Abstract
INTRODUCTION:
Canine visceral leishmaniasis (CVL) is an endemic disease in Brazil, and integrated control actions have been adopted by the Brazilian Ministry of Health to control its spread. However, the transmission profile is unknown in areas with recent CVL cases, including Itaúna, located in the Brazilian state of Minas Gerais, where the present study was carried out.
METHODS:
A total of 2,302 dogs from 12 neighborhoods were serologically tested for canine VL using the current diagnostic protocol adopted by the Brazilian Ministry of Health. Test positivity rate (TPR) and CVL prevalence were determined for each neighborhood. The presence of Leishmania was assessed in 60 seropositive dogs which had been recommended for euthanasia. Twenty-two of them (37%) were asymptomatic, and 38 (63%) were symptomatic for CVL. Parasitological (myeloculture and smear/imprint) and molecular (PCR) methods were employed for Leishmania detection in bone marrow, spleen, mesenteric lymph nodes, and ear skin. The infecting Leishmania species was identified by DNA sequencing.
RESULTS:
CVL prevalence (per 1,000 dogs) varied from 0.0-166.67, depending on the neighborhood, with a mean of 68.96 (SD 51.38). Leishmania DNA was detected in at least one tissue from all seropositive dogs, with comparable TPR among tissues. Leishmania parasites were identified in most (54/60) seropositive dogs, and the infecting parasite was identified as Leishmania infantum in all of these.
CONCLUSIONS:
Prevalence of CVL is a contributor to the spread of visceral leishmaniasis in Itaúna.
Keywords: Leishmaniasis, Visceral leishmaniasis, Leishmania, Canine leishmaniasis
INTRODUCTION
Visceral leishmaniasis (VL) is a neglected tropical zoonotic disease that is potentially fatal to humans and poses major public health concerns in developing countries. Brazil accounts for approximately 96% of VL cases reported in the Americas 1 . VL is caused by infection with L. infantum, and is transmitted through the bite of infected female phlebotomine sand flies; in Brazil, the main vector of L. infantum is Lutzomyia longipalpis (Diptera: Psychodidae: Phlebotominae). In urban areas, dogs are the most important domestic reservoirs of these parasites, and Leishmania amastigotes frequently present in the skin of infected dogs are a source of infection by L. longipalpis 2 - 5 . Canine VL (CVL) has been reported in a number of epidemiological studies in urban areas with active VL transmission. Since CVL frequently precedes the onset of human cases, dogs might be considered sentinel signaling markers of VL 6 - 9 .
In an attempt to control the increasing geographic expansion and urbanization of VL in Brazil, the Ministry of Health adopted the Surveillance and Control Program of Visceral Leishmaniasis (SCPVL) 10 . The program comprises integrated strategies intended to aid the early diagnosis and treatment of human cases, chemical and environmental control of phlebotomine sand fly vectors, health education, and screening and culling of infected dogs. Based on the average number of human cases (n) reported to the National System of Diseases with Compulsory Notification (SINAN) in the last three years, an epidemiological transmission risk (ETR) score is assigned to different geographical regions, municipalities, or even neighborhoods, as follows: high ETR for n ≥ 4, moderate ETR for 2.4 ≤ n < 4, and sporadic ETR for n < 2.4. SCPVL actions are intensified in areas with high and medium ETRs.
Concerning the screening and culling strategy, every dog presenting positive results by immunochromatography (TR-DPP) testing and enzyme-linked immunosorbent assays (ELISAs) the serial tests currently adopted for CVL diagnosis in Brazil, is considered Leishmania-infected and recommended for euthanasia 10 .
Our study was performed in a Brazilian municipality (Itáuna, Minas Gerais) with recent VL transmission and sporadic ETRs, where systematic control measures have not yet been implemented. In the last few years, however, public health officials from the local Zoonoses Control Center (ZCC) noticed an increase in the number of CVL-positive dogs submitted for serological testing by owners concerned about their dog’s health, termed ‘spontaneous owner’s demand’ (SOD). In a previous study we evaluated parasite-vector relationships through a phlebotomine sand fly survey in Itaúna 11 , due to the lack of local epidemiological data regarding the parasite-vector-reservoir triad involved in the VL transmission cycle. In the present study, we focused on the domestic reservoir, aiming to: a) perform a canine census survey in selected neighborhoods of Itaúna; b) determine the prevalence of CVL in selected neighborhoods using current serological diagnostic tests; c) confirm the presence of Leishmania in seropositive dogs using parasitological and molecular-based methods; and d) identify the infecting Leishmania species in seropositive dogs.
METHODS
Study area
The study was conducted in Itaúna (20° 4′ 26″ S, 44° 34′ 24″ W), in the Brazilian state of Minas Gerais, which is an important city in terms of steel and mining activities. Itaúna is located in the center-west region of Minas Gerais, 80 km from the state capital Belo Horizonte. The city occupies an area of 495,769 km2 and is divided into 52 neighborhoods. The local population is 92,561 inhabitants in total, according to the latest estimate by the Brazilian Institute of Geography and Statistics 12 .
Canine samples
Our sample comprised 2,302 dogs, of which 1,690 were from a canine census survey (CCS) performed in 2016 in neighborhoods with previous reports of human and/or canine cases of VL. The canine survey was performed by trained health agents of the local Zoonoses Control Center (ZCC), and included the following neighborhoods: Centro, Chácara do Quitão, Cidade Leonane, Cidade Nova, Garcias, Graças, Morada Nova, Nogueirinha, Novo Horizonte, Olaria, Parque Jardim Santanense, Três Marias, and Vila Nazaré. The remaining dogs (612 animals) were from SOD submissions, in the same year and from the same neighborhoods.
CVL diagnosis
All dogs were screened for CVL using the Rapid Dual Path Platform, which detects anti-Leishmania antibodies for the Leishmania donovani complex via immunochromatography (TR-DPP® LVC, Bio-Manguinhos, Rio de Janeiro, Brazil). The test was performed at the canine owner’s homes after collecting a drop of blood from the ear tip. In cases producing positive results, a new blood sample (3 mL) was collected by puncturing the venal or cephalic vein for examination in a laboratory. The blood serum was separated via centrifugation at 3000 × g for 10 min and tested by ELISAs, which quantify anti-Leishmania antibodies. In this test, purified proteins from in vitro cultures of a Leishmania major-like strain (MHOM/BR/71/BH121) were used as antigens (EIE® LVC).
Diagnostic CVL assays were performed by a certified public health institution (Fundação Ezequiel Dias) in accordance with the SCPVL protocol. Dogs with positive immunochromatography and ELISA results were diagnosed as seropositive for CVL and recommended for euthanasia, according to the current policy of the Brazilian Ministry of Health. The owner’s domiciles were georeferenced using a Garmin eTrex hand-held global positioning unit.
Subsamples of CVL seropositive dogs
CVL-positive dogs (n = 60) were randomly selected for clinical examination by veterinary physicians. A standard form was completed with the dog’s history, including previous vaccinations against rabies and/or VL. Dogs displaying at least one clinical sign attributable to Leishmania infection were considered symptomatic for CVL. The clinical signs observed were as follows: lymphadenopathy, corneal opacification, cushion hyperkeratinization, weight loss, ascites, cutaneous alterations (alopecia, furfuraceous eczema, ulcers, snout hyperkeratinization, ear-tip dermatitis), onychogryphosis, keratoconjunctivitis, and hind limb paresis. The affected group was comprised of so-called sick dogs, that is, dogs that presented clinical signs and/or clinicopath abnormalities with confirmed infection 13 . In the absence of any such signs, dogs were diagnosed as asymptomatic for CVL, which corresponded to clinically healthy but infected dogs 13 . Diagnosis was performed as previously described.
Tissue and bone marrow collection from CVL-seropositive dogs
Dogs were sedated with xylazine (1.1-2.2 mg/kg body weight, given intramuscularly) and anesthetized with thionembutal (adjusted according to animal body weight), before bone marrow aspiration via sterile puncture of the tibial crest. Subsequently, the animals were euthanized using intravenous injection of 0.5 mg/kg of 20% potassium chloride. Biopsy samples were collected from the spleen, mesenteric lymph nodes, and ear skin, and used for preparation of smears or imprints, and for DNA extraction. A total of 240 samples (4 tissue samples per dog, in 60 dogs) were analyzed.
Investigation of Leishmania parasites
Bone marrow aspirates were seeded into tubes containing NNN (Novy-MacNeal/Nicolle) media enriched with LIT (liver infusion tryptose) and maintained at 25 ± 1°C in an incubator, in duplicate. The myelocultures were examined weekly for the presence of Leishmania promastigotes over six weeks (as an indirect parasitological test). The isolates from positive samples were cryopreserved and stored in the strain bank of the Laboratory of Leishmaniases of Instituto René Rachou/Fiocruz Minas. Negative cultures were discarded. Bone marrow aspirates were also used in the preparation of slide smears. Imprints of mesenteric lymph nodes, skin, and spleen were prepared by slide apposition of the respective tissue fragments. After Giemsa staining, slide smear/imprints were examined for the presence of Leishmania amastigotes (as a direct parasitological test).
Investigation of Leishmania DNA
Total DNA was extracted from skin, lymph nodes, and spleen fragments of CVL-seropositive dogs using a Genomic Prep™ Cell and Tissue DNA isolation kit (GE Healthcare, Uppsala, Sweden). GFXTM Genomic Blood DNA Purification (GE Healthcare) was used for DNA extraction from bone marrow aspirates. The procedures were performed according to the manufacturer's instructions. The quality and the mammalian origin of the purified DNA was assessed by PCR amplification of the interphotoreceptor retinoid-binding protein (IRBP) gene primed with IRBPfwd (5′-TCCAACACCACCACTGAGATCTGGAC-3′) and IRBPrev (5′-GTGAGGAAGAAATCGGACTGGCC-3′) oligonucleotides 14 . Negative (no DNA) and positive (DNA extracted from dogs not infected by Leishmania and living in non-endemic area) control samples were run in parallel. A 227 bp amplified fragment proved the mammal origin of the samples. After DNA assessment, the presence of Leishmania DNA was tested by Leishmania nested PCR (LnPCR) targeting the small subunit ribosomal RNA (SSUrRNA) gene 15 , 16 . In the first amplification step, 10-20 ng of extracted DNA was amplified in the presence of kinetoplastid-specific oligonucleotides using R221 (5′-GGTTCCTTTCCTGATTTACG-3′) and R332 (5′-GGCCGGTAAAGGCCGAATAG-3′) primers. In Leishmania-positive samples, a conserved 603 bp fragment was amplified. The PCR products were then diluted 1:40 in sterile water and used as a template in the second amplification step with Leishmania-specific primers R223 (5′-TCCCATCGCAACCTCGGTT-3′) and R333 (5′-AAAGCGGGCGCGGTGCTG-3′). Positive samples generated a 358 bp fragment. LnPCR cycling conditions were as previously described 15 , 17 . The amplified products were visualized under UV light after electrophoresis on 2% agarose gels and ethidium bromide staining. Negative (no DNA) and positive (20 ng of DNA extracted from Leishmania chagasi - MHOM/BR74/PP75) control samples were used as controls. In all PCR amplifications, we used PureTaq Ready-To-Go PCR Beads (GE Healthcare) in a Veriti 96 well Thermo Cycler (Applied Biosystems, Foster City, USA).
Identification of infecting Leishmania species
LnPCR-amplified products were excised and purified from gels using a QIAquick® PCR Purification Kit (Qiagen, Fort Frederick, USA). DNA sequencing was performed using the BigDye® Terminator v3.1 Cycle kit and an ABI 3730 automated DNA sequencing platform (Applied Biosytems) of Instituto René Rachou. Sequencing conditions are described elsewhere 11 . The consensus nucleotide sequence for each sample was aligned and compared to L. braziliensis (M80292.1), L. amazonensis (M80293.1), and L. infantum (M81430.1); the sequences were deposited in the GenBank® database. BioEdit (www.mbio.ncsu.edu/bioedit.bioedit.html), BLAST (www.ncbi.nlm.nih.gov/BLAST), and MacVector® (www.macvector.com, MacVector Inc.) tools were employed for multiple sequence alignments.
Statistical and spatial analyses
TPRs for CVL were calculated as follows:
Whenever a significant difference was suggested in the TPR data by multiple data comparisons, further analysis was performed in pairs using McNemar’s test with Bonferroni correction. When applicable, result proportions were compared using Fisher’s exact test, with a 95% confidence level (α = 0.05).
CVL prevalence per neighborhood was determined as follows:
The canine population per neighborhood was estimated to be 13.5% of the human population, based on the protocol used in canine anti-rabies vaccination campaigns in the state of Minas Gerais 18 . Human population data are available on the website of the Brazilian Institute of Geography and Statistics 12 .
Numbers of human VL cases since the first report in 2007 were kindly provided by the Municipal Health Department of Itaúna with respective georeferenced coordinates. L. longipalpis and Leishmania-infected L. longipalpis data were also represented to provide a panorama of VL concerning parasites, vectors, and domestic reservoirs 11 . The R software package was used for map locations 19 .
Ethical statements
This study was approved by the Committee on Ethics in Animal Experimentation of the Oswaldo Cruz Foundation (CEUA/Fiocruz) under license no. LW-2/15 (protocol P-68/14-3). The procedures complied with the technical norms established by the Federal Council of Veterinary Medicine (CFMV, Resolution No. 714 of June 20, 2002).
RESULTS
General canine samples
A total of 2,302 dogs were serologically tested for CVL, of which 1,690 were included via CCS and 612 via SOD (Table 1). A total of 358 dogs (21.2%) from the first sample source were positive in the screening test (DPP). Among these animals, CVL diagnosis was confirmed in 203 (56.7%) using ELISA. TPR for CVL was 12.0%, with a mean of 13.2% (SD 7.5%). In the SOD samples, TPR increased to 49.7% (304 of 612 tested using DPP). CVL diagnosis was confirmed in 203 dogs (66.8%) by ELISA. TPRs in the second group were higher (36.5%) than in the first, with a mean of 35.7% (SD 13.8%). Considering both sample sources (CCS plus SOD), TPRs for CVL varied from 10.5-48.0%, depending on the neighborhood, with a mean of 21.3% (SD 11.6%). CVL prevalence varied from 0.0-166.67, according to the neighborhood, with a mean of 68.96 (SD 51.38). SOD dogs were not included in the calculation of CVL prevalence to avoid biasing the results.
TABLE 1: Diagnostic test results and calculated prevalence of canine visceral leishmaniasis by Itaúna city neighborhood in the Brazilian state of Minas Gerais, 2016. CCS: canine census survey, SOD: spontaneous owner’s demand. TPR: test positivity rate.
| Neighborhood | CCS | SOD | Total (CCS plus SOD) | CVL prevalencea | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dogs | Diagnosis test results | TPR | Dogs | Diagnosis test results | TPR | Dogs | Diagnosis test results | TPR | |||||
| tested (no.) | DPP+ | DPP+ ELISA+ | (%) | tested (no.) | DPP+ | DPP+ELISA + | (%) | tested (no.) | DPP + | DPP+ ELISA+ | (%) | ||
| Centro | 254 | 60 | 21 | 8.3 | 61 | 18 | 16 | 26.2 | 315 | 78 | 37 | 11.7 | 21.4 |
| Chácara do Quitão | 44 | 10 | 8 | 18.2 | 79 | 26 | 19 | 24.1 | 123 | 36 | 27 | 22.0 | 100.0 |
| Cidade Nova | 343 | 64 | 39 | 11.4 | 92 | 52 | 26 | 28.3 | 435 | 116 | 65 | 14.9 | 116.8 |
| Cidade Leonane | 186 | 34 | 19 | 10.2 | 27 | 11 | 11 | 40.7 | 213 | 45 | 30 | 14.1 | 97.4 |
| Garcias | 71 | 6 | 5 | 7 | 32 | 28 | 19 | 59.4 | 103 | 34 | 24 | 23.3 | 13.9 |
| Graças | 166 | 43 | 23 | 13.9 | 25 | 14 | 10 | 40.0 | 191 | 57 | 33 | 17.3 | 62.7 |
| Morada Nova | 15 | 0 | 0 | 0 | 83 | 60 | 47 | 56.6 | 98 | 60 | 47 | 48.0 | 0.0 |
| Nogueirinha | 90 | 17 | 15 | 16.7 | 4 | 2 | 1 | 25.0 | 94 | 19 | 16 | 17.0 | 166.7 |
| Novo Horizonte | 143 | 45 | 15 | 10.5 | 0 | 0 | 0 | - | 143 | 45 | 15 | 10.5 | 115.4 |
| Olaria | 42 | 9 | 6 | 14.3 | 45 | 27 | 11 | 24.4 | 87 | 36 | 17 | 19.5 | 17.1 |
| P. J. Santanense | 163 | 22 | 16 | 9.8 | 116 | 37 | 22 | 19.0 | 279 | 59 | 38 | 13.6 | 29.3 |
| Três Marias | 154 | 39 | 30 | 19.5 | 21 | 8 | 7 | 33.3 | 175 | 47 | 37 | 21.1 | 104.5 |
| Vila Nazaré | 19 | 9 | 6 | 31.6 | 27 | 21 | 14 | 51.9 | 46 | 30 | 20 | 43.5 | 51.3 |
| Total | 1690 | 358 | 203 | 12.0 | 612 | 304 | 203 | 33.2 | 2302 | 662 | 406 | 17.6 | - |
| Mean ± SD | - | - | - | 13.2±7.5 | - | - | - | 35.7 ±13.8 | - | - | 21.3±11.6 | 69.0±51.4 | |
Calculated based on CCS data only.
Subsample of CVL seropositive dogs
Most seropositive dogs (n = 38, 63.4%) exhibited symptoms of the disease, according to the parameters used for clinical evaluation. The main phenotypic characteristics of the canine subsample are listed in Table 2.
TABLE 2: General characteristics of the subsample of CVL-seropositive dogs (n = 60) in an area of recent transmission of visceral leishmaniases. Itaúna, Minas Gerais state, Brazil, 2016.
| Variable | Dogs | |
|---|---|---|
| No. | % | |
| Clinical classification | ||
| Asymptomatic | 22 | 36.6 |
| Symptomatic | 38 | 63.4 |
| Gender | ||
| Female | 34 | 56.6 |
| Male | 26 | 43.4 |
| Breed | ||
| Defined 1 | 16 | 26.6 |
| Undefined | 44 | 73.4 |
| Hair length | ||
| Short | 44 | 73.4 |
| Long | 16 | 26.6 |
| Size | ||
| Small | 21 | 35.0 |
| Medium | 28 | 46.6 |
| Big | 11 | 18.4 |
Foxhound, Basset Hound, Blue Heeler, Cocker, Pinscher, Poodle, Pit Bull, Fila, Labrador and German Shepherd.
Splenomegaly and lymphadenopathy were observed in 100% of the seropositive dogs. In the symptomatic group, other suggestive clinical signs of CVL included muzzle hyperkeratinization (40%), weight loss (38%), onychogriphosis (37%), keratoconjunctivitis (30%), ear dermatitis (27%), generalized dermatitis (18%), hipped mucosae (5%), decubitus ulcer (3%), localized alopecia (3%), and ascites (2%). (data not shown).
Detection of Leishmania in subsampled VL-seropositive dogs
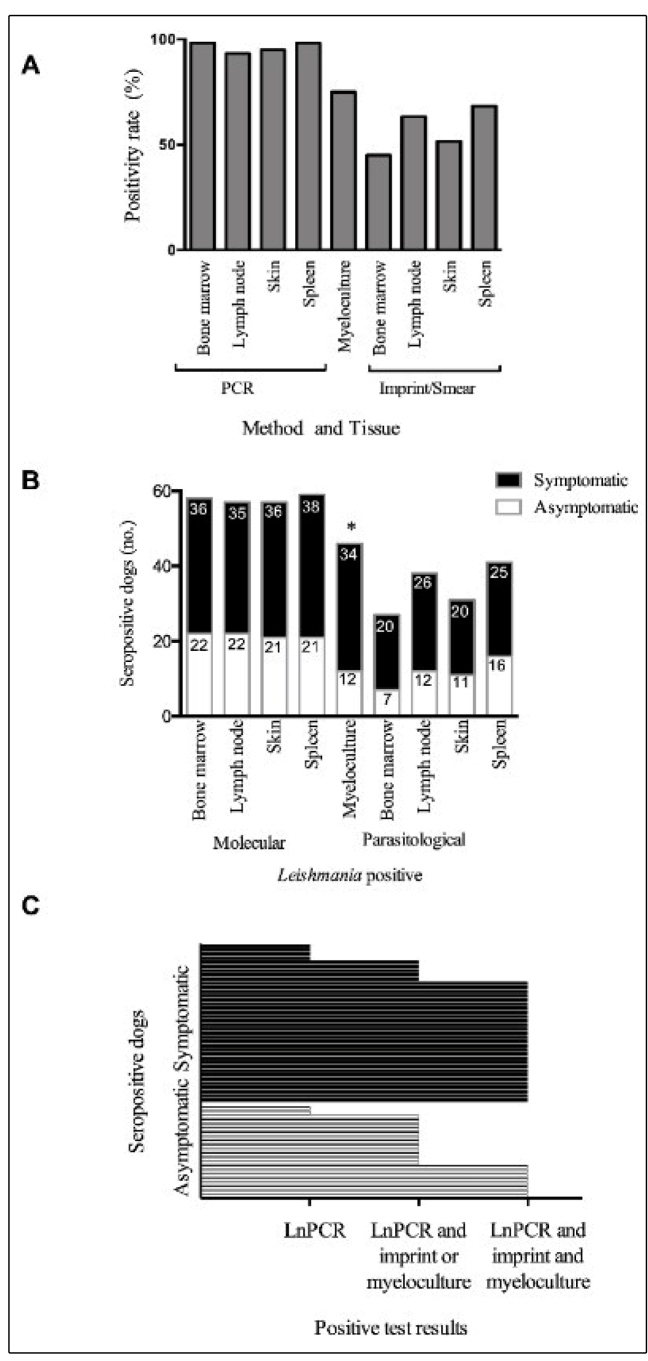
The results of molecular and parasitological tests for Leishmania in the subsample of dogs seropositive for CVL are presented in Table 3. Leishmania DNA was detected in at least one tissue from every dog, including six dogs with negative parasitological results (Table 3). The proportion of positive results for Leishmania DNA was comparable for any tissue used for LnPCR (p > 0.05) (Figure 1A). With regard to the presence of Leishmania DNA, no statistically significant difference was found between symptomatic and asymptomatic dogs (Figure 1B).
TABLE 3: Parasitological and molecular test results for Leishmania in CVL-seropositive dogs. Itaúna, Minas Gerais state, Brazil, 2016.
| Clinical status | Dog no. | Parasitological | Molecular | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slide imprint/smear | Bone | Lymph | Spleen | Ski n | Any | |||||||
| Myeloculture | Bone marrow | Lymph node | Spleen | Skin | Any tissue | marrow | node | tissue | ||||
| Asymp | 4 | - | - | + | + | - | + | + | + | + | + | + |
| 8 | - | - | + | + | + | + | + | + | + | + | + | |
| 9 | + | + | + | + | + | + | + | + | + | + | + | |
| 10 | + | - | - | + | + | + | + | + | + | + | + | |
| 11 | - | + | - | + | - | + | + | + | + | + | + | |
| 12 | + | + | + | + | + | + | + | + | + | + | + | |
| 15 | + | - | - | - | - | - | + | + | + | + | + | |
| 19 | + | - | - | - | - | - | + | + | + | + | + | |
| 22 | - | - | + | + | + | + | + | + | + | + | + | |
| 23 | - | - | - | - | - | - | + | + | + | + | + | |
| 27 | - | - | - | + | - | + | + | + | + | + | + | |
| 28 | + | - | + | + | + | + | + | + | + | + | + | |
| 32 | - | + | + | + | + | + | + | + | + | + | + | |
| 34 | + | + | + | + | + | + | + | + | + | + | + | |
| 48 | - | - | + | + | - | + | + | + | + | + | + | |
| 49 | - | - | + | + | + | + | + | + | + | - | + | |
| 51 | + | - | - | - | - | - | + | + | + | + | + | |
| 52 | + | + | + | + | + | + | + | + | + | + | + | |
| 53 | + | - | - | + | - | + | + | + | + | + | + | |
| 55 | + | - | - | - | - | - | + | + | + | + | + | |
| 57 | - | - | - | - | - | - | + | + | - | + | + | |
| 60 | + | + | + | + | + | + | + | + | + | + | + | |
| Symp | 1 | + | - | - | - | - | - | + | + | + | + | + |
| 2 | + | - | - | - | - | - | + | + | + | + | + | |
| 3 | + | - | + | - | + | + | + | + | + | + | + | |
| 5 | + | - | - | - | + | + | + | + | + | + | + | |
| 6 | + | + | + | + | + | + | + | + | + | + | + | |
| 7 | - | + | + | + | + | + | + | + | + | + | + | |
| 13 | + | - | - | - | - | - | + | + | + | + | + | |
| 14 | + | - | - | - | - | - | + | + | + | + | + | |
| 16 | + | + | + | + | + | + | + | + | + | + | + | |
| 17 | + | + | + | - | + | + | + | - | + | + | + | |
| 18 | + | + | + | + | - | + | + | + | + | + | + | |
| 20 | + | - | - | - | - | - | + | + | + | + | + | |
| 21 | + | + | + | + | + | + | + | + | + | - | + | |
| 24 | - | - | - | - | - | - | + | + | + | - | + | |
| 25 | + | + | + | + | - | + | n.d. | + | + | + | + | |
| 26 | + | - | + | + | + | + | + | + | + | + | + | |
| 29 | + | + | + | + | + | + | + | + | + | + | + | |
| 30 | + | + | + | + | + | + | + | + | + | + | + | |
| 31 | + | - | - | + | - | + | + | + | + | + | + | |
| 33 | + | + | + | + | - | + | + | + | + | + | + | |
| 35 | + | + | + | + | - | + | + | + | + | + | + | |
| 36 | + | + | + | - | + | + | + | + | + | + | + | |
| 37 | + | - | + | + | + | + | + | + | + | + | + | |
| 38 | + | + | + | + | + | + | + | + | + | + | + | |
| 39 | + | + | + | + | + | + | + | + | + | + | + | |
| 40 | + | + | + | + | + | + | + | + | + | + | + | |
| 41 | + | + | + | + | + | + | + | + | + | + | + | |
| 42 | - | - | - | - | - | - | + | + | + | + | + | |
| 43 | + | + | + | + | + | + | + | - | + | + | + | |
| 44 | + | + | + | + | + | + | + | + | + | + | + | |
| 45 | + | - | + | + | - | + | + | + | + | + | + | |
| 46 | + | - | - | + | - | + | + | + | + | + | + | |
| 47 | - | - | - | - | - | - | - | - | + | + | + | |
| 50 | + | + | + | + | + | + | + | + | + | + | + | |
| 54 | + | + | + | + | + | + | + | + | + | + | + | |
| 56 | - | - | - | - | - | - | + | + | + | + | + | |
| 58 | + | - | + | + | - | + | + | + | + | + | + | |
| 59 | + | - | + | + | - | + | + | - | + | + | + | |
| Positive/total | 45/60 | 27/60 | 38/60 | 41/60 | 31/60 | 45/60 | 58/59 | 56/60 | 59/60 | 57/60 | 60/60 | |
FIGURE 1: Leishmania detection in dogs seropositive for canine visceral leishmaniasis (CVL). (A) Positivity rates of parasitological and molecular tests. (B) Test positivity according to the clinical group. Statistically significant differences between the two groups were observed only for myelocultures (indicated by *) (p < 0.05). (C) Frequency of positive tests according to clinical group. Each horizontal bar represents one dog (n = 60). Itaúna, Minas Gerais state, Brazil, 2016.
Leishmania promastigotes or amastigotes were identified in every dog except for #23 (asymptomatic) and #24, 42, 47, 56, and #57 (symptomatic) (Table 3). Promastigote forms were detected in 45 of 60 (75%) myelocultures (Figure 1A). Leishmania amastigotes were present in 75% of the slide imprints/tissue smears (Figure 1A). Significantly higher TPRs for Leishmania amastigotes were obtained for spleen (68.3%) and lymph nodes (63.3%) than for bone marrow (45.0%) or skin (51.7%) samples (adjusted p-values < 0.05) (Figure 1A). TPRs for Leishmania amastigotes were significantly higher in the symptomatic group (86.6%) than in the asymptomatic group (54.4%) (p < 0.05) (Figure 1B).
In general, 60% (36/60) of seropositive dogs with CVL had Leishmania infection confirmed molecularly (LnPCR for Leishmania DNA) and by two parasitological methods, i.e., detection of Leishmania promastigotes by myeloculture, and Leishmania amastigotes using slide imprints/smears (Figure 1C). Leishmania DNA and Leishmania parasites (promastigotes or amastigotes) were observed in 18 dogs (30% of the samples). Parasitological tests failed to detect Leishmania infection in six dogs (10%), but Leishmania DNA was present in their tissues.
DNA sequencing identified L. infantum as the infecting species in 229 of 230 tissue samples containing Leishmania DNA. One tissue sample provided inconclusive results in DNA sequencing.
CVL prevalence
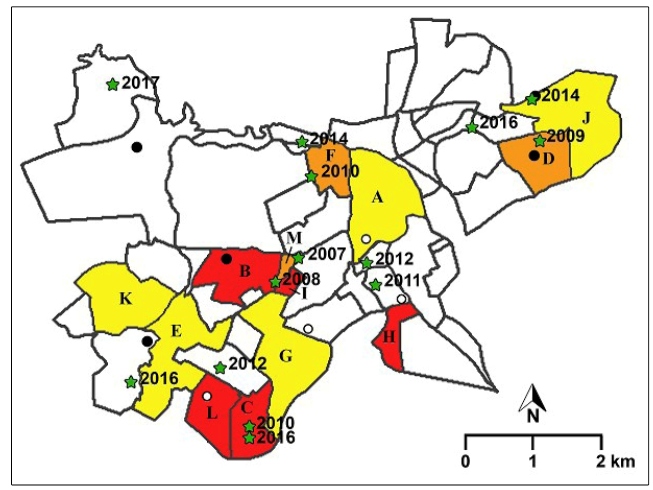
CVL prevalence per neighborhood was distributed into ranges as follows: < 50, 50-99, ≧ 100 (Figure 2). In an attempt to paint a more complete scenario of urban VL in the city, collection sites of L. longipalpis specimens and Leishmania-infected L. longipalpis (April 2015 to March 2016) 11 , as well as all human VL cases reported since 2007, are also shown in the figure.
FIGURE 2: Visceral leishmaniasis scenario in Itaúna (Minas Gerais state, Brazil), an area of sporadic epidemiological transmission risk (ETR) for the disease. Prevalence of canine visceral leishmaniasis (CVL) for each neighborhood is indicated by background color: < 50 (yellow), 50-99 (orange), ≥ 100 (red). A white background indicates non-studied districts. Human visceral leishmaniasis (VL) cases are indicated by green stars and year of report. Sites of L. longipalpis capture are represented by circles. Closed circles denote sites of L. longipalpis capture which were subsequently found to be infected by Leishmania 11 . Neighborhoods: A. Centro, B. Chácara do Quitão, C. Cidade Nova, D. Cidade Leonane, E. Garcias, F. Graças, G. Morada Nova, H. Nogueirinha, I. Novo Horizonte, J. Olaria, K. Parque Jardim Santanense, L. Três Marias, M. Vila Nazaré.
DISCUSSION
Dogs are the most important reservoirs of L. infantum in the domestic infection cycle, and their role in VL transmission in the northeast of Brazil has been recognized since the 1950s. Since then, canine euthanasia has been used as a control measure for VL in an attempt to interrupt the persistence of the parasite in the environment 20 , 21 . Euthanasia is still a major control strategy adopted by SCPVL, although is the least acceptable measure to the population 22 , 23 ; the effectiveness of this approach is controversial in the literature. Although there are reports of significant reductions in canine and human VL cases after the removal of seropositive dogs 24 - 26 , other studies have shown that massive euthanasia fail its purpose 22 , 27 - 30 . Mathematical modelling has suggested that culling of symptomatic dogs might be of some help in areas with low transmission rates, even under imperfect conditions (suboptimal screening, diagnosis, and euthanasia rates). Otherwise, the application of strategies integrated together has been suggested as the best choice 22 . Recently, it has been reported that more effective options than dog culling might be on the horizon for VL control in endemic countries 31 .
A major criticism of screening and culling policies, besides the culling of animals itself, is the insufficient sensitivity and specificity of diagnostic tests commonly employed, which lead to false-positive and false-negative results 32 - 35 . A recent study comparing previous (ELISA EIE plus IFI-CVL) and current (TR-DPP and ELISA EIE) diagnostic protocols in Brazil showed an increase in the performance of the latter, and a reduction in false positives 36 . However, a study of 405 asymptomatic dogs from a non-endemic area in Southern Brazil showed no agreement between serological and molecular (real-time PCR) results in cases of low parasite load 37 . In our study, Leishmania parasites and/or Leishmania DNA were confirmed in 100% of CVL seropositive dogs, regardless of clinical status.
With very few exceptions, Leishmania DNA was detected in every tissue from seropositive dogs in our study, with no statistical difference in TPRs between tissues (Table 3, Figure 1A). Using skin DNA as a template for amplification, we obtained a TPR of 95.0% for Leishmania DNA. Considering that skin sampling is minimally invasive and collection is simple, this tissue might be suggested for the molecular diagnosis of CVL. However, infected dogs with no lesions in their skins are still able to infect L. longipalpis 38 , 39 . It has been suggested that skin biopsies do not necessarily need to be punched only in lesioned skin, but also in clinically healthy skin for parasite detection 39 .
CVL control is a difficult task. Clinically asymptomatic dogs, and infected dogs with divergent serological results (as shown by parasitological and molecular diagnostic methods), are maintained in people’s homes, where they act as potential sources for Leishmania vector infection, and facilitate the disease transmission cycle 4 , 38 - 41 . The operational and ethical implications associated with euthanasia of free-roaming dogs are an additional impediment to CVL control. Interventions promoting responsible pet ownership have been suggested to be an effective strategy 42 . It is important to note that the highest TPR in the screening test (TR-DPP) came from dogs submitted via SOD, compared with CCS canines (Table 1). In addition, all of the positive dogs in the screening test were confirmed to be positive by ELISA. This finding suggested that dog owners are currently attentive to the clinical signs of CVL. However, we cannot discount the possibility of bias in our subsample of seropositive dogs, since most of them were short-haired (Table 2). It has been suggested that this characteristic makes dogs more vulnerable to Leishmania infection by making them preferred targets of bites by Leishmania vectors 43 , 44 .
Although our study was restricted to selected neighborhoods, it was possible to confirm favorable conditions for the spread of human VL in Itaúna, such as the concomitant or close presence of a high population of L. longipalpis, L. infantum-infected L. longipalpis, or L. infantum-infected dogs 11 . In a recent ecological study on phlebotomine sand flies 45 , the predominance of L. longipalpis among flies captured in Itaúna was confirmed. The species represented 90.4% of the 1,260 specimens captured there. Most importantly, L. longipalpis was captured in urban, rural, and forest areas, with a synanthropy index of +95.8. This index measures the degree of association of any given species with a human-modified environment, and varies from -100 (limit of negative association) to +100 (limit of positive association). The value of +95.8 reflects the high adaptation of the Leishmania vector in Itaúna to human presence.
In conclusion, although ETRs have been sporadic until now, the cycle of Leishmania transmission is active in Itaúna, and is favored by the presence of parasites, vectors, domestic reservoirs, and human cases of VL. The possibility of applying integrated SCPVL control actions should be evaluated in an attempt to minimize the spread of VL in this and other regions.
ACKNOWLEDGMENTS
We offer our deepest thanks to the inhabitants of Itaúna for allowing access to their houses and homes, and the Municipal Health Department for logistical support.
Footnotes
Financial Support: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) grant 443601/2014-3 to EMM, Doctoral fellowship to JVL, and undergraduate fellowship to NCLP. AJVL (undergraduate student) and AGMS (technical support) received fellowships from Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG). ESD is a productivity fellow from CNPq.
REFERENCES
- 1.Pan American Health Organization (PAHO) Leishmanioses. Informe Epidemiológico das Américas. 2018. http://iris.paho.org/xmlui/bitstream/handle/123456789/34857/LeishReport6_por.pdf?sequence=5
- 2.Carvalho CG, Junior, Teixeira RG, Neto, Lopes VV, Belo VS, Alves NR, de Paula TB, et al. Parasitism and inflammation in ear skin and in genital tissues of symptomatic and asymptomatic male dogs with visceral leishmaniasis. Parasitol Res. 2017;116(3):987–995. doi: 10.1007/s00436-017-5375-4. [DOI] [PubMed] [Google Scholar]
- 3.Giunchetti RC, Mayrink W, Genaro O, CM Carneiro, Correa Oliveira R, Martins-Filho OA, et al. Relationship between canine visceral leishmaniosis and the Leishmania (Leishmania) chagasi burden in dermal inflammatory foci. J Comp Pathol. 2006;135:100–107. doi: 10.1016/j.jcpa.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Michalsky EM, Rocha MF, da Rocha Lima AC, Franca-Silva JC, Pires MQ, Oliveira FS, et al. Infectivity of seropositive dogs, showing different clinical forms of leishmaniasis, to Lutzomyia longipalpis phlebotomine sand flies. Vet Parasitol. 2007;147(1-2):67–76. doi: 10.1016/j.vetpar.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Molina R, Amela C, Nieto J, San-Andrés M, González F, Castillo JA, et al. Infectivity of dogs naturally infected with Leishmania infantum to colonized Phlebotomus perniciosus. Trans R Soc Trop Med Hyg. 1994;88(4):491–493. doi: 10.1016/0035-9203(94)90446-4. [DOI] [PubMed] [Google Scholar]
- 6.Araújo VEM, Pinheiro LC, Almeida MCM, Menezes FC, Morais MHF, Reis IA, et al. Relative risk of visceral leishmaniasis in Brazil: a spatial analysis in urban area. PLoS Negl Trop Dis. 2013;7(11):e2540. doi: 10.1371/journal.pntd.0002540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belo VS, Werneck GL, Barbosa DS, Simões TC, Nascimento BWL, Silva ES, et al. Factors associated with visceral leishmaniasis in the Americas: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2013;7(4):e2182. doi: 10.1371/journal.pntd.0002182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monteiro EM, França-Silva JC, da Costa RT, Costa DC, Barata RA, Paula EV, et al. Visceral leishmaniasis: a study on phlebotomine sand flies and canine infection in Montes Claros, State of Minas Gerais. Rev Soc Bras Med Trop. 2005;38:147–152. doi: 10.1590/s0037-86822005000200004. [DOI] [PubMed] [Google Scholar]
- 9.Oliveira CL, Assunção RM, Reis IA, Proietti FA. Distribution of human and canine visceral leishmaniasis in Belo Horizonte, Minas Gerais State, Brazil, 1994-1997. Cad Saude Publica. 2001;17:1231–1239. doi: 10.1590/s0102-311x2001000500023. [DOI] [PubMed] [Google Scholar]
- 10.Ministério da Saúde (MS). Secretaria de Vigilância em Saúde. Coordenação Geral de Desenvolvimento da Epidemiologia em Serviços . Guia de Vigilância em Saúde. 2a edição. Brasília: MS; 2017. 705 p [Google Scholar]
- 11.Lopes JV, Michalsky EM, Pereira NCL, Paula AJV, Lara-Silva FO, Silva-Lana R, et al. Entomological studies in Itaúna, Brazil, an area with visceral leishmaniasis transmission: fauna survey, natural Leishmania infection, and molecular characterization of the species circulating in phlebotomine sand flies (Diptera: Psychodidae) J Med Entomol. 2019;56(5):1368–1376. doi: 10.1093/jme/tjz061. [DOI] [PubMed] [Google Scholar]
- 12.Instituto Brasileiro de Geografia e Estatísticas (IBGE) Itaúna. Minas Gerais, Brasil; 2018. https://cidades.ibge.gov.br/brasil/mg/itauna/panorama
- 13.Solano-Gallego L, Miró G, Koutinas A, Cardoso L, Pennisi MG, Ferrer L, et al. LeishVet guidelines for the practical management of canine leishmaniosis. Parasit Vectors. 2011;4:86–102. doi: 10.1186/1756-3305-4-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferreira EC, Gontijo CM, Cruz I, Melo MN, Silva AM. Alternative PCR protocol using a single primer set for assessing DNA quality in several tissues from a large variety of mammalian species living in areas endemic for leishmaniasis. Mem Inst Oswaldo Cruz. 2010;105(7):895–898. doi: 10.1590/s0074-02762010000700009. [DOI] [PubMed] [Google Scholar]
- 15.Cruz I, Cañavate C, Rubio JM, Morales MA, Chicharro C, Laguna F, et al. A nested polymerase chain reaction (Ln-PCR) for diagnosing and monitoring Leishmania infantum infection in patients co-infected with human immunodeficiency virus. Trans R Soc Trop Med Hyg. 2002;96(Suppl 1):S185–S189. doi: 10.1016/s0035-9203(02)90074-x. [DOI] [PubMed] [Google Scholar]
- 16.van Eys GJ, Schoone GJ, Kroon NC, Ebeling SB. Sequence analysis of small subunit ribosomal RNA genes and its use for detection and identification of Leishmania parasites. Mol Biochem Parasitol. 1992;51(1):133–142. doi: 10.1016/0166-6851(92)90208-2. [DOI] [PubMed] [Google Scholar]
- 17.Lins RMMA, Oliveira SG, Souza NA, de Queiroz RG, Justiniano SCB, Ward RD, et al. Molecular evolution of the cacophony IVS6 region in sandflies. Insect Mol Biol. 2002;11:117–122. doi: 10.1046/j.1365-2583.2002.00315.x. [DOI] [PubMed] [Google Scholar]
- 18.Brito MG, Chamone TL. Manual de condutas básicas na campanha de vacinação anti-rábica animal. 1st Edition. Belo Horizonte: Secretaria de Estado da Saúde de Minas Gerais; 2001. 16 p. http://www.pbh.gov.br/smsa/bibliografia/manual_vacinador.pdf [Google Scholar]
- 19.RCore Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2017. https://www.R.project.org/ [Google Scholar]
- 20.Alencar JE. Calazar canino. Contribuição para o estudo da epidemiologia do calazar no Brasil. Imprensa Oficial; 1959. pp. 1–342. Tese. [Google Scholar]
- 21.Deane LM, Deane MP, Alencar JE. Tipo de região e prevalência de leishmaniose visceral em uma região endêmica do Ceará. Rev Paul Med. 1955;46:130–131. [Google Scholar]
- 22.Costa DN, Codeço CT, Silva MA, Werneck GL. Culling dogs in scenarios of imperfect control: realistic impact on the prevalence of canine visceral leishmaniasis. PLoS Negl Trop Dis. 2013;7(8):e2355. doi: 10.1371/journal.pntd.0002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuben AP, Donalísio MR. Dificuldades na execução das diretrizes do Programa de Vigilância e Controle da Leishmaniose Visceral em grandes municípios brasileiros. Cad Saude Publ. 2016;32(6) doi: 10.1590/0102-311X00087415. S0102-311X2016000600401. [DOI] [PubMed] [Google Scholar]
- 24.Ashford DA, David JR, Freire M, David R, Sherlock I, Eulálio MC, et al. Studies on control of visceral leishmaniasis: impact of dog control on canine and human visceral leishmaniasis in Jacobina, Bahia, Brazil. Am J Trop Med Hyg. 1998;59:53–57. doi: 10.4269/ajtmh.1998.59.53. [DOI] [PubMed] [Google Scholar]
- 25.Costa CHN, Aguiar GB, Carvalho AS, Cavalcanti JC, Santos LS, Costa DL. Kala-azar is a slow-motion systemic inflammatory response syndrome: lessons from death; International Congress of Immunology; Rio de Janeiro, Brazil. 2007. [Google Scholar]
- 26.Palatnik-de-Sousa CB, Batista-de-Melo LM, Borja-Cabrera GP, Palatnik M, Lavor CC. Improving methods for epidemiological control of canine visceral leishmaniasis based on a mathematical model. Impact on the incidence of the canine and human disease. An Acad Bras Cienc. 2004;76(3):583–593. doi: 10.1590/s0001-37652004000300012. [DOI] [PubMed] [Google Scholar]
- 27.Costa CH. How effective is dog culling in controlling zoonotic visceral leishmaniasis? A critical evaluation of the science, politics and ethics behind this public health policy. Rev Soc Bras Med Trop. 2011;44(2):232–242. doi: 10.1590/s0037-86822011005000014. [DOI] [PubMed] [Google Scholar]
- 28.Dietze R, Barros GB, Teixeira L, Harris J, Michelson K, Falqueto A. Effect of eliminating seropositive canines on the transmission of visceral leishmaniasis in Brazil. Clin Infect Dis. 1997;25(5):1240–1242. doi: 10.1086/516096. [DOI] [PubMed] [Google Scholar]
- 29.Moreira ED, Jr, Mendes de Souza VM, Sreenivasan M, Nascimento EG, Pontes de Carvalho L. Assessment of an optimized dog-culling program in the dynamics of canine Leishmania transmission. Vet Parasitol. 2004;122(4):245–252. doi: 10.1016/j.vetpar.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 30.Romero GAS, Boelaert M. Control of visceral leishmaniasis in Latin America - a systematic review. PLoS Negl Trop Dis. 2010;4(1):e584. doi: 10.1371/journal.pntd.0000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dantas-Torres F, Miró G, Bowman DD, Gradoni L, Otranto D. Culling dogs for zoonotic visceral leishmaniasis control: the wind of change. Trends Parasitol. 2019;35(2):97–101. doi: 10.1016/j.pt.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 32.da Costa RT, França-Silva JC, Mayrink W, Nascimento E, Genaro O, Campos-Neto A. Standardization of a rapid immunochromatographic test with the recombinant antigens K39 and K26 for the diagnosis of canine visceral leishmaniasis. Trans R Soc Trop Med Hyg. 2003;97(6):678–682. doi: 10.1016/s0035-9203(03)80102-5. [DOI] [PubMed] [Google Scholar]
- 33.Grimaldi G, Jr, Teva A, Santos CB, Pinto IDS, de-Azevedo CT, Falqueto A. Evaluation of a novel chromatographic immunoassay based on Dual-Path Platform technology (DPP CLV rapid test) for the serodiagnosis of canine visceral leishmaniasis. Trans R Soc Trop Med Hyg. 2012;106:54–59. doi: 10.1016/j.trstmh.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Otranto D, Paradies P, Sasanelli M, Spinelli R, Brandonisio O. Rapid immunochromatographic test for serodiagnosis of canine leishmaniasis. J Clin Microbiol. 2004;42(6):2769–2770. doi: 10.1128/JCM.42.6.2769-2770.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reithinger R, Quinnell RJ, Alexander B, Davies CR. Rapid detection of Leishmania infantum infection in dogs: comparative study using an immunochromatographic dipstick test, enzyme-linked immunosorbent assay, and PCR. J Clin Microbiol. 2002;40(7):2352–2356. doi: 10.1128/JCM.40.7.2352-2356.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fraga DB, Pacheco LV, Borja LS, Tuy PG, Bastos LA, Solcà M, et al. The rapid test based on Leishmania infantum chimeric rk28 protein improves the diagnosis of canine visceral leishmaniasis by reducing the detection of false-positive dogs. PLoS Negl Trop Dis. 2016;10(1):e0004333. doi: 10.1371/journal.pntd.0004333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riboldi E, Carvalho F, Romão P, Barcellos RB, Bello GL, Ramos RR, et al. Molecular method confirms canine Leishmania infection detected by serological methods in non-endemic area of Brazil. Korean J Parasitol. 2018;56(1):11–19. doi: 10.3347/kjp.2018.56.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laurenti MD, Rossi CN, da Matta VL, Tomokane TY, Corbett CEP, Secundino NFC, et al. Asymptomatic dogs are highly competent to transmit Leishmania (Leishmania) infantum chagasi to the natural vector. Vet Parasitol. 2013;196(3-4):296–300. doi: 10.1016/j.vetpar.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 39.de Queiroz NM, da Silveira RC, de Noronha AC, Jr, Oliveira TM, Machado RZ, Starke-Buzetti WA. Detection of Leishmania (L.) chagasi in canine skin. Vet Parasitol. 2011;178(1-2):1–8. doi: 10.1016/j.vetpar.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 40.Dias ES, Regina-Silva S, França-Silva JC, Paz GF, Michalsky EM, Araújo SC, et al. Eco-epidemiology of visceral leishmaniasis in the urban area of Paracatu, state of Minas Gerais, Brazil. Vet Parasitol. 2011;176(2-3):101–101. doi: 10.1016/j.vetpar.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 41.Rocha MF, Michalsky EM, Lara-Silva FO, Valadão JL, França-Silva JC, Pinheiro LC, et al. Dogs with divergent serology for visceral leishmaniasis as sources of Leishmania infection for Lutzomyia longipalpis phlebotomine sand flies - an observational study in an endemic area in Brazil. PLoS Negl Trop Dis. 2020;14(2):e0008079. doi: 10.1371/journal.pntd.0008079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melo SN, Teixeira-Neto RG, Werneck GL, Struchiner CJ, Ribeiro R, Sousa LR, et al. Prevalence of visceral leishmaniasis in A population of free-roaming dogs as determined by multiple sampling efforts: A longitudinal study analyzing the effectiveness of euthanasia. Prev Vet Med. 2018;161:19–24. doi: 10.1016/j.prevetmed.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 43.França-Silva JC, da Costa RT, Siqueira AM, Machado-Coelho GL, da Costa CA, Mayrink W, et al. Epidemiology of canine visceral leishmaniosis in the endemic area of Montes Claros Municipality, Minas Gerais State, Brazil. Vet Parasitol. 2003;111:161–173. doi: 10.1016/s0304-4017(02)00351-5. [DOI] [PubMed] [Google Scholar]
- 44.Leal GGA, Carneiro M, Pinheiro ADC, Marques LA, Ker HG, Reis AB, et al. Risk profile for Leishmania infection in dogs coming from an area of visceral leishmaniasis reemergence. Prev Vet Med. 2018;150:1–7. doi: 10.1016/j.prevetmed.2017.11.022. [DOI] [PubMed] [Google Scholar]
- 45.Pereira NCL, Michalsky EM, Lara-Silva FO, Lana RS, Paula AJVP, Pereira DM, et al. Ecology of phlebotomine sand flies in a Brazilian area with recent leishmaniasis transmission (Itaúna, in Minas Gerais state) Rev Soc Bras Med Trop. 2020;53:e20190538. doi: 10.1590/0037-8682-2019-0538-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]