Abstract
Introduction:
Wharton’s jelly (WJ) is the mucoid connective tissue that surrounds the vessels in the human umbilical cord and provides protection from compression and torsion in response to fetal movement. WJ is known to be altered in the presence of pregnancy complications such as gestational diabetes mellitus and preeclampsia. The present study examined associations between the cross-sectional area of WJ measured by ultrasound and postpartum placental pathology and morphometry.
Methods:
The area of WJ was measured by ultrasound in 156 eligible participants between 23 to 37 weeks’ gestation. Morphometric assessment of fixed cord cross sections was conducted, together with assessment of the cord and placenta for specific pathologies using standard criteria.
Results:
From 156 participants, 123 ultrasound images met the data quality requirements and pathology reporting was completed for 99 placentas. 17 of the participants (14%) delivered a small for gestational age neonate and 32 of the 99 placentas examined (32%) had significant placental pathology findings. Area of WJ was associated with low birth weight (p = 0.002) and was associated with specific placental pathology (p = 0.01). WJ area was positively associated with placental dimensions such as width, length and surface area.
Discussion:
Decreased WJ area is associated with clinically-significant placental pathology and WJ area scales proportionally with placental size. These findings suggest that WJ area correlates with functional capacity of the placenta and thus merits further evaluation alongside currently-available tests of placental function in clinical practice.
Keywords: morphometry, placental pathology, ultrasound, umbilical cord, Wharton’s jelly
Introduction
A proper functioning placenta and umbilical cord (UC) are vital for normal fetal development, survival, and postnatal health. The UC, in conjunction with the placenta, works to provide oxygen and nutrients to the fetus in addition to facilitating the removal of waste products [1]. The UC is a coiled structure, composed of an umbilical vein, (typically two) umbilical arteries and a surrounding medium called Wharton’s jelly (WJ) [2]. The UC provides a vascular conduit between mother and fetus, and must be resilient to obstruction in the face of continuous fetal movements. The importance of UC morphology and structure to maintain fetal health is highlighted by the reported associations between a variety of abnormal cord anatomical findings, such as an abnormal placental insertion site, a single umbilical artery and abnormal cord coiling, and adverse perinatal outcomes of pregnancy [3-5]. Specifically, obstructive UC lesions associated with hypercoiling of the cord, or decreased presence of WJ, are the predominant risk factors for fetal vascular malperfusion lesions within the placenta [6].
WJ is a mucoid connective tissue that protects the vessels from torsion and compression in response to fetal motion. It contains mesenchymal stem cells and possesses contractile properties that are believed to aid in the regulation of blood flow [7]. In its absence, specifically around the umbilical arteries, case studies have reported incidences of perinatal death [8,9]. In a previous report from our institution of 497 pregnancies, the amount of WJ was associated with umbilical cord diameter [10]. Previous studies have produced nomograms and reference curves that display the healthy trajectory of WJ area expansion across gestation [11-15]. They suggest that early in gestation WJ area increases linearly with gestational age but beyond 32 weeks’ gestation, the area of WJ plateaus. However, there is evidence that this pattern of growth and the composition of the jelly are altered in pregnancy pathologies such as gestational diabetes mellitus [16] and preeclampsia [17]. Despite WJ being an integral part of a normal functioning umbilical cord and placenta, recent research has focused almost exclusively on its stem cell properties, and not on its potential role in fetal development [18-19]. Investigating the developmental role of WJ in healthy and adverse pregnancy outcomes may prove an important factor in our understanding of pregnancy risk, especially to understand the basis of stillbirth attributed to “cord accidents”.
To our knowledge there are no studies that have attempted to relate ultrasound measurements of WJ area in the umbilical cord in-utero to placental pathology findings at delivery. Therefore, the purpose of our study was to determine whether there was a relationship between the area of WJ and placental pathology in a population of low and high-risk human pregnancies. Additionally, we investigated associations of the area of WJ to placental morphometry and pregnancy outcome measures.
Methods
Subject recruitment.
The study was approved by the research ethics boards of The Hospital for Sick Children (Toronto, ON, Canada), Mount Sinai Hospital (Toronto, ON, Canada) and Johns Hopkins Medicine (Baltimore, MD, USA). Informed consent was obtained for each participant. A sample size calculation was performed based on the variation in the area of WJ during late gestation in low-risk pregnancies from Barbieri et al. [15]. With a sample size of 120 participants and allowing for a 5% chance of a false positive, we are powered at 0.8 to detect changes in the area of WJ on the order of 25% using ultrasound. 156 volunteers between 23 and 37 weeks of gestation, with a singleton pregnancy, body mass index < 45 kg/m2 and no significant maternal comorbidities (i.e. insulin-dependent diabetes, chronic hypertension) were recruited for fetal ultrasound examination.
Ultrasonography.
Ultrasound examinations were performed between 23-37 weeks’ gestation (mid/late gestation) by certified sonographers using either a Philips iU22 (Philips Healthcare Andover, MA, USA) or GE Voluson e10 (GE Healthcare, Chicago, IL, USA) ultrasound machine. The area of the umbilical cord was measured in a cross-sectional plane at a single location in the free loop. Biparietal diameter, head circumference, abdominal circumference and femur length were measured to calculate estimated fetal weight using Hadlock’s formula [20] and the growth centiles were classified using Hadlock et al. [21].
Image analysis.
The area of the vessels and cord were segmented by one experimenter using the software package Display (Montreal Neurological Institute, Montreal, Canada). The surface area of WJ was calculated by subtracting the cross-sectional areas of the vessels from the area of the cord. Datasets were subsequently excluded from analysis if it was not possible to accurately delineate greater than 25% of the UC margins due to poor contrast-to-noise with surrounding structures. The WJ area measurements were categorized by centile according to gestational age (in completed weeks) [15]. Adjusted WJ areas were delineated into two groups, low and high WJ. Low WJ area was classified as less than the 50th centile and high WJ area was greater than the 50th centile.
Pregnancy outcome.
Obstetrical data were obtained from the participants’ medical records. Birth weights were categorized into percentile groups according to neonatal sex and gestational age (in completed weeks) [22]. Small for gestational age (SGA) was classified as less than the 5th centile and large for gestational age (LGA) was greater than the 95th centile. Centiles between the 5th and 95th were considered appropriate for gestational age (AGA).
Placental pathology.
After delivery, placentas were fixed in 10% formaldehyde for 48 hours and then examined using the Amsterdam working group definitions for placental lesions [23]. Placental dimensions [longest axis, orthogonal axis and thickness (cm)] were recorded. The surface area of the placenta was calculated as longest axis x orthogonal axis x π/4. The umbilical cord, fetal membrane roll and placental disc were cut into a series of 2-cm wide sections for gross pathology examination. The sections were then paraffin-embedded, cut into 4-μm thick sections and stained with hematoxylin and eosin to assess for abnormalities. The sections were reviewed by experienced pediatric perinatal pathologists. Placental pathology was classified into six groups [24]: 1) no or minor pathology (i.e. small placenta without any additional pathology or acute chorioamnionitis), 2) chronic maternal inflammation (chronic villitis of unknown etiology, plasma cell deciduitis, and chronic intervillositis), 3) fetal vascular disease (large-vessel thrombosis, fetal thrombotic vasculopathy), 4) disorders of villus development, 5) massive perivillous fibrin deposition and 6) maternal vascular malperfusion. For the purposes of analysis, placental pathology was categorized as either minor findings (group 1) or diagnostic findings (groups 2-6).
Statistics.
All statistical tests were performed using the R statistical software package (www.r-project.org). With respect to analysis of placental morphometry, both WJ and placental area were treated as continuous variables. An ANOVA using linear model estimates was performed to compare the WJ area as a function of placental area. With respect to this model, the difference between the gestational age at the time of the ultrasound scan and the gestational age when the placental area was measured was included as a nuisance variable. For the analysis of estimated fetal weight (SGA, AGA, LGA), birth weight (SGA, AGA, LGA), placental pathology (minor, severe) and WJ area (low, high), all variables were categorical. The Pearson’s Chi-squared test was used to determine significant associations between WJ centile and estimated fetal weight, birth weight or placental pathologic findings. P values of <0.05 were considered significant and no correction for multiple comparisons was applied.
Results
Based on the image exclusion criteria (greater than 25% of the UC margins could not be delineated), 33 of the 156 participants were excluded. Table 1 provides a summary of patient characteristics and pregnancy outcome. While the most common cross-sectional shape of the UC was a circle (92 of the 123 UC images) (Figure 1A), the cord morphology was heterogeneous with significant deviations from circularity observed in the minority of cases (Figure 1B and 1C).
Table 1.
Characteristics of the study subjects meeting the inclusion criteria
| Characteristic N=121a |
Mean [Range] |
|---|---|
| Maternal age (years) | 34 [20-43] |
| Maternal prepregnancy body mass index (kg/m2) | 24 [17-37] |
| Gestational age at ultrasound scan (weeks) | 32 [23-37] |
| Gestational age at delivery (weeks) | 38 [29-41] |
| Birth weight (g) | 3105 [710-4750] |
| Infant male sex (%) | 55% |
| Number of live births (%) | 100% |
| Pregnancy outcomes | Total (%) |
| Vaginal | 70 (58%) |
| Intrapartum C-section | 23 (19%) |
| Pre-labor C-section | 28 (23%) |
| Small for gestational age | 17 (14%) |
| Gestational age < 37 weeks | 18 (15%) |
| Preeclampsia | 6 (5%) |
| Gestational hypertension | 5 (4%) |
Clinical data was missing from two subjects.
Figure 1. The cross-sectional area of the umbilical cord by ultrasound.
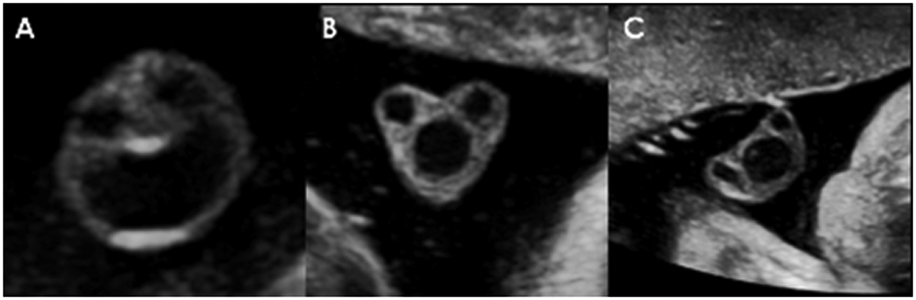
The umbilical cord morphology was typically circular (A); however, the shape deviated from circularity in several of the participants (B, C).
WJ area was significantly associated with estimated fetal weight centile at the time of the ultrasound scan (Pearson’s Chi-squared test, p = 0.02). 77% of estimated fetal weights < 5th centile had a low WJ area, compared to 36% in the > 5thcentile group, respectively. 17 of the 123 patients were classified as small for gestational age at birth. WJ area was significantly associated with birth weight centile (Pearson’s Chi-squared test, p = 0.002). 76% of birth weights < 5th centile (SGA) had a low WJ area, compared to 37% and 0% in the AGA and LGA groups, respectively.
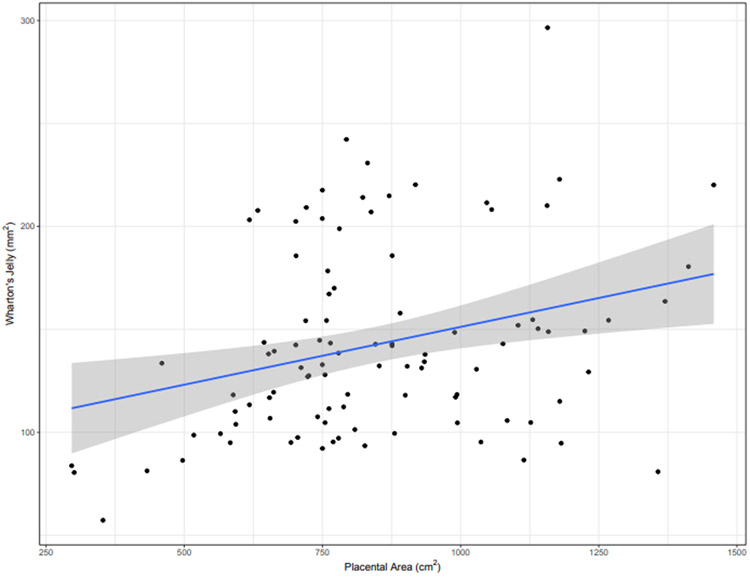
Placental length, width and surface area were found to be positively associated with WJ area (ANOVA, p = 0.02, p = 0.003, p = 0.007 respectively, Figure 2). Of the 99 placentas sent for examination, 32 indicated the presence of diagnostic pre-labor pathological findings. Table 2 summarizes the relevant categories of placental pathology with maternal-vascular malperfusion being the most common. Decreased WJ area was significantly associated with placental pathology (Chi-squared test, p=0.01). 63% of placentas with a diagnostic placental pathology had a low WJ area, compared to 33% in the non/minor pathology findings group. This corresponded to a relative risk of 1.90.
Figure 2. Association with placental morphometry.
The area of WJ (mm2) was positively associated with postpartum placental surface area (cm2) (n=99) (ANOVA, p = 0.007). The shaded gray area represents the 95% confidence interval.
Table 2.
Placental pathology findings.
| Placental pathology N=99a |
Total (%) |
|---|---|
| No major pathology identified | 67 (67.7%) |
| Chronic maternal inflammation | 8 (8.1%) |
| Fetal vascular disease | 7 (7.1%) |
| Disorders of villus development | 3 (3.0%) |
| Massive perivillous fibrin deposition | 2 (2.0%) |
| Maternal vascular malperfusion | 12 (12.1%) |
24 placentas did not have placental pathology assessed.
Discussion
The umbilical cord acts as a conduit connecting the growing fetus to the placenta and together they comprise the fetoplacental circulation [25]. The transfer of oxygen and nutrients between the maternal and fetal circulations relies in part on a sufficient amount of Wharton’s jelly to impart protection from cord compression due to fetal activity, thus ensuring continuous blood flow [2]. Several case studies have investigated associations between WJ area and adverse pregnancy outcomes [8, 9], but no studies to our knowledge have examined the relationship with placental pathology and morphometry.
Fetal growth restriction and postpartum placental examination indicative of severe pathology were significantly associated with a reduced area of WJ in the free loop of the UC in mid/late gestation. This is consistent with previous work regarding “lean” cord, where fetuses of normal weight and low WJ area at examination were at a greater risk for being SGA at delivery [26]. In the present study, 6 of the 104 fetuses that had appropriate growth based on estimated fetal weights at the time of the ultrasound examination were SGA at birth. Of these 6 fetuses, 4 of them had a low WJ area. While the sample size is small, this is consistent with the work of Raio et al [26]. One common cause of growth restriction is placental insufficiency that may arise from placental vascular abnormalities [27]. For example, cases of isolated single umbilical artery have been associated with decreased WJ area [28] and when born SGA are more likely to present with fetal vascular malperfusion abnormalities than infants born SGA but with a three-vessel cord [29]. In the current study, placental pathologies such as fetal and maternal vascular malperfusion were associated with WJ area, suggesting that low WJ is associated with the spectrum of placental insufficiency. Currently, the link between birth weight and placental disease in relation to WJ area is not well understood. It is possible that WJ loss is secondary to placental disease or that both are the consequence of a common early developmental etiology. Persistently low amounts of WJ across gestation compounded with placental structural defects may further exacerbate placental disease and negatively impact fetal growth potential.
SGA is associated with placental morphometry measures such as reduced placental size and villous surface area [30-31] and placental morphometry has been shown to be an effective clinical tool for quantifying placental efficiency. Salafia et al. [32] determined that abnormal placental morphology was associated with decreased efficiency, hypothesizing the irregularities in shape to be due to aberrant vasculature formation [32]. Similar to the variability reported in placental morphometry, we found differences in the cross-sectional shape of the UC (Figure 1). While these differences were qualitative, it may be possible in future studies to correlate UC shape with hemodynamics or birth outcomes. In this current study measures of placental dimensions, such as width, length and surface area, were found to be significantly associated with WJ area. This relationship shows that proportionally, WJ area scales with placental size. In addition, the size of the umbilical vessels were associated with both the WJ area and the placental shape measures (data not shown), implying a role for the WJ in supporting both the growth of the placenta and subsequent increase in UC blood flow with gestational age.
A potential limitation of our study is the low number of patients within each of the five severe pathology groups. Thus, we were unable to determine if a specific subgroup of placental pathology was associated with low WJ area. Another limitation is that a subset of the placentas were not sent for pathological examination. Most of the missing placental pathology were from low-risk pregnancies and this selection bias accounts for the higher rate of pathological findings in comparison to other large cohort studies [24]. Another limitation is that WJ area measurements were taken at an undefined location in the free loop potentially increasing the variability relative to a study design with a prescribed measurement location. A final limitation is that the area of WJ was calculated from a single b-mode image at one gestational age for each subject. A single image per subject by one sonographer does not allow the measurement of intra- and inter-observer differences; however, we anticipate this variability to be small [33]. A longitudinal study design would have increased the statistical power of the study and allowed investigation of associations between the rate of change of WJ area over gestation and placental pathology.
In summary, the principal finding of this work is that decreased WJ area is associated with clinically-significant placental pathology. To determine if the area of WJ has diagnostic utility, a randomized, controlled trial is necessary to account for potential selection bias and to establish a direct relationship between WJ area and adverse outcome. In addition, future work could investigate potential alternations in the composition of the WJ components in the context of placental pathology.
Acknowledgements:
We thank the pregnant volunteers for participating in this study.
Funding: This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant U01-HD-087177-01 and the Canadian Institutes of Health Research Grant PJT-153202.
Abbreviations:
- AGA
appropriate for gestational age
- LGA
large for gestational age
- SGA
small for gestational age
- UC
umbilical cord
- WJ
Wharton’s jelly
Footnotes
Disclosures: No conflicts of interest are declared by the authors.
References
- [1].Gude NM, Roberts CT, Kalionis B, King RG, Growth and function of the normal human placenta, Thromb. Res 114 (2004) 397–407. doi: 10.1016/j.thromres.2004.06.038. [DOI] [PubMed] [Google Scholar]
- [2].Davies JE, Walker JT, Keating A, Concise Review: Wharton’s Jelly: The Rich, but Enigmatic, Source of Mesenchymal Stromal Cells, Stem Cells Transl. Med 6 (2017) 1620–1630. doi: 10.1002/sctm.16-0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ebbing C, Kiserud T, Johnsen SL, Albrechtsen S, Rasmussen S, Prevalence, Risk Factors and Outcomes of Velamentous and Marginal Cord Insertions: A Population-Based Study of 634,741 Pregnancies, PLoS One. 8 (2013) e70380. doi: 10.1371/journal.pone.0070380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Murphy-Kaulbeck L, Dodds L, Joseph KS, Van Den Hof M, Single Umbilical Artery Risk Factors and Pregnancy Outcomes, 116 (2010) 843–850. doi: 10.1097/AOG.0b013e3181f0bc08. [DOI] [PubMed] [Google Scholar]
- [5].Pergialiotis V, Kotrogianni P, Koutaki D, Christopoulos-Timogiannakis E, Papantoniou N, Daskalakis G, Umbilical cord coiling index for the prediction of adverse pregnancy outcomes: a meta-analysis and sequential analysis, J. Matern. Neonatal Med (2019) 1–8. doi: 10.1080/14767058.2019.1594187. [DOI] [PubMed] [Google Scholar]
- [6].Redline RW, Ravishankar S, Fetal vascular malperfusion, an update, APMIS. 126 (2018) 561–569. doi: 10.1111/apm.12849. [DOI] [PubMed] [Google Scholar]
- [7].Takechi K, Kuwabara Y, Mizuno M, Ultrastructural and Immunohistochemical Studies of Wharton’s Jelly Umbilical Cord Cells, 14 (1993) 235–45. doi 10.1016/s0143-4004(05)80264-4 [DOI] [PubMed] [Google Scholar]
- [8].Labarrere C, Sebastiani M, Siminovich M, Torassa E, Althabe O, Absence of Wharton’s Jelly around the Umbilical Arteries: an Unusual Cause of Perinatal Mortality, 6 (1985) 555–559. doi 10.1016/s0143-4004(85)80010-2. [DOI] [PubMed] [Google Scholar]
- [9].Thomson LL, Hoo JJ, Brief Clinical Report Linear Disruption of Umbilical Cord: A Rare Anomaly of the Cord Associated With Acute Fetal Distress and Perinatal Death/Profound Psychomotor Retardation, 24, (1996) 348–9. doi:(/) [DOI] [PubMed] [Google Scholar]
- [10].Proctor LK, Fitzgerald B, Whittle WL, Mokhtari N, Lee E, Machin G, Kingdom JCP, Keating SJ, Umbilical cord diameter percentile curves and their correlation to birth weight and placental pathology, Placenta. 34 (2013) 62–66. doi: 10.1016/j.placenta.2012.10.015. [DOI] [PubMed] [Google Scholar]
- [11].Weissman A, Jakobi P, Bronshtein M, Goldstein I, Sonographic measurements of the umbilical cord and vessels during normal pregnancies, J. Ultrasound Med. 13 (1994) 11–14. doi: 10.7863/jum.1994.13.1.11. [DOI] [PubMed] [Google Scholar]
- [12].Raio L, Ghezzi F, Di Naro E, Gomez R, Franchi M, Mazor M, Brühwiler H, Sonographic measurement of the umbilical cord and fetal anthropometric parameters, Eur. J. Obstet. Gynecol. Reprod. Biol 83 (1999) 131–135. doi: 10.1016/S0301-2115(98)00314-5. [DOI] [PubMed] [Google Scholar]
- [13].Ghezzi F, Raio L, Di Naro E, Franchi M, Balestreri D, D’Addario V, Nomogram of Wharton’s jelly as depicted in the sonographic cross section of the umbilical cord, Ultrasound Obstet. Gynecol 18 (2001) 121–125. doi: 10.1046/j.1469-0705.2001,00468.x. [DOI] [PubMed] [Google Scholar]
- [14].Togni FA, Araujo E, Vasques FAP, Moron AF, Torloni MR, Nardozza LMM, The cross-sectional area of umbilical cord components in normal pregnancy, Int. J. Gynecol. Obstet 96 (2007) 156–161. doi: 10.1016/j.ijgo.2006.10.003. [DOI] [PubMed] [Google Scholar]
- [15].Barbieri C, Cecatti JG, Surita FG, Costa ML, Marussi EF, Costa JV, Area of Wharton’s jelly as an estimate of the thickness of the umbilical cord and its relationship with estimated fetal weight, Reprod. Health 8 (2011) 32. doi: 10.1186/1742-4755-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Weissman A, Jakobi P, Sonographic measurements of the umbilical cord in pregnancies complicated by gestational diabetes, J. Ultrasound Med 16 (1997) 691–694. doi: 10.7863/jum.1997.16.10.691. [DOI] [PubMed] [Google Scholar]
- [17].Bańkowski E, Collagen of the umbilical cord and its alteration in EPH-gestosis (preeclampsia), Proc. Indian Acad. Sci. Chem. Sci 111 (1999) 207–213. 10.1007/BF02869910 [DOI] [Google Scholar]
- [18].Bhuvanalakshmi G, Arfuso F, Kumar AP, Dharmarajan A, Warrier S, Epigenetic reprogramming converts human Wharton’s jelly mesenchymal stem cells into functional cardiomyocytes by differential regulation of Wnt mediators, Stem Cell Res. Ther 8 (2017) 185. doi: 10.1186/s13287-017-0638-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Busto F, Sepúlveda H, Prieto CP, Carrasco M, Díaz L, Palma J, Lattus J, Montecino M, Palma V, Runt-Related Transcription Factor 2 Induction During Differentiation of Wharton’s Jelly Mesenchymal Stem Cells to Osteoblasts Is Regulated by Jumonji AT-Rich Interactive Domain 1B Histone Demethylase, Stem Cells. 35 (2017) 2430–2441. doi: 10.1002/stem.2704. [DOI] [PubMed] [Google Scholar]
- [20].Hadlock FP, Harrist RB, Sharman RS, Deter RL, Parks SK, Estimation of fetal weight with the use of head, body, and femur measurements -- a prospective study, Am. J. Obstet. Gynecol 151 (1985) 333–337. DOI: 10.1016/0002-9378(85)90298-4. [DOI] [PubMed] [Google Scholar]
- [21].Hadlock FP, Harrist RB, Martinez-Poyer J, In utero analysis of fetal growth: a sonographic weight standard, Radiology. 181 (1991) 129–133. DOI: 10.1148/radiology.181.1.1887021 [DOI] [PubMed] [Google Scholar]
- [22].Kramer MS, Platt RW, Wu Wen S, Joseph K, Allen A, Abrahamowicz M, Blondel B, Bréart G, A New and Improved Population-Based Canadian Reference for Birth Weight for Gestational Age, 108 (2001) E35 www.aappublications.org/news. [DOI] [PubMed] [Google Scholar]
- [23].Khong TY, Mooney EE, Ariel I, Balmus NCM, Boyd TK, Brundler MA, Derricott H, Evans MJ, Faye-Petersen OM, Gillan JE, Heazell AEP, Heller DS, Jacques SM, Keating S, Kelehan P, Maes A, McKay EM, Morgan TK, Nikkels PGJ, Parks WT, Redline RW, Scheimberg I, Schoots MH, Sebire NJ, Timmer A, Turowski G, Van Der Voorn JP, Van Lijnschoten I, Gordijn SJ, Sampling and definitions of placental lesions Amsterdam placental workshop group consensus statement, in: Arch. Pathol. Lab. Med, College of American Pathologists, 2016: pp. 698–713. doi: 10.5858/arpa.2015-0225-CC. [DOI] [PubMed] [Google Scholar]
- [24].Wright E, Audette MC, Ye XY, Keating S, Hoffman B, Lye SJ, Shah PS, Kingdom JC, Maternal vascular malperfusion and adverse perinatal outcomes in low-risk nulliparous women, Obstet. Gynecol 130 (2017) 1112–1120. doi: 10.1097/AOG.0000000000002264. [DOI] [PubMed] [Google Scholar]
- [25].Wang Y, Zhao S, Vascular Biology of the Placenta, 2010. doi: 10.4199/C00016ED1V01Y201008ISP009. [DOI] [PubMed] [Google Scholar]
- [26].Raio L, Ghezzi F, Di Naro E, Franchi M, Maymon E, Brühwiler H, Prenatal diagnosis of a lean umbilical cord: a simple marker for the fetus at risk of being small for gestational age at birth, Ultrasound Obstet Gynecol 13(1999) 176–180. DOI: 10.1046/j.1469-0705.1999.13030176.x [DOI] [PubMed] [Google Scholar]
- [27].Silver RM, Examining the link between placental pathology, growth restriction, and stillbirth, Best Pract. Res. Clin. Obstet. Gynaecol 49 (2018) 89–102. doi: 10.1016/j.bpobgyn.2018.03.004. [DOI] [PubMed] [Google Scholar]
- [28].Raio L, Ghezzi F, Di Naro E, Franchi M, Brühwiler H, Lüscher KP, Prenatal assessment of Wharton’s jelly in umbilical cords with single artery, Ultrasound Obstet. Gynecol 14 (1999) 42–46. DOI: 10.1046/j.1469-0705.1999.14010042.x [DOI] [PubMed] [Google Scholar]
- [29].Battarbee AN, Palatnik A, Ernst LM, Grobman WA, Placental abnormalities associated with isolated single umbilical artery in small-for-gestational-age births, Placenta. 59 (2017) 9–12. doi: 10.1016/j.placenta.2017.09.001. [DOI] [PubMed] [Google Scholar]
- [30].Proctor LK, Toal M, Keating S, Chitayat D, Okun N, Windrim RC, Smith GCS, Kingdom JCP, Placental size and the prediction of severe early-onset intrauterine growth restriction in women with low pregnancy-associated plasma protein-A, Ultrasound Obstet. Gynecol 34 (2009) 274–282. doi: 10.1002/uog.7308. [DOI] [PubMed] [Google Scholar]
- [31].Egbor M, Ansari T, Morris N, Green CJ, Sibbons PD, Pre-eclampsia and Fetal Growth Restriction: How Morphometrically Different is the Placenta?, Placenta. 27 (2006) 727–734. doi: 10.1016/j.placenta.2005.06.002. [DOI] [PubMed] [Google Scholar]
- [32].Salafia CM, Yampolsky M, Misra DP, Shlakhter O, Haas D, Eucker B, Thorp J, Placental surface shape, function, and effects of maternal and fetal vascular pathology, Placenta. 31 (2010) 958–962. doi: 10.1016/j.placenta.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Barbieri C, Cecatti JG, Souza et al CE. Inter- and intra-observer variability in Sonographic measurements of the cross-sectional diameters and area of the umbilical cord and its vessels during pregnancy, Reprod. Health, 5 (2008) doi: 10.1186/1742-4755-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]