Abstract
Objective
The objective of this open-label, randomized study was to compare dose-dense paclitaxel plus carboplatin (PCdd) with dose-dense epirubicin and cyclophosphamide followed by paclitaxel (ECdd-P) as an adjuvant chemotherapy for early triple-negative breast cancer (TNBC).
Methods
We included Chinese patients with high recurrence risk TNBC who underwent primary breast cancer surgery. They were randomly assigned to receive PCdd [paclitaxel 150 mg/m2 on d 1 and carboplatin, the area under the curve, (AUC)=3 on d 2] or ECdd-P (epirubicin 80 mg/m2 divided in 2 d and cyclophosphamide 600 mg/m2 on d 1 for 4 cycles followed by paclitaxel 175 mg/m2 on d 1 for 4 cycles) every 2 weeks with granulocyte colony-stimulating factor (G-CSF) support. The primary endpoint was 3-year disease-free survival (DFS); the secondary endpoints were overall survival (OS) and safety.
Results
The intent-to-treat population included 143 patients (70 in the PCdd arm and 73 in the ECdd-P arm). Compared with the ECdd-P arm, the PCdd arm had significantly higher 3-year DFS [93.9% vs. 79.1%; hazard ratio (HR)=0.310; 95% confidence interval (95% CI), 0.137−0.704; log-rank, P=0.005] and OS (98.5% vs. 92.9%; HR=0.142; 95% CI, 0.060−0.825; log-rank, P=0.028). Worse neutropenia (grade 3/4) was found in the ECdd-P than the PCdd arm (47.9% vs. 21.4%, P=0.001).
Conclusions
PCdd was superior to ECdd-P as an adjuvant chemotherapy for early TNBC with respect to improving the 3-year DFS and OS. PCdd also yielded lower hematological toxicity. Thus, PCdd might be a preferred regimen for early TNBC patients with a high recurrence risk.
Keywords: Triple-negative breast cancer, dose-dense adjuvant chemotherapy, carboplatin, paclitaxel
Introduction
Triple-negative breast cancer (TNBC) is characterized by the lack of expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor 2 (HER2) (1,2). TNBCs, which account for approximately 12%−20% of all invasive breast cancers, are resistant to endocrine and HER2-targeted therapy (3,4); their aggressive behavior and poor prognosis make them one of the most challenging cancers to treat (5).
Postoperative adjuvant therapy for early breast cancer, which is an important part of comprehensive treatment, can reduce the risk of recurrence and metastasis (6). At present, since the use of polygenic prognostic detection in domestic hospitals is low, the decision of adjuvant treatment for early breast cancer patients is relatively conservative (7,8). The clinical application of an anthracycline sequential taxane regimen and aromatase inhibitors has also reached an expert consensus (9).
Systemic chemotherapy is generally recommended by guidelines and is, thus, currently considered as a mainstay of TNBC management (10). However, the proposed chemotherapy regimens remain controversial (10,11). In routine clinical practice, anthracycline and taxane-containing regimens (12,13) are the most commonly used systemic cytotoxic regimens for TNBC patients (14,15). Adding platinum to neoadjuvant chemotherapy regimens not only substantially increases the pathological complete response (pCR) rate (16,17) but may also improve the event-free survival (EFS) or overall survival (OS) of TNBC patients according to previous trials (18-20). Platinum-based neoadjuvant chemotherapy may be recommended as an option in TNBC patients with the cost of higher hematological toxicity incidence (18). However, there is limited direct evidence regarding an appropriate platinum-based adjuvant chemotherapy (18). Furthermore, determination of the optimal regimen balancing well-tolerated adverse toxicity with high efficacy is difficult (14).
Underlying genetic conditions appear to play an important role in TNBC (21). BRCA1-positive tumors show distinct clinic pathological characteristics (22). Seventy percent of all BRCA1-positive breast cancers and up to 23% of BRCA2 carriers have a TNBC phenotype (23). TNBC tumors with germline BRCA (gBRCA) mutation are associated with a better response to DNA-damaging systemic regimens (24) such as the platinum agents (25).
Dose-dense chemotherapy (i.e., a chemotherapy regimen in which each cycle has a shortened treatment interval) is associated with significant improvements in survival (26,27) and has been considered for use in the adjuvant setting for TNBC. With granulocyte colony-stimulating factor (G-CSF) support (28-30), dose-dense chemotherapy regimens at the optimal dose have been permitted at two-week intervals rather than the conventional three-week cycle in early breast cancer regimens (13).
Data supporting platinum-based adjuvant regimens for TNBC are scarce and are based mostly on retrospective research. Given the lack of well-established prospective or randomized studies, we conducted this study to compare the efficacy and safety of dose-dense paclitaxel plus carboplatin (PCdd) with those of the commonly used dose-dense epirubicin and cyclophosphamide followed by paclitaxel (ECdd-P) as adjuvant chemotherapy treatment in Chinese TNBC patients with high recurrence risk.
Materials and methods
Study design
This was a randomized, open-label, single-center study conducted in Chinese females with TNBC at high recurrence risk. The study was approved by the Independent Ethics Committee of the National Cancer Center/Cancer Hospital (No. CH-BC-012). All interventions were performed in accordance with the Declaration of Helsinki, guidelines of the International Conference for Harmonization/Good Clinical Practice. The study was registered with the ClinicalTrials.gov (No. NCT01378533).
Participants
All participating patients provided written informed consent. Female patients aged 18−65 years who had undergone primary breast surgery for confirmed ER-negative, PR-negative, and HER2-negative breast cancer were eligible. ER, PR and HER2 status were determined by immunohistochemistry (IHC) on patients’ tumor sections. The IHC cutoff for ER-negative and PR-negative status was 1% or less positive tumor cells with nuclear staining. HER2-negative status was determined by IHC by giving a score of 0 or 1 or by the absence ofHER2 amplification (HER2/CEP17 ratio <2.0 and HER2 copies <4.0) upon fluorescence in situ hybridization (FISH) analysis. ER, PR and HER2 analyses were performed centrally in a single laboratory of National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences. Patients were selected with positive axillary lymph or with other high-risk factors for recurrence (e.g., age <35 years, grade III disease, and intravascular cancer embolus). Further details regarding this study protocol are available in the Supplementary Table S1 .
S1. Synopsis of study protocol.
| Item | Description |
| ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor 2; ANC, absolute neutrophil count; AST, aspartate aminotransferase; ALT, alanine aminotransferase; AUC, area under the curve; 5-HT, 5-hydroxytryptamine; G-CSF, granulocyte colony-stimulating factor. | |
| Study ID | CH-BC-012 |
| Study title | Randomized phase III trial comparing dose-dense epirubicin and cyclophosphamide followed by paclitaxel with paclitaxel plus carboplatin as adjuvant therapy for triple-negative breast cancer |
| Protocol date | 4/20/2011 |
| Trial stage principal | Phase III |
| Investigator | Binghe Xu, M.D. & PhD. National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. Email: xubinghe@medmail.com.cn;
Qing Li, B.S.Med. National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. Email: cheryliqing@126.com |
| Participating study left | National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China |
| Objectives | To compare the efficacy and safety of dose-dense epirubicin and cyclophosphamide (ECdd) followed by paclitaxel (P) with dose-dense paclitaxel plus carboplatin (PCdd) as adjuvant therapy for patients with triple-negative breast cancer (TNBC) at high risk of recurrence
Primary objective: • Compare 3-year disease-free survival (DFS) of early TNBC patients at high risk treated with PCdd to those treated with ECdd-P regimens Secondary objectives: • Compare 3-year overall survival (OS) in the same population • Compare the toxicity of the PCdd to the ECdd-P in patients with TNBC at high risk of recurrence |
| Study population | Patients with early TNBC at high risk of recurrence |
| Study design | This is a single-left, open label, randomized, comparative phase III trial. The trial includes two groups: ECdd-P and PCdd.
Eligible participants will be randomly assigned in a 1:1 ratio to the PCdd group or the ECdd-P group. Randomization was conducted with no stratification factors. Eligible patients will be continually enrolled into the study until the total number of patients reached the planned sample size. The patients, medical staff and investigators were aware of treatment allocation. Sample size was determined based on a superiority test of 3-year DFS rate. To detect a difference of an approximate higher proportion of 0.10 between the two regimens (result of our preliminary clinical research demonstrated the proportion surviving in the ECdd-P regimen was 80.0%), an overall sample size of 133 subjects (66 in the ECdd-P arm and 67 in the PCdd arm) was calculated to achieve 80.0% power at a one-sided 0.050 significance level, with a 10% dropout rate (5% in each control/treatment arm). The accrual pattern across time periods was uniform (all periods equal). Primary and secondary efficacy analyses include the intent-to-treat (ITT) population of all randomly assigned patients. The safety analysis population includes all patients who received at least one dose of treatment. |
| Eligibility | Inclusion criteria: 1) Patient must accept the primary breast surgery; 2) Patients with histologically confirmed ER (−), PR (−) and HER2 (−),i.e., <1% positive tumor cells with nuclear staining in IHC and no HER2 overexpression; 3) Positive axillary lymph nodes; negative axillary lymph node with age <35 years or III grade or intravascular cancer embolus; 4) Age between 18 years to 65 years; 5) Able to give informed consent; 6) Patients with an Eastern Cooperative Oncology Group (ECOG) performance score of 0 or 1; 7) Not pregnant, and on appropriate birth control if of child-bearing potential; 8) Adequate bone marrow reserve with ANC >1.5×10 9/L and platelets >100×10 9/L; 9) Adequate renal function with serum creatinine <2.0× the upper limit of normal; 10) Adequate hepatic reserve with serum bilirubin <2.0× the upper limit of normal, AST/ALT <2× the upper limit of normal, and alkaline phosphatase < 5× the upper limit of normal. Serum bilirubin >2.0 is acceptable in the setting of known Gilbert’s syndrome; and 11) No active major medical or psychosocial problems that could be complicated by study participation.
Exclusion criteria: 1) Received neo-adjuvant therapy; 2) cardiac dysfunction documented by an ejection fraction less than the lower limit of the facility normal by multi-gated acquisition (MUGA) scan, or 45% by echocardiogram; 3) uncontrolled medical problems; 4) evidence of active acute or chronic infection; 5) pregnant or breast feeding; or 6) hepatic, renal or bone marrow dysfunction as detailed above. |
| Sample size calculation | The target sample size was calculated based on the primary endpoint, i.e., 3-year DFS rate. To detect a difference of 0.13 between the two regimens (result of our preliminary clinical research demonstrated the proportion surviving in the ECdd-P regimen was 80.0%), an overall sample size of 133 subjects (66 in the ECdd-P arm and 67 in the PCdd arm) was calculated to achieve 80.0% power at a one-sided 0.050 significance level. The accrual pattern across time periods was uniform (all periods equal). The proportion of drop out in the control and treatment group was 0.1000 (each 0.05). |
| Randomization | Upon meeting the eligibility criteria, patients will be randomised under concealment, by the study lead investigator (Cancer Hospital, Chinese Academy of Medical Sciences), according to prespecified randomisation number lists to receive ECdd-P or PCdd. |
| Treatment | Administration: Patients in both study groups received treatment in 14-day cycles. Patients assigned to the PCdd arm received paclitaxel 150 mg/m2 on d 1 plus carboplatin AUC=3 on d 2 for 8 cycles. Patients assigned to the ECdd-P arm received epirubicin 80 mg/m2 divided in 2 d and cyclophosphamide 600 mg/m2 on d 1 for 4 cycles followed by paclitaxel 175 mg/m2 on d 1 for 4 cycles. Prophylactic antiemetic measures, including 5-HT3 receptor antagonists, and dexamethasone, were allowed. Premedication with dexamethasone and histamine antagonists was administered before paclitaxel to prevent hypersensitivity reactions. Prophylactic G-CSF 3 µg/kg in d 5−9 was given for each chemotherapy cycle. |
| Safety assessments and dose modifications | Safety assessments included 12-lead electrocardiograms, vital sign taking and clinical laboratory evaluations every cycle. Adverse events (AEs) were recorded at each treatment cycle until 28 follow-up d after the end of study visit. Toxicity was graded by using the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 (NCI-CTCAE, version 3.0). Febrile neutropenia was managed according to institutional treatment guidelines in China. Toxicities were managed through dose delays of up to 3 weeks, and dose reductions were permitted in the following events: grade 4 hematological, grade 3 or 4 non-hematological, or other protocol-specified toxic effects. |
| Study drugs | Drug: epirubicin, cyclophosphamide, paclitaxel, carboplatin, G-CSF epirubicin 80 mg/m2 iv divide in 2 d cyclophosphamide 600 mg/m2 iv d 1 G-CSF 3 µg/kg in d 5−9 q14d ×4 cycles paclitaxel 175 mg/m2 iv d 1 G-CSF 3 µg/kg in d 5−9 q14d ×4 cycles paclitaxel 150 mg/m2 iv d 1 carboplatin AUC=3 iv d 2 G-CSF 3 µg/kg in d 5−9 q14d ×8 cycles. |
| Concomitant medications | 1. Antiemetics can be prescribed to patients who are vomiting due to administration of treatment drug(s);
2. Patients experiencing peripheral neuropathy can be treated with neurotropic supplements such as duloxetine, vitamin B, etc.; 3. Analgesics can be used for patients who have pain affecting quality of life; 4. Patients with constipation, diarrhea, or other conditions can be treated using appropriate medication for their respective condition; 5. Prophylactic antiemetic measures, including 5-HT3 receptor antagonists, and dexamethasone, were allowed. 6. Premedication with dexamethasone and histamine antagonists was administered before paclitaxel to prevent hypersensitivity reactions. |
| Outcome measures | Primary outcome measure:
The primary endpoint is 3-year DFS rate. DFS was calculated from the date of randomization to the date of the first local/distant recurrence (without second primary malignancies), according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Secondary outcome measures: Secondary endpoints include 3-year OS (defined as the time from randomization to death due to any cause) and safety of the treatment. Toxicity was graded by using the NCI- CTCAE, version 3.0. |
| Safety parameters | AEs, vital signs and clinical laboratory tests |
| Statistical analysis | All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) software (Version 22.0; IBM Corp., New York, USA). Data on clinical characteristics, chemotherapy, recurrence, and survival were analyzed. Data were presented as the number (%) or the mean standard deviation. Continuous variables were compared using the Student’s t test, while categorical variables were compared using the χ2 or Fisher’s exact test.
The proportion of patients remaining event-free over time will be displayed using the Kaplan-Meier method and analyzed using a two-sided log-rank test. All statistical tests were two-sided, and a P value of <0.05 was considered statistically significant. The safety population will include all patients who received at least one dose of treatment. For safety analysis, AEs will be coded using the Medical Dictionary for Regulatory Activities (MedDRA). Analysis of AEs will be based on treatment-emergent adverse events (TEAEs). TEAEs are AEs not present prior to medical treatment, or are already present and worsen either in intensity or frequency following treatment. The incidence rate of TEAEs will be described according to system organ class (SOC) and preferred term (PT). Meanwhile, serious AEs (SAEs) and AEs leading to study discontinuation will be similarly summarized and tabulated. Laboratory tests will be analyzed using descriptive statistical analysis. |
| Follow-up | All treated patients will be followed-up with once every 3 months to collect survival information for DFS and OS. Patients who discontinue treatment due to any causes will be followed-up with once every 3 months until disease recurrence or death. After disease recurrence, patient follow up can be conducted by phone or as general clinical visits until death. |
Randomization and masking
The patients were randomly assigned to receive either the PCdd or the ECdd-P regimen. Simple randomization was conducted with no stratification factors and was carried out by using random allocation sequence. The patients, medical staff, and investigators were aware of treatment allocation and assessing outcomes.
Procedures
Patients in both study arms received treatment in two-week cycles. Patients assigned to the PCdd arm received paclitaxel 150 mg/m2 on d 1 plus carboplatin AUC=3 on d 2 for 8 cycles. Patients assigned to the ECdd-P arm received epirubicin 80 mg/m2 divided in 2 d and cyclophosphamide 600 mg/m2 on d 1 for 4 cycles followed by paclitaxel 175 mg/m2 on d 1 for 4 cycles. Prophylactic G-CSF 3 µg/kg was administered during each cycle according to European Society for Medical Oncology (ESMO) and American Society of Clinical Oncology (ASCO) guidelines in the dose-dense setting. Toxicities were managed through dose delays of up to 3 weeks, and dose reductions were permitted in the following events: grade 4 hematological, grade 3 or 4 non-hematological, or other protocol-specified toxic effects. Safety was monitored with adverse events (AEs) reports, physical examinations, regular laboratory tests and electrocardiogram assessments at the end of each cycle until the 30th day of the last follow-up cycle.
Outcomes
The primary efficacy endpoint was the 3-year disease-free survival (DFS) rate, which was calculated from the date of randomization to the date of the first local/distant recurrence (in the absence of other primary malignancies). Secondary objectives included OS and safety. OS was defined as the time from randomization to death due to any cause. We analyzed the DFS and OS in patients who received at least one dose of the study treatment (intention-to-treat population, ITT). In the safety analysis, we evaluated the numbers and proportions of patients in each treatment arm who had any AEs, delay of chemotherapy, and dose reduction. AEs were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 (NCI-CTCAE, version 3.0).
In addition, we conducted exploratory subgroup analyses according to age (≤40 vs. >40 years), Ki-67 index (≤30 vs. >30), tumor size (<2 cm vs. ≥2 cm), nodal status (negative vs. positive), and surgery-chemotherapy interval (<30 dvs. ≥30 d) to investigate whether the treatment effect varied by subgroup.
Sample size computation
The sample size was calculated based on the primary endpoint, i.e., 3-year DFS rate. Assuming an approximate higher proportion of 0.10 as a primary outcome in PCdd regimen (results of our preliminary clinical research demonstrated the proportion achieving 3-year DFS in the ECdd-P regimen was 80.0%), an overall sample size of 133 participants (66 in the ECdd-P arm and 67 in the PCdd arm) was calculated to achieve 80.0% power with an alpha level at 0.05, with a 5% dropout rate in each control/treatment arm. Since the censoring proportion during the course of the study might be higher than expected; therefore, the sample size was increased to 143 patients to ensure the target number of events would be reached in a reasonable time frame.
Statistical analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) software (Version 22.0; IBM Corp., New York, USA). Data were presented as numbers (%) or as the mean standard deviation. Frequency tables were analyzed by using the χ2 test. The survival analysis was estimated using the Kaplan-Meier product-limit method in the ITT population. The hazard ratio (HR) and 95% confidence interval (95% CI) were estimated using the Cox proportional hazard model. Patients not showing progression were censored at the study cutoff date. The multivariable Cox model was used for subgroup analysis to explore the influence of clinical characteristics on the 3-year DFS. The safety analysis set included all randomized patients who received at least one dose of the study treatment and underwent at least one post-baseline safety assessment. A P value of <0.05 was considered statistically significant.
Results
Patients
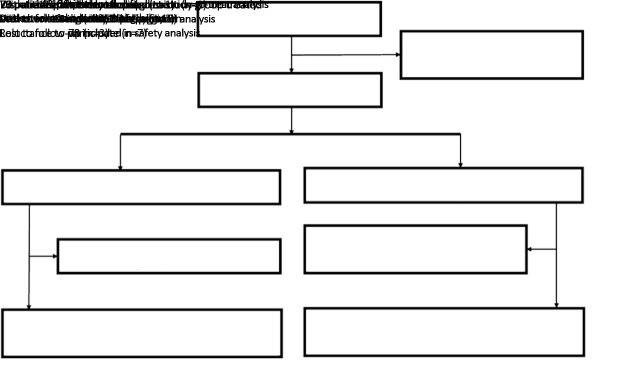
From June 2011 to December 2015, 143 patients were randomly enrolled in the PCdd arm (n=70) or the ECdd-P arm (n=73). After excluding 11 patients [treatment discontinuation due to tumor progression (n=1), withdrawal after chemotherapy (n=3), and lost to follow-up (n=7)], 132 patients who completed the planned eight cycles of chemotherapy were included in the per-protocol analysis (Figure 1 ). The data cutoff for the primary analysis was November 30th, 2018. Baseline characteristics were balanced between arms (Table 1 ). All enrolled patients had an Eastern Cooperative Oncology Group (ECOG) performance status score of 0−1, and 62.2% were postmenopausal. The median age was 49 (range, 22−64) years. In total, 111 patients (77.6%) were aged older than 40 years. Most patients had stage II or III disease (n=92, 64.3%), and 90.9% of patients had invasive ductal carcinoma. More than 42% had T2−T4 tumors, and 53 patients (37.1%) were clinically node positive. More than 75% of patients had a Ki-67 proliferation index >30%.
1.
Flow diagram of study design. ECdd-P, dose-dense epirubicin and cyclophosphamide followed by paclitaxel; PCdd, dose-dense paclitaxel plus carboplatin.
1. Baseline characteristics of patients with triple-negative breast cancer.
| Variable | ECdd-P arm (N=73) [n (%)] | PCdd arm (N=70) [n (%)] | P |
| ECdd-P, dose-dense epirubicin and cyclophosphamide followed by paclitaxel; PCdd, dose-dense paclitaxel plus carboplatin; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; MRM, modified radical mastectomy; BCS, breast conservative surgery; SLN, simple mastectomy and sentinel lymph node biopsy; SCI, surgery chemotherapy interval. | |||
| Age [mean (range)] (year) | 46 (26−64) | 49 (22−63) | 0.216 |
| ≤40 | 20 (27.4) | 12 (17.1) | 0.163 |
| >40 | 53 (72.6) | 58 (82.9) | |
| Menopause at diagnosis | |||
| Post-menopause | 50 (68.5) | 39 (55.7) | 0.124 |
| Pre-menopause | 23 (31.5) | 31 (44.3) | |
| Pathology | 0.114 | ||
| IDC | 63 (86.3) | 67 (95.7) | |
| ILC | 2 (2.7) | 0 (0) | |
| Other type | 8 (11.0) | 3 (4.3) | |
| Tumor size (cm) | 0.179 | ||
| <2 | 27 (37.0) | 34 (48.6) | |
| ≥2 | 46 (63.0) | 36 (51.4) | |
| Lymph node metastasis | 0.604 | ||
| Yes | 29 (39.7) | 24 (34.3) | |
| No | 44 (60.3) | 46 (65.7) | |
| Intravascular cancer embolus | 0.167 | ||
| Yes | 16 (21.9) | 10 (14.3) | |
| No | 57 (78.1) | 60 (85.7) | |
| Nuclear grade | 0.999 | ||
| Grade 1, 2 | 23 (31.5) | 22 (31.4) | |
| Grade 3 | 50 (68.5) | 48 (68.6) | |
| Ki-67 | 0.108 | ||
| ≤30 | 12 (16.4) | 20 (28.6) | |
| >30 | 61 (83.6) | 50 (71.4) | |
| TNM stage | 0.104 | ||
| I | 24 (32.9) | 27 (38.6) | |
| II/III | 49 (67.1) | 43 (61.4) | |
| Type of surgery | 0.309 | ||
| MRM | 57 (78.1) | 54 (77.1) | |
| BCS | 13 (17.8) | 9 (12.9) | |
| SLN | 3 (4.1) | 7 (10.0) | |
| Radiotherapy | 0.141 | ||
| Yes | 42 (57.5) | 33 (47.1) | |
| No | 31 (42.5) | 37 (52.9) | |
| SCI (d) | 0.609 | ||
| <30 | 47 (64.4) | 42 (60.0) | |
| ≥30 | 26 (35.6) | 28 (40.0) | |
Survival outcomes
As of cutoff date, the median duration of follow-up was 57.3 (range, 1.2−98.6) months, with 58.1 months in the PCdd arm and 56.1 months in the ECdd-P arm. In total, 98 patients (74.2%) were followed up over 4 years. During the study period, 23 relapse events were recorded, 5 in the PCdd arm and 18 in the ECdd-P arm. Most events (96.2%) were observed during the first 3 years after first diagnosis.
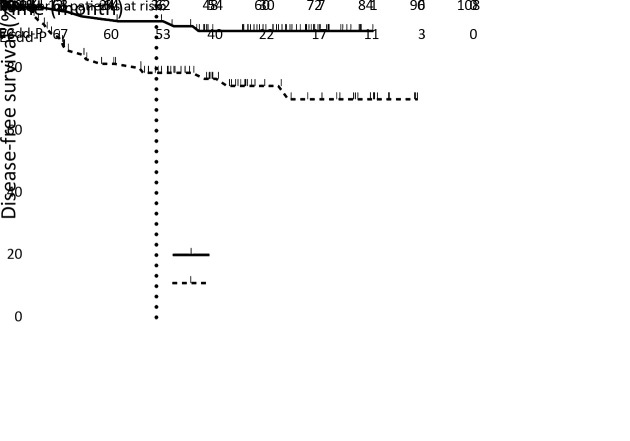
In the full analysis of ITT population, patients had significantly fewer DFS events in the PCdd arm than in the ECdd-P arm (5 vs. 18; HR=0.310; 95% CI, 0.137−0.704; log-rank, P=0.005). The 3-year DFS was 93.9% (95% CI, 88.2%−99.6%) in the PCdd arm and 79.1% (95% CI, 69.7−88.5%) in the ECdd-P arm. The Kaplan-Meier curves for DFS remained separated for the rest of the 3-year follow-up (Figure 2 ).
2.
Kaplan-Meier plot of disease-free survival (DFS). Cross marks indicate censored observations. Data for the intention-to-treat population. Hazard ratio (HR), 0.310, 95% confidence interval (95% CI), 0.137−0.704; Log-rank P=0.005; ECdd-P, dose-dense epirubicin and cyclophosphamide followed by paclitaxel; PCdd, dose-dense paclitaxel plus carboplatin.
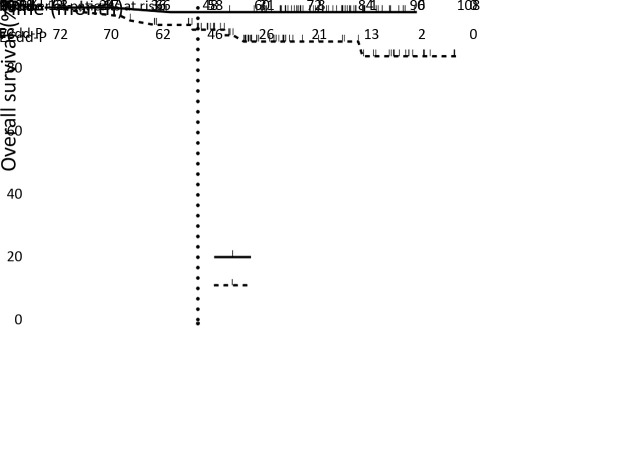
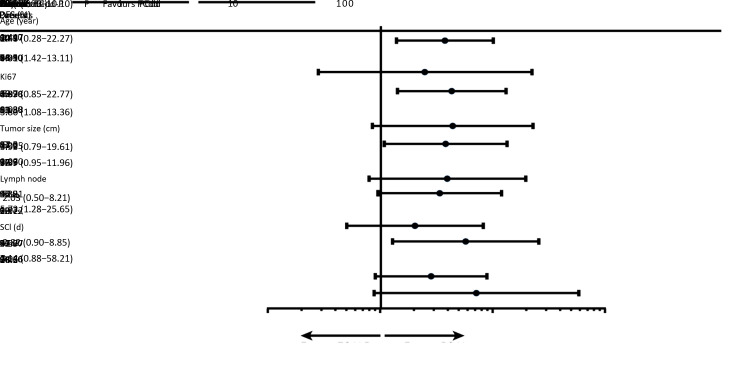
Data on OS were immature. Eight patients died in the ECdd-P arm, whereas only one died in the PCdd arm; all deaths were cancer related. Preliminary data showed a potential trend on a higher 3-year OS rate in the PCdd arm (98.5% vs. 92.9%; HR=0.142; 95% CI, 0.060−0.825; log-rank, P=0.028) (Figure 3 ). Subgroup analyses showed a consistent DFS benefit in the PCdd arm, with the difference reaching statistical significance in the following subgroups: age >40 years (HR=4.31; 95% CI, 1.42−13.11; P=0.010), Ki-67 index >30% (HR=3.80; 95% CI, 1.08−13.36; P=0.038), and clinically evaluated lymph nodes (HR=5.73; 95% CI, 1.28−25.65; P=0.022) ( Figure 4 ).
3.
Kaplan-Meier plot of overall survival (OS). Cross marks indicate censored observations. Data for the intention-to-treat population. Hazard ratio (HR), 0.142, 95% confidence interval (95% CI), 0.060−0.825, Log-rank P=0.028; ECdd-P, dose-dense epirubicin and cyclophosphamide followed by paclitaxel; PCdd, dose-dense paclitaxel plus carboplatin.
4.
Subgroup analyses of disease-free survival (DFS). The analyses of two arm patients were stratified for modified intention-to-treat population in clinically relevant subgroups. ECdd-P, dose-dense epirubicin and cyclophosphamide followed by paclitaxel; PCdd, dose-dense paclitaxel plus carboplatin; SCI, surgery-chemotherapy interval; HR, hazard ratio; 95% CI, 95% confidence interval.
AEs
Overall, both regimens were well tolerated with manageable AEs. There were more patients who experienced chemotherapy delay [25 (35.7%) vs. 23 (31.5%), P=0.361] and dose reduction [16 (22.9%) vs. 14 (19.2%), P=0.369] in the PCdd arm than in the ECdd-P arm, but the difference was not significant (Table 2 ).
2. Treatment exposure in TNBC patients treated with ECdd-P/PCdd chemotherapy.
| Variables | n (%) | P | |
| ECdd-P Arm (N=73) | PCdd Arm (N=70) | ||
| TNBC, triple-negative breast cancer; ECdd-P, dose-dense epirubicin and cyclophosphamide followed by paclitaxel; PCdd, dose-dense paclitaxel plus carboplatin. | |||
| Follow-up time [Median (range)] (month) | 56.1 (2.8−98.6) | 58.1 (1.2−76.6) | 0.320 |
| Number of chemotherapy cycles | |||
| Total | 573 | 552 | |
| Median | 8 (3−8) | 8 (2−8) | 0.783 |
| Delay of chemotherapy | 0.361 | ||
| Yes | 23 (31.5) | 25 (35.7) | |
| No | 50 (68.5) | 45 (64.3) | |
| Dose reduction | 0.369 | ||
| Yes | 14 (19.2) | 16 (22.9) | |
| No | 59 (80.8) | 54 (77.1) | |
The most frequent AEs were neutropenia, nausea and emesis. The incidence of grade 3 or 4 neutropenia was significantly higher in the ECdd-P arm than that in the PCdd arm [35 (47.9%) vs. 15 (21.4%), P=0.001], while the incidence of other grade 3 and 4 AEs was similar between the two arms. There was also no significant difference in the incidence of peripheral neuropathy between the two arms (Table 3 ). No death or life-threatening event was recorded during the study or within 30 days after the last cycle of treatment.
3. Common adverse events in TNBC patients treated with ECdd-P/PCdd chemotherapy.
| Adverse events | n (%) | P* | |||
| ECdd-P arm (n=73) | PCdd arm (n=70) | ||||
| A patient could have experienced more than one specific toxicity. TNBC, triple-negative breast cancer; ECdd-P, dose-dense epirubicin and cyclophosphamide followed by paclitaxel; PCdd, dose-dense paclitaxel plus carboplatin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; TBIL, total bilirubin; CRE, creatinine; *, P values for differences in two arms are tested by χ2 test or Fisher exact test. | |||||
| Hematologic toxicities | Grade 1/2 | Grade 3/4 | Grade 1/2 | Grade 3/4 | Grade 3/4 |
| Anemia | 28 (38.4) | 0 (0) | 14 (20.0) | 0 (0) | − |
| Leukopenia | 39 (53.4) | 26 (35.6) | 39 (55.7) | 12 (17.1) | 0.010 |
| Neutropenia | 30 (41.1) | 35 (47.9) | 31 (44.3) | 15 (21.4) | 0.001 |
| Thrombocytopenia | 8 (11.0) | 0 (0) | 9 (12.9) | 2 (2.9) | 0.238 |
| Non-hematologic toxicities | Grade 1/2 | Grade 3/4 | Grade 1/2 | Grade 3/4 | Grade 3/4 |
| Alopecia | 36 (49.3) | 8 (11.0) | 32 (45.7) | 4 (5.7) | 0.204 |
| Stomatitis | 38 (52.1) | 0 (0) | 29 (41.4) | 0 (0) | − |
| Nausea emesis | 65 (89.0) | 0 (0) | 56 (80.0) | 1 (1.4) | 0.490 |
| Diarrhea | 5 (6.8) | 1 (1.4) | 1 (1.4) | 0 (0) | 0.490 |
| Mucositis/cutaneous | 3 (4.1) | 1 (1.4) | 1 (1.4) | 0 (0) | 0.490 |
| Peripheral neuropathy | 28 (38.4) | 1 (1.4) | 31 (44.3) | 4 (5.7) | 0.170 |
| Foot and hand syndrome | 6 (8.2) | 0 (0) | 1 (1.4) | 0 (0) | − |
| Myalgia/arthralgia | 12 (16.4) | 1 (1.4) | 11 (15.7) | 0 (0) | 0.490 |
| Asthenia | 8 (11.0) | 1 (1.4) | 6 (8.6) | 0 (0) | 0.490 |
| Allergic | 1 (1.4) | 0 (0) | 3 (4.3) | 0 (0) | − |
| Cardiac toxicity | 3 (4.1) | 0 (0) | 2 (2.9) | 0 (0) | − |
| ALT elevation | 25 (34.2) | 3 (4.1) | 19 (27.1) | 1 (1.4) | 0.326 |
| AST elevation | 30 (41.1) | 0 (0) | 26 (37.1) | 0 (0) | − |
| TBIL elevation | 29 (39.7) | 0 (0) | 26 (37.1) | 0 (0) | − |
| CRE elevation | 3 (4.1) | 0 (0) | 7 (10.0) | 0 (0) | − |
Discussion
This open-label, randomized study achieved its primary endpoint, with a statistically significant difference in the 3-year DFS rate in patients randomized to receive PCdd as adjuvant chemotherapy for high-risk early TNBC vs. ECdd-P (93.9% vs. 79.1%; HR=0.310; 95% CI, 0.137−0.704; log-rank P=0.005). Further, PCdd was better tolerated than ECdd-P, with fewer hematological toxicities (grade 3/4) (21.4% and 47.9%, respectively). Collectively, these results indicate that PCdd might be an appropriate regimen for TNBC. PCdd not only is superior to ECdd-P as adjuvant chemotherapy with respect to improving the 3-year DFS and OS rates but also yields lower chemotherapy-related toxicities in early TNBC patients regardless of theBRCA mutation status. Thus, PCdd might be a beneficial standard adjuvant regimen for early TNBC patients at a high recurrence risk, as indicated herein by the clinically meaningful improvement in survival and safety. To our knowledge, this is an innovative randomized clinical study to evaluate the efficacy of a dose-dense carboplatin-based regimen in the adjuvant setting for TNBC with high recurrence risk.
TNBC may be more sensitive to platinum-based regimens (18). Carboplatin increased the pCR rate from 41% to 54% in the CALGB40603 trial (31) and from 36.9% to 53.2% in the GeparSixto trial (32). In the GeparSixto study, the improved pCR rate significantly increased the 3-year DFS rate from 76.1% to 85.8% (HR=0.56; 95% CI, 0.33−0.96; P=0.024) (33). However, in the CALGB40603 study, the 5-year distant recurrence-free interval was 76.3% with no significant difference (34). The randomized phase III clinical trial EA 1131 (NCT02445391) has also been designed to prove the efficacy of adjuvant cisplatin or carboplatin following neoadjuvant chemotherapy in patients with residual TNBC (35). The BrighTNess study has also confirmed that carboplatin-containing regimen appears to have a favorable risk-to-benefit profile for patients with high-risk TNBC in the neoadjuvant setting (36). However, the clinical benefit of adjuvant carboplatin in TNBC has not been well-investigated (37). For an adjuvant scenario, a retrospective, single-center study in a Swiss breast cancer center reported a 5-year relapse-free survival (RFS) of 90% in patients treated with carboplatin (38). In the present study, the PCdd regimen achieved significantly better survival benefit (3-year DFS and OS rates) for TNBC patients in the adjuvant setting compared with historical data from standard chemotherapy regimens (60%−80% with taxane-based regimens, 65%−85% with anthracycline- and taxane-based therapy, and 83.7% with anthracycline-based chemotherapy plus bevacizumab) (39-41).
A dose-dense regimen has been hypothesized to minimize residual tumor burden compared to dose escalation and serve as a more effective method for high-risk breast cancer (27). In the CALGB9741 trial (42), the 4-year DFS rate was 82% in the dose-dense group. A previous study from our institution also compared the epirubicin and cyclophosphamide followed by paclitaxel (EC-P) or epirubicin plus paclitaxel (EP) dose-dense group and the EP regular group regarding postoperative adjuvant treatment for high-risk breast cancer. The dose-dense group had higher 3-year RFS rates (84.1% vs. 80.0%, P=0.501) and OS rates (95.6% vs. 90.0%, P=0.153) (43). Our trial is a novel prospective study showing significant improvements in the 3-year DFS and OS rates by using a dose-dense anthracycline-free platinum-based adjuvant chemotherapy regimen for TNBC regardless of the BRCA mutation status. The 3-year DFS (93.9%) and OS (98.5%) rates in the PCdd arm were also superior to those of a dose-dense regimen reported by the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (13). Although the survival data in our study are immature at present, a relatively long follow-up time will allow us to report a beneficial trend in OS. In addition, these data are comparable to previous data on anthracycline- and taxane-based dose-dense regimens.
Because the TNBC phenotype is closely associated with hereditary breast cancer, the administration of platinum-based regimens has received a new impetus (44,45). However, in the Chinese population, BRCA1/2 mutations are prevalent in only 10.5% of TNBC patients younger than 50 years (46). The benefit of adjuvant carboplatin in TNBC with BRCA1/2 mutation(s) is still controversial. The GeparSixto trial showed that carboplatin is more effective in TNBC patients (33); however, a secondary analysis of the GeparSixto demonstrated that TNBC patients without BRCA1 and BRCA2 germline mutations would also benefit from the addition of carboplatin, which increased the DFS rate (85.3% in the carboplatin group and 73.5% in the non-carboplatin group; HR=0.53; 95% CI, 0.29−0.96; P=0.04) (33). The BRCA1/2 mutation status plays an important role for tumor identification in TNBC patients with higher response rate of platinum-based neoadjuvant therapy. However, other studies have shown that the clinical use of the homologous recombination deficiency (HRD) test may also have the potential to identify patients with TNBC that may respond to the treatment of DNA damage, in excess of those currently identified by gBRCA1/2 mutational screening (47,48).
It has been suggested that tumors carrying gBRCA mutations may be sensitive to DNA-damaging chemotherapeutic drugs, including platinum (49). In the present study, we found that for early TNBC patients, the addition of carboplatin to paclitaxel was superior to epirubicin plus paclitaxel with respect to the 3-year DFS among BRCA1/2 unselected patients. To analyze the trends in adjuvant regimens for TNBC and to explore the factors influencing efficacy, we demonstrated that patients aged >40 years, with Ki-67 index >30%, and clinically evaluated lymph nodes were found to have a survival advantage from the PCdd regimen. Future refinement of platinum-sensitive subgroups for targeting specific tumor biomarkers in TNBC is warranted ( 50).
With respect to tolerance, previous trials (42) showed a high incidence of AEs and an increasing discontinuation rate for dose-dense chemotherapy of TNBC. The PCdd regimen, which yields fewer adverse toxicities, may be considered a better alternative for the high-risk group of patients in our study, particularly for older patients. The toxicity profile in our study was as anticipated: gastrointestinal toxic effects were more common in the PCdd arm, while grade 3/4 hematological toxicity was more common in the ECdd-P arm. All gastrointestinal toxic effects were manageable and self-limiting. These findings indicate that the PCdd regimen can be recommended to reduce unnecessary toxicities.
Our study has some limitations, including its small sample size and the potential investigator bias from a single-center institutional experience. Further, we had limited statistical power to show a significant OS benefit. A longer follow-up time is necessary, and the median OS should be further evaluated. In addition, given the financial and technical limitations during the study period, the BRCA mutation status was not analyzed to identify whether the gBRCA subgroup will benefit from the PCdd regimen. Further prospective trials to evaluate other platinum-based regimens in the adjuvant setting for TNBC are warranted, particularly to define a sensitive population. An ongoing phase III trial in National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences (NCT03876886 at http://ClinicalTrials.gov) might provide further insight to evaluate the incorporation of platinum in the adjuvant setting, to detect HRD, and to identify specific TNBC patients who might benefit from carboplatin-based therapy.
Conclusions
PCdd not only is superior to ECdd-P as adjuvant chemotherapy with respect to improving 3-year DFS and OS rates but also yields lower chemotherapy-related toxicities in early TNBC patients regardless of the BRCA mutation status. Thus, PCdd might be a beneficial standard adjuvant regimen for early TNBC patients at a high recurrence risk, with clinically meaningful improvement in survival and safety data.
Acknowledgements
This work was supported by National Key Research and Development Program of China (No. 2018YFC1312101) and Chinese Academy of Medical Science Initiative for Innovative Medicine (No. CAMS-2016-I2M-1-010).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare
References
- 1.Hammond ME, Hayes DF, Dowsett M, et al American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–95. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar P, Aggarwal R An overview of triple-negative breast cancer. Arch Gynecol Obstet. 2016;293:247–69. doi: 10.1007/s00404-015-3859-y. [DOI] [PubMed] [Google Scholar]
- 3.Collignon J, Lousberg L, Schroeder H, et al Triple-negative breast cancer: treatment challenges and solutions. Breast Cancer (Dove Med Press) 2016;8:93–107. doi: 10.2147/BCTT.S69488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dent R, Trudeau M, Pritchard KI, et al Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429–34. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 5.Foulkes WD, Smith IE, Reis-Filho JS Triple-negative breast cancer. N Engl J Med. 2010;363:1938–48. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 6.National Health Commission of The People’s Republic of China Chinese guidelines for diagnosis and treatment of breast cancer 2018 (English version) Chin J Cancer Res 2019;31:259-77.
- 7.Lüftner D, Bauerfeind I, Braun M, et al Treatment of early breast cancer patients: Evidence, controversies, consensus: Focusing on systemic therapy - German Experts’ Opinions for the 16th International St. Gallen Consensus Conference (Vienna 2019) Breast Care (Basel) 2019;14:315–24. doi: 10.1159/000502603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burstein HJ, Curigliano G, Loibl S, et al Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann Oncol. 2019;30:1541–57. doi: 10.1093/annonc/mdz235. [DOI] [PubMed] [Google Scholar]
- 9.Balic M, Thomssen C, Würstlein R, et al St. Gallen/Vienna 2019: A brief summary of the consensus discussion on the optimal primary breast cancer treatment. Breast Care (Basel) 2019;14:103–10. doi: 10.1159/000499931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Apuri S Neoadjuvant and adjuvant therapies for breast cancer. South Med J. 2017;110:638–42. doi: 10.14423/SMJ.0000000000000703. [DOI] [PubMed] [Google Scholar]
- 11.Hong J, Chen XS, Wu JY, et al Analysis of the factors influencing adjuvant chemotherapy decisions for triple negative breast cancer. Zhonghua Zhong Liu Za Zhi. 2017;39:39–43. doi: 10.3760/cma.j.issn.0253-3766.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Caparica R, Bruzzone M, Poggio F, et al Anthracycline and taxane-based chemotherapy versus docetaxel and cyclophosphamide in the adjuvant treatment of HER2-negative breast cancer patients: a systematic review and meta-analysis of randomized controlled trials. Breast Cancer Res Treat. 2019;174:27–37. doi: 10.1007/s10549-018-5055-9. [DOI] [PubMed] [Google Scholar]
- 13.Early Breast Cancer Trialists’ Collaborative G Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: a patient-level meta-analysis of 37 298 women with early breast cancer in 26 randomised trials. Lancet. 2019;393:1440–52. doi: 10.1016/S0140-6736(18)33137-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lebert JM, Lester R, Powell E, et al Advances in the systemic treatment of triple-negative breast cancer. Curr Oncol. 2018;25:S142–S150. doi: 10.3747/co.25.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Locatelli MA, Curigliano G, Eniu A Extended adjuvant chemotherapy in triple-negative breast cancer. Breast care (Basel) 2017;12:152–8. doi: 10.1159/000478087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silver DP, Richardson AL, Eklund AC, et al Efficacy of neoadjuvant cisplatin in triple-negative breast cancer. J Clin Oncol. 2010;28:1145–53. doi: 10.1200/JCO.2009.22.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Minckwitz G, Schneeweiss A, Loibl S, et al Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014;15:747–56. doi: 10.1016/S1470-2045(14)70160-3. [DOI] [PubMed] [Google Scholar]
- 18.Poggio F, Bruzzone M, Ceppi M, et al Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: a systematic review and meta-analysis. Ann Oncol. 2018;29:1497–508. doi: 10.1093/annonc/mdy127. [DOI] [PubMed] [Google Scholar]
- 19.Schneeweiss A, Möbus V, Tesch H, et al Intense dose-dense epirubicin, paclitaxel, cyclophosphamide versus weekly paclitaxel, liposomal doxorubicin (plus carboplatin in triple-negative breast cancer) for neoadjuvant treatment of high-risk early breast cancer (GeparOcto-GBG 84): A randomised phase III trial. Eur J Cancer. 2019;106:181–92. doi: 10.1016/j.ejca.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 20.Zhang P, Yin Y, Mo H, et al Better pathologic complete response and relapse-free survival after carboplatin plus paclitaxel compared with epirubicin plus paclitaxel as neoadjuvant chemotherapy for locally advanced triple-negative breast cancer: a randomized phase 2 trial. Oncotarget. 2016;7:60647–56. doi: 10.18632/oncotarget.10607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sporikova Z, Koudelakova V, Trojanec R, et al Genetic markers in triple-negative breast cancer. Clin Breast Cancer. 2018;18:e841–e850. doi: 10.1016/j.clbc.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 22.Lips EH, Mulder L, Oonk A, et al Triple-negative breast cancer: BRCAness and concordance of clinical features with BRCA1-mutation carriers. Br J Cancer. 2013;108:2172–7. doi: 10.1038/bjc.2013.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie Y, Gou Q, Wang Q, et al The role of BRCA status on prognosis in patients with triple-negative breast cancer. Oncotarget. 2017;8:87151–62. doi: 10.18632/oncotarget.19895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang N, Li K, Huang W, et al Efficacy of platinum in advanced triple-negative breast cancer with germline BRCA mutation determined by next generation sequencing. Chin J Cancer Res. 2020;32:149–62. doi: 10.21147/j.issn.1000-9604.2020.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clifton K, Gutierrez-Barrera A, Ma J, et al Adjuvant versus neoadjuvant chemotherapy in triple-negative breast cancer patients with BRCA mutations. Breast Cancer Res Treat. 2018;170:101–9. doi: 10.1007/s10549-018-4727-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bayraktar S, Arun B Dose-dense chemotherapy for breast cancer. Breast J. 2012;18:261–6. doi: 10.1111/j.1524-4741.2012.01236.x. [DOI] [PubMed] [Google Scholar]
- 27.Hudis C, Dang C The development of dose-dense adjuvant chemotherapy. Breast J. 2015;21:42–51. doi: 10.1111/tbj.12364. [DOI] [PubMed] [Google Scholar]
- 28.Do T, Medhekar R, Bhat R, et al The risk of febrile neutropenia and need for G-CSF primary prophylaxis with the docetaxel and cyclophosphamide regimen in early-stage breast cancer patients: a meta-analysis. Breast Cancer Res Treat. 2015;153:591–7. doi: 10.1007/s10549-015-3531-z. [DOI] [PubMed] [Google Scholar]
- 29.Mäenpää J, Varthalitis I, Erdkamp F, et al The use of granulocyte colony stimulating factor (G-CSF) and management of chemotherapy delivery during adjuvant treatment for early-stage breast cancer – further observations from the IMPACT solid study. Breast. 2016;25:27–33. doi: 10.1016/j.breast.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Lambertini M, Bruzzi P, Poggio F, et al Pegfilgrastim administration after 24 or 72 or 96 h to allow dose-dense anthracycline- and taxane-based chemotherapy in breast cancer patients: a single-center experience within the GIM2 randomized phase III trial. Support Care Cancer. 2016;24:1285–94. doi: 10.1007/s00520-015-2907-2. [DOI] [PubMed] [Google Scholar]
- 31.Sikov WM, Berry DA, Perou CM, et al Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance) J Clin Oncol. 2015;33:13–21. doi: 10.1200/JCO.2014.57.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Minckwitz G, Loibl S, Schneeweiss A, et al Early survival analysis of the randomized phase II trial investigating the addition of carboplatin to neoadjuvant therapy for triple-negative and HER2-positive early breast cancer (GeparSixto) Cancer Res. 2016;76(4 suppl):S2–04. doi: 10.1158/1538-7445.SABCS15-S2-04. [DOI] [Google Scholar]
- 33.Hahnen E, Lederer B, Hauke J, et al Germline mutation status, pathological complete response, and disease-free survival in triple-negative breast cancer: Secondary analysis of the GeparSixto randomized clinical trial. JAMA Oncol. 2017;3:1378–85. doi: 10.1001/jamaoncol.2017.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sikov WM, Berry DA, Perou CM, et al Event-free and overall survival following neoadjuvant weekly paclitaxel and dose-dense AC +/- carboplatin and/or bevacizumab in triple-negative breast cancer: Outcomes from CALGB 40603 (Alliance) Cancer Res. 2016;76(4 suppl):S2–05. doi: 10.1158/1538-7445.SABCS15-S2-05. [DOI] [Google Scholar]
- 35.Platinum based chemotherapy or capecitabine in treating patients with residual triple-negative basal-like breast cancer following neoadjuvant chemotherapy (NCT02445391). Available online: https://clinicaltrials.gov/ct2/show/NCT02445391?term=02445391&draw=2&rank=1
- 36.Loibl S, O’Shaughnessy J, Untch M, et al Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. Lancet Oncol. 2018;19:497–509. doi: 10.1016/S1470-2045(18)30111-6. [DOI] [PubMed] [Google Scholar]
- 37.Pandy JGP, Balolong-Garcia JC, Cruz-Ordinario MVB, et al Triple negative breast cancer and platinum-based systemic treatment: a meta-analysis and systematic review. BMC Cancer. 2019;19:1065. doi: 10.1186/s12885-019-6253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vetter M, Fokas S, Biskup E, et al Efficacy of adjuvant chemotherapy with carboplatin for early triple negative breast cancer: a single center experience. Oncotarget. 2017;8:75617–26. doi: 10.18632/oncotarget.18118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bell R, Brown J, Parmar M, et al Final efficacy and updated safety results of the randomized phase III BEATRICE trial evaluating adjuvant bevacizumab-containing therapy in triple-negative early breast cancer. Ann Oncol. 2017;28:754–60. doi: 10.1093/annonc/mdw665. [DOI] [PubMed] [Google Scholar]
- 40.Budd GT, Barlow WE, Moore HC, et al SWOG S0221: a phase III trial comparing chemotherapy schedules in high-risk early-stage breast cancer. J Clin Oncol. 2015;33:58–64. doi: 10.1200/JCO.2014.56.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sparano JA, Wang M, Martino S, et al Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–71. doi: 10.1056/NEJMoa0707056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Citron ML, Berry DA, Cirrincione C, et al Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:1431–9. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 43.Wu WH, Li Q, Xu BH, et al Clinical contrast study on dose dense and regular chemotherapy in the postoperative adjuvant treatment for breast cancer. Zhongguo Zhong Liu Lin Chuang. 2009;36:493–6. doi: 10.1016/j.ceramint.2007.09.109>. [DOI] [Google Scholar]
- 44.Ellsworth DL, Turner CE, Ellsworth RE A review of the hereditary component of triple negative breast cancer: High- and moderate-penetrance breast cancer genes, low-penetrance loci, and the role of nontraditional genetic elements. J Oncol. 2019;2019:4382606. doi: 10.1155/2019/4382606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shimelis H, LaDuca H, Hu C, et al Triple-negative breast cancer risk genes identified by multigene hereditary cancer panel testing. J Natl Cancer Inst. 2018;110:855–62. doi: 10.1093/jnci/djy106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang J, Pei R, Pang Z, et al Prevalence and characterization of BRCA1 and BRCA2 germline mutations in Chinese women with familial breast cancer. Breast Cancer Res Treat. 2012;132:421–8. doi: 10.1007/s10549-011-1596-x. [DOI] [PubMed] [Google Scholar]
- 47.Telli ML, Timms KM, Reid J, et al Homologous recombination deficiency (HRD) score predicts response to platinum-containing neoadjuvant chemotherapy in patients with triple-negative breast cancer. Clin Cancer Res. 2016;22:3764–73. doi: 10.1158/1078-0432.CCR-15-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Belli C, Duso BA, Ferraro E, et al Homologous recombination deficiency in triple negative breast cancer. Breast. 2019;45:15–21. doi: 10.1016/j.breast.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 49.Torrisi R, Zuradelli M, Agostinetto E, et al Platinum salts in the treatment of BRCA-associated breast cancer: A true targeted chemotherapy? Crit Rev Oncol Hematol. 2019;135:66–75. doi: 10.1016/j.critrevonc.2019.01.016. [DOI] [PubMed] [Google Scholar]
- 50.Jin J, Zhang W, Ji W, et al Predictive biomarkers for triple negative breast cancer treated with platinum-based chemotherapy. Cancer Biol Ther. 2017;18:369–78. doi: 10.1080/15384047.2017.1323582. [DOI] [PMC free article] [PubMed] [Google Scholar]