Abstract
Objective
Fluoroscopy guidance is generally required for endobronchial ultrasonography with guide sheath (EBUS-GS) in peripheral pulmonary lesions (PPLs). Virtual bronchoscopic navigation (VBN) can guide the bronchoscope by creating virtual images of the bronchial route to the lesion. The diagnostic yield and safety profiles of VBN without fluoroscopy for PPLs have not been evaluated in inexperienced pulmonologist performing EBUS-GS.
Methods
Between January 2016 and June 2017, consecutive patients with PPLs referred for EBUS-GS at a single cancer center were enrolled. The diagnostic yield as well as safety profiles was retrospectively analyzed, and our preliminary experience was shared.
Results
A total of 109 patients with 109 lesions were included, 99 (90.8%) lesions were visible on EBUS imaging. According to the procedure time needed to locate the lesion on EBUS, 24.8% (27/109) were deemed technically difficult procedures; however, no significant relationships were identified between candidate parameters and technically difficult procedures. The overall diagnosis yield was 74.3% (81/109), and the diagnostic yield of malignancy was 83.7% (77/92). Lesions larger than 20 mm [odds ratio (OR), 2.758; 95% confidence interval (95% CI), 1.077−7.062; P=0.034] and probe of within type (OR, 3.174; 95% CI, 1.151−8.757, P=0.026) were independent factors leading to a better diagnostic yield in multivariate analysis. About 30 practice procedures were needed to achieve a stable diagnostic yield, and the proportion of technically difficult procedures decreased and stabilized after 70 practice procedures. Regarding complications, one patient (0.9%) had intraoperative hemorrhage (100 mL) which was managed under endoscopy.
Conclusions
VBN without fluoroscopy guidance is still useful and safe for PPLs diagnosis, especially for malignant diseases when performed by pulmonologist without previous experience of EBUS-GS. VBN may simplify the process of lesion positioning and further multi-center randomized studies are warranted.
Keywords: Endobronchial ultrasonography, fluoroscopy, lung neoplasms, peripheral pulmonary lesion, virtual bronchoscopic navigation
Introduction
Lung cancer is the most common incident cancer and the leading cause of cancer deaths in China and worldwide (1,2). Peripheral pulmonary lesions (PPLs) are defined as lesions surrounded by normal lung parenchyma, located beyond the segmental bronchus and unlikely to be visualized using bronchoscopy (3). More PPLs are being detected with increased use of chest computed tomography (CT), and some of these are indeed malignancies (4). Early diagnosis of these lesions is of great importance to reduce mortality owing to lung cancer (5). In recent years, endobronchial ultrasonography with guide sheath (EBUS-GS) has produced favorable results in the diagnosis of PPLs (6). However, EBUS-GS is only designed to confirm arrival at the lesion, fluoroscopy is still needed for guidance before the confirmation (7). As has been reported, without fluoroscopy and other navigation techniques, it takes 3 years or around 400 procedures to achieve a better and stable performance of this technique (8). While under fluoroscopy guidance, EBUS-GS could produce an acceptable diagnostic yield, even when performed by a novice pulmonologist (9). However, fluoroscopy guidance involves the requirement of X-ray fluoroscopy equipment in a special room, and the radiation exposure for both patients and operators during examination (10). Virtual bronchoscopic navigation (VBN) is a technology that can create a virtual bronchoscopic image and guide a pathway to the lesion (11). Use of VBN could shorten the total examination time and duration of radiation exposure for both patients and operators, some literatures reported that VBN can increase the diagnostic yield of EBUS-GS for PPLs (3,12). The feasibility of VBN assisted EBUS-GS without fluoroscopy guidance in PPLs for pulmonologist with no previous experience has seldom been discussed. As a local cancer center, we began to perform VBN assisted EBUS-GS without fluoroscopy guidance since 2016. In the present study, we evaluated the diagnostic performance as well as safety of this procedure; herein, we shared our experience. These results may further support the use of this approach in PPLs diagnosis.
Materials and methods
Patients
This study was approved by the Institutional Ethics Committee of Peking University Cancer Hospital (No. 2016KT15), and individual patient consent was waived. We retrieved information from a prospectively maintained database of EBUS-GS for consecutive patients admitted to Peking University Cancer Hospital from January 2016 to June 2017.
The inclusion criteria were: 1) patients with PPLs suspected to be malignancies on CT; and 2) in routine bronchoscopy prior to the EBUS-GS procedure, the PPLs were invisible and no definite diagnosis was obtained. The exclusion criteria were: 1) lesions observed as ground glass opacities (GGO); or 2) a final definite pathological diagnosis or clinical diagnosis in follow-up was unavailable.
EBUS-GS examination
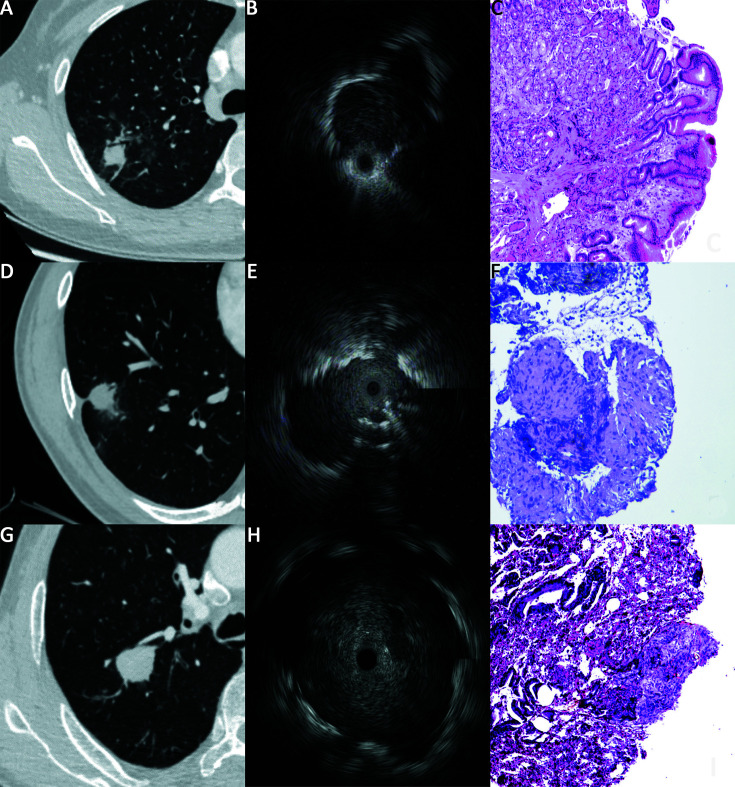
All chest CT scans were performed within 2 weeks prior to EBUS-GS, the imaging parameters were 120 kV and 55−275 mA. CT scans were reviewed by a chest radiologist with 12 years of experience in the interpretation of thoracic CT. The size of each PPL was measured based on its mean diameter using the axial lung window setting. Lesion’s location in the lung field was divided into two groups based on the study by Iwanoet al. (13). A lesion with its center located within 2.5 cm of the chest wall was considered to be in the lateral band, and lesion in the inner area was considered to be in the intermediate band. The presence or absence of a bronchus sign (presence of a bronchus directly leading to the target lesion on CT) was recorded (Figure 1 ).
1.
Endobronchial ultrasonography with guide sheath (EBUS-GS) for peripheral pulmonary lesions (PPLs). (A) Solid lesions with bronchus signs, measuring 16 mm × 14 mm, located in lateral band of the right upper lobe; (B) Probe position was adjacent type on EBUS imaging; (C) Pathology section indicating adenocarcinoma of lung (400×); (D) Solid lesions with bronchus signs, measuring 22 mm × 21 mm, located in lateral band of the right lower lobe; (E) Probe position was within type on EBUS imaging; (F) Pathology section indicating caseous granuloma, suggesting tuberculosis (100×); (G) Solid lesions without bronchus signs, measuring 27 mm × 24 mm, located in intermediate band of the right upper lobe; (H) Probe position was within type on EBUS imaging; (I) Pathology section indicating adenocarcinoma metastasis from colon (100×).
All examinations were conducted by one pulmonologist with 5 years of experience in performing convex probe EBUS, but no previous experience of EBUS-GS, using a BF-P260F flexible bronchoscope (Olympus Ltd., Tokyo, Japan), an endoscopic ultrasound system (EU-ME1, Olympus Ltd., Tokyo, Japan), an ultrasound processor (MAJ-935, Olympus Ltd., Tokyo, Japan), and a radial 20 MHz EBUS miniature probe (UM-S20-17s, Olympus Ltd., Tokyo, Japan). The guide sheath kit (K-201) including a guide sheath of 1.95 mm in diameter, a biopsy forcep, and a bronchus brush. Before the procedure, the CT data (slice thickness 0.6−0.7 mm) were imported into the DirectPath system (Cybernet System Inc., Tokyo, Japan) in DICOM format. The appropriate guidance path for VBN was generated and selected. The procedure was conducted under local anesthesia with aerosol inhalation and intratracheal spray of 2% lidocaine. According to VBN guidance, the bronchoscope was inserted and maneuvered to the suspected bronchi as far as possible. Then the radial probe with sheath tube was inserted through the biopsy tube. The position of the sheath tube and probe was adjusted until a satisfactory EBUS image was obtained. The sheath tube was fixed and the radial probe withdrawn. According to characteristics of the lesion and the patient’s tolerance for the procedure, sampling including brush smear (repeated three times), forceps biopsy (repeated five times or until two adequate samples were retrieved), and alveolar lavage (40 mL saline wash) was performed alone or in combination. If no EBUS image of the lesion could be obtained with repeated attempts, only alveolar lavage was performed at the end of the bronchial orifice reached by the bronchoscope. According to the relationship between the probe and lesion on EBUS imaging, the probe position on EBUS can be classified into three patterns as previously reported (7): within type (probe in a bronchus that ran inside the lesion), adjacent type (probe in a bronchus that ran alongside the lesion), and without type (probe in a bronchus that far from the lesion or could not access the lesion by EBUS exploration) (Figure 1 ). The procedure times to locate the lesions were recorded, considering those beyond the 75th percentile as technically difficult procedures. Patient with mediastinal or hilar lymphadenopathy received endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) or endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) synchronously. During the operation, patients received supplemental oxygen through a nasal catheter and vital signs were monitored. Complications were recorded during and after each procedure.
Pathological examination
The specimens obtained were sent for cytological (acquired by brush smear and alveolar lavage) or histological (acquired by forceps biopsy) analysis. All pathological results were determined by two experienced pathologists. The definite diagnosis was recorded to calculate the diagnostic yield. A definite diagnosis meant either proof of malignancy or a defined benign pathology (tuberculosis and so on) on histological or cytological analysis. As for nondiagnostic results, such as non-specific inflammatory changes, the patient was referred for CT-guided transthoracic needle biopsy (TTNB) or thoracoscopic surgery. Patients were followed up for at least 2 years if they refused further examination.
Statistics analysis
Statistical analyses were carried out using IBM SPSS Statistics (Version 20.0; IBM Corp., New York, USA). Continuous variables are presented as
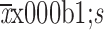 or median (range) according to their normality of distribution. Categorical data are presented as numbers and frequencies, and Fisher’s exact test was used for statistical comparisons. Spearman’s rank correlation was used to identify the association between two ordinal variables as follows: 0−0.40, weakly correlated; 0.41−0.75, moderately correlated, 0.76−1.00, strongly correlated. Univariate logistic regression analysis was used to identify factors associated with diagnostic yield and difficult procedure independent of other variables. Variables with a P value of <0.2 were entered into the multivariate analysis. A two-sided significance level of 5% and a two-sided confidence level of 95% were used to determine significances between the groups.
or median (range) according to their normality of distribution. Categorical data are presented as numbers and frequencies, and Fisher’s exact test was used for statistical comparisons. Spearman’s rank correlation was used to identify the association between two ordinal variables as follows: 0−0.40, weakly correlated; 0.41−0.75, moderately correlated, 0.76−1.00, strongly correlated. Univariate logistic regression analysis was used to identify factors associated with diagnostic yield and difficult procedure independent of other variables. Variables with a P value of <0.2 were entered into the multivariate analysis. A two-sided significance level of 5% and a two-sided confidence level of 95% were used to determine significances between the groups.
Results
Study population
During the study period, 117 patients with 117 PPLs received EBUS-GS examinations. Of these lesions, 8 were excluded, 3 were GGO, 3 obtained a cytological malignant diagnosis using alveolar lavage on routine bronchoscopy prior to the EBUS-GS procedure, a final definite diagnosis was unavailable in 2 (1 died from a heart attack within 2 months of follow-up, and 1 refused regular follow-up). Finally, a total of 109 patients with 109 lesions were enrolled.
The baseline of patients characteristics and lesions features are provided in Table 1 . The average patient age was 58.3±10.1 years, and 55.0% (60/109) were male patients. Most PPLs (60.6%) were located in upper lobes. On CT imaging, the median diameter of PPLs was 24.0 (range, 7.0−68.0) mm, and the median distance to chest wall was 26.1 (range, 11.4−59.0) mm, 75.2% (82/109) of PPLs had bronchus sign. On EBUS images, 99 (90.8%) lesions were visible. Sixty-three (57.8%) were within type, and 34 (31.2%) were adjacent type. Probe of within type on EBUS had a weak correlation with bronchus sign present on CT (Spearman rank correlation coefficient r=0.327, P=0.001).
1. Baseline of patient characteristics and lesion features (N=109).
| Characteristics | n (%) |
| CT, computed tomography; EBUS, endobronchial ultrasonography; Within type, the probe was placed in a bronchus that ran inside the lesion; Adjacent type, the probe was placed in a bronchus that ran alongside the lesion; Without type, the probe was placed in a bronchus that far from the lesion or the probe could not access the lesion for exploration. | |
| Gender, male | 60 (55.0) |
Age (
 ) (year)
) (year)
|
58.3±10.1 |
| Lesion location | |
| Left upper lobe | 30 (27.5) |
| Left lower lobe | 8 (7.3) |
| Right upper lobe | 36 (33.0) |
| Right middle lobe | 11 (10.1) |
| Right lower lobe | 24 (22.0) |
| Lesion size on CT | |
| Diameter [median (range)] (mm) | 24.0 (7.0−68.0) |
| Diameter ≤30 mm | 85 (78.0) |
| Diameter ≤20 mm | 44 (40.4) |
| Bronchus sign on CT | |
| Presence | 82 (75.2) |
| Absence | 27 (24.8) |
| Lung field on CT | |
| Distance to chest wall [median (range)] (mm) | 26.1 (11.4−59.0) |
| Lateral band | 49 (45.0) |
| Intermediate band | 60 (55.0) |
| Probe position on EBUS | |
| Within type | 63 (57.8) |
| Adjacent type | 34 (31.2) |
| Without type | 12 (11.0) |
| Time to locate the lesion on EBUS [median (range)] (min) | 5.0 (2.0−27.0) |
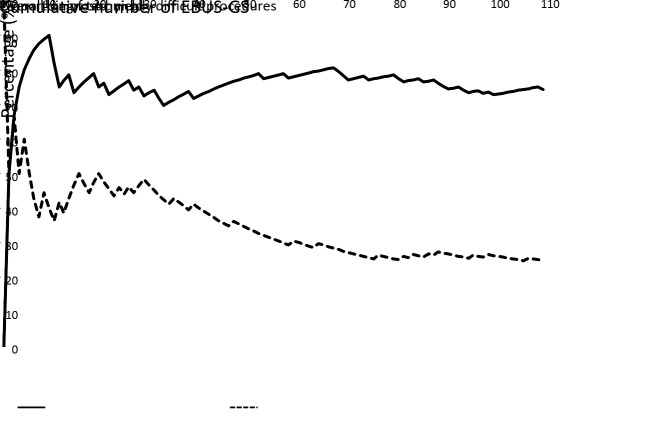
The median procedure time to locate the lesion on EBUS was 5 (range, 2−27) min. Given that procedure times beyond the 75th percentile qualified as technically difficult procedures, 24.8% (27/109) of procedures were deemed technically difficult, requiring more than 9.5 min to locate the lesion. The proportion of technically difficult procedures decreased with cumulative number of EBUS-GS procedures growing up, and reached a plateau of about 25% after 70 practice procedures (Figure 2 ). Factors associated with technically difficult procedures to locate the PPLs on EBUS were analyzed (Table 2 ); however, no significant relationships were found between candidate parameters and technically difficult locating procedures, although male sex and the absence of bronchus sign on CT were identified in univariate analysis with P<0.2.
2.
Diagnostic yield of endobronchial ultrasonography with guide sheath (EBUS-GS) and proportion of technically difficult procedures according to cumulative number of EBUS-GS procedures.
2. Factors affecting technical difficulty of procedures to locate PPLs on EBUS (N=109).
| Variables | Total (N) | Technical difficulty [n (%)] | Univariate analysis | Multivariate analysis | |||
| OR (95% CI) | P | OR (95% CI) | P | ||||
| EBUS, endobronchial ultrasonography; PPL, peripheral pulmonary lesion; CT, computed tomography; OR, odds ratio; 95% CI, 95% confidence interval. | |||||||
| Sex | |||||||
| Female | 49 | 8 (16.3) | 1 | ||||
| Male | 60 | 19 (31.7) | 2.375 (0.935−6.035) | 0.069 | 2.150 (0.832−5.555) | 0.114 | |
| Age (year) | |||||||
| ≤60 | 59 | 13 (22.0) | 1 | ||||
| >60 | 50 | 14 (28.0) | 1.376 (0.575−3.291) | 0.473 | |||
| Lesion size on CT (mm) | |||||||
| >20 | 65 | 16 (24.6) | 1 | ||||
| ≤20 | 44 | 11 (25.0) | 1.021 (0.421−2.475) | 0.964 | |||
| Lobe location on CT | |||||||
| Middle lobe | 11 | 2 (18.2) | 1 | ||||
| Upper lobe | 66 | 16 (24.2) | 1.440 (0.281−7.367) | 0.662 | |||
| Lower lobe | 32 | 9 (28.1) | 1.761 (0.317−9.785) | 0.518 | |||
| Lung field on CT | |||||||
| Intermediate band | 60 | 12 (20.0) | 1 | ||||
| Lateral band | 49 | 15 (30.6) | 1.765 (0.734−4.242) | 0.204 | |||
| Bronchus sign on CT | |||||||
| Present | 82 | 17 (20.7) | 1 | ||||
| Absent | 27 | 10 (37.0) | 2.249 (0.873−5.793) | 0.093 | 1.972 (0.750−5.190) | 0.169 | |
| Final diagnosis | |||||||
| Malignant diagnosis | 92 | 22 (23.9) | 1 | ||||
| Benign diagnosis | 17 | 5 (29.4) | 1.326 (0.421−4.179) | 0.630 | |||
Diagnostic yield
Table 3 provides a summary of diagnoses of this study. A final malignant diagnosis was made in 92 lesions, on the basis of EBUS-GS in 77, surgical resection in 6, EBUS-TBNA in 5, and CT guided-TTNB in 4. A final benign diagnosis was established in 17 lesions, including 4 using EBUS-GS (3 tuberculosis and 1 sarcoidosis), 4 using CT-guided TTNB (2 organizing pneumonia, 1 tuberculosis and 1 fungal pneumonia), and 1 using surgical resection (organizing pneumonia); 4 lesions disappeared after 2−4 weeks’ antibiotic therapy, and 4 remained stable during 2-year follow-up. As for the 16 lesions diagnosed as non-small cell lung carcinoma (NSCLC) using EBUS-GS, further surgical resections were performed; 14 adenocarcinomas and 2 squamous cell carcinomas were established subsequently. The overall diagnostic yield of EBUS-GS was 74.3% (81/109), it reached a plateau of around 75% after cumulative number of EBUS-GS procedures grew up to 30 (Figure 2 ). The diagnostic yield was significantly higher in malignant lesions than in benign lesions (83.7% vs. 23.5%, P<0.001). The diagnostic yield of EBUS-GS was correlated with the final diagnosis (Kappa=0.616, P<0.001).
3. Summary of results obtained by EBUS-GS and final diagnosis (N=109).
| Diagnosis | EBUS-GS diagnosis | Final diagnosis |
| EBUS-GS, endobronchial ultrasonography with guide sheath; NSCLC, non-small cell lung cancer not otherwise specified. | ||
| Malignant diagnosis | 77 | 92 |
| Squamous cell carcinoma | 5 | 8 |
| Adenocarcinoma | 45 | 71 |
| Small cell carcinoma | 9 | 9 |
| NSCLC | 16 | − |
| Large cell carcinoma | − | 1 |
| Metastatic cancer | 2 | 3 |
| Benign diagnosis | 32 | 17 |
| Tuberculosis | 3 | 4 |
| Sarcoidosis | 1 | 1 |
| Fungal pneumonia | − | 1 |
| Organizing pneumonia | − | 3 |
| Non-specific inflammatory change | 28 | − |
| Clinically benign in follow-up | − | 8 |
Factors associated with overall diagnostic yield were investigated (Table 4 ). In univariates analysis, larger size [>20 mm; odds ratio (OR), 3.091; 95% confidence interval (95% CI), 1.272−7.521; P=0.013], bronchus sign present on CT (OR, 2.629; 95% CI, 1.032−6.696, P=0.043), probe of within type (OR, 4.222; 95% CI, 1.686−10.572, P=0.002), and combined use of sample methods (OR, 2.545; 95% CI, 0.902−7.183, P=0.078) were identified with higher overall diagnostic yield. In multivariate analysis, larger PPL size (OR, 2.758; 95% CI, 1.077−7.062; P=0.034) and probe of within type (OR, 3.174; 95% CI, 1.151−8.757, P=0.026) remained independent factors leading to better overall diagnostic yield.
4. Factors affecting overall diagnostic yield of EBUS-GS in PPLs (N=109).
| Variables | Total (N) | Diagnostic yield by
EBUS-GS [n (%)] |
Univariate analysis | Multivariate analysis | |||
| OR (95% CI) | P | OR (95% CI) | P | ||||
| EBUS-GS, endobronchial ultrasonography with guide sheath; PPL, peripheral pulmonary lesion; CT, computed tomography; Within type, the probe was placed in a bronchus that ran inside the lesion; Adjacent type, the probe was placed in a bronchus that ran alongside the lesion; Without type, the probe was placed in a bronchus that far from the lesion or the probe could not access the lesion for exploration; OR, odds ratio; 95% CI, 95% confidence interval. | |||||||
| Sex | |||||||
| Female | 49 | 37 (75.5) | 1 | ||||
| Male | 60 | 44 (77.3) | 1.121 (0.471−2.668) | 0.796 | |||
| Age (year) | |||||||
| >60 | 50 | 35 (70.0) | 1 | ||||
| ≤60 | 59 | 46 (78.0) | 1.516 (0.640−3.595) | 0.344 | |||
| Lesion size on CT (mm) | |||||||
| ≤20 | 44 | 27 (61.4) | 1 | ||||
| >20 | 65 | 54 (83.1) | 3.091 (1.272−7.521) | 0.013 | 2.758 (1.077−7.062) | 0.034 | |
| Lobe location on CT | |||||||
| Lower lobe | 32 | 21 (65.6) | 1 | ||||
| Upper lobe | 66 | 51 (77.3) | 1.781 (0.703−4.511) | 0.224 | |||
| Middle lobe | 11 | 9 (81.8) | 2.357 (0.432−12.864) | 0.322 | |||
| Lung field on CT | |||||||
| Intermediate band | 60 | 43 (71.7) | 1 | ||||
| Lateral band | 49 | 38 (77.6) | 1.366 (0.569−3.276) | 0.485 | |||
| Bronchus sign on CT | |||||||
| Absent | 27 | 16 (59.3) | 1 | ||||
| Present | 82 | 65 (79.3) | 2.629 (1.032−6.696) | 0.043 | 1.679 (0.582−4.842) | 0.337 | |
| Probe position on EBUS | |||||||
| Adjacent or without type | 46 | 27 (58.7) | 1 | ||||
| Within type | 63 | 54 (85.7) | 4.222 (1.686−10.572) | 0.002 | 3.174 (1.151−8.757) | 0.026 | |
| Sample methods | |||||||
| Single use | 19 | 11 (57.9) | 1 | ||||
| Combined use | 90 | 67 (74.4) | 2.545 (0.902−7.183) | 0.078 | 1.350 (0.410−4.445) | 0.622 | |
In subgroup analysis for the 92 malignancies, the diagnostic yield of forceps biopsy, brush smear and alveolar lavage was 67.6% (48/71), 77.3% (51/66) and 63.5% (54/85), respectively. Combined sampling methods (88.2%, 67/76) had a significantly higher diagnostic yield than that of single methods (62.5%, 10/16; P=0.021).
Safety
One patient (0.9%) experienced hemorrhage (about 100 mL blood loss) during brush smear. A total 67 patients (61.5%) experienced mild hemoptysis immediately after examination, which resolved within 24−48 h, with no need for intervention. No patients had pneumothorax or infections. During the study period, two radial ultrasonic probes were damaged when the cumulative procedures number reached 41 and 30 for each probe.
Discussion
To the best of our knowledge this is the first report on the use of VBN assisted EBUS-GS without fluoroscopy guidance in PPLs performed by an inexperienced pulmonologist. We demonstrated that, even conducted by a pulmonologist with no previous experience of EBUS-GS, the procedure was still useful and safe in PPL diagnosis, especially for malignant diseases.
With the combined use of fluoroscopy, the overall diagnostic yield and malignancy diagnostic yield of EBUS-GS were reported to be 62%−79.7% and 70.7%−85.1% (7,14-17). When combined with VBN and fluoroscopy, the overall diagnostic yield and malignancy diagnostic yield were reported as 67.1%−84.2% and 78.3%−85.2% (12,16-18). In the present study, we demonstrated an overall diagnostic yield and malignancy diagnostic yield of 74.3% and 83.7%, which were consistent with the results mentioned above. Our findings implied that, performance of VBN without fluoroscopy might be equivalent to that with fluoroscopy guidance in diagnosis of PPLs.
Probe position of within type on EBUS (7,12,18,19), larger lesion size (mean diameter >30 mm or >20 mm) ( 13,20), and presence of a bronchus sign on CT (20,21) were found to be associated with successful diagnosis using EBUS-GS. However, some studies reported that neither lesion size nor bronchus sign affected the diagnostic yield (12,13,19). In our study, these three factors were associated with higher overall diagnostic yield in univariate analysis, whereas probe position of within type and lesions size larger than 20 mm remained independent factors leading to a better overall diagnostic yield. We assumed that larger lesions may have more connections with nearby bronchi, and the presence of a bronchus sign indicates a connection between the lesion and bronchus, these would make it easier for the guide sheath or probe to find the correct pathway to get closer to or enter the lesion. The finding of a correlation between a probe of within type on EBUS and bronchus sign present on CT in our study may support the above assumption.
The influence of lung field location (13,19) and lobe location (7,18,22) on the diagnostic yield varied among previous studies. Although lesions in the intermediate band seemed to be easily accessed, we did not observe a significant difference in diagnostic yield in our study. We found that lesions in the middle lobe had the highest diagnostic yield and those in the lower lobe had the lowest without significance. We considered that this may be related to fewer effects owing to respiratory motion and heartbeat fluctuation on the lower and left lung lobes.
The diagnosis yield using forceps biopsy was higher than that using other sampling methods, and the yield from alveolar lavage was the lowest (15,23), the combination of multiple sampling methods could provide the best diagnostic yield (15). In our study, the use of combined sampling methods showed the highest diagnosis yield, but brush smear was the single method with the highest diagnosis rate. Considering this, we did not use fluoroscopy monitoring, so it was impossible to determine the open/closed status of the forceps, this might result in a relatively small specimen size and difficulty with pathological diagnosis, and even failure to obtain histological specimens. As moderate sedation was unavailable in Endoscopy Center, Peking University Cancer Hospital, all procedures were performed under local anesthesia only, some patients did not receive all three sampling methods because of their poor tolerance to the procedures, and this bias in the data may affect our diagnostic yield.
Mastering the EBUS-GS technique requires continuous practice. An analysis showed that with the guidance of fluoroscopy, diagnostic yields were stable, even when the procedure was performed by a novice (9). Another study concluded that without fluoroscopy guidance, about 3 years or around 400 procedures were needed to achieve skill mastery and stable diagnostic performance; in that study, improvements in diagnostic yield over time was mainly observed in PPLs smaller than 2 cm and probe of without type (8). VBN was not employed in either of the above studies. In our study, the overall diagnostic yield quickly stabilized after about 30 EBUS-GS procedures; the proportion of technically difficult procedures begun to decrease after 30 practice procedures and plateaued after 70. We believe this was owing to the use of VBN, which simplified the operation, and especially the process of lesion positioning, which can be difficult for beginners. But no factor was identified to be associated with technically difficult procedures, that may owe to our low volume of cases.
EBUS-GS has extremely few complications. The literature reported that the total complication rate was 0−6.7%. The most common complication is pneumothorax, with an incidence of 0−7.5%, only 0.6% of patients need chest tube drainage, and the incidence of infection is about 0.5% (6,17,24,25). No patients with massive bleeding or operation-related deaths have been reported. In this study, only one patient had a relatively large amount of bleeding, but this was successfully managed under endoscopy, and no complications such as pneumothorax or infection occurred.
During the study period, we did encounter some problems in performing EBUS-GS without fluoroscopy. Even with the help of VBN, nearly 10% of lesions could not be detected by EBUS, and in some lesions the probe position failed to achieve a within type from an adjacent type. As we discussed above, real-time status of the biopsy instruments cannot be obtained without fluoroscopy; therefore, insufficient specimens or biopsy failures were not unusual. For example, 16 lesions were diagnosed as NSCLC because of inadequate specimens for immunohistochemistry. Although the life span of the radial probe was reported to be 50−100 EBUS-GS procedures (26), the durability was poorer in our study. The probe may have been bent too much during the procedure, as we could not observe its real-time status owing to the absence of fluoroscopy. Moreover, the diagnostic yield did not yield a further improvement with the operator’s experience growing. We supposed that this might be improved with the addition of fluoroscopy guidance.
This study has several limitations. First, this was a retrospective study conducted at a single center. Although we collected the data prospectively, selection bias might have influenced our results. For example, at the beginning of the study, we tended to select cases that appeared to be easier (larger, closer to the hilum or with bronchus sign present), which may lead to a higher diagnostic yield. There was a decrease in the diagnostic yield when the cumulative number of procedures reached 20 in comparison with the first 10 cases, which may indicate bias. Second, as Peking University Cancer Hospital is a local cancer center, it was impossible to include as many benign lesions as malignant ones; additional information about the application in benign lesions was lacking. Third, owing to different duties for each staff member of Endoscopy Center, Peking University Cancer Hospital, all procedures in this study were performed by a single pulmonologist, this might also cause bias when evaluating our experience in skill mastery. Further studies are needed to clarify the role of VBN without fluoroscopy performed by inexperienced pulmonologists in PPLs.
Conclusions
This study highlights that even when performed by a pulmonologist without previous experience of EBUS-GS, VBN without fluoroscopy guidance is still useful and safe in the diagnosis of PPLs, especially for malignant disease. VBN may simplified the process of lesion positioning and further multicenter randomized studies are warranted.
Acknowledgements
This study was supported by Beijing Municipal Hospital Scientific Research Cultivation Program (No. PX2016057). We thank Xiaoping Kang for her help in data analysis.
Footnote
Conflict of interest: The authors have no conflicts of interest to declare.
References
- 1.Chen W, Sun K, Zheng R, et al Cancer incidence and mortality in China, 2014. Chin J Cancer Res. 2018;30:1–12. doi: 10.21147/j.issn.1000-9604.2018.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Health Commission of the People’s Republic of China Chinese guidelines for diagnosis and treatment of primary lung cancer 2018 (English version) Chin J Cancer Res. 2019;31:1–28. doi: 10.21147/j.issn.1000-9604.2019.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishida T, Asano F, Yamazaki K, et al Virtual bronchoscopic navigation combined with endobronchial ultrasound to diagnose small peripheral pulmonary lesions: a randomised trial. Thorax. 2011;66:1072–7. doi: 10.1136/thx.2010.145490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McWilliams A, Tammemagi MC, Mayo JR, et al Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med. 2013;369:910–9. doi: 10.1056/NEJMoa1214726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alberg AJ, Brock MV, Ford JG, et al Epidemiology of lung cancer: Diagnosis and management of lung cancer, 3rd ed. American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(suppl 5):e1S–29S. doi: 10.1378/chest.12-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang L, Wu H, Wang G Endobronchial ultrasonography using a guide sheath technique for diagnosis of peripheral pulmonary lesions. Endosc Ultrasound. 2017;6:292–9. doi: 10.4103/eus.eus_48_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurimoto N, Miyazawa T, Okimasa S, et al Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest. 2004;126:959–65. doi: 10.1378/chest.126.3.959. [DOI] [PubMed] [Google Scholar]
- 8.Huang CT, Ruan SY, Tsai YJ, et al Experience improves the performance of endobronchial ultrasound-guided transbronchial biopsy for peripheral pulmonary lesions: A learning curve at a medical centre. PLoS One. 2017;12:e0179719. doi: 10.1371/journal.pone.0179719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eom JS, Mok JH, Kim I, et al Radial probe endobronchial ultrasound using a guide sheath for peripheral lung lesions in beginners. BMC Pulm Med. 2018;18:137. doi: 10.1186/s12890-018-0704-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berrington de Gonzalez A, Darby S Risk of cancer from diagnostic X-rays: estimates for the UK and 14 other countries. Lancet. 2004;363:345–51. doi: 10.1016/s0140-6736(04)15433-0. [DOI] [PubMed] [Google Scholar]
- 11.Asano F, Eberhardt R, Herth FJ Virtual bronchoscopic navigation for peripheral pulmonary lesions. Respiration. 2014;88:430–40. doi: 10.1159/000367900. [DOI] [PubMed] [Google Scholar]
- 12.Asano F, Shinagawa N, Ishida T, et al Virtual bronchoscopic navigation combined with ultrathin bronchoscopy. A randomized clinical trial. Am J Respir Crit Care Med. 2013;188:327–33. doi: 10.1164/rccm.201211-2104OC. [DOI] [PubMed] [Google Scholar]
- 13.Iwano S, Imaizumi K, Okada T, et al Virtual bronchoscopy-guided transbronchial biopsy for aiding the diagnosis of peripheral lung cancer. Eur J Radiol. 2011;79:155–9. doi: 10.1016/j.ejrad.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 14.Oki M, Saka H, Kitagawa C, et al Randomized study of endobronchial ultrasound-guided transbronchial biopsy: thin bronchoscopic method versus guide sheath method. J Thorac Oncol. 2012;7:535–41. doi: 10.1097/JTO.0b013e3182417e60. [DOI] [PubMed] [Google Scholar]
- 15.Boonsarngsuk V, Kanoksil W, Laungdamerongchai S Diagnosis of peripheral pulmonary lesions with radial probe endobronchial ultrasound-guided bronchoscopy. Arch Bronconeumol (in En, Spanish) 2014;50:379–83. doi: 10.1016/j.arbres.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Oshige M, Shirakawa T, Nakamura M, et al Clinical application of virtual bronchoscopic navigation system for peripheral lung lesions. J Bronchology Interv Pulmonol. 2011;18:196–202. doi: 10.1097/LBR.0b013e3182198f24. [DOI] [PubMed] [Google Scholar]
- 17.Jiang S, Xie F, Mao X, et al The value of navigation bronchoscopy in the diagnosis of peripheral pulmonary lesions: A meta-analysis. Thorac Cancer. 2020;11:1191–201. doi: 10.1111/1759-7714.13373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamiya M, Okamoto N, Sasada S, et al Diagnostic yield of combined bronchoscopy and endobronchial ultrasonography, under LungPoint guidance for small peripheral pulmonary lesions. Respirology. 2013;18:834–9. doi: 10.1111/resp.12095. [DOI] [PubMed] [Google Scholar]
- 19.Chavez C, Sasada S, Izumo T, et al Endobronchial ultrasound with a guide sheath for small malignant pulmonary nodules: a retrospective comparison between central and peripheral locations. J Thorac Dis. 2015;7:596–602. doi: 10.3978/j.issn.2072-1439.2015.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minezawa T, Okamura T, Yatsuya H, et al Bronchus sign on thin-section computed tomography is a powerful predictive factor for successful transbronchial biopsy using endobronchial ultrasound with a guide sheath for small peripheral lung lesions: a retrospective observational study. BMC Med Imaging. 2015;15:21. doi: 10.1186/s12880-015-0060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okachi S, Imai N, Imaizumi K, et al Factors affecting the diagnostic yield of transbronchial biopsy using endobronchial ultrasonography with a guide sheath in peripheral lung cancer. Intern Med. 2016;55:1705–12. doi: 10.2169/internalmedicine.55.6341. [DOI] [PubMed] [Google Scholar]
- 22.Yoshikawa M, Sukoh N, Yamazaki K, et al Diagnostic value of endobronchial ultrasonography with a guide sheath for peripheral pulmonary lesions without X-ray fluoroscopy. Chest. 2007;131:1788–93. doi: 10.1378/chest.06-2506. [DOI] [PubMed] [Google Scholar]
- 23.Izumo T, Sasada S, Chavez C, et al The diagnostic value of histology and cytology samples during endobronchial ultrasound with a guide sheath. Jpn J Clin Oncol. 2015;45:362–6. doi: 10.1093/jjco/hyv004. [DOI] [PubMed] [Google Scholar]
- 24.Hayama M, Izumo T, Matsumoto Y, et al Complications with endobronchial ultrasound with a guide sheath for the diagnosis of peripheral pulmonary lesions. Respiration. 2015;90:129–35. doi: 10.1159/000431383. [DOI] [PubMed] [Google Scholar]
- 25.Slavova-Azmanova NS, Lizama C, Johnson CE, et al Impact of the introduction of EBUS on time to management decision, complications, and invasive modalities used to diagnose and stage lung cancer: a pragmatic pre-post study. BMC Cancer. 2016;16:44. doi: 10.1186/s12885-016-2081-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eberhardt R, Anantham D, Ernst A, et al Multimodality bronchoscopic diagnosis of peripheral lung lesions: a randomized controlled trial. Am J Respir Crit Care Med. 2007;176:36–41. doi: 10.1164/rccm.200612-1866OC. [DOI] [PubMed] [Google Scholar]