Abstract
Background
Intensive studies have failed to identify an etiologic agent in >50% cases of community-acquired pneumonia (CAP). Bacterial pneumonia follows aspiration of recognized bacterial pathogens (RBPs) such as Streptococcus pneumoniae, Haemophilus influenzae, and Staphylococcus aureus after they have colonize the nasopharynx. We hypothesized that aspiration of normal respiratory flora (NRF) might also cause CAP.
Methods
We studied 120 patients hospitalized for CAP who provided a high-quality sputum specimen at, or soon after admission, using Gram stain, quantitative sputum culture, bacterial speciation by matrix-assisted laser desorption ionization time-of-flight, and viral polymerase chain reaction. Thresholds for diagnosis of bacterial infection were ≥105 colony-forming units (cfu)/mL sputum for RBPs and ≥106 cfu for NRF.
Results
Recognized bacterial pathogens were found in 68 of 120 (56.7%) patients; 14 (20.1%) of these had a coinfecting respiratory virus. Normal respiratory flora were found in 31 (25.8%) patients; 10 (32.2%) had a coinfecting respiratory virus. Infection by ≥2 RBPs occurred in 10 cases and by NRF together with RBPs in 13 cases. Among NRF, organisms identified as Streptococcus mitis, which share many genetic features of S pneumoniae, predominated. A respiratory virus alone was found in 16 of 120 (13.3%) patients. Overall, an etiologic diagnosis was established in 95.8% of cases.
Conclusions
Normal respiratory flora, with or without viral coinfection, appear to have caused one quarter of cases of CAP and may have played a contributory role in an additional 10.8% of cases caused by RBPs. An etiology for CAP was identified in >95% of patients who provided a high-quality sputum at, or soon after, the time of admission.
Keywords: community-acquired pneumonia, etiology, lower respiratory infection, normal respiratory flora
Pneumonia results from aspiration of pathogenic bacteria that colonize the nasopharynx. Using Gram stain, quantitative cultures, and viral PCR in hospitalized patients who provided a valid sputum at admission, we show that normal respiratory flora contribute importantly to community-acquired pneumonia.
Intense prospective studies using conventional microbiologic techniques and viral polymerase chain reaction (PCR) have failed to establish an etiologic diagnosis in approximately one half of cases of community-acquired pneumonia (CAP) [1–5]. Studies utilizing molecular techniques with “high-quality” sputum samples have identified a causative organism in a much higher proportion of cases [6, 7]; the reliance on high-quality sputum helps, in part, to explain the discrepancy, but, even with this technology, no pathogen has been identified in up to 13% of cases [6, 7].
Colonization of the upper airways by recognized bacterial pathogens (RBPs) such as Streptococcus pneumoniae, Haemophilus influenzae, or Staphylococcus aureus is thought to be the initial step in the pathogenesis of bacterial pneumonia. Colonization may be followed by microaspiration of bacteria into the lower airways, a regularly occurring event even in healthy adults [8]. In the absence of good clearance mechanisms and effective innate or acquired immune responses, such aspiration may be followed by the development of pneumonia.
Consistent with the findings of Bartlett and Finegold [9], we hypothesized that microaspiration of so-called normal respiratory flora (NRF)—bacteria that normally colonize the upper airways—might be responsible for some proportion of cases of CAP [10]. Techniques used to date would not identify these bacteria: (1) microbiology laboratories regularly report NRF but do not attempt further identification of these bacteria and cannot distinguish colonizing from infecting organisms; and (2) quantitative molecular techniques have not used primers that might detect NRF. To our knowledge, no previous study has systematically examined the hypothesis that NRF plays an etiologic role in CAP. The present study applied quantitative microbiologic methods in a prospective study of patients hospitalized for CAP who were able to provide a high-quality expectorated sputum at the time of, or soon after, admission to examine the potential etiologic role of NRF in pneumonia.
METHODS
Study Design
We carried out a prospective study of a convenience sample of patients admitted to the Michael E. DeBakey VA Medical Center (MEDVAMC) between September 1, 2017 and April 30, 2019. On days selected for study, Gram stains of all sputum samples that had been submitted to the clinical microbiology laboratory in the preceding 24 hours were examined. For every sputum categorized as high quality (≥20 white blood cells [WBCs] per epithelial cell), a higher standard than that usually accepted [11], electronic medical records were reviewed to identify patients who had been admitted from the community with ≥2 of the following findings: (1) fever, increased cough, sputum production or shortness of breath, pleuritic chest pain, rales or confusion; (2) on imaging had a newly recognized pulmonary infiltrate; and (3) submitted a sputum sample within 16 hours of antibiotics being begun. A final reading of the sputum Gram stain was made by 2 observers without knowledge of the culture results, and agreement was reached by consensus. To minimize selection bias, on each day selected for study, we included every patient who met inclusion criteria. This research was approved by Review Boards at Baylor College of Medicine and MEDVAMC.
Diagnostic Studies
One-milliliter micropipetters, with the tips cut to enlarge the orifice, were used to draw up 0.5–1 mL purulent sputum that was then liquefied with 2% N-acetyl cysteine and diluted serially; 0.01 mL aliquots were streaked onto blood and chocolate agar and incubated under 5% CO2. Recognized bacterial pathogens were identified by standard microbiologic techniques. Streptococcus pneumoniae was identified by screening for sensitivity to optochin and verified by bile solubility (if colony morphology was suspicious but the optochin test was negative, bile solubility was done anyway). Organisms that are generally identified only as “normal respiratory flora” but met quantitative criteria (as defined below) were further studied by matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF). White blood cells per milliliter in liquefied sputum were counted in a hemocytometer. Almost all patients had blood cultures, nasopharyngeal swab PCR for respiratory viruses, Mycoplasma pneumoniae and Chlamydia pneumoniae, urine for pneumococcal and Legionella antigens, plasma procalcitonin, and B-natriuretic peptide.
Definitions
Patients whose sputum contained ≥105 colony-forming units (cfu)/mL of a RBP were categorized as having pneumonia due to a RBP [7, 12–14]. For diagnosing pneumonia due to NRF, we used more stringent criteria. Patients (1) whose sputum contained ≥106 cfu/mL of organism(s) that are not generally regarded as a cause of pneumonia, for example, Streptococcus mitis and other viridans streptococci, Corynebacteria, Lactobacillus, or Candida sp and (2) in whom the reading of the sputum Gram stain was consistent with these culture results were categorized as having pneumonia due to NRF. Cases in which PCR on a nasopharyngeal swab revealed a respiratory virus were diagnosed with viral pneumonia. Patients with a positive viral PCR who met criteria for RBP or NRF pneumonia were regarded as having viral/bacterial coinfection.
These criteria were used to stratify pneumonia into 6 etiologic groups: pneumonia due to (1) RBPs; (2) respiratory viruses; (3) coinfection by RBPs and a respiratory virus; (4) NRF; (5) coinfection by NRF and a respiratory virus; and (6) cause undetermined. Patients infected with RBPs whose sputum also contained >106 cfu/mL NRF will be discussed below but, to follow convention, were categorized under RBP.
Statistics
Categorical values were compared using Fisher’s exact test. Mean values among groups were compared using analysis of variance. Median values were compared using the Kruskal-Wallis test.
RESULTS
Patients
Of 163 patients whose sputum Gram stain met initial inclusion criteria, 43 were excluded for the following reasons: the official reading of the chest x-ray or a subsequent computed tomography did not confirm the presence of a pulmonary infiltrate (22 cases); antibiotics had been given for >16 hours (9); sputum was judged inadequate (8); and infection was thought not to be present (pulmonary edema in 3, diffuse alveolar hemorrhage in 1). In 79 of 120 (65.8%) cases, antibiotics had been given for ≤2 hours.
Etiology: Recognized Respiratory Pathogens
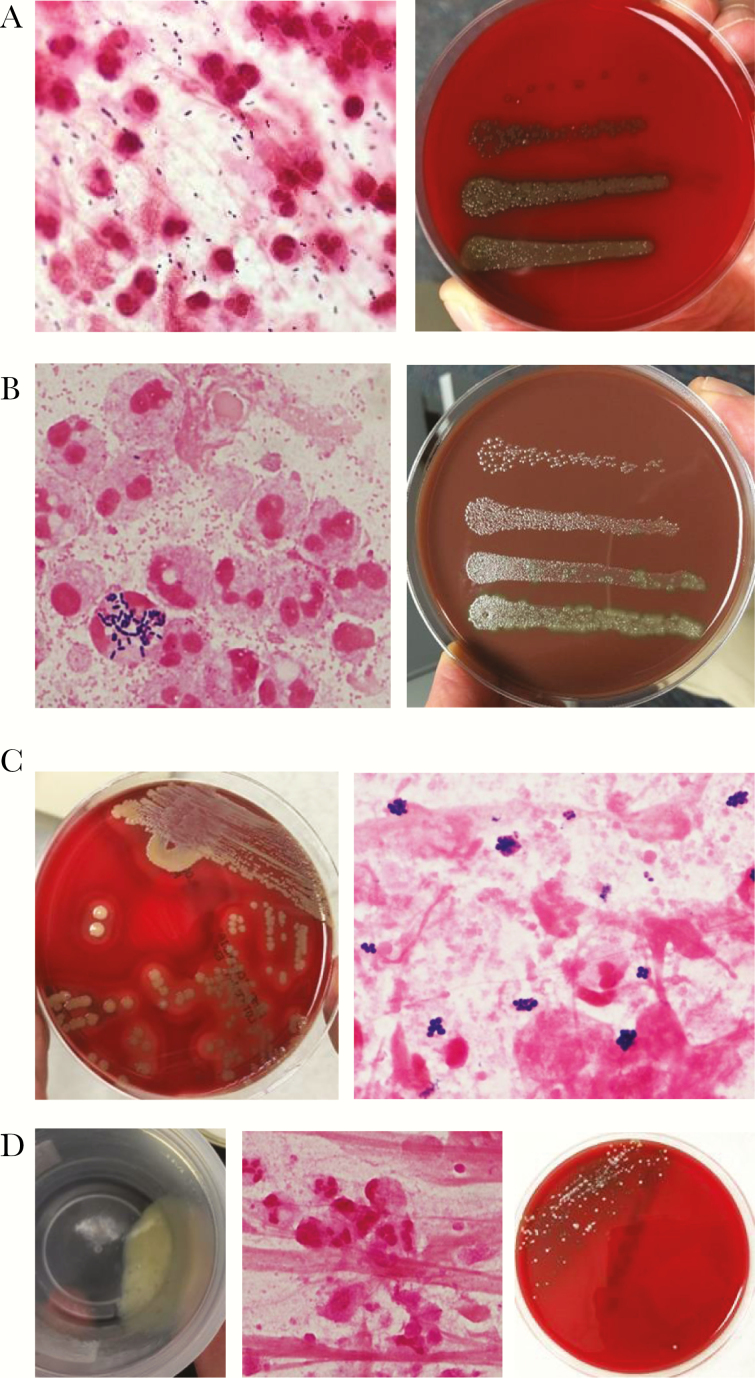
One or more RBPs were identified in sputum from 68 of 120 (56.7%) patients (Table 1); representative Gram stains and quantitative bacteriologic results are shown in Figure 1. Streptococcus pneumoniae was present in 26 of 120 (21.7%) cases—as the sole bacterial isolate in 20 (Figure 1A) and together with another RBP in 6. Haemophilus influenzae was detected in 27 (22.5%) cases, alone in 21 (Figure 1B) and together with another RBP in 6. Staphylococcus aureus (Figure 1C) and Moraxella catarrhalis were detected alone or as coinfecting bacterial agents in 11 and 7 cases, respectively. Median colony-forming units per milliliter for S pneumoniae, H influenzae, M catarrhalis, and S aureus were 2 × 106, 4 × 106, 7 × 107, and 3 × 106, respectively, and, after final review, Gram stain results were consistent with quantitative bacterial cultures in all but 4 of 68 (5.9%) cases. Cases in which Gram stain results did not match culture results were ones in which relatively small numbers of RBP’s and large numbers of NRF were detected, so it was easy to overlook the RBPs. Bacterial counts exceeded 106 cfu/mL in 65 of 68 cases of pneumonia attributed to RBPs.
Table 1.
Recognized Bacterial Pathogens in 120 Cases of Community-Acquired Pneumonia
| Bacteria | RBP Alone | RBP + Respiratory Virus |
|---|---|---|
| Streptococcus pneumoniae | 15 | 5 |
| S pneumoniae + Haemophilus influenzae | 2 | 2 |
| S pneumoniae + Staphylococcus aureus | - | 2 |
| H influenzae | 16 | 5 |
| H influenzae + Moraxella | 2 | 0 |
| S aureus | 7 | - |
| S aureus + Moraxella | 1 | - |
| S aureus + Klebsiella | 1 | - |
| Moraxella catarrhalis | 4 | - |
| Pseudomonas | 4 | - |
| Other | 2a | - |
| Total | 54 | 14 |
Abbreviations: RBP, recognized bacterial pathogen.
aOne case each of Pasteurella multocida and Mycobacterium avium/intracellulare
Figure 1.
Recognized respiratory pathogens. (A) Pneumococcal pneumonia. Gram-positive cocci on Gram stain (left). Quantitative culture yielded 1.6 × 107 Streptococcus pneumoniae per milliliter of sputum (right); the figure shows colony-forming units in 0.01-mL aliquots of sputum that had been diluted by 10–1 to 10–4 after an initial 1:2 dilution with 4% N-acetyl cysteine in 0.9% saline. (B) Haemophilus pneumonia. Gram stain (left) shows overwhelmingly predominant small Gram-negative coccobacilli. Haemophilus influenzae. It is interesting to note that occasional polymorphonuclear leukocytes (PMN) are laden with intracellular streptococci. Quantitative culture (right) revealed 2.3 × 107 Haemophilus influenzae and 5 × 104 viridans streptococci (not further speciated). In this case, the streptococci were disregarded because the number fell below the defined threshold. (C) Staphylococcal pneumonia. Sputum cultured on blood agar (left) shows nearly pure growth of Staphylococcus aureus on sputum culture. Gram stain (right) shows many Gram-positive cocci in clusters. Quantitative culture (data not shown ) yielded 2 × 106 S aureus/mL. (D) Influenza A virus pneumonia, no bacterial coinfection. Polymerase chain reaction on a nasopharyngeal swab was positive for influenza A virus. Despite absence of detectable bacteria, sputum is purulent (left, shown in collection cup) and contained 3 × 107 white blood cells per mL. Gram stain (right) is typical of the findings in viral pneumonia, showing many PMN and no bacteria. Sputum culture on blood agar showed scant growth in first quadrant only. Quantitative culture revealed 7 × 104 viridans streptococci and 3 × 104 Stomatococcus.
A respiratory virus (Table 1 and Figure 1D) was identified by PCR in 40 of 120 (33.3%) cases of CAP; in 14 cases, there was coinfection with a RBP. Thus, in these 120 cases of CAP, ≥1 recognized bacterial and/or viral pathogen(s) was/were identified in 94 (78.3%) cases.
Etiology: Normal Respiratory Flora
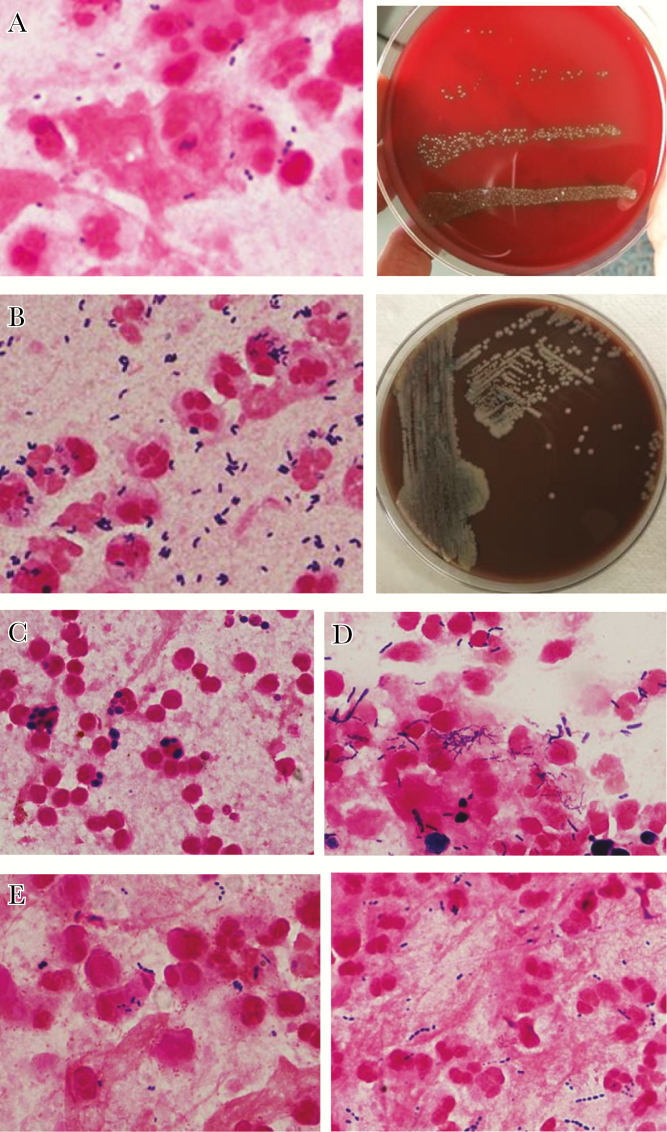
Quantitative sputum cultures from 31 of 120 (25.8%) cases of CAP yielded ≥106 cfu/mL NRF (Table 3). In 21 (17.5%) cases, the viral PCR was negative and no RBP were recognized; in these cases, the cause was attributed solely to NRF. In every case in which Gram stain showed >20 polymorphonuclear leukocytes (PMNs) per epithelial cells and large numbers of organisms including many within PMN, bacterial counts exceeded 106/mL, and no RBP was recognized even on quantitative culture (which allows for closer examination for minority populations). A coinfecting respiratory virus was documented in 10 cases (8.3% of the total 120 cases and 32% of patients infected with NRF). Organisms identified by MALDI-TOF as S mitis (oralis), alone or together with other NRF, predominated (14 of 31 [45.2%] cases; Figure 2A); the median cfu/mL of these streptococci was 5 × 106. Other NRF included viridans streptococci other than S mitis, Corynebacteria (Figure 2B), Lactobacillus sp, and Candida sp (Figure 2C). Chronic aspiration was cited in the medical records of 5 patients, including 3 of the 4 whose sputum contained >106 Candida per mL; in all of these patients, many PMNs contained yeast forms, and, in 2, the serum assay for 1,3-beta-d-glucan was strongly positive (>500 pgm/mL). All samples that had large numbers of Candida were polymicrobial. After final review of sputum Gram stains in cases attributed to NRF, microscopic readings matched quantitative cultures in every case but 2. In these 2 cases, large numbers of Gram-positive cocci were seen by Gram stain, but quantitative cultures yielded <105 cfu per mL; we attributed infection in these cases to anaerobic organisms and categorized them as due to NRF.
Table 3.
Etiologic Role of Normal Respiratory Flora in 120 Cases of Community-Acquired Pneumonia
| Microorganisms | NRF Alone | NRF + Respiratory Virus |
|---|---|---|
| Streptococcus mitis (oralis) | 3 | 3 |
| S mitis (oralis) + other(s)a | 7 | 1 |
| Other (sole isolate) | ||
| Streptococcus | 3 | 1 |
| Corynebacterium | 0 | 0 |
| Lactobacillus | 1 | 1 |
| Candida albicans | 1 | 0 |
| Others,a no S mitis | 6 | 4 |
| Total | 21 | 10 |
Abbreviations: NRF, normal respiratory flora.
aOthers include the following: Streptococcus sanguinis, Streptococcus parasanguinis, and Streptococcus salivarius; Corynebacterium propinquum and Corynebacterium pseudodiphtheriticum; Lactobacillus fermentarium; Actinomyces odontolyticus; Rothia mucilagenosa; Candida albicans and Candida glabrata.
Figure 2.
Normal respiratory flora (NRF). (A) Pneumonia due to Streptococcus mitis (oralis). Gram stain (left) shows many polymorphonuclear leukocytes and Gram-positive cocci. Quantitative culture (right) revealed 6 × 106 S mitis (oralis) with <104/mL other bacteria. (B) Pneumonia due to Corynebacterium pseudodiphtheriticum. Sputum Gram stain (left) showed profuse short Gram-positive rods, some suggesting “Chinese lettering.” Routine sputum culture on admission (chocolate agar plate, right) showed with nearly pure growth, and quantitative culture yielded 2 × 107 colony-forming units C pseudodiphtheriticum per mL. The reading of Gram stain by the microbiology laboratory was “mixed Gram-positive organisms,” and the final culture report was “normal respiratory flora.” (C) Pneumonia due to Candida glabrata. Patient was suspected to have intermittent aspiration. Gram stain shows large numbers of yeast, many of which appear to be intracellular, and some Gram-positive cocci. Quantitative sputum culture showed 2 × 107 C glabrata and 2 × 105 viridans streptococci per mL. Serum (1,3) beta-d-glucan level was not done. (D) Coinfection by respiratory virus (influenza) and NRF. In this patient with pneumonia who was noted to be chronically aspirating, polymerase chain reaction was positive for influenza virus. Gram stain showed many Gram-positive rods and yeast, many of which are cell-associated. Quantitative culture revealed 5 × 107 Lactobacillus gasseri and 5 × 10 < 6 Candida albicans. Serum (1,3) beta-d-glucan level was >500 pcg/mL (strongly positive). Fine, beaded Gram-positive filamentous bacteria did not grow in aerobic cultures. (E) Coinfection by RBP and NRF. Haemophilus influenzae and S mitis (left). Gram stain shows many small Gram-negative coccobacilli and Gram-positive cocci, many of which were cell-associated. Quantitative culture yielded 1.8 × 106 S mitis (oralis) and 1.2 × 106 H influenzae. Streptococcus pneumoniae and S mitis (right). Gram stain shows characteristic pairs of Gram-positive cocci suggestive of S pneumoniae as well as long chains of streptococci that are not consistent with pneumococcus. Quantitative culture revealed 3 × 106 S pneumoniae and 1 × 106 S mitis (oralis) per mL.
Viral Infection and Viral/Bacterial Coinfections
Polymerase chain reaction identified a respiratory virus in 40 of 120 (33.3%). In 16 cases, rare or no bacteria were seen on Gram stain (Figure 1D is representative), and sputum contained <105 bacteria per mL; in these cases, pneumonia was attributed to the virus alone. Bacterial coinfection was present in 24 of 40 (60%) cases with viral detection: 14 cases with RBP and 10 with NRF. Fourteen of 68 (20.5%) patients with RBP and 10 of 31 (32.3%) with NRF had viral coinfection (P = 0.2), consistent with the hypothesis that NRF, on their own, may cause pneumonia.
Bacterial/Bacterial Coinfections
Sputum culture from 10 of 68 (14.7%) RBP pneumonias yielded ≥2 RBPs (Table 1). An additional 13 of 68 (19.1%) patients with RBP pneumonia had ≥1 × 106 cfu of NRF per mL sputum (Figure 2E); although, in these cases, NRF may have contributed to infection, we followed convention by listing them in Tables 1, 2, and 4 as pneumonia due to RBP (see Discussion).
Table 2.
Respiratory Viruses in 120 Cases of Community-Acquired Pneumonia
| Virus | Virus Alone | Virus + RBP | Virus + NRF |
|---|---|---|---|
| Influenza virus A | 5 | 8 | 2 |
| Influenza virus B | 1 | - | 1 |
| Rhinovirus | 6 | 2 | 4 |
| Human metapneumovirus | 1 | 2 | 2 |
| Respiratory syncytial virus | 2 | 1 | - |
| Adenovirus | 1 | 1 | - |
| Parainfluenza virus | - | - | 1 |
| Total | 16 | 14 | 10 |
Abbreviations: NRF, normal respiratory flora; RBP, recognized bacterial pathogen.
Table 4.
Final Identification of Bacterial and Viral Etiology in 120 Cases of Community-Acquired Pneumonia
| Recognized bacterial pathogen | 54 (45.0%)a |
| Recognized bacterial infection + viral coinfection | 14 (11.7%) |
| Respiratory virus alone | 16 (13.3%) |
| Normal respiratory flora | 21 (17.5%) |
| Normal respiratory flora + viral coinfection | 10 (8.3%) |
| Undetermined | 5 (4.2%)b |
| Total | 120 (100%) |
aFollowing accepted convention, and, to be able to relate these numbers to those in prior reports, we included in this category13 cases in which a recognized bacterial pathogen was isolated but, based on Gram stain and quantitative culture results, coinfection with normal respiratory flora was thought to play a role.
bIncludes 2 cases in which polymerase chain reaction (PCR) for influenza virus and respiratory syncytial virus were negative but the full viral respiratory PCR was not done.
Etiology, Total
In total (Table 4), RBPs caused CAP in 68 (56.7%) of 120 patients; 14 (20.6%) were coinfected with a respiratory virus. Normal respiratory flora caused CAP in 31 (25.8%) cases; 10 (32.3%) of these had viral coinfection. This difference in the rate of viral coinfection was not significant (P = .26). Bacterial coinfection by ≥2 RBP or by RBP plus NRF was seen in 23 (19.2%) cases.
A respiratory virus was found in 40 (33.3%) of 120 cases of CAP. More importantly, for purposes of treatment, 24 (60%) of all patients with a positive PCR for a respiratory virus had evidence for bacterial coinfection, whether by RBP or NRF. Overall, an etiologic agent was identified in 115 (95.8%) of 120 cases of CAP.
Charlson Comorbidity Index
Using the Charlson comorbidity index (CCI), we sought to determine whether patients with pneumonia due to NRF were more likely than those with RBP to be susceptible to pneumonia due to the presence of comorbid conditions. The CCI for all patients with NRF pneumonia was 6.6 vs 5.3 for those with RBP pneumonia and 4.2 for those with viral pneumonia alone (P = .01).
Laboratory Data
Blood cultures were positive in 6 (5.0%) cases, including 6 of 68 (8.8%) patients with pneumonia due to RBP and 0 of 31 (0%) due to NRF (P = .17). The median WBC count in peripheral blood of patients with pneumonia due to recognized bacteria (with or without viral coinfection) was 13 100/mm3, compared with 11 200 in patients with pneumonia due to NRF (with or without viral coinfection, P = .06) and 8400 in those with viral pneumonia alone (Table 5); in patients with viral infection, peripheral WBC count was significantly lower than in bacterial pneumonia (P = .01). The intensity of the inflammatory response in the lungs, as measured by median WBC per milliliter in liquefied sputum, was slightly greater in pneumonia due to recognized pathogens than pneumonia due to NRF (1.7 × 107 vs 1.0 × 107 per mL, P = .04), and far greater when all bacterial pneumonias were compared with viral pneumonias (1.5 × 107 vs 3.2 × 106, P = .01). Median procalcitonin levels were similar in these groups of patients, as was 14-day mortality.
Table 5.
Certain Clinical Features Related to Etiology
| Clinical Feature | Bacterial Pathogen ± Virus | Normal Flora ± Virus | Virus Only | P Value |
|---|---|---|---|---|
| Peripheral WBC (per mm3)a | 13 100 | 11 200 | 8400 | .01b |
| Procalcitonin (ng/mL)a | 0.33 | 0.15 | 0.2 | .13c |
| Sputum WBC (per mL)a | 1.7 × 107 | 1.0 × 107 | 3.2 × 106 | .003d |
| 14-day mortality (%) | 3.0% | 3.2% | 12.5% | .44e |
Abbreviations: WBC, white blood cells.
aData presented as medians.
bOverall comparison, P = .01; bacterial pathogen vs normal respiratory flora (NRF), P = .06; all bacterial vs viral, P = .01 (Kruskal-Wallis).
cOverall comparison, P = .13; bacterial pathogen vs NRF, P = .06; all bacterial vs viral, P = .22 (Kruskal-Wallis).
dOverall comparison, P = .003; bacterial pathogen vs NRF, P = .04; all bacterial vs viral, P = .01 (Kruskal-Wallis).
eOverall comparison, P = .44; bacterial pathogen vs NRF, P = .38; all bacterial vs viral, P = .31 (Fisher’s exact).
DISCUSSION
The novel finding in this study is that bacteria that are generally reported as NRF appears to play a causative role in 25.8% of cases of CAP. In 17.5% of adults hospitalized for CAP who provided a high-quality sputum, pneumonia appeared to be caused by NRF alone and, in 8.3%, by coinfection with NRF and a respiratory virus. In an additional 19.1% of patients whose sputum yielded RBP, coinfection with NRF may have played a contributory role; mixed bacterial infections will be discussed in detail below. These results support the hypothesis that, just as aspiration of RBPs cause pneumonia after colonization of the nasopharynx, in some proportion of cases, aspiration NRF that colonize the nasopharynx may do the same.
The present study identified a recognized bacterial and/or viral pathogen in 78.3% of cases of CAP, a result strikingly different from other recent studies (including ours [1]) that found a bacterial cause in <30% and failed to identify any cause in >50% of CAP [1–5, 15, 16]. The explanation for this difference is that the present study included only patients who expectorated a high-quality sputum and who had not received antibiotics for >16 hours; in fact, in two thirds of cases, antibiotics had been given for ≤2 hours. Although only a minority of pneumonia patients produce such a sputum in timely fashion, the sensitivity and specificity of Gram stain and culture of such specimens for RBP have previously been shown to be quite good [17–21]. Forty of 120 (33.3%) patients had PCR evidence for a viral infection, 14 (35.0%) of whom were coinfected with RBP; using different criteria, Falsey et al [22] found that, of 348 patients who were hospitalized for respiratory illness, a similar proportion (136 [39.1%]) had evidence for concurrent viral and bacterial infection. Using quantitative PCR, Gadsby et al [7] demonstrated an RBP in 87% of CAP; these authors did not use primers that could detect NRF. Including results for RBP, NRF, and viruses, the present study identified an etiologic CAP in 95.8% of CAP.
We are unaware of any previous study that has systematically sought a role for NRF in CAP. These organisms have been detected in transtracheal aspirates or bronchoalveolar lavage of patients with pneumonia, but investigators (including ourselves [12]) have paid little attention to them [12, 14, 23, 24]. Most microbiology laboratories do not speciate or otherwise identify NRF in sputum cultures, even when they are the predominant isolate (eg, Figure 2B). Nonetheless, a pathogenic role for NRF, including S mitis [25] and Rothia [26], has been demonstrated. We recently described a series of cases of pneumonia due to Corynebacterium sp [27], and Garg et al [28] documented bacteremia due to viridans streptococci and Corynebacteria in patients with influenza virus pneumonia.
The principal objection to the results of our study (aside from the fact that it goes against years of tradition in the world of microbiology and infectious diseases) is that it is not possible to obtain a sputum sample that is not heavily contaminated by oral bacteria. The following factors show that this is not the case and support the validity of our results. (1) Using semiquantitative methods, Chodosh [29, 30] reported that, during infection-free intervals, Gram-stained sputum from patients with chronic bronchitis contained very few bacteria. (2) Sputum from patients with viral pneumonia had rare or no bacteria on Gram stain and <3 × 105 cfu/mL NRF on quantitative culture (Figure 1D). (3) Sputum from patients with RBP were often remarkably free of other bacteria on Gram stain and quantitative culture (Figure 1A–C). (4) When NRF were implicated, Gram stains and cultures were similarly free of other organisms (Figure 2A and B). (5) The median number of NRF per milliliter sputum in CAP patients (7 × 106 per mL) was strikingly similar to that observed in patients with pneumonia due to RBP (8 × 106).
Among our patients with CAP, streptococci in the mitis group, generally identified by MALDI-TOF as S mitis (oralis), were identified (with or without a respiratory virus) as the sole bacterial pathogen in 6 patients and together with other NRF in an additional 8 patients, thereby potentially implicating this group of organisms as the third most common bacterial cause of CAP (after S pneumoniae and H influenzae). The taxonomy of the mitis streptococci has become much more complicated with careful genetic analysis [31], but, in this study, we only identified alpha-hemolytic streptococci to the level of mitis after carefully excluding S pneumoniae, the limit to which most microbiology laboratories can go at the present time. Streptococcus mitis shares molecular characteristics of S pneumoniae, including the capacity to make capsule, and ample evidence shows their capacity to cause serious infection in humans [25, 32, 33]. Not surprisingly, NRF appeared to be less virulent than RBPs. Blood cultures were uniformly negative in NRF pneumonia (it should be noted that blood cultures are generally negative in pneumonia caused nontypeable H influenzae or Moraxella). In addition, the peripheral WBC count was lower in pneumonia due to NRF. The CCI was significantly greater in patients with NRF pneumonia, consistent with the concept that these individuals were more susceptible to pneumonia caused by less virulent bacteria, and 32.3% of patients with NRF pneumonia had viral coinfection compared with 14.7% in patients with RBPs, suggesting that a second insult may be necessary to allow NRF to cause pneumonia.
Of patients with RBP pneumonia, sputum from 14.7% had ≥2 RBPs. Mixed bacterial infections in pneumonia were well documented in the past [34, 35], although this phenomenon has not received much attention from modern clinicians. Recent studies using molecular techniques also have recognized multiple bacterial pathogens in high-quality sputum samples [6, 7, 36]. These studies have all reported coinfections only with RBPs. We found that 13 of 68 (19.1%) patients infected with RBPs were coinfected with NRF, based on the presence of equal or higher numbers of NRF in their sputum, suggesting a pathogenic role for NRF in an even larger proportion of patients with CAP.
We specifically did not exclude patients who were identified by clinicians as having aspiration pneumonia (generally chronic aspiration in neurologically impaired and/or bedridden individuals) because our underlying hypothesis is that microaspiration plays a central role in the pathogenesis of all bacterial pneumonia. Chronic (macro)aspiration was noted clinically in only 5 patients in this series. Consistent with the concept that aspiration of bacteria of low pathogenicity by patients who are unable to clear secretions may cause pneumonia, these 5 patients had only NRF in their sputum or tracheal secretions, including several with large numbers of Candida and/or Lactobacillus species. Although the teaching has been that Candida does not cause CAP, the presence in sputum of large numbers of yeast forms within PMN (Figure 2C), high counts of Candida (8 × 106 per mL), and positive tests for beta-d-glucan indicate that they do.
Our results validate the reliability of Gram stain under the conditions stated, namely that the sputum sample be of good quality and antibiotics not have been given for >16 hours. They suggest that a Gram stain showing rare or no bacteria in a patient with a positive viral PCR who has not received antibiotics would justify withholding antibiotic therapy, a suggestion that opposes current guidelines [37]. However, absent such a Gram stain, our findings support recommendations by the guidelines for empiric antibiotics for patients hospitalized for CAP even if a viral PCR is a positive because fully 60% of our patients with a positive viral PCR had bacterial coinfection.
The present study, a single-center study with mainly male patients, was confined to patients who provided a high-quality expectorated sputum. We sought to minimize selection bias by selecting days to investigate during a 19-month period and, on those days, studying every patient who submitted a sputum during the preceding 24 hours. Because we were studying patients who were acutely infected, we focused on organisms that could be identified by culture and did not address the lung microbiome [38], although NRF are clearly an important component of that biome and the microbiome is a likely determinant of what organisms emerge to cause bacterial pneumonia. If pneumococci had been present in very small numbers in sputum, for example 105 per mL in the presence of 5 × 106 S mitis, we might not have been able to detect them. However, their presence in such small numbers relative to other bacteria might then raise serious question about their relevance.
CONCLUSIONS
In conclusion, this study shows that NRF, alone or with viral coinfection, cause approximately one quarter of cases of CAP and may also contribute to another 11% of cases caused by RBPs. By limiting this study to patients who could provide a high-quality sputum specimen at, or shortly after admission, we found that (1) a causative organism could be identified in >95% of patients hospitalized for CAP, (2) bacteria, whether RBP or NRF, played a causative role in 82.5% of cases, and (3) when PCR demonstrates a respiratory virus, 35% of patients have bacterial coinfection due to RBP and another 25% to NRF. These results appear to validate current guidelines [37] that recommend empiric antibiotic therapy for all patients hospitalized for pneumonia.
Acknowledgments
We are deeply indebted to the technologists of the Microbiology Laboratory at the Michael E. DeBakey VA Medical Center, without whose gracious and expert assistance this work could not have been done. Dr. Duc T. Nguyen kindly assisted us with statistical analysis.
Potential conflicts of interest. Since the completion of the work reported in this study, but during the time the manuscript was in preparation, D. N. C. has received salary from Merck & Co. As the data-gathering was nearing completion, D. N. C. left Baylor College of Medicine to take a position with Merck, where he receives a salary and stock options, but his work in no way presents a conflict of interest with the present study. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Musher DM, Roig IL, Cazares G, et al. Can an etiologic agent be identified in adults who are hospitalized for community-acquired pneumonia: results of a one-year study. J Infect 2013; 67:11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jain S, Self WH, Wunderink RG, et al. ; CDC EPIC Study Team Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015; 373:415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ieven M, Coenen S, Loens K, et al. ; GRACE consortium Aetiology of lower respiratory tract infection in adults in primary care: a prospective study in 11 European countries. Clin Microbiol Infect 2018; 24:1158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. File TM, Goldberg L, Das A, et al. Efficacy and safety of intravenous-to-oral lefamulin, a pleuromutilin antibiotic, for the treatment of community-acquired bacterial pneumonia: the Phase III Lefamulin Evaluation Against Pneumonia (LEAP 1) Trial. Clin Infect Dis 2019; 69:1856–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johansson N, Kalin M, Tiveljung-Lindell A, et al. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis 2010; 50:202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wolff BJ, Bramley AM, Thurman KA, et al. Improved detection of respiratory pathogens by use of high-quality sputum with TaqMan array card technology. J Clin Microbiol 2017; 55:110–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gadsby NJ, Russell CD, McHugh MP, et al. Comprehensive molecular testing for respiratory pathogens in community-acquired pneumonia. Clin Infect Dis 2016; 62:817–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mandell LA, Niederman MS. Aspiration pneumonia. N Engl J Med 2019; 380:651–63. [DOI] [PubMed] [Google Scholar]
- 9. Bartlett JG, Finegold SM. Anaerobic pleuropulmonary infections. Medicine (Baltimore) 1972; 51:413–50. [DOI] [PubMed] [Google Scholar]
- 10. Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med 2014; 371:1619–28. [DOI] [PubMed] [Google Scholar]
- 11. Murray PR, Washington JA. Microscopic and baceriologic analysis of expectorated sputum. Mayo Clin Proc 1975; 50:339–44. [PubMed] [Google Scholar]
- 12. Thorsteinsson SB, Musher DM, Fagan T. The diagnostic value of sputum culture in acute pneumonia. JAMA 1975; 233:894–5. [PubMed] [Google Scholar]
- 13. Musher DM, Kubitschek KR, Crennan J, Baughn RE. Pneumonia and acute febrile tracheobronchitis due to Haemophilus influenzae. Ann Intern Med 1983; 99:444–50. [DOI] [PubMed] [Google Scholar]
- 14. Jordan GW, Wong GA, Hoeprich PD. Bacteriology of the lower respiratory tract as determined by fiber-optic bronchoscopy and transtracheal aspiration. J Infect Dis 1976; 134:428–35. [DOI] [PubMed] [Google Scholar]
- 15. Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015; 372:1114–25. [DOI] [PubMed] [Google Scholar]
- 16. File TM Jr, Low DE, Eckburg PB, et al. Integrated analysis of FOCUS 1 and FOCUS 2: randomized, doubled-blinded, multicenter phase 3 trials of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in patients with community-acquired pneumonia. Clin Infect Dis 2010; 51:1395–405. [DOI] [PubMed] [Google Scholar]
- 17. Musher DM, Montoya R, Wanahita A. Diagnostic value of microscopic examination of Gram-stained sputum and sputum cultures in patients with bacteremic pneumococcal pneumonia. Clin Infect Dis 2004; 39:165–9. [DOI] [PubMed] [Google Scholar]
- 18. Fukuyama H, Yamashiro S, Kinjo K, et al. Validation of sputum Gram stain for treatment of community-acquired pneumonia and healthcare-associated pneumonia: a prospective observational study. BMC Infect Dis 2014; 14:534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gleckman R, DeVita J, Hibert D, et al. Sputum gram stain assessment in community-acquired bacteremic pneumonia. J Clin Microbiol 1988; 26:846–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van der Eerden MM, Vlaspolder F, de Graaff CS, et al. Value of intensive diagnostic microbiological investigation in low- and high-risk patients with community-acquired pneumonia. Eur J Clin Microbiol Infect Dis 2005; 24:241–9. [DOI] [PubMed] [Google Scholar]
- 21. Ogawa H, Kitsios GD, Iwata M, Terasawa T. Sputum Gram stain for bacterial pathogen diagnosis in community-acquired pneumonia: a systematic review and Bayesian meta-analysis of diagnostic accuracy and yield. Clin Infect Dis 2020; 71:499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Falsey AR, Becker KL, Swinburne AJ, et al. Bacterial complications of respiratory tract viral illness: a comprehensive evaluation. J Infect Dis 2013; 208:432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ries K, Levison ME, Kaye D. Transtracheal aspiration in pulmonary infection. Arch Intern Med 1974; 133:453–8. [PubMed] [Google Scholar]
- 24. Bartlett JG. Diagnostic accuracy of transtracheal aspiration bacteriologic studies. Am Rev Respir Dis 1977; 115:777–82. [DOI] [PubMed] [Google Scholar]
- 25. Shelburne SA, Sahasrabhojane P, Saldana M, et al. Streptococcus mitis strains causing severe clinical disease in cancer patients. Emerg Infect Dis 2014; 20:762–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ramanan P, Barreto JN, Osmon DR, Tosh PK. Rothia bacteremia: a 10-year experience at Mayo Clinic, Rochester, Minnesota. J Clin Microbiol 2014; 52:3184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang K, Kruse RL, Lin WV, Musher DM. Corynebacteria as a cause of pulmonary infection: a case series and literature review. Pneumonia (Nathan) 2018; 10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garg S, Jain S, Dawood FS, et al. Pneumonia among adults hospitalized with laboratory-confirmed seasonal influenza virus infection-United States, 2005–2008. BMC Infect Dis 2015; 15:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chodosh S. Clinical significance of the infection-free interval in the management of acute bacterial exacerbations of chronic bronchitis. Chest 2005; 127:2231–6. [DOI] [PubMed] [Google Scholar]
- 30. Chodosh S. Acute bacterial exacerbations in bronchitis and asthma. Am J Med 1987; 82:154–63. [PubMed] [Google Scholar]
- 31. Jensen A, Scholz CFP, Kilian M. Re-evaluation of the taxonomy of the Mitis group of the genus Streptococcus based on whole genome phylogenetic analyses, and proposed reclassification of Streptococcus dentisani as Streptococcus oralis subsp. dentisani comb. nov., Streptococcus tigurinus as Streptococcus oralis subsp. tigurinus comb. nov., and Streptococcus oligofermentans as a later synonym of Streptococcus cristatus. Int J Syst Evol Microbiol 2016; 66:4803–20. [DOI] [PubMed] [Google Scholar]
- 32. Lessa FC, Milucky J, Rouphael NG, et al. Streptococcus mitis expressing pneumococcal serotype 1 capsule. Sci Rep 2018; 8:17959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kilian M, Riley DR, Jensen A, et al. Parallel evolution of Streptococcus pneumoniae and Streptococcus mitis to pathogenic and mutualistic lifestyles. mBio 2014; 5:e01490–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sheng ZM, Chertow DS, Ambroggio X, et al. Autopsy series of 68 cases dying before and during the 1918 influenza pandemic peak. Proc Natl Acad Sci U S A 2011; 108:16416–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Finland M. The significance of mixed infections in pneumococci pneumonia. JAMA 1934; 103:1681–6. [Google Scholar]
- 36. Gadsby NJ, McHugh MP, Forbes C, et al. Comparison of Unyvero P55 pneumonia cartridge, in-house PCR and culture for the identification of respiratory pathogens and antibiotic resistance in bronchoalveolar lavage fluids in the critical care setting. Eur J Clin Microbiol Infect Dis 2019; 38:1171–8. [DOI] [PubMed] [Google Scholar]
- 37. Metlay JP, Waterer G, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Resp Crit Care Med 2019; 200:e45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dickson RP, Huffnagle GB. The lung microbiome: new principles for respiratory bacteriology in health and disease. PLoS Pathog 2015; 11:e1004923. [DOI] [PMC free article] [PubMed] [Google Scholar]