Abstract
Introduction:
Take-home naloxone (THN) is a clinically effective and cost-effective means of reducing opioid overdose fatality. Nonetheless, naloxone administration that successfully saves a person’s life can still produce undesirable and harmful effects.
Aim:
To better understand factors associated with two widely reported adverse outcomes following naloxone administration; namely the person resuscitated displays: i. withdrawal symptoms and ii. anger.
Methods:
A mixed methods study combining a randomized controlled trial of overdose education and naloxone prescribing to people with opioid use disorder and semi-structured qualitative interviews with trial participants who had responded to an overdose whilst in the trial. All data were collected in New York City (2014–2019). A dataset (comprising demographic, pharmacological, situational, interpersonal, and overdose training related variables) was generated by transforming qualitative interview data from 47 overdose events into dichotomous variables and then combining these with quantitative demographic and overdose training related data from the main trial. Associations between variables within the dataset and reports of: i. withdrawal symptoms and ii. anger were explored using chi-squared tests, t-tests, and logistic regressions.
Results:
A multivariate logistic regression found that people who had overdosed were significantly more likely to display anger if the person resuscitating them criticized, berated or chastised them during resuscitation (adjusted OR = 27 [95% CI = 4.0 – 295]). In contrast, they were significantly less likely to display anger if the person resuscitating them communicated positively with them (OR = 0.10 [95% CI = 0.01 – 0.78]). Both positive and negative communication styles were independently associated with anger, and communication was associated with 59% of the variance in anger. There was no evidence that people who displayed withdrawal symptoms were more likely to display anger than those not displaying withdrawal symptoms, and neither displaying withdrawal symptoms nor displaying anger were associated with using more drugs after resuscitation.
Conclusions:
Contrary to common assumptions, withdrawal symptoms and anger following naloxone administration may be unrelated phenomena. Findings are consistent with previous research that has suggested that a lay responder’s positive or reassuring communication style may lessen anger post overdose. Implications for improving THN programmes and naloxone administration are discussed.
Keywords: Naloxone, Overdose, Opioids, Withdrawal, Anger, Mixed Methods
1. INTRODUCTION
Opioid overdose deaths have been increasing in the United States and internationally (Kim et al., 2009; Rudd et al., 2016; Hedegaard et al., 2017; Roxburgh et al., 2017; Ciccarone, 2019). The timely administration of the opioid antagonist naloxone can prevent fatalities by temporarily counteracting the central nervous system-mediated respiratory depression associated with opioid overdose (Strang et al., 1996; Faulkner-Gurnstein, 2017; Strang et al., 2019). Naloxone reverses all types of opioid overdoses, including those caused by heroin and pharmaceutical or synthetic opioid drugs; although its effectiveness in reversing overdoses involving fentanyl and/ or its analogs is unclear under some circumstances (Sommerville et al., 2017; Bardsley, 2019; Moe et al., 2020).
Medical staff in emergency departments and ambulance personnel have used naloxone to treat opioid overdoses for many years (Sporer et al., 1996; Clarke et al., 2005; Lenton et al., 2015). More recently, the drug has been made available to non-medically trained people, including people who use opioids and their friends and family, to use in the community wherever an overdose may occur (Strang et al., 1996; Darke and Hall, 1997; Mueller et al., 2015; McDonald et al., 2017; Behar et al., 2018). This pre-provision of naloxone to laypersons, in conjunction with instruction on how to manage an overdose whilst waiting for emergency medical care to arrive, is known as take-home naloxone (THN) (Strang et al., 1996).
The rate of successful overdose reversal following naloxone administration by laypersons has historically been very high (nearly 100% in some studies) (Dettmer et al., 2001; Clark et al., 2014; EMCDDA, 2015; Giglio et al., 2015; McDonald and Strang, 2016; Fairbairn et al. 2017) and THN is widely recognised as being effective and cost-effective in reducing opioid overdose mortality (Coffin and Sullivan, 2013; McDonald and Strang, 2016; Langham et al., 2018). Nonetheless, laws governing the possession of drugs, weak local and national support for harm reduction, and the stigma associated with addiction can all impede the successful implementation of THN (Dwyer et al., 2016; Mitchell and Higgens, 2016; Winstanley et al., 2016; Holloway et al., 2018). Other identified barriers to naloxone use include limited professional and lay person awareness of the availability of THN (Dietze et al., 2015; Mitchell and Higgens, 2016), as well as witnesses’ reluctance to intervene because they are uncertain about how to identify an overdose (Richert, 2015; Dwyer et al., 2016; Heavey et al., 2018; Holloway et al., 2018) or are worried about police involvement, needle stick injuries, being personally too intoxicated to respond, or intervening when a person who has overdosed does not want help (Lagu et al., 2006; Wright et al., 2006; Richert, 2015; Neale et al., 2019).
One further, and frequently reported, factor undermining layperson preparedness to administer naloxone is concern that the person who has overdosed will respond by becoming angry, aggressive or even violent on regaining consciousness (Worthington et al., 2006; Sporer and Kral, 2007; Neale and Strang, 2015; Olsen et al., 2015; Richert, 2015; Sondhi et al., 2016; Faulkner-Gurstein, 2017; Heavey et al., 2018; Holloway et al., 2018; McAuley et al., 2018; Bessen et al., 2019). Within the literature, this phenomenon has been identified as resulting from ‘over-antagonism’; that is, the dose of the antagonist naloxone given was more than pharmacologically needed to reverse the effects of the illicit agonists consumed (Neale and Strang, 2015). The anger of the person who has been resuscitated is variously attributed to the naloxone causing them to lose their ‘high’, to feel that they have wasted their drugs or money, or to experience uncomfortable or painful withdrawal symptoms (known as ‘acute withdrawal syndrome’) (Worthington et al., 2006; Kerr et al., 2008; Sporer and Kral, 2007; Neale and Strang 2015; Richert, 2015; Sondhi et al., 2016; UKMi, 2017; Heavey et al., 2018; Holloway et al., 2018; McAuley et al., 2018).
The reasons why people who have overdosed are given more naloxone than necessary have not been well studied. However, they are likely to include responder uncertainties regarding the amount and combination of drugs the person who has overdosed has consumed, poor understanding of naloxone pharmacology, first responder protocols that advocate a high naloxone dose, inability to titrate the dose of the different naloxone products, variable time periods before naloxone is first administered and then between any subsequent administrations, and other confounders such as the gender, weight and opioid tolerance of the person being resuscitated (Cantwell et al., 2005; Clarke et al., 2005; Lankenau et al., 2013; Neale and Strang, 2015; Fairbairn et al., 2017; Farrugia et al., 2019). In addition, lay responders may panic, forget to follow the dosing instructions they have been given, and accidentally ‘over treat’ or ‘over-antagonise’ the person who has overdosed (Neale et al., 2019).
Despite these various risk factors for over-antagonism, adverse events are not an inevitable outcome of naloxone administration and they may be mitigated by better information and guidelines on titrating naloxone dose against response (Neale and Strang, 2015) and by deploying routes of administration that arouse the person who has overdosed more slowly (i.e. subcutaneous or intramuscular rather than intravenous injections) (Horowitz, 1998; Wanger et al., 1998; Buajordet et al., 2004). Supporting this, research has found that some lay responders already actively seek to avoid inducing withdrawal by incrementally administering naloxone in small doses (Lankenau et al., 2013; Winston et al., 2015; Farrugia et al 2019; Neale et al., 2019) and that strategies for dose titration can be successfully built into overdose response training (Dettmer et al., 2001; Madah-Amiri et al., 2019). In addition, it has been suggested that adverse events could be prevented by better communication about naloxone, how it works, and its potential side effects at the point of treatment (Horowitz, 1998; Neale and Strang, 2015; Bessen et al., 2019). Reflecting this, Farrugia et al. (2019) found that lay responders seemed to manage conflict when administering naloxone by talking to, and reassuring, people as they were being resuscitated.
A naloxone reversal that successfully saves a person’s life can thus still produce suboptimal, undesirable, and harmful effects in the form of precipitated withdrawal symptoms, anger and aggression. These can undermine the effectiveness of THN programmes by prompting people who have overdosed to refuse or resist treatment and by increasing the likelihood that they might re-use opioids post resuscitation (Watson et al., 1998; Worthington et al., 2006; Richert, 2015; Neale and Strang, 2015; Black et al., 2017; UKMi, 2017; Bessen et al., 2019; Greene et al., 2019). Additionally, lay responders can become anxious or reluctant to administer naloxone in case they encounter hostility or aggression from the person being treated (Neale and Strang, 2015; Strang et al., 2018). Responding to such problems, the aim of this paper is to better understand factors associated with two widely reported adverse outcomes following naloxone administration; namely, the person resuscitated displays: i. withdrawal symptoms and ii. anger. In so doing, our objective is to identify ways of potentially improving both THN programmes and responses to emergency overdose events.
2. METHODS
2.1. Data sources
Our data derive from a convergent mixed methods study (Creswell and Plano Clark, 2018), combining quantitative data from a randomized controlled trial (RCT) of overdose education and naloxone prescribing to individuals with opioid use disorder and qualitative data from semi-structured interviews conducted with trial participants who had responded to an overdose whilst in the trial. Both the trial and the qualitative study received ethical approval from the New York State Psychiatric Institute (NYSPI) Institutional Review Board (IRB# 6723) and were conducted in the New York City (NYC) metropolitan area. Core information about each study component is described below.
2.1.1. RCT of overdose education and naloxone prescribing
The RCT (entitled: “Risks and benefits of overdose education and naloxone prescribing to heroin users”) was conducted between September 2014 and October 2019 and funded by the National Institute on Drug Abuse (NIDA) (R01DA035207; NCT02535494). The primary aim was to determine whether more extensive overdose education could improve overdose outcomes compared to brief overdose education. Men and women (21-65 years of age) were eligible to participate provided that they met DSM-IV (American Psychiatric Association, 2000) criteria for opioid use disorder within the last 6 months regardless of current treatment status; spoke/read English; had no active psychiatric disorder that might interfere with participation; and had had no naloxone or cardiopulmonary resuscitation training within the past two years. In total, 403 participants were enrolled in the trial, of whom 228 completed follow up interviews at 1, 3, 6, and 12 months. Among other measures, follow up interviews assessed retention of naloxone training knowledge and captured key information surrounding any overdose events witnessed or experienced.
In NYC, organizations that work with people who use illicit opioids routinely offer largely standardized, brief overdose training that addresses risk factors, how to recognize an opioid overdose, and how to use naloxone. Trial participants either received the brief training (20-30 minutes) or an extended training that comprised the brief training plus a further two-hour session. The extended training reiterated and reinforced key information from the brief training, but additionally provided information about non-opioid overdose and taught cardiopulmonary resuscitation. Both the brief and extended training were conducted at Columbia University Irving Medical Center by research assistants who followed a written script to ensure that every participant received exactly the same information.
On completion of the training, all trial participants were given the option of receiving either intramuscular (IM) or intranasal (IN) naloxone as part of a naloxone rescue kit. All rescue kits included two doses of naloxone (regardless of formulation) plus a pair of latex gloves, a face shield for rescue breathing, sterile wipes, and an instructional handout. At the start of the trial, participants were offered the choice of an IM naloxone formulation comprising a needle and syringe with two glass vials (where the dose concentration was 0.4mg naloxone per 1ml solution in each vial, yielding a total dose of 0.8mg) or a multi-step atomized nasal naloxone spray assembled by combining a pre-filled luer-lock syringe with a nasal atomizer and spare vial (where the dose was 2mg/2ml in each vial [1mg/1ml to be administered to each nostril] yielding a total possible dose of 4mg). In November 2015, the U.S. Food and Drug Administration approved a new concentrated nasal naloxone formulation (Narcan® nasal spray) that administers a single dose with a concentration of 4mg/0.1ml into one nostril (provided in a twin-pack yielding a total dose of 8mg). On NIDA’s recommendation, the trial changed from providing the multistep IN naloxone to the single step Narcan® nasal spray in August 2017, although participants were still offered the option of receiving the IM device instead.
2.1.2. Semi-structured interviews
Between January 2016 and December 2018, 62 semi-structured interviews were conducted with 52 trial participants who reported being present at an overdose during one of their follow up assessments. Seven trial participants were interviewed more than once as they reported being present at multiple overdoses whilst in the trial: five were interviewed twice, one was interviewed three times, and one was interviewed four times. Sampling ensured a mix of males, females, overdose experiences, and naloxone formulations used. The time between the overdose event reported and the interview was also minimized as much as possible to maximize recall (most interviews were conducted within 3 months of the witnessed overdose; range 1 day to 6 months).
The primary aim of the qualitative interviews was to supplement the RCT data by collecting and analysing more detailed first-hand accounts of overdoses occurring post training in order to better understand and potentially improve the effectiveness of current overdose prevention programmes in NYC. Participation in the qualitative study was optional and followed a separate information giving and consenting process from the trial. All qualitative interviews were audio-recorded and conducted in private offices at the NYSPI. They lasted 20-65 minutes and were guided by an interview schedule that covered: demographic and psychosocial information, substance use and treatment, pre-trial overdoses experienced and witnessed, the last overdose witnessed since joining the trial (including how the person who overdosed responded to naloxone), and views on overdose training. Interview schedule questions were supplemented by prompts and probes to follow up issues raised by the participants. On completion of a qualitative interview, participants were compensated US$40.
2.2. Data handling and management
All quantitative trial data were entered, cleaned and stored at Columbia University, NYC. The audio files from the qualitative interviews were transcribed verbatim in the U.S. but the transcriptions were then encrypted and emailed to research team members in London, UK. Here, they were entered into the qualitative software programme MAXQDA v18 (VERBI Software, 2019) and coded by two team members who worked together to ensure consistency. The coding frame used was co-developed with a third team member and comprised deductive codes based on the interview schedule and more inductive codes emerging from the data. The coded data were then extracted from the qualitative software programme into Microsoft Word documents ready for analyses.
2.3. Analyses
2.3.1. Variable construction
Preliminary readings of the interview transcriptions and coded data revealed that although the study had a very high rate of successful reversals (96% of trial reversals were successful), there was widespread evidence of ‘over-antagonism’. Specifically, participants often reported that the people they had resuscitated had displayed signs of opioid withdrawal and had become angry or aggressive. Meanwhile, further qualitative analyses of the interview data indicated that withdrawal symptoms and anger/aggression did not always occur together and, when they did occur together, they did not always follow the same sequential order (Parkin et al., 2020). As further qualitative analysis was unable to systematically test factors that might be associated with over-antagonism, we transformed key information from the qualitative interviews into dichotomous quantitative variables so that we could then combine these with variables from the trial and undertake a mixed methods analysis (Creswell and Plano Clark, 2018). In the mixed methods literature, this process of transforming qualitative data into numbers has been termed ‘quantitization’ (Tashakkori and Teddlie, 1998; Driscoll et al., 2007). Quantitization can, inter alia, show regularities and patterns in qualitative data that might otherwise be difficult to see, communicate or verify (Sandelowski et al., 2009).
To begin, the coded qualitative data relating to the last overdose event reported at each interview (n=62) were reviewed to ascertain whether (or not) withdrawal symptoms and, separately, whether (or not) anger, including aggression or violence, were reported. These results were then entered as two dichotomous variables (‘withdrawal’ [yes/no] and ‘anger’ [yes/no]) into the statistical software programme R (version 3.5.0) (R Core Team, 2013). The coded qualitative data were next further reviewed to create additional variables that were also entered into the R database along with quantitative variables relating to the same participants and overdoses from the main trial.
Variables were developed and selected based on the available study data and factors that, according to the team’s reading of the literature and clinical understanding of the topic, seemed likely to make an overdose difficult to manage and/or to provoke either withdrawal symptoms or an angry reaction. They included demographic, pharmacological, situational, interpersonal, and overdose training related factors. One variable that was considered but reluctantly excluded was a naloxone dose variable. Whilst it would have been theoretically possible to convert the naloxone given at each overdose into a standardised dose, this would likely have produced misleading data as the amount of naloxone absorbed from different kits and formulations varies significantly (McDonald et al., 2018; Krieter et al., 2019). After several attempts to calculate a variable for ‘naloxone dose absorbed’, the team were not satisfied that the results were sufficiently reliable and so decided to use variables for ‘intramuscular versus intranasal administration’ and ‘one naloxone dose versus more than one naloxone dose’ instead. Table 1 describes the variables used in the analyses and the data source (interview or main trial) from which each variable was derived.
TABLE 1:
VARIABLES CONSTRUCTED FOR THE MIXED METHODS ANALYSIS
| Variable | Data source1 |
|---|---|
| Demographic | |
| Sex of person who overdosed is male v female | Interview |
| Age of person who overdosed (years) | Main trial |
| Pharmacological | |
| Person who overdosed consumed fentanyl prior to overdosing (no v yes) | Interview |
| Person who overdosed consumed heroin and no other substances prior to overdosing (no v yes) | Interview |
| Person who overdosed injected drugs prior to overdosing (no v yes) | Interview |
| Intramuscular naloxone was used to reverse the overdose (no v yes) | Interview |
| More than one dose of naloxone (IM or IN) was administered to the person who overdosed (no v yes) | Interview |
| Person who overdosed was known or presumed to have gone on to use more drugs post reversal (no v yes) | Interview |
| Situational | |
| Emergency services (911) attended the overdose event (no v yes) | Interview |
| Person who overdosed expressed concern about loss of money or drugs post resuscitation (no v yes) | Interview |
| Interpersonal | |
| Participant had a pre-existing relationship with the person who overdosed as they were a family member or friend (no v yes) | Interview |
| Participant communicated positively with the person who overdosed during the reversal process (no v yes)2 | Interview |
| Participant communicated negatively with the person who overdosed during the reversal process (no v yes)3 | Interview |
| Overdose training related | |
| Participant received extended (rather than brief) overdose training (no v yes) | Main trial |
| Participant’s Opioid Overdose Knowledge Scale (OOKS) score at their last assessment prior to the overdose (range 0-45)4 | Main trial |
| Participant completed 8 or more of 11 standard revival procedures (no v yes)5 | Interview |
Responses are based on participant self-reports (via their follow up assessments for the trial or the qualitative interviews) unless otherwise stated.
Participant talked to the person who had overdosed, explained what was happening and/ or tried to calm them down (based on researcher assessment of the interview data).
Participant criticized, berated or chastised the person who had overdosed or reacted angrily when the person who had overdose complained or reacted negatively (based on researcher assessment of the interview data).
The Opioid Overdose Knowledge Scale (Williams et al., 2014) is a validated self-completed instrument used to assess the level of knowledge of opioid overdose management among addiction professionals, patients and family members. It records knowledge about risk factors for having an opioid overdose, signs of an opioid overdose, actions to be taken in an overdose situation, naloxone effects and administration, adverse effects, and aftercare procedures.
Participant completed 8 or more of the following 11 standard overdose response procedures (based on researcher assessment of the interview data): 1. Recognized overdose symptoms; 2. Called out the name of the person who had overdosed; 3. Shaked, touched, shouted at the person who had overdosed to waken them; 4. Checked the eyes, pulse and breathing of the person who had overdosed; 5. Checked the person who had overdosed for the colour of their skin, lips, fingernails; 6. Called the Emergency Services before administering naloxone; 7. Placed the person who had overdosed in the recovery position; 8. Administered cardiopulmonary resuscitation; 9. Gave rescue breathing; 10. Successfully assembled the naloxone kit; 11. Delivered naloxone.
At this point, 15/62 overdose events (cases) had to be excluded because of missing information relating to how the person who had overdosed had responded to naloxone. Specific reasons for these missing data are shown in Figure 1. This left 47 overdose events described by 40 participants: 11 cases of withdrawal only; 9 cases of anger only; 6 cases of withdrawal with anger; and 21 cases where neither withdrawal symptoms nor anger were reported. An analysis plan was then developed to explore associations between each of the variables of interest and reports of i. withdrawal (with or without anger; 17 overdoses: 11+6) and ii. anger (with or without withdrawal; 15 overdoses: 9+6).
FIGURE 1:
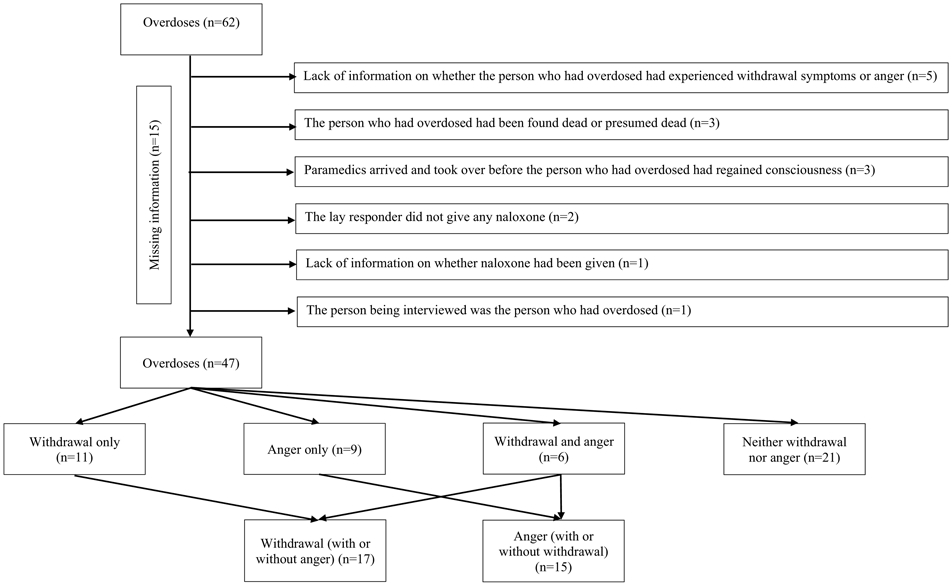
SELECTION OF OVERDOSE EVENTS FOR INCLUSION IN THE ANALYSES
2.3.2. Statistical analyses
All analyses were undertaken in R (version 3.5.0) (R Core Team, 2013). Descriptive analyses of variables were first conducted using counts and proportions for categorical variables and means and standard deviations for continuous variables (Table 2).
TABLE 2:
FREQUENCY OF VARIABLES
| Categorical variables | ||||||
|---|---|---|---|---|---|---|
| Variable | No | Yes | Missing | |||
| n | % | n | % | n | % | |
| Outcomes | ||||||
| Withdrawal | 30 | 63.8 | 17 | 36.1 | 0 | 0 |
| Anger | 32 | 68.1 | 15 | 32.0 | 0 | 0 |
| Demographic | ||||||
| Sex of person who overdosed | Male | Female | ||||
| 34 | 72.3 | 13 | 27.7 | 0 | 0 | |
| Pharmacological | ||||||
| Fentanyl taken | 36 | 76.6 | 10 | 21.3 | 1 | 2.1 |
| Heroin only taken | 24 | 51.0 | 22 | 46.8 | 2 | 2.1 |
| Drugs injected | 12 | 25.5 | 28 | 59.6 | 7 | 14.9 |
| IM naloxone given | 36 | 76.6 | 10 | 21.3 | 1 | 2.1 |
| More than one dose of naloxone given | 21 | 44.7 | 25 | 53.2 | 1 | 2.1 |
| Known or presumed re-use of drugs post resuscitation | 28 | 59.6 | 7 | 14.9 | 12 | 25.5 |
| Situational | ||||||
| Emergency services attended | 16 | 34.0 | 30 | 63.8 | 1 | 2.1 |
| Person who overdosed expressed concern about loss of money or drugs | 29 | 61.7 | 18 | 38.2 | 0 | 0 |
| Interpersonal | ||||||
| Pre-existing relationship between participant and person who overdosed | 25 | 53.2 | 22 | 46.8 | 0 | 0 |
| Positive communication during the resuscitation process | 14 | 29.8 | 28 | 59.6 | 5 | 10.6 |
| Negative communication during the resuscitation process | 28 | 59.6 | 14 | 29.8 | 5 | 10.6 |
| Overdose training related | ||||||
| Participant received extended overdose training | 13 | 27.7 | 34 | 72.3 | 0 | 0 |
| 8 or more standard revival procedures completed | 31 | 66.0 | 15 | 31.9 | 1 | 2.1 |
| Continuous variables | ||||||
| Variable | Mean | SD | Median | Range | Missing n |
Missing % |
| Age of person who overdosed | 40.5 | 12.49 | 40 | 19-62 | 0 | 0 |
| Opioid Overdose Knowledge Scale (OOKS) score | 39.3 | 3.46 | 40 | 25-44 | 6.4 | 3 |
Exploratory analyses of variables associated with i. withdrawal and ii anger were then conducted. Yates’ chi-squared tests (Yates, 1934) were used to identify possible associations between categorical independent variables and outcomes where values existed in all combinations of fields. This was not possible for one variable (‘emergency services [911] attended the overdose event’) as there were no instances where an ambulance did not attend and anger was not present. Continuous variables were examined for normality and t-tests were used to examine differences in means. The Bonferroni-corrected threshold for significance was set at p = 0.0018 (Bonferroni, 1936).
Associations that reached Bonferroni-corrected statistical significance were quantified using univariate logistic regressions, before these variables were combined in a single multivariate logistic regression. The model results are presented as regression coefficients and odds ratios (OR) with 95% confidence intervals (CI). The multivariate model was subsequently compared with the univariate model using Aikaike Information Criteria and a chi-squared test using the deviance and deviance residuals of both models. The variables were examined for multicollinearity by calculating the variance inhibitory factor.
Because of the small sample size, imputation of missing data was not reliable, so a complete case analysis was undertaken. Logistic regression was used to confirm that missing data were random or completely random. No predictor variable was significantly associated with complete case status.
3. RESULTS
3.1. Withdrawal symptoms
Withdrawal symptoms were more likely to be reported when more than one dose of naloxone was administered to the person who had overdosed (X2 [df = 1, N = 46] = 4.0, p = 0.046); however, this did not meet the significance threshold following correction for multiple comparisons. Other pharmacological variables – fentanyl consumption, consumption of heroin only, injection of drugs, route of naloxone administration, subsequent use of drugs – were not associated with withdrawal. Additionally, no demographic, situational, interpersonal or overdose training related variable – sex of the person who had overdosed, person who had overdosed being concerned about loss of money or drugs, a pre-existing relationship between the participant and the person who had overdosed, participant’s positive or negative communication during the reversal process, participant having received brief or extended overdose training, and participant completing eight or more standard revival procedures – was associated with withdrawal (Table 3).
TABLE 3:
TEST STATISTICS FOR WITHDRAWAL
| Variable | X2 | df | N | p |
|---|---|---|---|---|
| Demographic | ||||
| Sex of person who overdosed | 0.29 | 1 | 47 | 0.588 |
| Pharmacological | ||||
| Person who overdosed consumed fentanyl prior to overdosing | 0.36 | 1 | 46 | 0.554 |
| Person who overdosed consumed heroin and no other substances prior to overdosing | 2.59 | 1 | 45 | 0.100 |
| Person who overdosed injected drugs prior to overdosing | 0.05 | 1 | 40 | 0.833 |
| Intramuscular naloxone was used to reverse the overdose | 0.02 | 1 | 46 | 0.885 |
| More than one dose of naloxone (IM or IN) was administered to the person who overdosed | 4.0 | 1 | 46 | 0.046* |
| Person who overdosed was known or presumed to have gone on to use more drugs post reversal | 0.96 | 1 | 35 | 0.327 |
| Situational | ||||
| Person who overdosed expressed concern about loss of money or drugs on resuscitation | 0.38 | 1 | 47 | 0.911 |
| Interpersonal | ||||
| Participant had a pre-existing relationship with the person who overdosed as they were a family member or friend | 0.88 | 1 | 47 | 0.588 |
| Participant communicated positively with the person who overdosed during the reversal process | 0.01 | 1 | 42 | 0.911 |
| Participant communicated negatively with the person who overdosed during the reversal process | 0.01 | 1 | 42 | 0.911 |
| Overdose training related | ||||
| Participant received extended overdose training | 0.02 | 1 | 47 | 0.891 |
| Participant completed 8 or more of 11 standard revival procedures | 1.6 | 1 | 46 | 0.202 |
| Variable | t | Mean (SD) Withdrawal − |
Mean (SD) Withdrawal + |
p |
| Age of person who overdosed (years) | −0.14547 | 40 (13) | 41 (11) | 0.885 |
| Opioid Overdose Knowledge Scale (OOKS) score | 0.20035 | 39 (2.7) | 40 (4.6) | 0.843 |
There were also no associations between whether (or not) withdrawal was reported and either the age of person who had overdosed or the participant’s Opioid Overdose Knowledge Scale (OOKS) score (Williams et al., 2013) (Table 3).
3.2. Anger
Anger was less likely to be reported when participants communicated positively with the person who had overdosed by talking to them, explaining what had happened, and/or trying to calm them down (X2 [df = 1, N = 42] = 14 p < 0.001). In contrast, anger was more likely to be reported when the participant communicated negatively with the person who had overdosed by criticizing, berating or chastising them during the resuscitation process (X2 [df = 1, N = 42] = 20, p < 0.001). Anger was also more frequent when the person who had overdosed was concerned about loss of money or drugs after they had been resuscitated (X2 [df = 1, N =47] = 5.8 p = 0.016), but the significance of the association did not survive correction for multiple comparisons. No other demographic, pharmacological, interpersonal or overdose training related variables – including sex of the person who had overdosed, consumption of fentanyl, consumption of heroin only, injection of drugs, route of naloxone administration, subsequent use of drugs, a pre-existing relationship between the participant and the person who had overdosed, the participant having received brief or extended overdose training, and the participant completing eight or more standard revival procedures – were associated with anger (Table 4).
TABLE 4:
TEST STATISTICS FOR ANGER
| Variable | X2 | df | N | p |
|---|---|---|---|---|
| Demographic | ||||
| Sex of person who overdosed | 1 | 47 | ||
| Pharmacological | ||||
| Person who overdosed exhibited withdrawal symptoms | 0.002 | 1 | 47 | 0.961 |
| Person who overdosed consumed fentanyl prior to overdosing | 4.9 x 10−31 | 1 | 46 | 1 |
| Person who overdosed consumed heroin and no other substances prior to overdosing | 1.9 | 1 | 45 | 0.160 |
| Person who overdosed injected drugs prior to overdosing | 3.1 | 1 | 40 | 0.077 |
| Intramuscular naloxone was used to reverse the overdose | 0.12 | 1 | 46 | 0.723 |
| More than one dose of naloxone (IM or IN) was administered to the person who overdosed | 0.51 | 1 | 46 | 0.476 |
| Person who overdosed was known or presumed to have gone on to use more drugs post reversal | 0.46 | 1 | 35 | 0.499 |
| Situational | 1 | |||
| Person who overdosed expressed concern about loss of money or drugs post resuscitation | 5.8 | 1 | 47 | 0.016 |
| Interpersonal | ||||
| Participant had a pre-existing relationship with the person who overdosed as they were a family member or friend | 0.11 | 1 | 47 | 0.744 |
| Participant communicated positively with the person who overdosed during the reversal process | 14 | 1 | 42 | <0.001 |
| Participant communicated negatively with the person who overdosed during the reversal process | 20 | 1 | 42 | <0.001 |
| Overdose training related | ||||
| Participant received extended overdose training | 1.3x10−31 | 1 | 47 | 1 |
| Participant completed 8 or more of 11 standard revival procedures | 0.53 | 1 | 46 | 0.467 |
| Variable | t | Mean (SD) Anger − |
Mean (SD) Anger + |
p |
| Age of person who overdosed (years) | 1.52 | 42 (12) | 37 (12) | 0.139 |
| Opioid Overdose Knowledge Scale (OOKS) score | −1.28 | 39 (3.9) | 40 (23.0) | 0.207 |
There was also no association between anger and withdrawal symptoms or between anger and either the age of person who had overdosed or the participant’s Opioid Overdose Knowledge Scale (OOKS) score (Williams et al., 2013) (Table 4).
Univariate logistic regression estimated the odds of the person who had overdosed displaying anger in reversals where there was positive communication as OR = 0.045 (95% CI 0.0072 – 0.21). The model was significant (X2 [1] = 17.23, p <0.001), explaining 37% of the variance (Nagelkerke R2) (Table 5). Univariate logistic regression estimated the odds of the person displaying anger in reversals where there was negative communication as OR = 50 (95% CI 8.9 – 457). The model was also significant (X2 [1] = 24.20, p <0.001), explaining 50% of the variance (Nagelkerke R2) (Table 5).
TABLE 5:
UNIVARIATE LOGISTIC REGRESSION – COMMUNICATION AND ANGER
| Variable | B (SE) | 95% CI for odds ratio | ||
|---|---|---|---|---|
| Lower | Odds ratio | Upper | ||
| Constant | 1.3 (0.65) | |||
| Positive communication | −3.1 (0.85) | 0.0072 | 0.045 | 0.21* |
| Constant | −2.1 (0.61) | |||
| Negative communication | 3.9 (1.0) | 8.9 | 50 | 457** |
R2 = 0.31 (Hosmer-Lemeshow), 0.14 (Cox-Snell), 0.37 (Nagelkerke). Model X2 (1) = 17.23, p <0.001
R2 = 0.44 (Hosmer-Lemeshow), 0.19 (Cox –Snell), 0.50 (Nagelkerke). Model X2 (1) = 24.20, p <0.001
When positive and negative communication were combined in a single model, the association between both variables and anger continued to be significant. The adjusted odds ratio was 0.10 (95% CI 0.01 – 0.78) for positive communication and 27 (95% CI 4 – 295) for negative communication. Notably, the upper bound of the adjusted 95% confidence interval reflected more than a 20% reduction in odds of anger where there was positive communication and the lower bound of the adjusted 95% confidence interval represented an almost four-fold increase in the odds of anger where there was negative communication. The model was significant (X2 [2] = 29.00, p <0.001) and accounted for 59% of the variance (Nagelkerke R2) (Table 6).
TABLE 6:
MULTIVARIATE LOGISTIC REGRESSION – COMMUNICATION AND ANGER
| Variable | B (SE) | 95% CI for odds ratio | ||
|---|---|---|---|---|
| Lower | Odds ratio | Upper | ||
| Constant | −0.46 (0.95) | |||
| Positive communication | −2.3* (1.1) | 0.010 | 0.10 | 0.78 |
| Negative communication | 3.3** (1.1) | 4.0 | 27 | 295 |
R2 = 0.53 (Hosmer-Lemeshow), 0.23 (Cox –Snell), 0.59 (Nagelkerke). Model X2 (2) = 29.00, p <0.001.
p <0.05
p < 0.01
VIF 1.002
When compared to the univariate model with negative communication as the sole predictor, the model including positive and negative communication provided a better fit (X2 [1] = 11.7 p <0.001) and had a smaller Akaike Information Criterion (AIC [negative communication] = 34.6; AIC [positive communication model] = 41.5; AIC [all communication model] = 31.8), suggesting that both positive and negative communication were associated with anger. The model was examined for multicollinearity and the variance inflation factor was 1.002; demonstrating that multicollinearity was not present.
4. DISCUSSION
Our analyses were exploratory and inevitably have limitations. The sample size was initially small and cases then had to be deleted due to missing information on how the person who had overdosed had responded to receiving naloxone. A logistic regression including many variables was not possible, only very large effects could be detected and quantified, and the 95% confidence intervals of the point estimates for the odds ratios were wide. In addition, the analyses undertaken were neither pre-specified nor pre-registered; rather they were undertaken in response to inductive analyses of the qualitative data occurring whilst the trial was in progress. Accordingly, the variables used were based on the available data, rather than ideal measures, and focused on factors that seemed likely to be relevant from the team’s reading of the literature and clinical understanding of the topic. This may have introduced bias into the analyses and we caution that other factors not explored, such as the naloxone dose absorbed, may also be relevant in explaining withdrawal and anger.
Despite these weaknesses, we believe that this the first research to combine demographic, pharmacological, situational, interpersonal and overdose training related factors to better understand why people often respond so negatively when their lives have just been saved by naloxone. We also note that it would be impossible to prospectively create a robust quantitative dataset that included all the variables that might be of interest given that sufficient real life emergency overdose events would be difficult to observe within the context of a research study; the accounts of those who overdosed, those who responded, and other bystanders would almost certainly yield inconsistencies; and there would be no reliable way of knowing what exact quantities and combination of substances and adulterants an individual had consumed prior to overdosing, their opioid tolerance at the point of overdose, or precisely how long intervals were between opioid use, first naloxone administration, and subsequent administrations.
Our analyses add to the existing literature by seeming to challenge a common assumption about opioid overdose reversals: that anger and aggression after a person has received naloxone occur in response to them experiencing withdrawal symptoms (Gaddis and Watson, 1992; Horowitz, 1998; Neale & Strang, 2015; Richert, 2015; Heavey et al., 2018; McAuley et al., 2018; Bessen et al., 2019; Farrugia et al., 2019). Instead, our findings suggest that withdrawal and anger could be independent phenomena. Withdrawal symptoms were potentially associated with receiving more than one naloxone dose, which seems logical given that withdrawal symptoms are likely to have strong pharmacological and physiological origins. Nonetheless, withdrawal was not associated with any other pharmacological or demographic variables. This seems surprising, but may be explained by small effects which could not be detected using this size of sample, lack of current understanding regarding optimal dosing, and differences between the dosages and pharmacokinetics of the various naloxone products used (Goldfrank et al., 1986; Horowitz, 1998; Cantwell et al., 2005; Clarke et al., 2005; Fairbairn et al., 2017; UKMi, 2017).
In contrast, our findings indicate that anger may be more of a social phenomenon and less of a pharmacological or physiological response to naloxone administration. Consistent with previous research (Neale and Strang, 2015; Richert, 2015; Farrugia et al., 2019), we found that anger was potentially associated with a person who had overdosed being concerned about losing their money and drugs. Additionally, anger was strongly associated with the responder’s positive or negative communication style (Neale and Strang, 2015; Bessen et al., 2019; Farrugia et al., 2019). Management of potential anger did not, however, appear to be affected by a responder’s knowledge or attitude to overdose as measured by the Opioid Overdose Knowledge Scale (Williams et al., 2013), by their practical skills in managing an overdose (as measured by completion of 8 or more of 11 standard revival procedures), or by the training an individual had received in the study trial (brief or extended). Equally, and contra Farrugia et al. (2019), anger was not associated with whether the person who had overdosed knew the responder.
As previously stated, the primary purpose of naloxone administration is to prevent death. Nonetheless, it is also important to address any unintended harms associated with naloxone use (Neale and Strang, 2015; Strang et al., 2018). The risk of death from precipitated withdrawal symptoms and rebound toxicity is believed to be small (1 per cent or less), but it still exists (Worthington et al., 2006; UKMi, 2017). Withdrawal was reported in 17/47 (36%) of the overdose events we studied. However, we found no evidence that either withdrawal symptoms or anger were associated with people who had overdosed being known or presumed to go on to use more drugs post reversal. Despite this, people who had overdosed were known or presumed to have later re-used drugs in 7/35 (20%) of the overdose events for which data were available (Table 2). Reasons for post resuscitation substance use thus require further investigation and strategies to prevent it are urgently needed.
Anger was reported in 15/47 (32%) of the overdose events studied. According to previous research, anger and aggression can result in first responders being abused and attacked, can cause them to feel resentful about the ingratitude of those they have resuscitated, and can leave them anxious or reluctant to administer naloxone again (Neale and Strang, 2015; McAuley et al., 2018). Our analyses showed that anger was strongly linked to how well lay responders communicated with the person who had overdosed. It is therefore possible that lay responders may be able to placate a person they have resuscitated by explanation and reassurance (Neale and Strang, 2015; Farrugia et al., 2019). Equally, they might provoke them by criticism or rebuke. On the face of it, expecting people who have received relatively limited training to ‘socially’ manage complex and potentially aggressive and violent medical emergencies involving acquaintances or even strangers seems unrealistic. Yet, THN appears to be premised on the assumption that lay responders will behave in this highly skilful way and our data confirmed that this was, in fact, how they often reacted (Farrugia et al., 2019; Neale et al., 2019).
5. CONCLUSIONS
Delivering enough naloxone to ensure adequate resuscitation whilst avoiding the precipitation of withdrawal symptoms has been likened to walking a medical tightrope (Clarke et al., 2005). The increasing range of THN products and the emergence of more potent and faster-acting synthetic opioids, such as fentanyl, only serve to compound the administration complexity (Fairbairn et al., 2017). Unfortunately, our analyses do not yield any new suggestions for preventing withdrawal symptoms whilst guaranteeing a successful overdose reversal. Nonetheless, the data we have presented seem broadly supportive of existing recommendations to deliver naloxone carefully in incremental doses, to choose products that support the delivery of low incremental doses, and to provide training on the specific product selected for supply locally (WHO, 2014; Neale and Strang, 2015; UKMi, 2016).
Although more research is needed, our findings indicate ways that THN programmes might be improved to help address the angry outbursts that can occur after naloxone administration. Most obviously, more time and resources could be invested in training lay responders to understand how people who experience an opioid overdose may feel post resuscitation, why they may react negatively, and also how it may be possible to manage the situation and prevent problems from escalating by using positive communication and reassurance (Neale and Strang, 2015; Farrugia et al., 2019). This must not be presented in a way that either makes lay responders unnecessarily afraid that anger or violence will occur or leads them to feel that they have failed if the person who is being resuscitated cannot be placated. Nonetheless, training needs to recognize that people who have overdosed might react angrily. Moreover, it seems important to offer people who have been present at an opioid overdose support in talking through what happened, particularly any anger, aggression or violence directed at them.
HIGHLIGHTS.
Withdrawal symptoms and anger can undermine willingness to administer naloxone
Withdrawal symptoms and anger after naloxone administration may be unrelated
Positive communication style is associated with less anger after naloxone
Negative communication style is associated with more anger after naloxone
Training programmes should support lay responders in managing anger after naloxone
ACKNOWLEDGEMENTS
The authors would like to thank all study participants for sharing their experiences; Verena Metz for conducting some of the interviews; and Gregory Cortorreal, Rebecca Abbott, Benjamin Foote, Suky Martinez, and Richie Eisenberg for their technical assistance on the trial.
FUNDING SOURCE
The randomized controlled trial (“Risks and benefits of overdose education and naloxone prescribing to heroin users”) was funded by the National Institute on Drug Abuse (NIDA) (R01DA035207; Comer – Principal Investigator). J.N., N.J.K. and S.P. are part-funded by, and J.S. is supported by, the NIHR (National Institute for Health Research) Maudsley Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London. N.J.K. is also employed by the South London and Maudsley NHS Foundation Trust and S.P. is also part-funded by the Pilgrim Trust. L.B. is funded by an Erwin Schroedinger Fellowship by the Austrian Science Fund (ASF). The views expressed are those of the authors and not necessarily those of NIDA, the NHS, the NIHR, the ASF, the Department of Health, or the Pilgrim Trust.
Footnotes
DECLARATIONS OF INTEREST
In the last 3 years, J.N. has received, through her University, research funding from Mundipharma Research Ltd and Camurus AB. N.J.K’s PhD (2010 – 2015) was supported by a Wellcome Trust GlaxoSmithKline Translational Medicine Training Fellowship and, during this time, she received funds for travel, training and research materials from GlaxoSmithKline. N.J.K. has also been supported in attending educational meetings by the Lundbeck Institute (2010). J.S. is a researcher and clinician who has advocated for wider pre-provision of take-home naloxone, using several types of naloxone. He has also worked with pharmaceutical companies to seek to identify new or improved treatments (including forms of naloxone) from whom, within the last 3 years, his employer (King’s College London) has received honoraria, travel costs and/or consultancy payments. This includes work with, during past 3 years, Martindale, Indivior, MundiPharma, Braeburn/Camurus and trial medication supply from iGen and from Camurus. His employer (King’s College London) has registered intellectual property on a novel buccal naloxone formulation and he has also been named in a patent registration by a Pharma company regarding a concentrated nasal naloxone spray. For a fuller account, see J.S.’s web-page at http://www.kcl.ac.uk/ioppn/depts/addictions/people/hod.aspx. Within the past three years, S.D.C. has received research funding from Alkermes, Braeburn Pharmaceuticals, Cerecor Inc., Corbus, Go Medical, Intra-cellular Therapies, and Lyndra. In addition, S.D.C has consulted for: Alkermes, Charleston Labs, Clinilabs, Collegium, Depomed, Epiodyne, Inspirion Delivery Sciences, Janssen, KemPharm, Mallinckrodt, Nektar, Newron, Opiant, Otsuka, Pfizer, and Sun Pharma. She also has received honoraria from the World Health Organization. S.P., C.B., L.B., A.N.C.C., and J.D.J. have no disclosures to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders (4th ed.). Washington, DC: Author. [Google Scholar]
- Bardsley R (2019). Higher naloxone dosing may be required for opioid overdose. American Journal of Health-System Pharmacy, 76, 1835–1837. [DOI] [PubMed] [Google Scholar]
- Behar E, Bagnulo R, & Coffin P (2018). Acceptability and feasibility of naloxone prescribing in primary care settings: a systematic review. Preventative Medicine, 114, 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessen S, Metcalf SA, Saunders EC, Moore SK, Meier A, McLeman B, Walsh O, & Marsch LA (2019). Barriers to naloxone use and acceptance among opioid users, first responders, and emergency department providers in New Hampshire, USA. International Journal of Drug Policy, 74, 144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black L, Connolly I, Getty M, Hogan C, Lennon P, McCusker M, Neale J, & Strang J (2017). Poor implementation of naloxone needs to be better understood in order to save lives. Addiction, 112, 911–912. [DOI] [PubMed] [Google Scholar]
- Bonferroni CE (1936). Teoria statistica delle classi e calcolo delle probabilit `a. Pubblicazioni del R Istituto Superiore di Scienze Economiche e Commerciali di Firenze, 8, 3–62. [Google Scholar]
- Buajordet I, Næss A, Jacobsen D, & Brørs O (2004). Adverse events after naloxone treatment of episodes of suspected acute opioid overdose. European Journal of Emergency Medicine, 11, 19–23. [DOI] [PubMed] [Google Scholar]
- Cantwell K, Dietze P, & Flander L (2005). The relationship between naloxone dose and key patient variables in the treatment of non-fatal heroin overdose in the prehospital setting. Resuscitation, 64, 315–319. [DOI] [PubMed] [Google Scholar]
- Ciccarone D (2019). The triple wave epidemic: supply and demand drivers of the US opioid overdose crisis. International Journal of Drug Policy, 71, 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AK, Wilder CM, & Winstanley EL (2014). A systematic review of community opioid overdose prevention and naloxone distribution programmes. Journal Addiction Medicine, 8, 153–163. [DOI] [PubMed] [Google Scholar]
- Clarke SF, Dargan PL, & Jones AL (2005). Naloxone in opioid poisoning: walking the tightrope. Emergency Medicine Journal, 22, 612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin PO, & Sullivan SD (2013). Cost-effectiveness of distributing naloxone to heroin users for lay overdose reversal. Annals of Internal Medicine, 158, 1–9. [DOI] [PubMed] [Google Scholar]
- Creswell JW, & Plano Clark VP (2018). Designing and conducting mixed methods research. (3rd ed.) Thousand Oaks, California: Sage Publications, Inc. [Google Scholar]
- Darke S, & Hall W (1997). The distribution of naloxone to heroin users. Addiction, 92, 1195–1199. [PubMed] [Google Scholar]
- Dettmer K, Saunders B, & Strang J (2001). Take home naloxone and the prevention of deaths from opiate overdose: two pilot schemes. BMJ, 322, 895–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietze P, Cogger S, Malandkar D, Olsen A, & Lenton S (2015). Knowledge of naloxone and take-home naloxone programs among a sample of people who inject drugs in Australia Illicit Drugs Reporting System Drug Trends Bulletin April 2015. Sydney, Australia: National Drug and Alcohol Research Centre, UNSW. [Google Scholar]
- Driscoll DL, Appiah-Yeboah A, Salib P, & Rupert DJ (2007). Merging qualitative and quantitative data in mixed methods research: how to and why not. Ecological and Environmental Anthropology, 3, 19–28. [Google Scholar]
- Dwyer R, Fraser S, & Dietze P (2016). Benefits and barriers to expanding the availability of take-home naloxone in Australia: a qualitative study. Drugs: Education, Prevention and Policy, 23, 388–396. [Google Scholar]
- EMCDDA. (2015). Preventing fatal overdoses: a systematic review of the effectiveness of take-home naloxone. EMCDDA Papers. Lisbon: EMCDDA. [Google Scholar]
- Fairbairn N, Coffin PO, & Walley A (2017). Naloxone for heroin, prescription opioid and illicitly made fentanyl overdoses: challenges and innovations responding to a dynamic epidemic. International Journal of Drug Policy, 46, 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrugia A, Neale J, Dwyer R, Fomiatti R, Fraser S, Strang J, & Dietze P (2019). Conflict and communication: managing the multiple affordances of take-home naloxone administration events in Australia. Addiction Research and Theory. 10.1080/16066359.2019.1571193. [DOI] [Google Scholar]
- Faulkner-Gurstein R (2017). The social logic of naloxone: peer administration, harm reduction, and the transformation of social policy. Social Science & Medicine, 180, 20–27. [DOI] [PubMed] [Google Scholar]
- Gaddis GM, & Watson WA (1992). Naloxone- associated patient violence: an overlooked toxicity? Annals of Pharmacotherapy, 26, 196–198. [DOI] [PubMed] [Google Scholar]
- Giglio RE, Li G, & DiMaggio CJ (2015). Effectiveness of bystander naloxone administration and overdose education programs: a meta-analysis. Injury Epidemiology, 2, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfrank L, Weisman RS, Errick JK, & Lo MW (1986). A dosing nomogram for continuous infusion intravenous naloxone. Annals of Emergency Medicine, 15, 566–570. [DOI] [PubMed] [Google Scholar]
- Greene J, Deveau B, Dol J, & Butler M (2019). Incidence of mortality due to rebound toxicity after ‘treat and release’ practices in prehospital opioid overdose care: a systematic review. Emergency Medical Journal, 26, 219–224. [DOI] [PubMed] [Google Scholar]
- Heavey SC, Chang Y-P, Vest BM, Collins RL, Wieczorek W, & Homish GG (2018). ‘I have it just in case’ – Naloxone access and changes in opioid use behaviours. International Journal of Drug Policy, 51, 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedegaard H, Warner M, & Miniño AM (2017). Drug overdose deaths in the United States, 1999–2016. NCHS Data Brief, no 294. Hyattsville, MD: National Center for Health Statistics. [Google Scholar]
- Holloway K, Hills R, & May T (2018). Fatal and non-fatal overdose among opiate users in South Wales: a qualitative study of peer responses. International Journal of Drug Policy, 56, 56–63. [DOI] [PubMed] [Google Scholar]
- Horowitz Z (1998). Commentary. Subcutaneous naloxone. A less rude awakening? Academic Emergency Medicine, 5, 4, 283–285. [DOI] [PubMed] [Google Scholar]
- Kerr D, Dietze P, Kelly A-M, & Jolley D (2008). Attitudes of Australian heroin users to peer distribution of naloxone for heroin overdose: perspectives on intranasal administration. Journal of Urban Health, 85, 352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Irwin KS, & Khoshnood K (2009). Expanded access to naloxone: options for critical response to the epidemic of opioid overdose mortality. American Journal of Public Health, 99, 402–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieter PA, Chiang CN, Gyaw S, & McCann DJ (2019). Comparison of the pharmacokinetic properties of naloxone following the use of FDA- approved intranasal and intramuscular devices versus a common improvised nasal naloxone device. The Journal of Clinical Pharmacology, 59, 1078–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagu T, Anderson B, & Stein M (2006). Overdoses among friends: drug users are willing to administer naloxone to others. Journal of Substance Abuse Treatment, 30, 129–133. [DOI] [PubMed] [Google Scholar]
- Langham S, Wright A, Kenworthy J, Grieve R, & Dunlop WCN (2018). Cost-effectiveness of take-home naloxone for the prevention of overdose fatalities among heroin users in the United Kingdom. Value Health, 21, 407–415. [DOI] [PubMed] [Google Scholar]
- Lankenau S, Wagner K, Silva K, Kecojevic A, Iverson E, McNeely M, & Kral A (2013). Injection drug users trained by overdose prevention programs: responses to witnessed overdoses. Journal of Community Health, 38, 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenton S, Dietze P, Olsen A, Wiggins N, McDonald D, & Fowlie C (2015). Working together: expanding the availability of naloxone for peer administration to prevent opioid overdose deaths in the Australian Capital Territory and beyond. Drug and Alcohol Review, 34, 404–411. [DOI] [PubMed] [Google Scholar]
- Madah-Amiri D, Gjersing L, & Clausen T (2019). Naloxone distribution and possession following a large-scale naloxone programme. Addiction, 114, 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley A, Munro A, & Taylor A (2018). Once I'd done it once it was like writing your name’: lived experience of take- home naloxone administration by people who inject drugs. International Journal of Drug Policy, 58, 46–54. [DOI] [PubMed] [Google Scholar]
- McDonald R, & Strang J (2016). Are take-home naloxone programmes effective? Systematic review utilizing application of the Bradford Hill criteria. Addiction, 111, 1177–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald R, Campbell N, & Strang J (2017). Twenty years of take-home naloxone for the prevention of overdose deaths from heroin and other opioids — conception and maturation. Drug and Alcohol Dependence, 178, 176–187. [DOI] [PubMed] [Google Scholar]
- McDonald R, Danielsson Glende Ø, Dale O, & Strang J (2018). International patent applications for non-injectable naloxone for opioid overdose reversal: exploratory search and retrieve analysis of the PatentScope database. Drug and Alcohol Review, 37, 205–215. [DOI] [PubMed] [Google Scholar]
- Mitchell KD, & Higgins LJ (2016). Combating opioid overdose with public access to naloxone. Journal of Addictions Nursing, 27, 160–179. [DOI] [PubMed] [Google Scholar]
- Moe J, Godwin J, Purssell R, O’Sullivan F, Hau JP, Purssell E, Curran J, Doyle-Waters MM, Brasher PMA, Buxton JA, & Hohl CM (2020). Naloxone dosing in the era of ultra-potent opioid overdoses: a systematic review. Canadian Journal of Emergency Medicine – Journal Canadien de la Médecine d’Urgence, 1–9. doi: 10.1017/cem.2019.471 [DOI] [PubMed] [Google Scholar]
- Mueller SR, Walley AY, Calcaterra SL, Glanz JM, & Binswanger IA (2015). A review of opioid overdose prevention and naloxone prescribing: implications for translating community programming into clinical practice. Substance Abuse, 36, 240–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale J, & Strang J (2015). Naloxone – does over-antagonism matter? Evidence of iatrogenic harm after emergency treatment of heroin/opioid overdose. Addiction, 110, 1644–1652. [DOI] [PubMed] [Google Scholar]
- Neale J, Brown C, Campbell ANC, Jones JD, Metz VE, Strang J, & Comer SD (2019). How competent are people who use opioids at responding to overdoses? Qualitative analyses of actions and decisions taken during overdose emergencies. Addiction, 114, 708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen A, McDonald D, Lenton S, & Dietze P (2015). Independent evaluation of the ‘Implementing Expanded Naloxone Availability in the ACT (I-ENAACT)‘ Program, 2011-2014. Final report. Canberra: ACT Health. [Google Scholar]
- Parkin S, Neale J, Brown C, Brandt L, Campbell ANC, Castillo F, Jones JD, Strang J, & Comer SD (2020). Opioid overdose reversals in New York City by laypersons using naloxone: implications for public health and harm reduction approaches from a qualitative study. International Journal of Drug Policy, 79, 102751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2013). R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; http://www.R-project.org/ Accessed 10 January 2020. [Google Scholar]
- Richert T (2015). Wasted, overdosed, or beyond saving — To act or not to act? Heroin users’ views, assessments, and responses to witnessed overdoses in Malmo, Sweden. International Journal of Drug Policy, 26, 92–99. [DOI] [PubMed] [Google Scholar]
- Roxburgh A, Hall WD, Dobbins T, Gisev N, Pearson S, & Degenhardt L (2017). Trends in heroin and pharmaceutical opioid overdose deaths in Australia. Drug & Alcohol Dependence, 179, 291–298. [DOI] [PubMed] [Google Scholar]
- Rudd RA, Seth P, David F, & Scholl L (2016). Increases in drug and opioid-involved overdose deaths — United States, 2010–2015. Morbidity and Mortality Weekly Report, 65, 1445–1452. [DOI] [PubMed] [Google Scholar]
- Sandelowski M, Voils CI, & Knafl G (2009). On Quantitizing. Journal of Mixed Methods Research, 3, 208–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville NJ, O’Donnell J, Gladden RM, Zibbell JE, Green TC, Younkin M, Ruiz S, Babakhanlou-Chase H, Chan M, Callis BP, Kuramoto-Crawford J, Nields HM, & Walley AY (2017). Characteristics of fentanyl overdose - Massachusetts, 2014-2016. Morbidity and Mortality Weekly Report, 66, 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondhi A, Ryan G, & Day E (2016). Stakeholder perceptions and operational barriers in the training and distribution of take-home naloxone within prisons in England. Harm Reduction Journal, 13, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporer K, & Kral A (2007). Prescription naloxone: a novel approach to heroin overdose prevention. Annals of Emergency Medicine, 49, 172–177. [DOI] [PubMed] [Google Scholar]
- Sporer KA, Firestone J, & Isaacs SM (1996). Out-of-hospital treatment of opioid overdoses in an urban setting. Academic Emergency Medicine, 3, 660–667. [DOI] [PubMed] [Google Scholar]
- Strang J, Darke S, Hall W, Farrell M, & Ali R (1996). Heroin overdose: the case for take-home naloxone. British Medical Journal, 312, 1435–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang J, Neale J, McDonald R, & Kalk N (2018). Toxicity: exploring and expanding the concept. Editorial, Addiction, 113, 592–594. [DOI] [PubMed] [Google Scholar]
- Strang J, McDonald R, Campbell G, Degenhardt L, Nielsen S, Ritter A, & Dale O (2019). Take-Home naloxone for the emergency interim management of opioid overdose: the public health application of an emergency medicine. Drugs, 79, 1395–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashakkori A, & Teddlie C (1998). Mixed methodology: combining qualitative and quantitative approaches. Thousand Oaks, CA: Sage Publications. [Google Scholar]
- UKMi (UK Medicines Information). (2016). In use product safety assessment report: naloxone products for emergency opiate reversal in non-medical settings. https://www.sps.nhs.uk/articles/in-use-product-safety-assessment-report-naloxone-products-for-emergency-opiate-reversal-in-non-medical-settings/ Accessed 2 January 2020.
- UKMi (UK Medicines Information). (2017). What naloxone doses should be used in adults to reverse urgently the effects of opioids or opiates? https://www.evidence.nhs.uk/document?id=881093&returnUrl=Search%3Fq%3Dopiate%2Btoxicity&q=opiate+toxicity Accessed 2 January 2020.
- VERBI Software. (2019). MAXQDA 2019. Computer program. Berlin: VERBI Software. [Google Scholar]
- Wanger K, Brough L, MacMillan I, Goulding J, MacPhail I, & Christenson J (1998). Intravenous vs. subcutaneous naloxone for out-of-hospital management of presumed opioid overdose. Academic Emergency Medicine, 5, 293–299. [DOI] [PubMed] [Google Scholar]
- Watson WA, Steele MT, Muelleman RL, & Rush MD (1998). Opioid toxicity recurrence after an initial response to naloxone. Clinical Toxicology, 36, 11–17. [DOI] [PubMed] [Google Scholar]
- Williams AV, Strang J & Marsden J (2013). Development of Opioid Overdose Knowledge (OOKS) and Attitudes (OOAS) Scales for take-home naloxone training evaluation. Drug Alcohol Dependence, 132, 383–386. [DOI] [PubMed] [Google Scholar]
- Winstanley EL, Clark A, Feinberg J, & Wilder CM (2016). Barriers to implementation of opioid overdose prevention programs in Ohio. Substance Abuse, 37, 42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. (2014). Community management of opioid overdose. Geneva, Switzerland: World Health Organization. [PubMed] [Google Scholar]
- Winston I, McDonald R, Tas B, & Strang J (2015). Heroin overdose resuscitation with naloxone: patient uses own prescribed supply to save the life of a peer. BMJ Case Report, doi: 10.1136/bcr-2015-210391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington N, Piper T, Galea S, & Rosenthal D (2006). Opiate users’ knowledge about overdose prevention and naloxone in New York City: a focus group study. Harm Reduction Journal, 3, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright N, Oldham N, Francis K, & Jones L (2006). Homeless drug users’ awareness and risk perception of ‘Take Home Naloxone’ use – a qualitative study. Substances Abuse Treatment, Prevention and Policy, 1, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates F (1934). Contingency table involving small numbers and the χ2 test. Supplement to the Journal of the Royal Statistical Society, 1, 217–235. [Google Scholar]


