1. Overview
The incidence and mortality of colorectal cancer (CRC) in China have been on the rise. The Cancer Statistics Report of China in 2018 showed that the incidence and mortality of CRC ranked third and fifth among all malignant tumors, with 376,000 new cases and 191,000 deaths. Among them, the number in cities was much higher than that in rural areas, and the incidence of colon cancer had increased significantly. Most patients were already in advanced stage at the time of diagnosis.
CRC can be prevented and diagnosed early through screening. The screening of CRC in people over 50 years old and high-risk groups in Guangzhou, Shanghai, Tianjin and Beijing showed that the incidence of CRC continued to increase. The early diagnosis rate was increased and the mortality was decreased through screening. The main methods included screening high-risk groups based on age, family history and fecal occult blood tests (FOBTs), followed by endoscopic screening.
The diagnosis and treatment of CRC may involve surgery, chemotherapy, radiotherapy, imaging evaluation, pathological evaluation and endoscopy, etc. Studies have shown that the model of multi-disciplinary team (MDT) can improve the diagnosis and treatment of CRC. To further standardize the diagnosis and treatment of CRC in China, improve the level of medical institutions, improve the prognosis of CRC patients, and ensure the quality and safety of medical treatment, this guideline is formulated.
2. Diagnosis
2.1 Clinical manifestations
Early CRC may have no obvious symptoms, and the following symptoms may occur when the disease develops to a certain extent:
(1) Changes in bowel habits;
(2) Changes in stool characteristics (thinning, bloody stools, mucus stools, etc);
(3) Abdominal pain or discomfort;
(4) Abdominal mass;
(5) Symptoms related to intestinal obstruction;
(6) Systemic symptoms: such as anemia, weight loss, fatigue, low fever.
2.2 Disease history and family history
(1) The incidence of CRC may be related to the following diseases: ulcerative colitis, colorectal polyps, colorectal adenoma, Crohn’s disease, schistosomiasis, etc. The patient’s relevant disease history should be asked in detail.
(2) The incidence of hereditary CRC accounts for about 6% of the total incidence of CRC. Patients should be asked in detail about family history: Lynch syndrome, familial adenomatous polyposis (FAP), etc.
2.3 Physical examination
(1) General condition evaluation, superficial lymph nodes throughout the body, especially the inguinal and supraclavicular lymph nodes.
(2) Abdominal inspection and palpation to check for intestinal type, intestinal peristaltic wave and abdominal mass; abdominal percussion and auscultation to check for shifting dullness and abnormal bowel sounds.
(3) Digital rectal examination (DRE): For those with suspected CRC, a DRE must be routinely performed to clear the size, shape, texture, range of intestinal wall circumference of the rectal tumor, the basal activity, the distance of the lower margin of the tumor from the anal margin, the tumor’s invasion into the intestine, the relationship with the surrounding organs, and the presence of pelvic floor implants, while observing if there is blood on the finger stall.
(4) Vagino-recto-abdominal examination: For female patients with rectal cancer suspected of having tumors invading the vaginal wall, a vagino-recto-abdominal examination is recommended to clear the relationship between the mass and the posterior vaginal wall.
2.4 Clinical laboratory examinations
(1) Blood routine test: Check for anemia.
(2) Urine routine test: Test for hematuria and combined with urinary imaging examination to understand whether the tumor invades the urinary system.
(3) Stool routine test: Test for red blood cells and white blood cells.
(4) FOBT: It has great value in the diagnosis of small amount of gastrointestinal bleeding.
(5) Test for blood biochemistry, blood electrolytes, liver and renal function.
(6) Peripheral blood carcinoma embryonic antigen (CEA) and carbohydrate antigen (CA)19-9 must be detected in patients with CRC at the time of diagnosis, before treatment, estimating curative effect and follow-up. Alpha fetoprotein detection is recommended for patients with liver metastases. CA125 detection is recommended for patients with suspected peritoneal and ovarian metastases.
2.5 Endoscopy
Rectoscopy and sigmoidoscopy are suitable for lower colorectal lesions. Colonoscopy is recommended for all suspected CRC patients, except:
(1) Poor general conditions, difficult to tolerate;
(2) Acute peritonitis, intestinal perforation, extensive adhesion in abdominal cavity;
(3) Perianal or severe intestinal infection.
The endoscopic report must include: depth of view, size of the tumor, distance from the anal margin, morphology and extent of local infiltration. Pathological biopsies must be performed on suspicious lesions.
Since the colon may be wrinkled during examination, errors maybe exist in the distance between the distal side of the tumor and the anal margin in endoscopy. It is recommended that the lesion site should be determined with computed tomography (CT), magnetic resonance imaging (MRI) or barium enema.
2.6 Imaging
2.6.1 Common testing
(1) CT: Chest/whole abdominal/pelvic contrast-enhanced CT is recommended for the following aspects:
1) TNM staging of colon cancer; screening for anastomotic recurrence and distant metastasis of CRC during follow-up.
2) To judge the efficacy of adjuvant or conversion therapy for primary and metastatic colon cancer.
3) To identify the internal structure of internal and external oppressive lesions found by Barium enema or endoscopy, and to clarify their nature.
4) Rectal cancer patients who have contraindications for MRI. However, it is important to understand that CT is of limited value in evaluating the status of mesorectal fascia (MRF), especially in patients with low rectal cancer.
(2) MRI
1) MRI is recommended as a routine examination for rectal cancer.
For patients with locally advanced rectal cancer (LARC), baseline and preoperative MRI should be performed before and after neoadjuvant therapy, in order to evaluate the efficacy of neoadjuvant therapy.
If there are no contraindications, intramuscular injection of anisodamine is recommended to perform before MRI scan for rectal cancer to inhibit intestinal peristalsis. Non-fat suppression, small field of view axial high resolution T2WI scanning is recommended. Diffusion weighted imaging (DWI) scanning is recommended, especially for patients with rectal cancer after neoadjuvant therapy. For patients with contraindications to MRI, contrast-enhanced CT scan is feasible.
2) When liver metastases are suspected by clinical or ultrasound/CT, hepatic contrast-enhanced MRI is recommended (Gd-EOB-DTPA, a hepatocyte specific contrast agent, is recommended).
(3) Ultrasound: Transrectal ultrasound (TRUS) is recommended for staging diagnosis of early rectal cancer (stage T2 and below).
(4) X-ray: Barium gas double X-ray can be used as a method for CRC diagnosis, but not for staging. Patients suspected of colonic obstruction should be careful in choosing this method.
(5) Positron emission tomography (PET)-CT: It is not recommended for routine use but can be used as an effective auxiliary test for patients with complicated conditions and cannot be clearly diagnosed by routine examination. It is recommended to be used on patients whose preoperative examination results indicate stage III and above to discover distant metastases.
(6) Excretory urography: It is not recommended as a routine preoperative test. It is only applicable to patients with large tumors that may invade the urinary tract.
2.6.2 Imaging evaluation of clinical crucial issues in colon cancer
Whole abdomen plus pelvic CT scan (plain scan + contrast-enhanced) is recommended, which can take into account of both the tumor itself and the prone site of metastatic tumor, referring to liver. Imaging physicians are supposed to evaluate TNM staging of colon cancer and the presence or absence of extramural vascular invasion (EMVI). Chest CT is recommended for screening other distant metastatic tumors, such as lung metastatic tumors. PET-CT is helpful in screening for metastatic tumors throughout the body.
2.6.3 Imaging evaluation of clinical crucial issues in rectal cancer
(1) Pelvic MRI is recommended for patients with rectal cancer. Tumor location, TNM stage, MRF status and the presence of EMVI should be defined by imaging.
(2) For the screening of distant metastatic tumors in other locations, such as the lung, chest CT is recommended. For liver, hepatic contrast-enhanced MRI, CT or ultrasound examination is recommended, and if it is available, the first choice is MRI. PET-CT is recommended for metastatic tumors throughout the body.
2.6.4 MRI structural report of rectal cancer is recommended
The report template is shown in Table 1 .
1. MRI structural report of rectal cancer.
| Name Sex Age Imaging number Date of examination
Test item Rectal MRI Clinical diagnosis | ||||||
| MRI, magnetic resonance imaging; LN, lymph node; MRF, mesenteric fascia; EMVI, extramural vascular invasion of rectum. | ||||||
| T-staging of tumor | ||||||
| Lesion localization | ||||||
| Retroperitoneal fold | [ ] Above the retroperitoneal fold and not involved
[ ] Below the retroperitoneal fold and not involved [ ] Across the retroperitoneal fold and not involved [ ] The retroperitoneal fold is involved |
|||||
| Location referring to the distance from lower margin of tumor to the margin of anus | [ ] Upper rectal cancer: within 10−15 cm
[ ] Middle rectal cancer: within 5−10 cm [ ] Lower rectal cancer: within 5 cm |
|||||
| Distance between the lower margin of tumor and the anal rectum ring (cm) | ||||||
| Size measurement | ||||||
| Mass type | Oblique axial position measurement: __ mm × __ mm | Sagittal position measurement (longitudinal diameter): __ mm | ||||
| Intestinal wall infiltration | The thickest intestinal wall measured at oblique axis: __ mm | Sagittal position measurement (longitudinal diameter): __ mm | ||||
| Perimeter of lesions surrounding the intestine | <1/4 loop 1/4−1/2 loop 1/2−3/4 loop 3/4−1 loop | |||||
| Description of T-staging with tumor invasion degree | ||||||
| T1: Tumor invades submucosa | ||||||
| T2: Tumor invades muscularis propria but does not penetrate it | ||||||
| T3: Tumor invades through the outer membrane of the muscularis propria and reaches into perirectal mesenteric fat [ ] __ mm | ||||||
| T3a: Tumor invades through muscularis <5 mm | ||||||
| T3b: Tumor invades through muscularis for 5−10 mm | ||||||
| T3c: Tumor invades through muscularis >10 mm | ||||||
| T4a: Tumor penetrates the peritoneum or serous membrane (upper rectum) | ||||||
| T4b: Tumor invades adjacent organs | ||||||
| Remark: | ||||||
| N-staging of lymph nodes (evaluation of lymph node margins, morphology and internal signal characteristics should be integrated) | ||||||
| [ ] LN around the superior rectal artery | No. of suspicious LNs: | Maximum short diameter: | ||||
| [ ] LN inside the mesenteric fascia | No. of suspicious LNs: | Maximum short diameter: | ||||
| [ ] LN adjacent to the internal iliac vessels | No. of suspicious LNs: | Maximum short diameter: | ||||
| Lateral LN | ||||||
| [ ] Obturator artery LN | No. of suspicious LNs: | Maximum short diameter: | ||||
| [ ] Internal iliac vessels LN | No. of suspicious LNs: | Maximum short diameter: | ||||
| Remark: | ||||||
| M-staging | ||||||
| [ ] Groin LN | No. of suspicious LNs: | Maximum short diameter: | ||||
| Remark: | ||||||
| MRF status | [ ] Positive: front,
back, left, right |
Causes of MRF positive: tumor, lymph nodes, cancer nodules, positive EMVI | ||||
| [ ] Negative | ||||||
| Remark: | ||||||
| EMVI | [ ] Positive: front,
back, left, right |
Location: referring tumor location (upper, top and lower segments) | ||||
| [ ] Negative | ||||||
| Remark: | ||||||
| Other abnormal signs [ ] suggest the possibility of mucinous adenocarcinoma | ||||||
| Diagnostic comments: mrT __ N __ M __, MRF ( ), EMVI ( ). | ||||||
2.6.5 CT structural report of colon cancer can be used
The report template is shown in Table 2 .
2. CT structure report of colon cancer.
| Name Sex Age Image number Date of examination
Inspection item Colon CT Clinical diagnosis | |||
| CT, computed tomography; RSM, retroperitoneal surgical margin; EVMI, extramural vascular invasion. | |||
| Tumor location | |||
| Left colon [ ] | Right colon | [ ] | |
| Cecum | [ ] | ||
| Ascending colon | [ ] | ||
| Hepatic flexure of colon | [ ] | ||
| Transverse colon | [ ] | ||
| Splenic flexure of colon | [ ] | ||
| Descending colon | [ ] | ||
| Sigmoid colon | [ ] | ||
| Size measurement | |||
| Mass type | Size of mass: __ mm × __ mm | ||
| Intestinal wall infiltration | The thickest layer of tumor: __ mm | ||
| RSM (only applicable to the ascending/descending segment) | [ ] | ||
| Tumor staging | [ ] | ||
| Invasion to submucosa (T1) | [ ] | ||
| Tumor invades the muscularis propria but does not penetrate into the muscularis propria (T2) | [ ] | ||
| Tumor breaks through muscularis propria (T3) <5 mm | [ ] | ||
| Tumor breaks through muscularis propria (T3) ≥3 mm | [ ] | ||
| Tumor invades beyond peritoneal coverage (T4a) | [ ] | ||
| Invasion of adjacent organs (T4b) | [ ] | ||
| Lymph node | [ ] | ||
| No. of suspected positive lymph nodes in the region ___ Maximum short diameter ____ | |||
| No. of suspected positive retroperitoneal lymph node ___ Maximum short diameter ____ | |||
| EMVI | [ ] | ||
| Distant metastases | |||
| Liver metastases | [ ] | ||
| Lung metastases | Left lung [ ] | Right lung [ ] | |
| Peritoneal implant metastases | [ ] | ||
| Other metastatic lesions | [ ] | ||
| Other abnormal signs | |||
| Tumor perforation | [ ] | ||
| Intestinal obstruction | [ ] | ||
| Diagnostic comments: ctT __ N __ M, EMVI ( ). | |||
For cases with suspected liver metastases in abdominal examination, CT and MRI structural reports for liver metastases can be used. The report templates are shown in Table 3 ,4 .
3. CT structure report of hepatic metastatic tumor.
| 1. Fatty liver: Yes [ ] No [ ] | |||||||||
| CT, computed tomography; LN, lymph node; MRI, magnetic resonance imaging. | |||||||||
| 2. Number of liver metastatic tumors: 1−3 [ ] 4−7 [ ] 8 or more [ ] | |||||||||
| 3. Size of hepatic metastatic tumor: The largest lesion ______ mm is located in _______ segment | |||||||||
| 4. Distribution of lesions: | |||||||||
| Caudate | S1 [ ] | ||||||||
| Left lobe | S2 [ ] | S3 [ ] | S4 [ ] | ||||||
| Right lobe | S5 [ ] | S6 [ ] | S7 [ ] | S8 [ ] | |||||
| 5. Relationship with important blood vessels: | |||||||||
| Right portal vein | Trunk | Not shown [ ] | Shift [ ] | Next to [ ] | Well-defined [ ] | ||||
| Branch | Not shown [ ] | Shift [ ] | Next to [ ] | Well-defined [ ] | |||||
| Left portal vein | Trunk | Not shown [ ] | Shift [ ] | Next to [ ] | Well-defined [ ] | ||||
| Branch | Not shown [ ] | Shift [ ] | Next to [ ] | Well-defined [ ] | |||||
| Right hepatic vein | Not shown [ ] | Shift [ ] | Next to [ ] | Well-defined [ ] | |||||
| Middle hepatic vein | Not shown [ ] | Shift [ ] | Next to [ ] | Well-defined [ ] | |||||
| Left hepatic vein | Not shown [ ] | Shift [ ] | Next to [ ] | Well-defined [ ] | |||||
| Inferior vena cava | Not shown [ ] | Shift [ ] | Next to [ ] | Well-defined [ ] | |||||
| 6. Hilar lymph nodes: Yes [ ] No [ ] | |||||||||
| Size of the largest LN ___ mm × ___ mm | |||||||||
| 7. Origin of vascular variation: | |||||||||
| Left hepatic artery | Arteria hepatica propria [ ] | Left gastric artery [ ] | |||||||
| Right hepatic artery | Arteria hepatica propria [ ] | Superior mesenteric artery [ ] | |||||||
| Common hepatic artery | Celiac trunk [ ] | Superior mesenteric artery [ ] | Abdominal aorta [ ] | ||||||
| 8. Uncertain transfer lesion: Yes [ ] No [ ] | |||||||||
| 9. Location distribution of uncertain transfer lesion: | |||||||||
| Caudate | S1 [ ] | ||||||||
| Left lobe | S2 [ ] | S3 [ ] | S4 [ ] | ||||||
| Right lobe | S5 [ ] | S6 [ ] | S7 [ ] | S8 [ ] | |||||
| Suggestions: For the lesions less than 10 mm according to CT results, it is recommended that indeterminate metastatic lesions in other cases should be included and further evaluation by hepatic contrast-enhanced MRI should be done except for those with typical metastatic tumor manifestations. | |||||||||
| 10. Others | |||||||||
4. MRI structure report of hepatic metastatic tumor*.
| 1. No. of liver metastatic tumors: 1−3 [ ] 4−7 [ ] 8 or more [ ] | |||||||||
| *, only applicable to cases with hepatic metastasis considered by abdominal contrast-enhanced MRI. Not applicable for patients with liver metastasis after treatment. MRI, magnetic resonance imaging; LN, lymph node; CT, computed tomography. | |||||||||
| 2. Size of hepatic metastatic tumor: The largest lesion ______ mm is located in _______ segment | |||||||||
| 3. Distribution of lesions: | |||||||||
| Caudate | S1 [ ] | ||||||||
| Left lobe | S2 [ ] | S3 [ ] | S4 [ ] | ||||||
| Right lobe | S5 [ ] | S6 [ ] | S7 [ ] | S8 [ ] | |||||
| 4. Relationship with important blood vessels: | |||||||||
| Right portal vein | Trunk | Not shown [ ] | Shift [ ] | Next to [ ] | Well-defined [ ] | ||||
| Branch | Not shown [ ] | Shift [ ] | Next to [ ] | Well-defined [ ] | |||||
| Left portal vein | Trunk | Not shown [ ] | Shift [ ] | Next to [ ] | Well-defined [ ] | ||||
| Branch | Not shown [ ] | Shift [ ] | Next to [ ] | Well-defined [ ] | |||||
| Right hepatic vein | Not shown [ ] | Shift [ ] | Next to [ ] | Well-defined [ ] | |||||
| Middle hepatic vein | Not shown [ ] | Shift [ ] | Next to [ ] | Well-defined [ ] | |||||
| Left hepatic vein | Not shown [ ] | Shift [ ] | Next to [ ] | Well-defined [ ] | |||||
| Inferior vena cava | Not shown [ ] | Shift [ ] | Next to [ ] | Well-defined [ ] | |||||
| 5. Hilar lymph nodes: Yes [ ] No [ ] | |||||||||
| Size of the largest LN ___ mm × ___ mm | |||||||||
| 6. Origin of vascular variation: | |||||||||
| Left hepatic artery | Arteria hepatica propria [ ] | Left gastric artery [ ] | |||||||
| Right hepatic artery | Arteria hepatica propria [ ] | Superior mesenteric artery [ ] | |||||||
| Common hepatic artery | Celiac trunk [ ] | Superior mesenteric artery [ ] | Abdominal aorta [ ] | ||||||
| 7. Uncertain transfer lesions: Yes [ ] No [ ] | |||||||||
| 8. Location distribution of uncertain transfer lesions: | |||||||||
| Caudate | S1 [ ] | ||||||||
| Left lobe | S2 [ ] | S3 [ ] | S4 [ ] | ||||||
| Right lobe | S5 [ ] | S6 [ ] | S7 [ ] | S8 [ ] | |||||
| Suggestions: For the lesions less than 10 mm shown by CT results, it is recommended that indeterminate metastatic lesions should be classified and further evaluation by hepatic contrast-enhanced MRI should be done, except for those with typical metastatic tumor features | |||||||||
| 9. Others | |||||||||
2.7 Histopathological examination
The pathological biopsy report is the basis for the treatment of CRC. Patients diagnosed as invasive carcinoma of CRC by biopsy require standard therapy. Due to the limitation of biopsy sampling, biopsy pathology could not determine whether there is submucosal infiltration, and the diagnosis of high-grade intraepithelial neoplasia is made. It is suggested that clinicians should determine the treatment plan by integrating other clinical information, including the tumor size and invasion depth as evaluated by endoscopy or imaging, whether there is suspicious lymph node metastasis, etc. When low rectal tumors may involve anal preservation decisions, the pathologist is advised to note in the report whether the biopsy tissue has reached the “canceration” level. K-ras and N-ras gene mutations are recommended to detect in patients with clinically diagnosed recurrence or metastasis colorectal cancer (mCRC) to guide tumor targeted therapy. The assessment of BRAFV600E mutation status should be performed simultaneously during RAS testing to stratify the prognosis and guide clinical treatment. Mismatch repair (MMR) protein expression or microsatellite instability (MSI) detection is recommended to perform for all CRC patients for the screening of Lynch syndrome, prognostic stratification, and guided immunotherapy, etc. MMR-deficient (dMMR) tumors with MLH1 deletion should be tested for BRAFV600E mutant molecules and/or MLH1 methylation to assess the risk of Lynch syndrome. Some clinical studies on anti-human epidermal growth factor receptor (anti-HER2) treatment of CRC have achieved gratifying results, but there is no standard test and interpretation method at present. Qualified units may carry out relevant work appropriately.
2.8 Open surgery or laparoscopic exploration
In the following cases, laparotomy or laparoscopic exploration is recommended to perform:
(1) After various diagnostic methods, the diagnosis is still not clear and the colorectal tumor is highly suspected.
(2) Intestinal obstruction occurs and conservative treatment is not effective.
(3) Suspected bowel perforation.
(4) Massive hemorrhage of the lower gastrointestinal tract which is ineffective with conservative treatment.
3. Specimen collection and pathological evaluation
3.1 Specimen fixation criteria
(1) Fixative: 10% neutral buffered formalin fixative without heavy metals is recommended.
(2) Amount of fixative: ≥5 times the volume of the fixed specimen.
(3) Fixed temperature: normal room temperature.
(4) Fixated duration: The specimen should be dissected and fixed as soon as possible, and the time from isolation to fixation should not exceed 30 min. Surgical specimens must be standardized cut open and fixed. It is recommended that the pathologist should perform the dissection and fixation of the specimens.
Endoscopic specimens or biopsy specimens are recommended: in 6−48 h.
Surgical specimen: in 12−48 h.
3.2 Specimen collection requirements
3.2.1 Biopsy specimens
(1) Check the number of clinical specimens to be submitted and all specimens must be taken for biopsy.
(2) To avoid loss, wrap specimens in gauze or soft permeable paper.
(3) Each wax block was embedded with no more than 5 biopsy specimens and adjusted according to the size of the tissue.
3.2.2 Endoscopic resection specimens
(1) Specimen fixation is recommended to be normalized by clinicians: after the biopsy specimen is isolated, the endoscopist should attach the base surface of the biopsy mucosal tissue to the filter paper in time, and immediately immerse it in the fixative. After the mucosal resection under endoscopy in vitro, the endoscopist unfolds the specimen, and fixes the mucosa upward with a pin to the cork or foam board, marks the lateral margins of mouth and anus, and turns the mucosa downward into the fixating solution. For the resected polyps, place the pedunculated polyps directly into the fixator, and mark the resection margin with ink before putting the sessile polyps into the fixator.
(2) It is recommended that the size and morphological features of the specimen or the tumor lesion along with the distance from each orientation to the resection margin should be recorded.
(3) Sampling of resected polyp specimens: Firstly, clarify the resection margin of the polyp, the presence or absence of the peduncle, and the diameter of the peduncle. It is recommended that ink should be applied to the resection margin of the peduncle (pedunculated polyps) and cauterize the resection margin (sessile polyps). During the sampling, the fact that the resection margin and the infiltration of pedunculated polyps can be evaluated objectively and correctly should be taken into account.
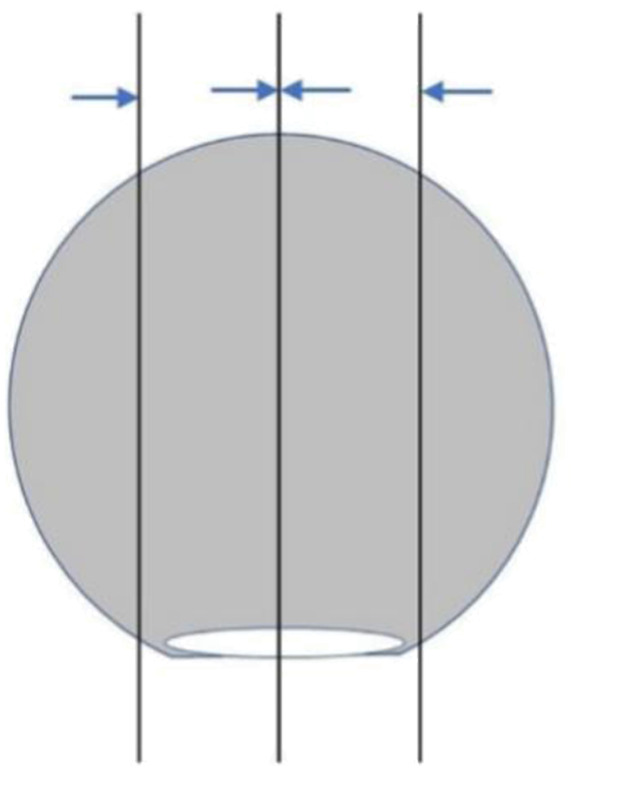
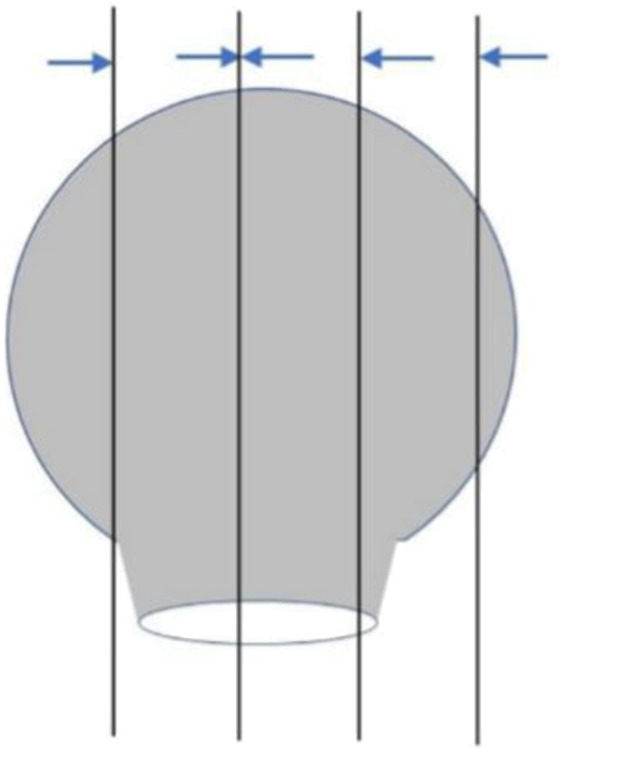
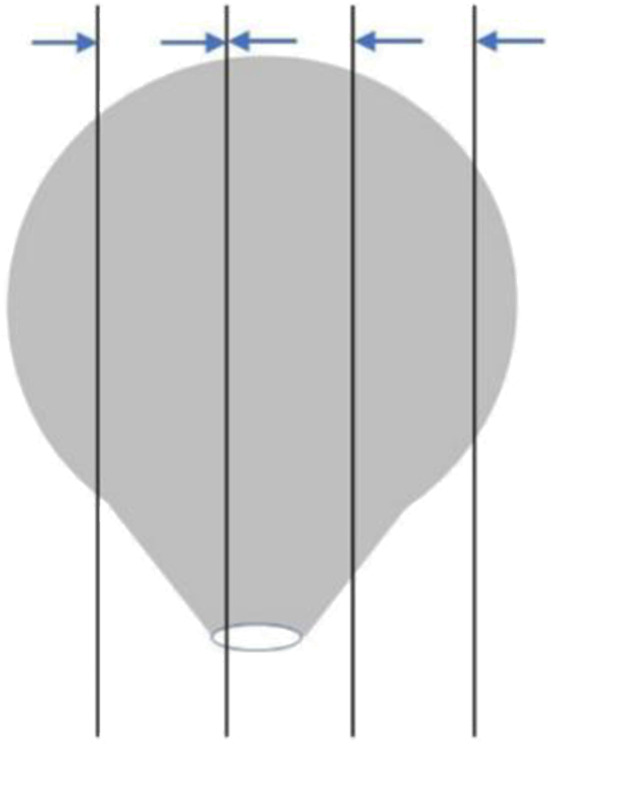
The material is suggested to take as follows: The sessile polyps are well taken in parallel from the base center of the resection margin to the left and right sides (Figure 1 ). For polyps with peduncle resection margin >2 mm in diameter, cut the specimen perpendicular to the plane of the peduncle resection margin slightly deviating from the center of the peduncle resection margin. Then paralleling to this section, take all specimens at intervals of 2−3 mm ( Figure 2 ); when the diameter of the peduncle resection margin is no more than 2 mm, sampling all specimens at intervals of 2−3 mm perpendicular to the plane of the resection margin, so that the pedunculated section serves as a separate wax block (Figure 3 ). All materials are recommended to take in the same embedding direction. Record the orientation corresponding to the tissue block.
1.
Sampling of sessile polyps. Cut in parallel with the base of the resection margin as the center, and take all materials to the left and right sides. The direction of the arrow is the embedding direction recommended.
2.
Sampling of pedunculated polyps with broad base (>2 mm). Sampling specimens at intervals of 2−3 mm perpendicular to the plane of the peduncle resection margin. The direction of the arrow is the embedding direction recommended.
3.
Sampling of pedunculated polyps with narrow base (≤2 mm). Sampling specimens at intervals of 2−3 mm perpendicular to the plane of the peduncle resection margin. The direction of the arrow is the embedding direction recommended.
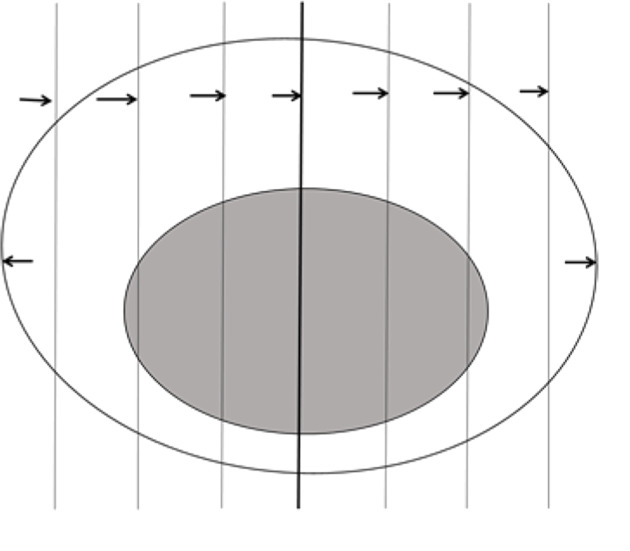
(4) Sampling of specimens from endoscopic mucosal resection and mucosal dissection: Assessment of the resection margin is particularly critical because the mass is generally close to the resection margin. The basal and lateral margins are suggested to mark with different pigments, so that we can locate the resection margin and evaluate the tumor resection margin in observation. Cut the specimen in parallel at intervals of 2−3 mm (Figure 4 ). The specimens with clinical special marks can be adjusted appropriately: divide them into tissue blocks of appropriate size, collect and embed all in the same direction.
4.
Sampling of specimens from endoscopic mucosal resection and mucosal dissection. Cut the specimen in parallel at intervals of 2−3 mm, all taken and embedded in the same direction.
3.2.3 Surgical specimens
(1) General examination and recording: Describe and record the general characteristics of the intestinal tube and tumor, along with the distance from the tumor to the bilateral and radial (circumferential) resection margins. Ink is recommended to mark the radial (circumferential) resection margin of the serous area corresponding to the tumor so as to accurately assess the depth of tumor infiltration and distance from the resection margin. Lymph node sampling should be grouped according to the direction of lymphatic drainage. It is recommended that clinicians group the lymph nodes for examination (pathologists cannot distinguish lymph node groups in vitro).
(2) Sampling
1) Cut the intestinal tube open along the long axis of the intestinal wall and fully excise the tumor specimen perpendicular to the intestinal wall. Sampling tumor tissues according to size, depth of infiltration, texture and color separately. Take one more full-layer tumor and intestinal wall tissue in the deepest part to evaluate the deepest degree of tumor invasion. Observe the serous membrane involvement carefully. When the tumor approaches or invades the serous membrane, take the area of serous membrane suspected to judge accurately the serous membrane involvement under microscope. Cut tissues that can show the relationship between tumor and adjacent mucosa.
2) Excise the distal and proximal surgical margins. The mesangial/circumferential margins are recommended to cut. For cases with suspected positive mesenteric/circumferential margins, it is recommended that the surgeon cut the part marked with ink and manage to distinguish marks for different resection margins.
3) If the specimen contains ileocecal, anal canal or anus, it should be taken from ileocecal flap, dentate line and anal margin. If the tumor invades the above sites, the tissue block which fully shows the degree of lesion should be cut. The samples of appendix were taken routinely.
4) Complete mesorectum excision is required for radical resection of middle and low rectal cancer. Pathologists are advised to conduct systematic examination and evaluation on the surgical specimens, including the integrity of the mesentery membrane and existence/absence of tumor invasion to the circumferential margin. Pathologic examination is the most intuitive method to evaluate the integrity of mesorectum.
5) Lymph nodes: Embed all the lymph nodes detected. Large lymph nodes would be dissected and then embedded. At least 12 lymph nodes should be detected in radical specimens without neoadjuvant treatment.
6) As for surgical specimens of rectal cancer after neoadjuvant treatment, the changes of the original tumor site should be observed and recorded. If the mass is obvious still, take it according to the routine sampling. If the tumor is small or there is no obvious tumor with the naked eye, the original tumor should be taken altogether based on the colonoscopy description before treatment.
(3) Size of specimen recommended: No more than 2 cm × 1.5 cm × 0.3 cm.
3.3 Principles of post-sampling specimen process and retention time
(1) Preservation of the remaining specimens. The remaining tissue should be stored in the standard fixative solution, and the sufficient amount of fixation and formaldehyde concentration should be maintained at all times to avoid tissue decay caused by the dry-up of specimen or decrease in quantity or concentration of fixed solution; in order to prepare materials whenever needed in line with observation and diagnosis through microscope; or to review the general specimen or supplement the materials when receiving clinical feedback after the pathological report is issued.
(2) Retention time for remaining specimens. It is suggested that 2 weeks after the issuance of the pathological diagnosis report, the hospital can handle it in accordance with the relevant regulations, when no clinical feedback information has been received, and no cases such as requesting a review due to the disagreement of the consultation opinions of the external hospital have occurred.
(3) Retain fresh tissues at low temperatures for further study use if permitted.
3.4 Pathological types
3.4.1 Early-stage (pT1) CRC
Cancer cells penetrating the muscularis mucosae of colorectum and infiltrating into the submucosa without involving the muscularis propria, are known as early CRC (pT1). Carcinoma with severe dysplasia of the epithelium and no penetration of the muscularis mucosa is called high-grade intraepithelial neoplasia, including intramucosal carcinoma confined to the mucosal layer but with membrane propria infiltration.
The submucosal infiltration depth of early CRC should be measured and graded for local resection specimens under endoscope or by transanal operation. For flat lesions, when the depth of submucosal infiltration is ≤1,000 μm, it is a shallow submucosal infiltration, which is an indication of endoscopic therapy. When the depth of submucosal infiltration is greater than 1,000 μm, it is deep submucosal infiltration, and other factors as well as clinical conditions need to be considered as to whether to perform surgical operation to expand the resection range.
When the muscularis mucosae is clear, the depth of infiltration is measured from the lower edge of the muscularis mucosae to the deepest infiltration. When the muscularis mucosae disappears completely, the depth of submucosal infiltration is measured from the surface. There are two cases of pedunculated lesions: if the muscularis mucosae grows in a branch, the line between the two sides of the tumor and the non-tumor junction is taken as the baseline. Infiltration above the baseline is regarded as head infiltration, which is the indicator of endoscopic treatment; if the infiltration is below the baseline, it is regarded as pedunculated infiltration, which is equivalent to the deep infiltration of the submucosa. The treatment principle is the same as above. When the muscularis mucosae with pedunculated lesion could be localized or not branching, the depth of infiltration is measured as a flat lesion.
3.4.2 General types of advanced CRC
(1) Bulge type: Anyone whose tumor protrudes into the intestinal cavity belongs to this type.
(2) Ulcer type: This type is found in those with deep ulcerations or penetrating the muscularis.
(3) Type of infiltration: The tumor infiltrates into the whole layers of the intestinal wall, and the local intestinal wall thickens, but there is no obvious ulcer or bulge on the surface.
3.4.3 Histological type
World Health Organization (WHO) classification of digestive system tumors (4th edition) is taken as the reference. The proportion of common adenocarcinoma containing specific histological types such as mucinous adenocarcinoma or signet ring cell carcinoma should be noted.
(1) Adenocarcinoma, non-special type;
(2) Adenocarcinoma, special type, including mucinous adenocarcinoma, signet ring cell carcinoma, serrated adenocarcinoma, micropapillary adenocarcinoma, medullary carcinoma, sieve-like acne adenocarcinoma;
(3) Adenosquamous carcinoma;
(4) Squamous cell carcinoma;
(5) Spindle cell carcinoma/sarcoma-like carcinoma;
(6) Undifferentiated carcinoma;
(7) Other special types;
(8) Cancer, uncertain type.
3.4.4 Histological grade
For colorectal adenocarcinoma (common type), histological grade criteria are shown in Table 5 .
5. Histological grade criteria of colorectal cancer (based on version WHO 2010).
| Criterion | Differentiation category | Numerical grade* | Descriptive grade |
| *, undifferentiated carcinoma (grade 4) is reserved for carcinomas with no gland formation, mucin production, neuroendocrine, squamous or sarcomatoid differentiation; WHO, World Health Organization; MSI-H, high-level microsatellite instability. | |||
| >95% with gland formation | Well differentiated | 1 | Low level |
| 50%−95% with gland formation | Moderately differentiated | 2 | Low level |
| 0−49% with gland formation | Poorly differentiated | 3 | High level |
| MSI-H | Variable | Variable | Low level |
3.5 Contents of pathological report
3.5.1 Contents and requirements of pathological reports for biopsy specimens
(1) Basic information of patients and inspection information.
(2) Report the grade for intraepithelial neoplasia (dysplasia). For the diagnosis of low rectal tumor with high-grade intraepithelial neoplasia, it is suggested that the pathologist note in the report whether the biopsy tissue has reached the degree of “canceration”, because this may involve clinical treatment decision.
(3) Distinguish the histological type in cases of invasive carcinoma.
(4) When identified as CRC, the detection of MMR protein (MLH1, PMS2, MSH2, MSH6) expression is recommended. For unresectable CRC, it is recommended that K-ras and N-ras genes, BRAF gene mutations and other related gene status should be detected.
Clinicians should understand the limitations of biopsy specimens. If the biopsy pathology cannot fully determine the presence of submucosal infiltration, it is diagnosed as high-level intraepithelial neoplasia. Then the tumor body may be an invasive cancer.
3.5.2 Contents and requirements of pathological report for endoscopic resection specimens
(1) Basic information of patients and inspection information.
(2) Size of the sample or tumor.
(3) Grading of intraepithelial neoplasia (dysplasia).
(4) In the case of invasive carcinoma that penetrates into the muscularis mucosae through the submucosal layer, histological classification, grade, depth of submucosal invasion, vascular invasion, nerve invasion, horizontal and vertical resection margins should be reported. It is recommended that MMR protein (MLH1, MSH2, MSH6, PMS2) expression and tumor budding grade should be reported.
If the cancer has the 3rd or the 4th grade differentiation, deep submucosal infiltration, vascular invasion, positive basal resection margin (the distance between tumor and resection margin is less than 1 mm, and adenoma/low-grade dysplasia can be seen on the horizontal margin, then the margin is considered as negative, but need to be marked) and other high-risk factors, clinicians need to take re-surgery into consideration.
3.5.3 Contents and requirements of pathological reports for surgical specimens
(1) Basic information of patients and inspection information.
(2) General condition: tumor size, general type, depth of infiltration with the naked eye, presence/absence of perforation, distance from tumor to both sides of resection margin.
(3) Degree of tumor differentiation (tumor classification, grade).
(4) Depth of tumor invasion (pT stage) (pT stage or ypT is determined by the viable tumor cells, while acellular mucus lake in specimens after neoadjuvant treatment is not considered to be residual tumor).
(5) For stage I and stage II CRC, tumor budding is a poor prognostic factor. Tumor budding grade is recommended to report in CRC cases without lymph node metastasis. Tumor budding is a cluster of tumor cells less than 5 cells located at the leading edge of tumor infiltration. The total number and grade of buds should be reported from a selected hot spot measuring 0.785 mm2 (20 times visual field). The grade criteria are shown in Table 6 .
6. Grade for tumor budding.
| Grade | Total No. of buds
(0.785 mm2, visual field of 20 times) |
| Low | 0−4 |
| Intermediate | 5−9 |
| High | 10 or more |
(6) Detect the number of lymph nodes and the number of positive lymph nodes, as well as tumor deposit (TD) (pN stage) outside the lymph nodes. The latter refers to the solid deposit of cancer cells that are not connected with the primary tumor in the perienteric adipose tissue. Cancer cell deposition but no residual lymph node can be seen through microscope. When there’s no lymph node metastasis, but there are TDs, it is reported as pN1c stage, and the number of TD should be reported; when lymph node metastasis exists, the pN stage is based on the number of positive lymph nodes, without considering cancer deposit, but the number of TD should also be presented in the pathological report.
(7) Status of the proximal and distal resection margins.
(8) It is recommended that the condition of the mesangial/circumferential margin should be reported (if the tumor is close to the resection margin, the distance between the tumor and the resection margin should be measured and reported under a microscope, and it is reported as positive resection margin when the distance between tumor and resection margin is less than 1 mm).
(9) Tumor response grade (TRG), used to assess the efficacy of preoperative neoadjuvant therapy, is shown in Table 7 .
7. Tumor response grade (TRG).
| Grade | Level of regression | Degree description |
| TRG score is limited to primary tumor lesions; tumor cells refer to active tumor cells, excluding degenerative and necrotic tumor cells; cell-free mucus lake after radiotherapy/chemotherapy is not considered as residue tumors. | ||
| Level 0 | Complete response | No residual cancer cells |
| Level 1 | Moderate response | Only small clusters or single cancer cells remaining |
| Level 2 | Minimal response | Residual cancer cells, but with predominant fibrosis |
| Level 3 | Poor response | Extensive residual cancer cells, minimal or no tumor cell necrosis |
(10) Vascular invasion (V stands for blood vessels, V1 for microscopic blood vessel infiltration, V2 for blood vessel infiltration with the naked eye, and L for lymphatic vessel). It is recommended that blood vessel should be distinguished from lymphatic vessel infiltration as much as possible.
(11) Nerve bundle invasion.
(12) Expression of MMR protein (MLH1, PMS2, MSH2, MSH6). The mutation and methylation status of MMR genes are suggested to detect according to immunohistochemical results.
(13) Detection of gene status such as K-ras, N-ras and BRAF is recommended when determined as relapsed or metastatic CRC. Without surgical resection specimens, they can be measured from biopsy specimens.
A complete pathological report is based on a detailed pathological diagnostic application form filled by the clinician, with detailed surgical findings, results of related clinical auxiliary examination, and clearly marked lymph nodes. The mutual communication, trust and cooperation between clinicians and pathologists are the basis for establishing correct staging and guiding clinical treatment. The templates of pathological report of endoscopic resection specimens and surgical specimens are shown in Table 8 −10 .
8. Structural report of colorectal endoscopic resection specimens*.
| Name Sex Age Pathology number
Medical record number Inspection site | |
| *, For complete polyp or mucosal/intestinal wall excision specimens only. | |
| Specimen size | Maximum diameter: ___ cm; Other two diameters: ___ cm × ___ cm |
| Polyp size | Maximum diameter: ___ cm; Other two diameters: ___ cm × ___ cm |
| Polyp structure | □ Peduncle length ___ cm, diameter ___ cm
□ Broad base |
| Type of polyp | □ Glandular tubular adenoma
□ Villous adenoma □ Villous glandular adenoma □ Traditional serrated adenoma □ Sessile serrated polyp/adenoma □ Hamartoma-like polyps □ Other |
| High-level intraepithelial neoplasia | □ None
□ Yes □ Inherent membrane invasion (intramucosal carcinoma) |
| Invasive carcinoma (carcinoma infiltrating submucosa) | □ None
□ Yes |
| Size of invasive cancer | Maximum diameter: ___ cm
Other two diameters: ___ cm × ___ cm |
| Histological classification | □ Adenocarcinoma, non-special type
□ Adenocarcinoma, special type □ Mucinous adenocarcinoma □ Signet ring cell carcinoma □ Serrated adenocarcinoma □ Micropapillary carcinoma □ Medullary carcinoma □ Sieve-like acne carcinoma □ Adenosquamous carcinoma □ Squamous cell carcinoma □ Spindle cells/sarcoma-like carcinoma □ Undifferentiated carcinoma □ Other special types: _______________ □ Cancer, uncertain type |
| Histological grade | □ Uncertain
□ Low level (well/moderately differentiated) □ High level (poorly differentiated, undifferentiated) |
| Tumor invasion (deepest point) | □ Membrane propria
□ Muscularis mucosae □ Submucosal layer (<1,000 infiltration) □ Submucosal layer (>1,000 infiltration) □ Muscularis propria |
| Deep resection margin (pedunculated
resection margin) |
□ Cannot be assessed
□ Non-invasive cancer, invasive cancer distance from resection margin: ___ mm □ Invasive cancer involvement |
| Mucosal resection margin | □ Cannot be assessed
□ No intradermal tumor and dysplasia □ Adenoma (low-grade intraepithelial neoplasia/dysplasia) visible □ High-grade intraepithelial neoplasia/dysplasia or intramucosal carcinoma □ Invasive cancer involvement |
| Tumor budding | □ Low (0−4/20 times if visual field)
□ Medium (5−9/20 times of visual field) □ High (10 or more/20 times of visual field) □ Cannot be assessed |
| Vascular invasion | □ Invisible
□ Visible □ Uncertain |
| Immunohistochemistry of mismatch
repair protein |
MLH1 ( ) PMS2 ( )
MSH2 ( ) MSH6 ( ) |
10. Anatomical staging/prognosis group.
| Stage | T | N | M |
| cTNM is the clinical stage, and pTNM is the pathological stage; prefix y is used for tumor stage after neoadjuvant (preoperative) treatment (e.g. ypTNM). The stage for patients with complete pathological response is ypT0N0cM0, which may be similar to stage 0 or 1. The prefixe r is used in patients who relapse after a tumor-free interval gained by treatment (rTNM). | |||
| 0 | Tis | N0 | M0 |
| I | T1 | N0 | M0 |
| T2 | N0 | M0 | |
| IIA | T3 | N0 | M0 |
| IIB | T4a | N0 | M0 |
| IIC | T4b | N0 | M0 |
| IIIA | T1−2 | N1/N1c | M0 |
| T1 | N2a | M0 | |
| IIIB | T3−4a | N1/N1c | M0 |
| T2−3 | N2a | M0 | |
| T1−2 | N2b | M0 | |
| IIIC | T4a | N2a | M0 |
| T3−4a | N2b | M0 | |
| T4b | N1−2 | M0 | |
| IVA | Any T | Any N | M1a |
| IVB | Any T | Any N | M1b |
| IVC | Any T | Any N | M1c |
9. Structural report of surgical resection specimens of colorectum.
| Name Sex Age Pathology number
Medical record number Inspection site | |
| American Joint Committee on Cancer (AJCC)/International Union Against Cancer (UICC) Colorectal Cancer TNM Staging System (8th Edition, 2017)
Primary tumor (T) Tx Primary tumor cannot be evaluated T0 No evidence of primary tumor Tis Carcinoma in situ: intramucosal carcinoma (invades lamina propria, but without penetrating the muscularis mucosae) T1 Tumor invades submucosa T2 Tumor invades muscularis propria T3 Tumor invades through muscularis propria into pericolorectal tissues T4 Tumor invades the visceral peritoneum or invades/adheres to adjacent organs or structures T4a Tumor penetrates the visceral peritoneum (including tumor perforation of the gross intestine and continuous infiltration of the tumor through the inflammatory region) T4b Tumor directly invades or adheres to adjacent organs or structures Regional lymph nodes (N) Nx Regional lymph node invaluable N0 No regional lymph node metastasis N1 Metastasis in 1−3 regional lymph nodes (intra-node tumors ≥0.2 mm) or any number of tumor deposits and no metastasis of all identifiable lymph nodes N1a Metastasis in 1 regional lymph node N1b Metastasis in 2−3 regional lymph node N1c There is no regional lymph node metastasis, but there are tumor deposits in the following areas: Subserous, mesenteric or pericolic/rectal mesenteric tissue without peritoneum coverage N2 Metastasis in 4 or more regional lymph nodes N2a Metastasis in 4−6 regional lymph nodes N2b Metastasis in 7 or more regional lymph nodes Metastasis (M) M0 No distant metastasis M1 Metastasis confined to one or more distant sites or organs, or peritoneal metastasis confirmed M1a Metastasis in a site or organ without peritoneal metastasis M1b Metastasis in two or more sites or organs without peritoneal metastasis M1c Metastasis only in the peritoneal surface or along with other sites or organs | |
| Specimen size | Length: ___ cm Circumference: ___ cm |
| Tumor location | To the proximal end ___ cm, to the distal end ___ cm |
| General type | □ Bulge type
□ Ulcer type □ Invasive type |
| Tumor size | Maximum diameter: ___ cm; Other two diameters: ___ cm × ___ cm |
| Perforation of tumor | □ Visible
□ Invisible □ Uncertain |
| Histological classification | □ Adenocarcinoma, non-special type
□ Adenocarcinoma, special type □ Mucinous adenocarcinoma □ Signet ring cell carcinoma □ Serrated adenocarcinomav □ Micropapillary carcinoma □ Medullary carcinoma □ Sieve-like acne cancer □ Adenosquamous carcinoma □ Squamous cell carcinoma □ Spindle cells/sarcoma-like carcinoma □ Undifferentiated cancer □ Other special types: _______________ □ Cancer, uncertain type |
| Histological grade | □ Uncertain
□ Low level (well/moderately differentiated) □ High level (poorly differentiated, undifferentiated) |
| Tumor invasion (deepest point) | □ Cannot be assessed
□ No evidence of primary tumor □ No membrane propria infiltration □ Intramucosal carcinoma, invasion of the membrane propria/muscularis mucosae □ Tumor invades submucosal layer □ Tumor invades the muscularis propria □ Tumor penetrates the muscularis propria to subserosal adipose tissue or pericolic/perirectal soft tissue without peritoneum but not to the serosa surface □ Tumor penetrates the visceral peritoneum (serous membrane) (including tumor perforation of the gross intestine and continuous infiltration of the tumor through the inflammatory region) □ Adhesion of tumor to other organs or structures: _____________ □ Tumors directly invade nearby structures: _____________ |
| Proximal resection margin | □ Cannot be assessed
□ No intradermal tumor and dysplasia □ Adenoma (low-grade intraepithelial neoplasia/dysplasia) □ High-grade intraepithelial neoplasia/dysplasia or intramucosal carcinoma □ Invasive cancer involvement |
| Distal resection margin | □ Cannot be assessed
□ No intradermal tumor and dysplasia □ Adenoma (low-grade intraepithelial neoplasia/dysplasia) □ High-grade intraepithelial neoplasia/dysplasia or intramucosal carcinoma □ Invasive cancer involvement |
| Circumferential (radial) or mesangial margin | □ Inapplicable
□ Cannot be assessed □ Non-invasive cancer □ Invasive carcinoma (distance between tumor and resection margin less than 1 mm) |
| Therapeutic effects (for cancer after neoadjuvant treatment) | □ No pre-treatment
□ Therapeutic effect □ No residual tumor (grade 0, complete response) □ Moderate response (grade 1, minor residual tumor) □ Minimal response (grade 2) □ No certain response (grade 3, poor response) □ Uncertain |
| Tumor budding | □ Low (visual field of 0−4/20 times)
□ Medium (visual field of 5−9/20 times) □ High (visual field of 10 or more/20 times) □ Cannot be assessed |
| Vascular invasion | □ Invisible
□ Visible □ Uncertain |
| Nerve invasion | □ Invisible
□ Visible □ Uncertain |
| Lymph node | □ No lymph nodes submitted or no lymph nodes found
□ No. of lymph nodes examined ___ □ No. of lymph nodes involved ___ |
| Extranodal tumor deposits | □ Invisible
□ Visible (Number: ___) □ Uncertain |
| Immunohistochemistry of mismatch repair protein | MLH1 ( ) PMS2 ( )
MSH2 ( ) MSH6 ( ) |
| Pathological stage | □ m (multiple primary tumors)
□ r (relapse) □ y (after neoadjuvant treatment) T ___ N ___ M ___ |
4. Surgical treatment
4.1 Standards for surgical treatment of colon cancer
4.1.1 Principles of surgical treatment of colon cancer
(1) Thorough abdominal exploration from far to near is required. Liver, gastrointestinal tract, uterus and appendages, pelvic floor peritoneum, related mesentery, major paravascular lymph nodes and tumor adjacent organs must be explored and recorded.
(2) Routine extensive excision of intestine, dissection of regional lymph nodes and en bloc resection are recommended, and routine dissection of more than two stations of lymph nodes is recommended.
(3) Sharp dissection technology is recommended.
(4) The principle of tumor-free surgery is recommended to follow.
(5) For tumor that has lost the chance of radical surgery, if the patient has no bleeding, obstruction, perforation symptoms or symptoms caused by compression of adjacent organs, the need for resection of the primary lesion is determined based on multidisciplinary team (MDT) evaluation.
(6) For the colon neoplasm whose clinical diagnosis is highly suspicious of malignant tumor and whose pathological biopsy report indicates high-grade intraepithelial neoplasia, if patients can tolerate surgery, surgical exploration is recommended.
4.1.2 Treatment of early colon cancer cT1N0M0
Endoscopic resection, local excision or colectomy is recommended. For superficial submucosal invasion, endoscopic resection may be considered. Before making the decision, relevant information such as tumor size, depth of invasion and degree of tumor differentiation should be carefully evaluated. For the tumor which is staged as T1 after the assessment of preoperative endoscopic ultrasonography or pathologically confirmed as T1 after local excision, if it is removed completely with negative resection margins (including the base of the tumor) and favorable histologic features which indicate a good prognosis (such as well-differentiated, no vascular invasion), surgical resection is not recommended, whether the tumor is sessile or pedunculated. If the tumor specimen shows unfavorable histopathology, or if the specimen is fragmented, the margins cannot be assessed, colectomy with dissection of regional lymph nodes is recommended.
If endoscopic resection or local excision is performed, the following requirements must be met:
(1) Tumor size <3 cm;
(2) The perimeter of the lesion surrounding the intestine is less than 30%;
(3) Resection margin distance to tumor >3 mm;
(4) Mobile, not fixed;
(5) Only for stage T1 tumors;
(6) High-moderate differentiation;
(7) No sign of lymph node metastasis by pre-treatment imaging examination.
Note: Locally resected specimens must be flattened and fixed by the surgeon. The specimens must be marked and then sent for pathologic examination.
4.1.3 T2−4, N0−2, M0 colon cancer
(1) The preferred surgical methods are colectomy of the corresponding colon segment with dissection of regional lymph nodes. Regional lymph node dissection must include paracolic lymph nodes, mesenteric lymph nodes at central or root of the mesentery. Lymph nodes at the root of the mesentery is recommended to mark and send them for pathologic examination; if lymph nodes and deposits beyond the scope of dissection are suspected to have metastasis, complete resection is recommended. The incomplete resection is considered as palliative resection.
(2) If FAP has turned cancerous, total colorectal resection plus ileal pouch anal anastomosis, total colorectal resection with end-to-end ileorectal anastomosis or total colorectal resection with ileostomy should be performed according to the site of tumor. Patients who have not developed malignant tumor can choose total or segmental colorectal resection according to their condition. Selection between total and segmental colorectal resection combined with follow-up by colonoscopy should be made based on adequate communication with patients with Lynch syndrome.
(3) For the tumor that invades adjacent tissues or organs, multi-visceral resection is recommended. Colon cancer staged as T4 by preoperative imaging examination can be treated with colectomy after preoperative chemotherapy or chemoradiotherapy on the premise of MDT discussion.
(4) Laparoscopic-assisted colectomy is recommended to be conducted by surgeons experienced in laparoscopy as appropriate.
(5) For resectable colon cancer that has caused intestinal obstruction, it is recommended that I-stage resection and anastomosis, or I-stage resection with a proximal end colostomy and closure of the distal stump, or II-stage resection after the colostomy, or limited resection after stent implantation should be conducted. If the locally advanced tumor cannot be resected, palliative treatment including surgery is recommended, such as proximal end colostomy, bypass surgery and stent implantation.
4.2 Standards for surgical treatment of rectal cancer
The principle of abdominal exploration for rectal cancer surgery is the same as that for colon cancer.
4.2.1 Local excision of cT1N0M0 rectal cancer
The treatment principle of early rectal cancer (cT1N0M0) is the same as that of early colon cancer.
Transanal (non-laparoscopic and non-endoscopic) resection of early rectal cancer (cT1N0M0) must meet the following requirements:
(1) Tumor size <3 cm;
(2) The perimeter of the lesion surrounding the intestine is less than 30%;
(3) Resection margin distance to tumor >3 mm;
(4) Mobile, not fixed;
(5) Within 8 cm from the anal verge;
(6) Only for stage T1 tumors;
(7) No lymphovascular invasion (LVI) or perineural invasion (PNI);
(8) High-moderate differentiation;
(9) No signs of lymph node metastasis on pre-treatment imaging examination.
Note: Locally resected specimens must be flattened and fixed by the surgeon. The specimens must be marked and then sent for pathologic examination.
4.2.2 cT2−4, N0−2, M0 rectal cancer
Radical surgery is recommended. Low anterior resection (LAR) is recommended for middle or upper rectal cancer; abdominoperineal resection or sphincter preserving surgery (under careful selection) is recommended for lower rectal cancer. Resection of middle or lower rectal cancer must follow the principle of total mesorectal excision (TME), and the mesorectum should be dissected sharply if possible. Try to confirm the negative circumferential resection margin (CRM). For the suspected positive CRM, follow-up treatment should be added. Make sure the distance from distal resection margin of rectum wall to tumor is 1−2 cm, and the distance from the distal resection margin of the mesorectum to tumor is more than or equal to 5 cm or the whole mesorectum is resected. Intraoperative fast frozen-section biopsy should be performed to determine whether there are residual tumor cells on the resection margin if necessary. On the premise of radical cure of tumor, try to preserve anal sphincter function, urinary and sexual function. The principles of treatment are as follows:
(1) Excise the primary tumor and ensure adequate surgical margins. The distal resection margin should be at least 2 cm away from the distal end of the tumor. If the distal resection margin of distal rectal cancer (<5 cm from the anus) is 1−2 cm from the tumor, intraoperative fast frozen-section biopsy is recommended to confirm that the resection margin is negative. Make sure the distal resection margin of the mesorectum is ≥5 cm or the whole mesorectum is resected.
(2) Resect the lymphatic and adipose tissue in the mesorectum and suspected positive lateral lymph nodes.
(3) Try to preserve pelvic autonomic nerve.
(4) For locally advanced middle or upper rectal cancer staged as cT3−4 and/or N+ by preoperative imaging examination, preoperative chemoradiotherapy or preoperative chemotherapy is recommended. The interval between preoperative chemoradiotherapy and surgery is shown in the chemoradiotherapy section.
(5) For the tumor that invades adjacent tissues or organs, multi-visceral resection is recommended.
(6) For the rectal tumor complicated by intestinal obstruction which is clinically highly suspicious of malignancy without pathological diagnosis, exploratory laparotomy is recommended if the patient can tolerate surgery and does not have problem of sphincter preservation.
(7) For resectable rectal cancer that has caused intestinal obstruction, I-stage resection and anastomosis, or Hartmann surgery, or II-stage resection after colostomy, or limited resection after stent implantation is recommended to conduct to relieve obstruction. Intraoperative intestine lavage is recommended before I-stage resection and anastomosis. If the risk of anastomotic leakage is estimated to be high, Hartmann surgery or I-stage resection and anastomosis as well as preventive colostomy is recommended.
(8) If the locally advanced tumor cannot be resected or patient cannot tolerate surgery, palliative treatment including radiotherapy for uncontrollable bleeding and pain, and proximal double barrel colostomy or stent implantation for intestinal obstruction and supportive treatment are recommended to conduct.
(9) If there is a clear residual tumor during the surgery, it is recommended that metal surgical clips should be placed as a marker for subsequent radiotherapy.
(10) Laparoscopic-assisted radical resection for rectal cancer is recommended to be conducted by surgeons experienced in laparoscopy as appropriate.
5. Medical treatment
General principles of medical drug treatment: The purpose of treatment must be defined, and it must belong to preoperative treatment/postoperative adjuvant treatment or palliative treatment; the imaging baseline evaluation must be conducted before systemic treatment, and the relevant gene detection is recommended. It is recommended that K-ras and N-ras gene mutations should be detected in patients with clinically diagnosed recurrent or mCRC in order to guide tumor targeted therapy. The evaluation of BRAFV600E mutation status should be carried out simultaneously during Ras detection, so as to stratify the prognosis and guide the clinical treatment. It is recommended that the expression of MMR or MSI in all patients with CRC should be detected for Lynch syndrome screening, prognosis stratification and immunotherapy guidance. In order to evaluate the risk of Lynch syndrome, BRAFV600E mutation and/or MLH1 methylation should be performed in dMMR tumors with MLH1 deficiency. Some clinical studies on anti-HER2 treatment of CRC have obtained gratifying results, but there is no standard test and interpretation standard at present, and qualified units can properly carry out relevant work. In the course of treatment, we must evaluate the curative effect and side effects in time, and adjust the treatment target, drug and dosage according to the patient’s condition and physical strength score under the guidance of MDT. Pay attention to improving the quality of life of patients and treatment of complications, including pain, nutrition, mental and psychological needs.
5.1 Preoperative treatment of CRC
5.1.1 New adjuvant therapy for rectal cancer
The purpose of neoadjuvant therapy is to improve the resection rate, improve the rate of anus preservation and prolong the disease-free survival (DFS) of patients. It is recommended that neoadjuvant chemoradiotherapy (nCRT) should only be used for rectal cancer less than 12 cm from anus.
(1) Fluorouracil based nCRT is recommended for preoperative treatment of rectal cancer.
(2) Patients with T1−2N0M0 or contraindications of radiochemotherapy are recommended to surgical treatment directly, and new adjuvant therapy is not recommended.
(3) In principle, preoperative nCRT is recommended for T3 and/or N+ patients with resectable rectal cancer (see radiotherapy section for specific radiotherapy indications); after MDT discussion, neoadjuvant chemotherapy alone can also be considered, and then combined radiotherapy can be determined according to the efficacy evaluation.
(4) Patients with T4 or local advanced unresectable rectal cancer must receive preoperative radiochemotherapy. After treatment, resectability must be reevaluated, and the feasibility of surgery must be discussed by MDT.
In nCRT, capecitabine alone or continuous infusion of 5-fluorocrail (5-FU) or 5-FU/leucovorin (LV) is recommended as the first choice, and chemotherapy is carried out simultaneously during long-term radiotherapy. Please refer to 6. Radiotherapy for CRC .
(5) For patients who are not suitable for radiotherapy, whether to use neoadjuvant chemotherapy alone is recommended to decide under the discussion of MDT.
5.1.2 Preoperative treatment of T4b colon cancer
(1) For the initial local non-resectable T4b colon cancer, chemotherapy or chemotherapy combined with targeted therapy is recommended. If necessary, under the discussion of MDT, decide whether to add local radiotherapy or not.
(2) For the initial local resectable T4b colon cancer, whether to receive preoperative chemotherapy or direct surgery is recommended to decide under the discussion of MDT.
5.1.3 Preoperative treatment of liver and/or lung metastasis of CRC
CRC patients with liver and/or lung metastasis which is resectable or potentially resectable, please refer to the relevant sections. Preoperative chemotherapy or chemotherapy combined with targeted drug therapy is recommended under the discussion of MDT. Targeted drugs include cetuximab (recommended for K-ras, N-ras, BRAF genes wild-type patients), or combination of bevacizumab. Capeox (capecitabine + oxaliplatin), or FOLFOX (oxaliplatin + fluorouracil + hydrofolate), or FOLFIRI (irinotecan + fluorouracil + hydrofolate), or FOLFOXIRI (oxaliplatin + irinotecan + fluorouracil + hydrofolate) was recommended as the chemotherapy plan. It is suggested that the time limit of treatment be 2−3 months.
After treatment, it is necessary to reevaluate and consider the feasibility of local destructive treatment, including surgery, radiofrequency ablation and stereotactic radiotherapy.
5.2 Adjuvant treatment of CRC
The adjuvant therapy should be determined according to the primary site, pathological stage, molecular markers and postoperative recovery. It is recommended that adjuvant chemotherapy should be started about 4 weeks after operation (the patients with poor constitution should be prolonged appropriately), and the time limit of chemotherapy should be 3−6 months. During the treatment, we should adjust the dosage and/or shorten the chemotherapy cycle according to the patients’ physical condition, drug toxicity, postoperative TN stage and patients’ wishes. Adjuvant therapy is not recommended in patients with contraindications of radiotherapy and chemotherapy.
(1) Adjuvant therapy is not recommended for stage I (T1−2N0M0) CRC.
(2) Adjuvant chemotherapy for stage II colon cancer.
For stage II colon cancer, we should confirm whether there are the following high-risk factors: poor histological differentiation (grade III or IV), normal MMR, microsatellite stability (MSS), T4, vascular lymphatic infiltration, preoperative intestinal obstruction/intestinal perforation, insufficient lymph nodes (less than 12) detected in specimens, nerve invasion, positive or undeterminable resection margin .
1) If there are no high-risk factors, follow-up observation or single drug like fluorouracil chemotherapy is recommended.
2) If there are high-risk factors, adjuvant chemotherapy is recommended. Capeox or FOLFOX, based on oxaliplatin, or 5-FU/LV or capecitabine as a single drug, are recommended for chemotherapy. The treatment time is 3−6 months.
3) If the tumor tissue is dMMR or high-level microsatellite instability (MSI-H), postoperative adjuvant chemotherapy is not recommended.
(3) For stage II rectal cancer, please refer to 6. Radiotherapy for CRC .
(4) Adjuvant chemotherapy for stage III CRC
Adjuvant chemotherapy is recommended for patients with stage III CRC. Capeox, FOLFOX or capecitabine, 5-FU/LV is recommended for chemotherapy. For low-risk patients (T1−3N1), 3-month regimen with CapeOx can also be considered as adjuvant chemotherapy.
(5) Adjuvant radiotherapy and chemotherapy for rectal cancer
Rectal cancer which is T3−4 or N1−2 with distance to anal margin less than 12 cm is recommended to use preoperative neoadjuvant radiochemotherapy. If neoadjuvant radiotherapy is not performed before operation, the adjuvant radiotherapy and chemotherapy will be determined according to the postoperative pathological conditions, among which fluorouracil-based chemotherapy is recommended. Please refer to6. Radiotherapy for CRC .
(6) At present, irinotecan, tegafur, letetrexed and targeted drugs are not recommended in adjuvant chemotherapy.
5.3 Systemic treatment of recurrent/metastatic CRC
At present, 5-FU/LV, irinotecan, oxaliplatin, capecitabine, tripyridine and letetrexed are used in the treatment of advanced or metastatic CRC. Targeted drugs include cetuximab (recommended for K-ras, N-ras, BRAF genes wild-type patients), bevacizumab, regofinib, and furquitinib.
(1) Detection of K-ras, N-ras, BRAF genes and microsatellite status is recommended before treatment.
(2) Combined chemotherapy should be the first-line and second-line treatment for patients with metastatic CRC who can tolerate chemotherapy. The following chemotherapy regimens are recommended: FOLFOX/FOLFIRI ± cetuximab (recommended for K-ras, N-ras, BRAF genes wild-type patients), CapeOX/FOLFOX/FOLFIRI/ ± bevacizumab. The first-line treatment of FOLFOXIRI ± bevacizumab can also be considered for patients with large tumor burden, poor prognosis or need of transformation treatment, if the general situation allows. FOLFOXIRI + cetuximab can also be considered in the treatment of K-ras, N-ras, BRAF genes wild-type patients who need transformation.
(3) The prognosis of right colon cancer (from ileocecal to splenic curvature) is worse than that of left colon cancer and rectum (from splenic curvature to rectum). For K-ras, N-ras, BRAF genes wild-type patients, the first-line treatment of right colon cancer in the anti-vascular endothelial growth factor (anti-VEGF) monoclonal antibody (bevacizumab) combined chemotherapy is better than the anti-epidermal growth factor receptor (anti-EGFR) monoclonal antibody (cetuximab) combined chemotherapy, while in the left colon cancer and rectal cancer in the anti-EGFR monoclonal antibody combined chemotherapy is better than the anti VEGF monoclonal antibody combined chemotherapy.
(4) Patients with three or more lines of treatment are recommended to take regofinib or furquitinib or to participate in clinical trials, or to consider tripyrimidine. We can adjust the initial dose of regofinib in the first cycle treatment according to the patient’s condition and physical condition. For the patients who did not choose the targeted drugs in the first- and second-line treatment, cetuximab ± irinotecan (recommended for K-ras, N-ras, BRAF genes wild type) can also be considered.
(5) For the first-line patients receiving oxaliplatin, if the second-line treatment is chemotherapy plus bevacizumab, FOLFIRI or improved irinotecan plus capecitabine is recommended. For patients who cannot tolerate combined chemotherapy, 5-FU/LV or capecitabine single drug ± target drug is recommended. Letetrexed should be considered in patients with advanced CRC who are not suitable for 5-FU/LV.
(6) Patients with stable disease but no chance of R0 operation after 4−6 months of palliative treatment may be considered for maintenance treatment (such as 5-FU/LV or capecitabine single drug or combination of targeted treatment or suspension of systemic treatment with low toxicity), so as to reduce the toxicity of combined chemotherapy.
(7) For BRAFV600E mutation patients, if the general condition is good, the first-line treatment of FOLFOXIRI + bevacizumab can be considered.
(8) For the patients with dMMR or MSI-H, according to the patients’ condition and wishes, we can consider the treatment of immunosuppressive checkpoint inhibitors under the discussion of MDT.
(9) If the general condition or organ function of late patients with advanced CRC is poor, the best supportive treatment is recommended.
(10) If the metastasis is limited to the liver or/and lung, refer to 7. Treatment for liver metastases and 8. Treatment for lung metastases .
(11) For patients with local recurrence of CRC after surgery, multidisciplinary evaluation is recommended to conduct to determine whether there is a chance to resect, or to receive radiotherapy or radiofrequency ablation and other local treatment, so as to achieve the state without tumor evidence. If it is only suitable for systemic therapy, the above principles of drug treatment for advanced patients should be adopted.
5.4 Other treatments
On the premise that the above conventional treatment is not applicable, the patients in the advanced stage can choose local treatment, such as interventional treatment, tumor injection, physical treatment or Traditional Chinese Medicine treatment.
5.5 Best support treatment
The best supportive treatment should run through the whole process of patients’ treatment, and multidisciplinary comprehensive treatment is suggested. The best supportive treatment recommendations cover the following:
(1) Pain management: Accurately perfect pain assessment, comprehensive and reasonable measures to treat pain, recommend according to the principle of three-step pain treatment, actively prevent and deal with adverse reactions of painkillers, and pay attention to etiology treatment. Pay attention to the pain education and psychosocial support of patients and their families, and strengthen communication and follow-up.
(2) Nutritional support: It is suggested that the nutritional status should be assessed regularly, appropriate nutritional support be given and enteral nutrition support be advocated.
(3) Psychological intervention: It is suggested that oncology psychiatrists should provide psychological intervention and necessary psychotropic drug intervention in the areas where conditions permit.
5.6 Clinical trial research and new progress
Clinical trials may bring more benefits to patients based on the existing standard treatment. In view of the limitations of the therapeutic effect of standard drugs, it is suggested that patients should be encouraged to participate in the clinical trials in accordance with their conditions on a voluntary basis.
For advanced CRC with special gene mutation (such as BRAF gene mutation, HER2 amplification, K-ras, BRCA gene pathogenic mutation, NTRK gene fusion, etc.), there were early small sample studies abroad that show that the corresponding targeted treatment had a certain effect. First of all, this kind of patients is recommended to participate in the corresponding clinical research, and it can also be considered to try the treatment of special targets under the guidance of experienced oncologists. For patients with failed standard treatment, next-generation sequencing (NGS) can be considered in a qualified gene testing institution to find suitable clinical research or drug treatment. At present, for MSS or pMMR patients, immunosuppressant single drug has no significant effect. Patients should be encouraged to participate in the clinical study of the combination of immunosuppressive agents and other drugs based on their condition and wishes and with full knowledge.
6. Radiotherapy for CRC
6.1 Indications for radiotherapy for CRC
The main modes of radiotherapy or chemoradiotherapy for rectal cancer are neoadjuvant/adjuvant therapy, radical therapy, conversion therapy and palliative therapy.
Neoadjuvant radiotherapy indication is mainly for stage II−III mid-low rectal cancer (from the anus <12 cm): after the end of long-course concurrent chemoradiotherapy, radical surgery is recommended at an interval of 5−12 weeks. Short-course radiotherapy (SCRT) combined with immediate radical surgery (1 week after completion of radiotherapy) is recommended for MRI or ultrasound endoscopy diagnosis of surgically resectable stage T3 rectal cancer; The combination of SCRT with delayed radical surgery and the addition of neoadjuvant chemotherapy during the interval is recommended for stage II−III rectal cancer with high risk of recurrence. Adjuvant radiotherapy is mainly recommended for rectal cancer who have not received nCRT and have postoperative pathological stage II−III and are at high risk of local recurrence. Patients who need preoperative or postoperative radiotherapy should be recommended to hospitals with radiotherapy equipment and conditions for radiotherapy.
Patients with low rectal cancer who have a strong desire to preserve their anus may be recommended to undergo nCRT first. If the tumor is sensitive to chemoradiotherapy and achieves clinical complete response (cCR), the treatment strategy of Watch & Wait (W & W) may be considered (see 6.1.4 W & W strategy for details); Radical surgery is recommended if cCR is not achieved. For recurrent or metastatic rectal cancer patients who have the chance of radical resection, if the rectal lesion is local but difficult to resect, and dose not receive radiotherapy before, local radiotherapy can be considered to transform it into surgically resectable lesion; The indications of palliative radiotherapy for rectal cancer are local recurrence and/or distant metastasis tumor, or some patients who cannot tolerate surgery, and cannot be cured by radiotherapy and combined therapy. Postoperative radiotherapy may also be considered after palliative resection of colon cancer and labeling.
6.1.1 Radiotherapy for stage I rectal cancer
Radical surgery is recommended for patients with high-risk factors after local resection of stage I rectal cancer (see 4. Surgical treatment for details). If further radical surgery cannot be performed for some reasons, postoperative radiotherapy is recommended.
6.1.2 nCRT for stage II−III rectal cancer
The clinical diagnosis was stage II−III rectal cancer, and rectal MRI was the first choice for local examination (see2. Diagnostis for details). If the patient cannot receive MRI, rectal ultrasonography is recommended. Stratified treatment based on the location of the tumor in the rectum and the risk of recurrence suggested by MRI are recommended, as shown in Table 11 .
11. Neoadjuvant chemoradiotherapy stratification treatment recommendations for stage II−III rectal cancer.
| Risk stratification for recurrence of stage II−III rectal cancer | Treatments | Recommended level | |
| MRF, mesorectal fascia; EMVI, extramural vascular invasion; CRT, long-course concurrent chemoradiotherapy; TME, total mesorectal excision; SCRT, short-course radiotherapy. | |||
| Low-risk group, meeting all the following criteria | Direct TME surgery; Surgical quality evaluation of TME; Postoperative adjuvant therapy is determined by postoperative pathology; If high quality TME surgery is not assured, preoperative CRT + delayed surgery/SCRT + immediate surgery should be performed; | Recommended | |
| ≤cT3a/b | |||
| cN0−2 (no cancer deposits) | |||
| MRF (−) | |||
| The tumor is located in the posterior wall of the rectum; | Recommended | ||
| EMVI (−) | |||
| Middle-risk group, MRF (−) and meeting any or more of the following criteria | Preoperative CRT + delayed surgery/SCRT + immediate surgery | Recommended | |
| cT3c/d | |||
| Extremely low lesion | |||
| cN1−2 cancer deposits | |||
| EMVI (+) | |||
| High-risk group, meeting one or more of the following criteria | Preoperative CRT + delayed surgery/SCRT sequential neoadjuvant chemotherapy + delayed surgery | Recommended | |
| cT3 with MRF (+) | |||
| cT4 | |||
| The elevator ani muscle is invaded; Lateral lymph node (+) | |||
| Elderly patients with infirmity or severe complications who cannot tolerate CRT | SCRT + delayed surgery | Recommended | |
6.1.3 Adjuvant chemoradiotherapy for stage II−III rectal cancer
For postoperative pathological stage II−III rectal cancer without nCRT, stratified treatment was recommended according to TME surgical quality, CRM status and distance from the tumor to the anal margin, as shown inTable 12 .
12. Adjuvant chemoradiotherapy stratification treatment recommendations for stage II−III rectal cancer.
| Risk stratification for recurrence of stage II−III rectal cancer | Treatments | Recommended level |
| CRM, circumferential resection margin; TME, total mesorectal excision; MRF, mesorectal fascia. | ||
| Meeting any of the following criteria | Postoperative concurrent chemoradiotherapy | Recommended |
| CRM ≤1 mm | ||
| pT4b | ||
| pN2 with poor TME quality/mesorectum defect | ||
| Pathology stage II/III but the quality of TME operation could not be evaluated | ||
| The nerve infiltrates near MRF | ||
| Pathology stage II/III but unable to assess CRM status | ||
| Meeting all the following criteria | Postoperative concurrent chemoradiotherapy | Suggested |
| CRM is 1−2 mm | ||
| Circumferential obstruction tumor | ||
| Meeting all the following criteria | Postoperative concurrent chemoradiotherapy | Not suggested |
| TME quality is good/mesorectum smooth and complete | ||
| pT1−3 or pT4a above the peritoneal reflection | ||
| pN0−1 | ||
| CRM >2 mm | ||
6.1.4 W & W strategy
For low advanced rectal cancer (cT1N0, cT2N0, cT3−4 or N+) with difficulty in preserving the anal sphincter, preoperative concurrent chemoradiotherapy is recommended if the patient has a strong desire to preserve the anus. If cCR is obtained after nCRT, the W & W strategy can be adopted. The evaluation time of cCR is recommended to be 8−12 weeks after concurrent nCRT, and follow-up is recommended every 1−2 months for 1−2 years. cCR evaluation items are strongly recommended to include DRE, colonoscopy, rectal MRI and blood CEA level, all of which must meet the cCR evaluation criteria, as shown inTable 13 .
13. cCR criteria.
| Evaluation item | cCR criteria |
| cCR, clinical complete response; MRI, magnetic resonance imaging; CEA, carcinoembryonic antigen. | |
| Digital rectal examination | No clear mass was observed, and the intestinal wall was soft |
| Endoscopy | No clear residual tumor was observed, and only leukoplakia and/or telangiectasia could be seen in the original tumor area |
| Pelvic cavity MRI | No residual tumors or lymph nodes were observed, only fibrosis was observed |
| Blood CEA level | Normal |
6.1.5 Stage IV rectal cancer
For stage IV rectal cancer with resectable or potentially resectable metastatic lesions, chemotherapy ± radiotherapy or surgical resection for primary lesions is recommended; In the case of radiotherapy, resectability can be reevaluated 4 weeks after radiotherapy; If necessary, stereotactic radiotherapy or palliative radiotherapy should be applied.
6.1.6 Locally recurrent rectal cancer
For locally recurrent rectal cancer, if they have not received pelvic radiotherapy before, preoperative concurrent chemoradiotherapy is recommended. After chemoradiotherapy, the patient should be reevaluated and strive for surgical resection; If the patient has received pelvic radiotherapy before, the high risk of second-course radiotherapy should be carefully evaluated, and the treatment plan by MDT is recommended to determine.
6.2 Guidelines of radiotherapy for rectal cancer
According to different radiotherapy equipment in hospital, choose suitable radiotherapy technology, three-dimensional conformal radiation therapy or intensity modulated radiation therapy (IMRT) technology is recommended, compared with two-dimensional radiotherapy technology, it has better dose coverage, uniformity and conformality, and reduce the injectivity of the near endanger organs, so as to reduce the incidence of radiation related adverse events, increase patient’s tolerance to radiotherapy. CT simulation localization is recommended. If not, routine simulation localization must be performed. If IMRT is to be performed, planned validation must be performed. Intraoperative radiotherapy, intracavitary irradiation or external irradiation may be applied for local dosing. Radioactive seed implantation therapy is not routinely recommended.
6.2.1 Three-dimensional conformal radiation therapy and intensity modulated radiotherapy localization
(1) Preparation before localization: The bladder is recommended to empty 1 h before localization and then drink water to fill the bladder.
(2) Body position and body film fixation: Supine position or prone position can be adopted, and thermoplastic body film fixation. For patients who are recommended to undergo preoperative radiotherapy for rectal cancer or postoperative radiotherapy after LAR, lead marks can be placed in the anus to determine the position of the anal margin. The perineal scar was marked with fine lead wire in patients who were recommended for radiotherapy after combined abdominal perineal resection (APR) for rectal cancer.
(3) Simulated CT: The range of CT scan is recommended, the upper boundary is from the level of the 2nd to the 3rd lumbar vertebra, the lower boundary is to the upper 1/3 segment of the femur, and the thickness is 5 mm. Patients who are not allergic should have an enhanced venography scan to clearly show the tumor and blood vessels. For those who have received preoperative radiotherapy, it is recommended that qualified medical centers apply MRI localization CT/MRI fusion simultaneously to help clarify the tumor range so as to more accurately delineate the target area.
6.2.2 Definition of exposure range and target volume
Gross target volume (GTV) refers to gross tumor determined by clinical examination. Clinical target volume (CTV) includes GTV, the region with high risk of recurrence of the primary tumor and the region with lymphatic drainage, which must be irradiated. Planned target volume (PTV) is formed by CTV outward expansion, including CTV itself, and covers uncertain factors such as organ movement and daily positioning during irradiation.
(1) Regions with high risk of recurrence of primary tumor include tumor/tumor bed, mesorectal region and presacral region. Radiation fields are recommended to include tumor/tumor beds and safety margins of 2−5 cm.
(2) Regions with lymphatic drainage include the mesorectal region, the iliac endovascular lymphatic drainage region and the obturator lymphatic drainage region. When T4 tumors invade the anterior structure, the lymphatic drainage region of the external iliac vessels can be irradiated.
(3) For patients with tumors and/or residues, local shrinking field irradiation should be applied after total pelvic irradiation. Meanwhile, intestinal irradiation dose should be carefully considered.
(4) Definition of organs at risk: Pelvic small intestine, colon, bladder, bilateral femoral head, male and female genitalia, and female perineum are organs at risk in the preoperative/postoperative radiotherapy area of rectal cancer, the dose and volume of exposure are recommended to delineate and define.
(5) Radiotherapy for recurrent pelvic lesions: If there is no previous history of radiotherapy, radiotherapy for recurrent tumor and high-risk recurrence region is recommended, and local radiotherapy with increased dosage for tumor can be considered. If so, radiotherapy should be decided according to the situation.
(6) The specific target region was delineated and the definition of organ at risk was referred to the professional books on radiotherapy.
6.2.3 Radiotherapy dose and segmentation pattern
There must be a clear definition of radiation dose whether using conventional irradiation or three-dimensional conformal radiotherapy or IMRT. Three-dimensional conformal radiotherapy and IMRT must be defined by volume dose, and isocentric dose definition is recommended for routine irradiation.
Preoperative neoadjuvant radiotherapy segmentation mode: There are mainly two kinds of dose segmentation modes in preoperative neoadjuvant radiotherapy. 1) SCRT mode, 5 Gy × 5 radiotherapy is recommended for primary tumors and high-risk region. The SCRT segmentation model is not suitable for patients with rectal cancer who are MRF positive or T4 (i.e., LARC which cannot be initially resected at R0 or cannot be resected). For SCRT + immediate TME surgery, SCRT must be preceded by a MDT discussion and adequate communication with the surgeon (The connection between radiotherapy and operation time); 2) Long-course chemoradiotherapy mode, it is recommended that the primary tumor and high-risk area should be irradiated with DT 45.0−50.4 Gy, 1.8−2.0 Gy each time, for a total of 25−28 times. The long-course chemoradiotherapy mode of 5-Fu or capecitabine monotherapy during radiotherapy is suitable for all patients with stage II−III rectal cancer, which is conducive to full tumor regression; 3) If preoperative radiotherapy adopts other dose segmentation methods, effective biological dose (BED) must be ≥30 Gy.
Postoperative adjuvant chemoradiotherapy dose: For patients at stage II−III who did not receive radiotherapy before surgery, it is recommended that DT 45.0−50.4 Gy should be given to tumor bed and high-risk area after surgery, 1.8−2.0 Gy each time, a total of 25−28 times. 5-FU or capecitabine chemotherapy was given simultaneously during radiotherapy. For patients with residual tumor or positive resection margin, secondary surgery is recommended. If the second operation cannot be performed or the patient refuses the second operation, the radiation dose DT 10−20 Gy is suggested to add to the local shrinking field after the total pelvic irradiation. The method of synchronous dosage is not recommended when the intestinal tube is in the target area. And the intestinal exposure dose must be considered, especially the small intestine/colon dose in the radiation field (must be ≤DT 56−60 Gy).
6.2.4 Interval is recommended between neoadjuvant radiotherapy and surgery
The interval between neoadjuvant radiotherapy and surgery is different depending on the course of neoadjuvant radiotherapy: 1 week after SCRT (5 Gy × 5) (SCRT + immediate surgery mode) or 6−8 weeks after SCRT (SCRT + delayed surgery mode).
Surgery is recommended at 5−12 weeks after LCRT.
6.3 Principle of combined chemoradiotherapy for rectal cancer
6.3.1 Concurrent chemotherapy regimen
(1) Fluorouracil monotherapy is recommended for concurrent chemotherapy during long-course radiotherapy, and there are three specific types: 1) Capecitabine 825 mg/m2, bid, 5 d a week, oral medication on radiotherapy days is recommended; 2) 5-FU 225 mg/(m2·d), continuous intravenous infusion during radiotherapy, 24 h a day, 5−7 d a week; 3) 5-FU 400 mg/(m2·d) + LV 20 mg/(m2·d), d 1−d 4, intravenous infusion during the 1st and 5th week of radiotherapy.
(2) The combination of two drugs may increase the probability of tumor regression, but the level of evidence is low and is not recommended for use outside of clinical studies.
(3) Bevacizumab, cetuximab, panizumab and other targeted drugs are not recommended to add into preoperative chemoradiotherapy for rectal cancer.
(4) Concurrent chemotherapy and targeted therapy drugs are not recommended for SCRT.
6.3.2 Adding chemotherapy during interval between concurrent chemoradiotherapy or brachytherapy and surgery
For LARC, especially in patients with pretherapeutic assessment of MRF positive or T4b or lateral lymph node metastasis, chemotherapy according to tumor regression under MDT advice can be added after long-course concurrent chemoradiotherapy or SCRT, followed by surgery to increase the degree of tumor regression. FOLFOX, CapeOx or capecitabine monotherapy can be selected as chemotherapy regimens, and 2−6 cycles of chemotherapy are recommended in the interval.
6.3.3 Sequence of postoperative adjuvant chemoradiotherapy and adjuvant chemotherapy
For patients who need additional pelvic radiotherapy after radical resection of stage II−III rectal cancer, the first concurrent chemoradiotherapy followed by adjuvant chemotherapy, or the first 1−2 cycles of adjuvant chemotherapy, concurrent chemoradiotherapy followed by adjuvant chemotherapy is recommended. For patients with negative resection margins and pN2, the prior adjuvant chemotherapy followed by concurrent chemoradiotherapy can also be considered.
6.4 Radiotherapy for metastatic CRC
Radiotherapy for metastasis CRC is recommended to be discussed by MDT, and finally the most reasonable treatment plan is determined according to the following aspects: 1) The size, number and location of metastasis; 2) The patient’s situation of receiving other treatments; 3) The functional state of the metastatic organ, such as the liver; 4) The primary benefit of radiotherapy for metastatic CRC is the reduction of local symptoms and the radical treatment of a small or isolated number of lesions.
7. Treatment for liver metastases
7.1 CRC with initially radical resectable liver metastases
7.1.1 Definition
Synchronous liver metastases are defined as liver metastases detected at or before diagnosis of the primary tumor. Metachronous liver metastases are considered to be detected after radical resection of the primary CRC.
7.1.2 Multidisciplinary therapy is recommanded to patients with liver metastases
(1) Neoadjuvant chemotherapy.
1) CRC is diagnosed with initially radical resectable liver metastases: Preoperative chemotherapy is recommended when there is no bleeding, obstruction or perforation in the primary lesion, and liver metastasis has high-risk factors of recurrence after resection. The chemotherapy regimens are referred to medical treatment section.
2) Radical resectable liver metastases after radical resection of CRC: Preoperative chemotherapy can be used for patients who have not received chemotherapy after primary tumor resection, or who have completed chemotherapy 12 months ago and have high-risk factors of liver metastases recurrence after resection. The chemotherapy regimens are referred to medical treatment section; patients who had received chemotherapy within 12 months before the detection of liver metastases can have direct resection for liver metastases.
(2) For patients who have no evidence of disease after liver metastases resection, it is recommended that whether to receive postoperative adjuvant chemotherapy after MDT discussion should be decided according to preoperative treatment and postoperative pathology.
7.1.3 Local treatment
(1) Indications of surgery for liver metastasis:
1) Primary CRC can be or has been removed radically.
2) The remnant liver still has adequate function after liver metastases resection.
3) The general condition of patients who have no extrahepatic metastasis or only pulmonary nodular lesions allow.
(2) Contraindications of surgery for liver metastasis:
1) Primary CRC cannot be removed radically.
2) There are unresectable extrahepatic metastatic lesions.
3) The residual liver volume after surgery is expected to be insufficient.
4) The general condition of patients does not tolerate surgery.
(3) Surgical treatment:
1) If synchronous liver metastasis is allowed to get a radical resection, synchronous resection of primary CRC and liver metastasis is recommended.
2) Synchronous liver metastasis which could not satisfy the condition of simultaneous resection of primary lesions and liver metastases after preoperative evaluation: Surgical resection of primary lesions was performed firstly, and the resection of metastatic lesions can be delayed within 3 months after the resection of primary lesions; simultaneous resection of primary tumors and liver metastases is not recommended for emergency surgery.
3) Liver metastasis occurred after radical resection of CRCs. Previously, primary lesions got a radical resection without recurrence of the primary tumor. For these patients whose liver metastases can be completely resected and the volume of hepatectomy is <70% (without cirrhosis), surgical resection of liver metastases should be performed.
4) If metastatic lesions which recurred after excision of liver metastases have reached the operating conditions, liver metastases can be excised twice, three times or even many times.
(4) Ablation treatment: Radiofrequency ablation is also one of the methods to eradicate liver metastases. However, the local recurrence rate is higher than other therapies. In general, radiofrequency ablation can be performed for metastatic lesions with a residual diameter of <3 cm, and up to 3 liver metastases can be ablated during a single treatment session. Combined radiofrequency ablation is also recommended for metastatic lesions with a residual diameter of <3 cm if the estimated residual liver volume is too small during the hepatectomy.
(5) Stereotactic body radiation therapy (SBRT): SBRT is one of the optional radical treatments for liver metastasis. It is a non-invasive, well-tolerated and effective treatment and can give high precision and high dose irradiation to the lesion. Indications of liver metastases for SBRT:
1) The number of liver metastases ≤3, and maximum diameter of metastatic lesions ≤5 cm;
2) The primary lesion was in control without extrahepatic metastases or just small extrahepatic metastases;
3) Expected survival ≥3 months;
4) The liver has not been treated with radiotherapy, the normal liver size >700 mL;
5) The patients were generally in good condition, with normal serum liver enzyme levels or lower than 200% of the upper limit of normal value, normal coagulation function, and Child-Pugh grading A or B.
It is recommended that for most liver metastases, especially those with diameter ≤3 cm, BED should be ≥100 Gy on the premise of safety. SBRT is not suitable for liver metastasis which is close proximity to vital organs such as small intestine, stomach, duodenum and kidney. SBRT in hospitals and units without image-guided technology or respiratory control technology is not recommended to carry out.
7.2 Potentially resectable liver metastases treatment
Treatment plans must be developed through MDT discussions. Re-evaluation is recommended to conduct after systemic chemotherapy ± targeted drugs or other treatments. If the lesions convert to resectable liver metastasis, it can be treated as resectable treatment plans. If the lesions are still not be resectable, the content of chemotherapy for recurrent/metastatic CRC can be referred in the section of 5. Medical treatment .
7.3 Unresectable liver metastases treatment
7.3.1 Treatment of primary lesions
(1) When there is no bleeding, obstruction or perforation in the primary CRC, systemic chemotherapy can be performed, or the primary lesions can be excised first, followed by further treatment. (Note: It is still controversial whether primary tumor resection is necessary in CRC patients with primary lesions without bleeding, obstruction, or perforation accompanied by persistent unresectable liver/lung metastasis.)
(2) When the primary lesions of CRC have bleeding, obstruction or perforation, it should be removed first and followed by systemic chemotherapy. After treatment, the patient should be evaluated to determine the next course of treatment every 6−8 weeks.
7.3.2 Radiofrequency ablation
Radiofrequency ablation is recommended to consider in the following situations: 1) Patients with resectable liver metastases who are generally inappropriate or unwilling to receive surgical treatment; 2) If the residual liver volume is too small after hepatectomy, large liver metastases can be resected first, and then remaining metastatic lesions with the diameter <3 cm can be treated with radiofrequency ablation.
7.3.3 Radiotherapy
For unresectable liver metastases, radiotherapy can be considered if systemic chemotherapy, hepatic arterial infusion chemotherapy, or radiofrequency ablation are ineffective.
8. Treatment for lung metastases
General guidelines of treatment: Because the number, site, size, primary tumor, extrapulmonary metastases, and genotype of lung metastases all affect prognosis and treatment decisions. Comprehensive therapy should be conducted under the discussion of MDT. Therapeutic approaches include systemic therapy, radical local therapy (e.g., R0 surgical resection, SBRT and ablation therapy), and local palliative treatment. MDT discussion should combine the patient’s clinical characteristics with accessibility to medical resources to determine treatment goals and thereby formulate a rational and orderly comprehensive treatment strategy.
8.1 Resectable lung metastases treatment
8.1.1 Neoadjuvant and adjuvant therapy
See 7. Treatment for liver metastases . However, there is a controversy about the need for chemotherapy after resection of lung metastases.
8.1.2 Local treatment
Imaging diagnosis can be used as the basis for surgery without the need for evidence of histopathology and percutaneous needle biopsy. When imaging findings indicate that metastatic lesions are atypical or other conditions require, the metastatic lesions should be confirmed by histopathology or close observation.
(1) Indications of surgery
1) The primary lesion must be removed radically (R0).
2) Resection of lung metastases is not recommended if there are unresectable extrapulmonary lesions.
3) Adequate function must be maintained after pneumonectomy.
4) Fractional resection may be considered in some patients.
5) There are resectable metastatic lesions outside the lung, which can be treated at the same time or different stages.
(2) Surgery timing
The timing of resection of lung metastases has not been determined.
1) Immediate surgery: It can stop resectable lesions from developing into unresectable lesions, or tumor spreading.
2) Delayed surgery: Since multiple lung metastases are common, the observation period of 3 months can be reserved for single micro nodule, which may avoid repetitive surgery.
3) For patients who can tolerate simultaneous excision of lung and liver metastases, simultaneous hepatectomy and pneumonectomy are feasible. For patients who cannot tolerate simultaneous resection, the liver is suggested to resect first and then the lung.
(3) Surgery methods
Wedge resection was the most common method, followed by segmental resection, lobectomy and pneumonectomy. Nanometer laser excision is suitable for patients with multiple sites or deep metastases.
Lung metastasis has a high recurrence rate. If the recurrent lesion can be resected, patients with appropriate conditions can have secondary or even multiple resections, which can effectively prolong patient’s survival time.
(4) Radiofrequency ablation
For lung metastases with small size (maximum diameter <3 cm) and far from the large vessels, radiofrequency ablation shows a good local control rate (about 90%).
(5) SBRT
Indications of SBRT for lung metastases:
1) The number of lung metastases are 1−3, the number of small lesions ≤5; The maximal diameter ≤5 cm.
2) It will be optimal if the distribution of lung metastases was relatively limited and on one side; peripheral lung metastases are more suitable for SBRT.
3) The primary lesion is in control, and there are no extrapulmonary metastases or extrapulmonary metastases are under control.
4) The patient is generally in good condition with normal lung function.
5) Life expectancy ≥6 months.
It is recommended that BED ≥100 Gy on the premise of safety. Different techniques are recommended to use to limit or track the mobility of lung metastases, and to verify the exact location of lung metastases through an image-guided system before each SBRT. SBRT is not recommended in hospitals and units without image-guided or respiratory control techniques.
8.2 Unresectable lung metastases treatment
Refer to the 7. Treatment for liver metastases .
9. Treatment of peritoneal metastases
Synchronous peritoneal metastases are defined as peritoneal metastases detected at diagnosis of the primary tumor. Metachronous peritoneal metastases are considered to be peritoneal metastases detected after radical resection of the primary CRC.
In general, peritoneal metastases have a poor prognosis and are mainly treated with systemic therapy (specific drug options refers to 5.3 Systemic treatment of recurrent/metastatic CRC ). According to the patient’s tumor load, ascites fluid condition, and physical score, the following local treatments can be considered in experienced tumor center under the guidance of MDT: 1) cytoreductive surgery (CRS): total resection of the peritoneum (complete resection of anterior peritoneum, left and right lateral peritoneum, pelvic peritoneum, diaphragmatic peritoneal; resection of ligamentum teres hepatis, sickle ligaments, greater omentum and lesser omentum; and removal and burning of intestinal surface, mesenteric, and the tumor of visceral peritoneum), multi-visceral resection (stomach, small intestine, colorectal, part of the pancreas, spleen, gallbladder, liver, uterus, ovaries, kidney, ureter, etc.), etc. 2) hyperthermia intraperitoneal chemotherapy: open or closed intraperitoneal hyperthermia chemotherapy with or without CRS is recommended.
10. Treatment of local recurrence in rectal cancer
10.1 Classification
At present, the following methods are recommended for the classification of local recurrence: according to the anatomical site in pelvis, it can be divided into central type (including anastomotic stoma, mesorectum, soft tissue around the rectum, perineum after abdominoperineal resection), anterior type (infiltration into the urogenital system including the bladder, vagina, uterus, seminal vesicle, and prostate), posterior type (invasion into the sacrum, anterior sacral fascia), and lateral type (infiltration into the soft tissue of pelvic wall or bony pelvis).
10.2 Treatment principles
Multidisciplinary assessment based on condition of patients and lesions: comprehensive treatment of primary surgery in combination with perioperative chemoradiotherapy is recommended to patients who are initially resectable; chemoradiotherapy and/or systemic treatment is recommended to patients who are initially unresectable, and the surgical resectability should be assessed after treatment.
10.3 Surgical treatment
(1) Assessment of resectability
Preoperative assessment for the possibility of radical resection of recurrent lesions is neccessary. Whether preoperative chemoradiotherapy can be used depends on the extension of recurrance. The resectability of lesions should be verified according to the results of surgical exploration, and fast frozen pathology should be used if necessary. Unresectable local relapsed focus includes:
1) Extensive pelvic lateral wall invasion;
2) External iliac blood vessels involvement;
3) Greater sciatic notch and sciatic nerve invasion;
4) The second sacrum level and above invasion.
(2) Surgical principle
1) It is recommended that a specialist in colorectal surgery should choose proper surgical plan in combination with preoperative chemoradiotherapy, intraoperative radiotherapy, and adjuvant radiotherapy and chemotherapy according to the specific conditions of patients and lesions.
2) If necessary, surgical plans are recommended to formulate with physicians in urology, orthopedics, vascular surgery, obstetrics and gynecology.
3) Surgical exploration must be from far to near and pay attention to exclude distant metastases.
4) Must follow the principle of en bloc resection and try to achieve R0 resection.
5) Pay attention to protection of the ureter (discretionarily place the ureteral stent before surgery) and the urethra.
(3) Surgical methods of resectable lesions
The surgical methods include LAR, APR, Hartmann surgery and pelvic cavity dissection, etc.
1) Central type: APR is recommended to guarantee R0 resection; LAR can be considered in patients who have received anus-preserving operation if the lesion is limited. Transperineal or transsacral resection can be considered if the recurrent vulvar lesion is limited after APR.
2) Anterior type: If patients can tolerate surgery, the resection of invasive organs should be considered, and then posterior half-pelvic dissection or total pelvic organectomy may be performed.
3) Lateral type: Involved ureter, internal iliac blood vessels and piriformis should be resected.
4) Posterior type: Invaded sacrum was resected by combined abdominosacral resection. The incision of the perineum can be covered with omentum or primary suture. Use myocutaneous flaps or biomaterial patches if necessary.
(4) Principle of radiotherapy
Preoperative concurrent chemoradiotherapy (try to obtain the pathology of the recurrent lesion before radiotherapy) is recommended to the patients who have never received pelvic cavity radiotherapy, then the surgery can be considered. For the patients who have local resectable lesions, surgery should be considered first, and then whether postoperative radiotherapy/chemotherapy will be considered. Patients who have received pelvic cavity radiotherapy in principle no longer receive radiotherapy. It is recommended that the most reasonable treatment plan should be formulated through MDT discussion.
(5) Principles of medical treatment
Perioperative medical treatment plan depends on previous chemotherapy history for initial resectable patients. For unresectable recurrent patients, radiotherapy and/or systemic treatment should be decided under MDT discussion. After treatment, the resectability should be reevaluated through MDT discussion.
11. Rehabilitation treatment of colostomy
11.1 Personnel, tasks and architecture
Qualified hospitals are recommended to be equipped with enterostmal therapists (specialist nurses). The duties of an enterostmal therapist include preoperative and postoperative care of all ostomies (enterostomy, gastrostomy, urostomy, tracheostomy, etc), treatment of complex wounds, care of incontinence, setting up ostomy specialist outpatient clinic, liaison with patients and other professionals and ostomy merchants, organizing ostomy fraternities and conducting ostomy visitor activities.
11.2 Preoperative psychotherapy
The relevant diagnosis, surgery and nursing knowledge are recommended to fully explain to patients, so that patients can accept the facts of the disease and have a comprehensive understanding of what is about to happen.
11.3 Preoperative ostomy location
It is recommended that the site of ostomy should be selected by physicians, enterostmal therapists, family members and patients before operation.
(1) Requirements: Patients can see by themselves, convenient for nursing, have enough paste area, and there is no discomfort when the ostomy equipment is affixed to the ostomy skin.
(2)The common locations of enterostomy are shown in Figure 5 .
5.
Common locations of enterostomy.
11.4 Postoperative nursing of enterostomy
(1) After operation, we should pay attention to observe the blood supply of ostomy and whether there is retraction or not.
(2) The standard for the selection of ostomy products should be light, transparent, odor-proof, leak-proof and protect the surrounding skin, and suitable for patients to wear.
(3) Keep the skin around the enterostomy clean and dry. Patients who take antibiotics, immunosuppressants and hormones for a long time should pay special attention to fungal infection at the enterostomy site.
12. Follow-up
Regular follow-up is recommended after the treatment of CRC.
(1) Medical history, physical examination and CEA, CA19-9 surveillance, once every 3 months for 2 years, then once every 6 months for a total of 5 years, once a year after 5 years.
(2) Chest/abdominal/pelvic CT or MRI once every half a year for 2 years, and then once a year for 5 years.
(3) Enteroscopy within 1 year after operation, and if there is any abnormality, it should be reexamined within 1 year. If there is no polyp, it should be reexamined within 3 years, and then once every 5 years, resection of colorectal adenoma is recommended in follow-up examination. If the total colon examination is not completed by colonoscopy before operation, it is recommended that enteroscopy should be performed 3−6 months after operation.
(4) PET-CT is not a routine recommended examination. For patients with existing or suspected recurrence and distant metastasis, PET-CT examination can be considered to exclude recurrence and metastasis of CRC.
Working group members
Principal consultant: Yan Sun
Consultant: Shu Zheng, Desen Wan
General group leader: Jin Gu, Jianping Wang
Surgery Group
Group leader: Jin Gu, Jianping Wang, Suzhan Zhang, Sanjun Cai
Task group members (listed alphabetically by last name):
Xuedong Fang, Chuangang Fu, Baoqing Jia, Dalu Kong, Ping Lan, Zhizhong Pan, Haiping Pei, Huizhong Qiu, Chun Song, Guiying Wang, Xishan Wang, Ziqiang Wang, Jianmin Xu, Zhongfa Xu, Jin Yan, Yingjiang Ye, Yueming Yu, Zhongtao Zhang, Ren Zhao
Secretary (listed alphabetically by last name):
Lei Lian, Qian Liu, Yifan Peng
Medicine Group
Group leader: Lin Shen, Ruihua Xu, Jin Li
Task group members (listed alphabetically by last name):
Yi Ba, Chunmei Bai, Li Bai, Yanhong Deng, Jian Li, Tianshu Liu, Yunpeng Liu, Hongming Pan, Min Tao, Jianming Xu, Xianglin Yuan, Ying Yuan, Yanqiao Zhang, Aiping Zhou
Secretary (listed alphabetically by last name):
Feng Wang, Xicheng Wang
Radiotherapy Group
Group leader: Yexiong Li, Zhen Zhang
Task group members (listed alphabetically by last name):
Yong Cai, Yuanhong Gao, Jing Jin, Yongheng Li, Shixin Liu, Renben Wang, Junxin Wu, Hongyan Zhang, Li Zhu, Yuan Zhu
Secretary:
Yuan Tang
Pathology Group
Group leader: Zhiyong Liang
Task group members (listed alphabetically by last name):
Mulan Jin, Li Liang, Zhiqiang Qiu, Weiqi Sheng, Baocun Sun, Weicheng Xue
Secretary:
Weixun Zhou
Imaging Group
Group leader: Yingshi Sun
Task group members (listed alphabetically by last name):
Jiangning Dong, Yi Wang, Tao Yu, Xiaoyan Zhang, Zhiyang Zhou
Secretary (listed alphabetically by last name):
Ruijia Sun, Juan Wang
Secretary Group
Group leader: Yifan Peng, Xicheng Wang
Task group member (listed alphabetically by last name):
Lei Lian, Qian Liu, Ruijia Sun, Yuan Tang, Feng Wang, Juan Wang, Weixun Zhou
Translation Group
Group leader:Jin Gu, Yong Yang
Task group member (listed alphabetically by last name):
Jiajia Chen, Yongkang Chen, Yu Cheng, Zihan Han, An Huang, Dandan Huang, Xin Li, Yukun Li, Jingyi Shi, Mengyuan Shi, Can Song, Hanyang Wang, Jingxuan Xu