled among 3 counties in Jiangsu and Anhui province. The incidence rate of other 2 counties in Anhui province was almost similar to that of the whole country. For liver cancer, 13 counties were selected. The incidence rate of 1 county in Henan province was 2 times higher than that in the whole country. The incidence rate has doubled among 8 counties in the four provinces. The incidence rate of other 4 counties in Henan, Jiangsu and Shandong province were almost similar to that of the whole country.
Permanent residents aged 40−69 years living in the selected counties were approached by trained staffs by means of phone-calls and personal encounter. If there were high-risk sites for liver cancer, the residents aged 35−64 years were recruited. All had no history of cancer and serious disease reported by themselves. Counties broadcasting and science popularization lectures were used to raise the public awareness of esophageal, stomach and liver cancer screening program.
Sample size calculation
There were different calculation strategies for esophageal, stomach and liver cancers in the screening sites at selected counties. Generally, residents aged 40−69 years accounted for 30% of the total population in the screening sites, and the high-risk population of esophageal and stomach cancer accounts for nearly 30%. According to 70% participation rate and the financial funds for available screening, the coverage population of 26,460 was needed in each screening site.
For liver cancer screening, it is estimated that the target population accounts for about 40% of the total population. According to 70% participation rate and 10% high-risk rate, the coverage population was 71,400 in each screening site. Because this program is an ongoing national cancer screening program, and the sample size was adjusted according to the financial funds.
Study design
This ongoing population-based cancer screening program has been conducted on three types of cancer (esophageal, stomach and liver cancers) which are most prevalent in the selected rural counties. All participants needed to write informed consent. A two-step design with cancer risk assessment based on questionnaire interview, Hepatitis B surface antigen (HBsAg) test strip, and subsequent clinical intervention for high-risk populations was adopted free of charge at the local high-level hospitals designated in the program. Cancer risk assessment by questionnaire survey including risk factors and exposure rates of risk factors (including dietary habit, family history, digestive system diseases history, smoking, drinking, clinical symptoms, two-week drug history like aspirin) was basically on the guideline published in the English medical journals and adjusted according to the characteristics of Chinese population by experts’ discussion panel at the beginning of the program. Score scaled was used to evaluate the risk (Table 1).
1. Identified criteria of high-risk population in cancer screening program.
| Identified items | Score |
| *, Subjects were asked to reply the items above, and a summary score was added up for identification of high-risk individual. | |
| 1. Smoking: 20 cigarettes per day for more than 10 years | 1 |
| 2. Drinking: half a liter of liquor per week for more than 10 years | 1 |
| 3. Eating pickled vegetables often | 1 |
| 4. Eating processed meat often | 1 |
| 5. Hot-eating habit | 1 |
| 6. High-salt diet | 1 |
| 7. Family history of upper gastrointestinal cancer | 2 |
| 8. Family history of liver cancer | 2 |
| 9. Any current symptom of dysphagia, odynophagia, chest pain, back pain, neck pain | 2 |
| 10. Any current symptom of nausea and vomiting, anorexia, melena | 2 |
| 11. Any current symptom of abdominal distension, anorexia, nausea and vomiting | 2 |
| 12. Jaundice and edema | 2 |
| 13. Progressive emaciation | 2 |
| High-risk individual for esophageal cancer*: any two items among 1−5; or any one item of 7/9/13 | ≥2 |
| High-risk individual for stomach cancer*: any two items among 1−4 and 6; or any one item of 7/10/13 | ≥2 |
| High-risk individual for liver cancer*: any two items among 1−3; or any one item of 8/11/12/13 | ≥2 |
Esophageal cancer screening
All eligible participants aged 40−69 years were interviewed to complete the standardized epidemiological questionnaire to gather baseline information and identify whether they were high-risk population of esophageal cancer.
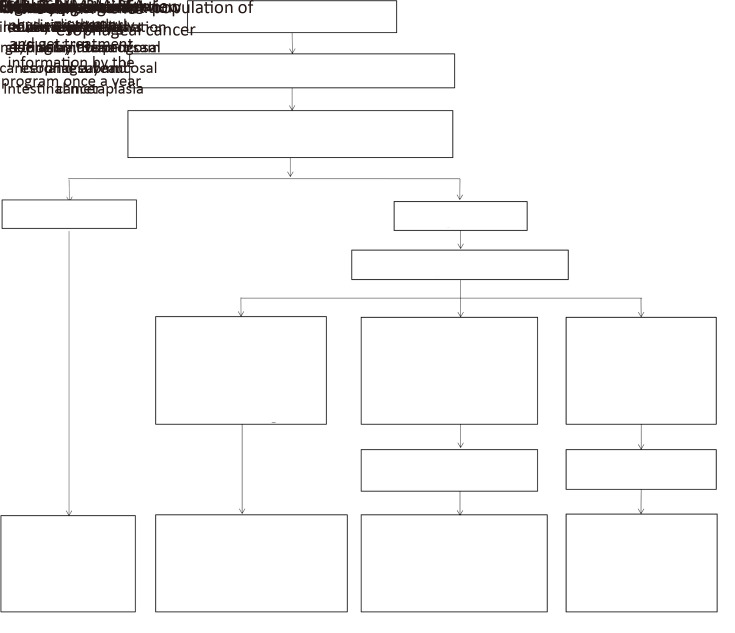
Subjects without risk or with low risk needed re-evaluation every 5 years. When high-risk population were identified, they were also considered as targeted population for endoscopy examination of esophageal cancer. Standard clinical procedures of the screening endoscope include appointment making, informed consent signing, routine blood test for infectious diseases such as hepatitis B virus (HBV), hepatitis C virus (HCV) and human immunodeficiency virus (HIV) infections, and endoscope examination. The entire esophagus was visually examined by experienced endoscopists, who have 5-year experience at least. If suspicious lesions were detected, tissue specimens were collected for diagnosis pathology to be confirmed. Subjects with the following pathology diagnoses were required in the following precautions: 1) subjects who were diagnosed as esophageal squamous intraepithelial neoplasia, Barrett’s esophageal and intestinal metaplasia needed to receive another new endoscopy examination by the program at least once every 3 years until positive results found; and then they were followed up actively; 2) subjects who were diagnosed as high-grade esophageal intraepithelial neoplasia, intra-mucosal cancer and sub-mucosal cancer needed endoscopic mucosal resection (EMR), endoscopic sub-mucosal dissection (ESD), endoscopic multiband mucosectomy (MBM) or radiofrequency ablation (RFA), which was not covered by the program. Besides these interventions, they were followed up actively to get information by the program at least once a year; and 3) subjects who were diagnosed as esophageal cancer, lesions infiltrating 1/3 submucosa needed to receive treatment intervention such as traditional surgery, chemotherapy and radiotherapy, which was not covered by the program. And they were followed up regularly by treating physician; and the detailed information of diagnosis and treatment was followed up by the program (Figure 1).
1.
Scheme of study design of esophageal cancer screening in esophageal, stomach and liver cancer screening program. EMR, endoscopic mucosal resection; ESD, endoscopic sub-mucosal dissection; MBM, endoscopic multiband mucosectomy; RFA, radiofrequency ablation.
Stomach cancer screening
All eligible participants aged 40−69 years were interviewed to complete the standardized epidemiological questionnaire to gather baseline information and identify whether they were high-risk population of stomach cancer.
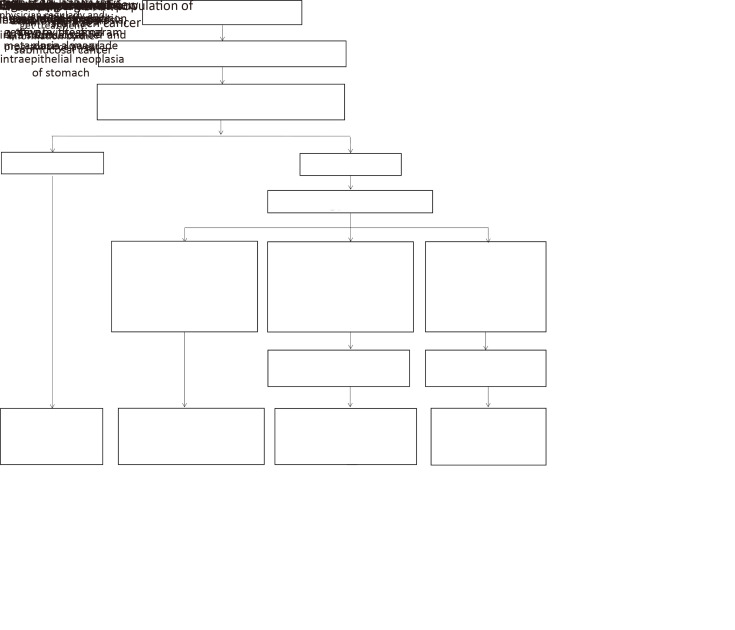
Subjects without risk or with low risk needed re-evaluation every 5 years. When high-risk population were identified, they were also considered as targeted population for endoscopy examination of stomach cancer. Standard clinical procedures of the screening endoscope were the same as esophageal cancer screening. Subjects with the following pathology diagnoses were required the following precautions: 1) subjects who were diagnosed as severe chronic atrophic gastritis of stomach, severe intestinal metaplasia, and low-grade intraepithelial neoplasia of stomach needed to receive another new endoscopy examination by the program at least once every 3 years until positive results found, and then they were followed up actively; 2) subjects who were diagnosed as high-grade stomach intraepithelial neoplasia, intra-mucosal cancer and sub-mucosal cancer needed the intervention of EMR, ESD, MBM or RFA out of the program. Besides these interventions, they were followed up actively to get information by the program at least once a year; and 3) subjects who were diagnosed as stomach cancer, lesions infiltrating 1/3 submucosa, needed to receive treatment intervention such as traditional surgery, chemotherapy and radiotherapy, which was not covered by the program. And they were followed up regularly by treating physician; and the detailed information of diagnosis and treatment was followed up by the program (Figure 2).
2.
Scheme of study design of stomach cancer screening in esophageal, stomach and liver cancer screening program. EMR, endoscopic mucosal resection; ESD, endoscopic sub-mucosal dissection; MBM, endoscopic multiband mucosectomy; RFA, radiofrequency ablation.
Liver cancer screening
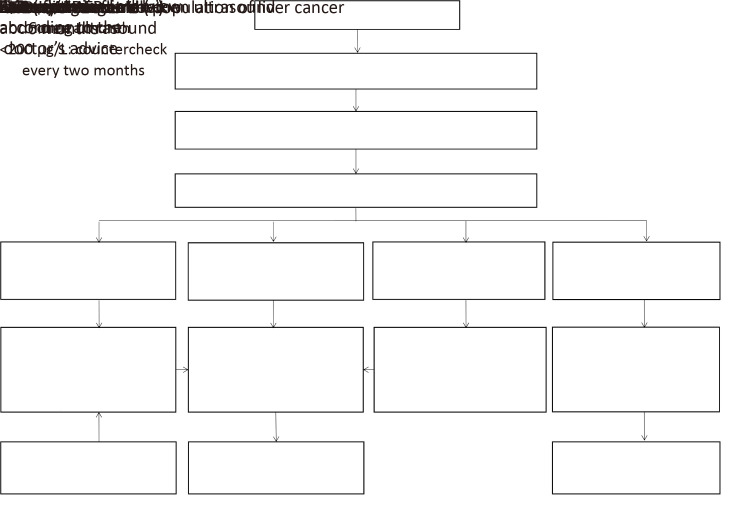
All females aged 45−64 years and males aged 35−64 years were approached through sequential method including the standardized epidemiological questionnaire and the detection of serum HBsAg. Clinical screening intervention was carried out for participants with positive HBsAg test or high-risk factors by questionnaire. Serum alpha-fetoprotein (AFP) and abdomen ultrasound jointly for liver cancer screening were used in the program. All the target population for liver cancer clinical screening were required to visit the hospital and undertake laboratory tests for AFP and abdomen ultrasound examination. The abdomen ultrasound is performed by attending physicians with at least 5-year ultrasonic experience. According to the results of AFP and ultrasound, different interventions were given inFigure 3.
3.
Scheme of study design of liver cancer screening in esophageal, stomach and liver cancer screening program. AFP, alpha-fetoprotein.
Data collection and management
A standardized epidemiological questionnaire collected information including sociodemographic factors, dietary habit, life style, history of personal disease, family history of malignancies, and psychological problems and administered by trained investigators in the screening sites for all participants recruited to assess the cancer risk. The printed questionnaire was filled and then entered into the data system by investigators.
Clinical information about the endoscopic findings and pathology diagnosis are collected using standardized form and documented which were formulated by panel discussion by clinical experts in the data system. All the forms are filled by physicians who performed the clinical examinations. The data in the collected forms are entered into the data system by trained staffs. The staffs who have done high-risk evaluation and clinical intervention in each screening sites have independent system account numbers. The staffs in National Cancer Center of China had one account to manage and analyze the whole data.
Outcome measures
The primary outcome is high-risk rate, screening compliance rate, participant rate and detection rate for esophageal cancer, stomach cancer and liver cancer at the end of every year. The secondary outcomes include incidence rate, mortality rate, detection rate for precancerous lesions, participation rate and clinical stage distribution. Besides, the exposure of risk factors and cost effectiveness was also calculated. The screening process would be repeated every year.
Follow-up management
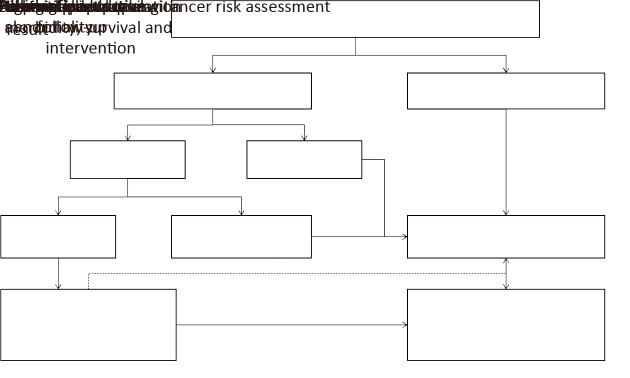
All participants recruited in the program need to be followed up by two methods (passive follow-up and active follow-up). Passive follow-up is conducted for all the participants by using data from cancer registry system and death surveillance system to collected information about cancer incidence (type, date of diagnosis, stage, etc.) and death (cause, date, etc.) each a year. Active follow-up targets patients with positive findings by screening and further diagnosis has been carried out to collect relevant information using a standardized form (Figure 4).
4.
Scheme of follow-up management in esophageal, stomach and liver cancer screening program.
The positive finding criteria for esophageal cancer and stomach cancer were the pathological diagnosis results and the study design in Figure 1,2 also gave the active follow-up management process after pathological diagnosis results. And for liver cancer, the combination criteria on AFP and abdominal ultrasound were used to judge the positive; and active follow-up management process was shown in the study design in Figure 3. All the relevant data would be therefore collected and documented in the data system. If the relevant data are not available, the eligible participants are interviewed by trained staffs by means of telephone call, home visit or other personal encounters. All positive patients need to be treated at their own expense.
Quality control
This was an ongoing population-based cancer screening program in the selected rural counties. Four steps were carried out to control the quality: 1) The technology process of screening including cancer risk assessment and clinical intervention was basically on the guideline published in the English medical journals and adjusted by native experts’ discussion panel; 2) All investigators were selected from communities and Centers for Disease Control and Prevention (CDC) in the screening sites. All investigators and experienced physicians from designated hospitals were trained by experts who has much more excellent experience on epidemiology and cancer screening from National Cancer Center of China; 3) 1% of negative results would be selected randomly to check for correctness. All positive result would be checked again; and 4) The combined strategy of passive and active follow-up was used to get high quality diagnosis and treatment information as much as possible.
Conclusions
This protocol described the design of population-based cancer (esophageal, stomach and liver cancers) screening cohort study in rural China. This program has been expected to improve the health services and reduce the burden and provide appropriate screening strategy in rural China in the future.
Acknowledgements
This study was sponsored by National Key R&D Program of China (No. 2018YFC1313100); Sanming Project of Medicine in Shenzhen (No. SZSM201911015) and CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2019-I2M-2-004).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Sun K, Zheng R, et al Cancer incidence and mortality in China, 2014. Chin J Cancer Res. 2018;30:1–12. doi: 10.21147/j.issn.1000-9604.2018.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torre LA, Bray F, Siegel RL, et al Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 5.Hamashima C, Shibuya D, Yamazaki H, et al The Japanese guidelines for stomach cancer screening. Jpn J Clin Oncol. 2008;38:259–67. doi: 10.1093/jjco/hyn017. [DOI] [PubMed] [Google Scholar]
- 6.Pasechnikov V, Chukov S, Fedorov E, et al Stomach cancer: prevention, screening and early diagnosis. World J Gastroenterol. 2014;20:13842–62. doi: 10.3748/wjg.v20.i38.13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim BJ, Heo C, Kim BK, et al Effectiveness of stomach cancer screening programs in South Korea: organized vs opportunistic models. World J Gastroenterol. 2013;19:736–41. doi: 10.3748/wjg.v19.i5.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Health Commission of the People’s Republic of China Chinese guidelines for diagnosis and treatment of gastric cancer 2018 (English version) Chin J Cancer Res. 2019;31:707–37. doi: 10.21147/j.issn.1000-9604.2019.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]