Abstract
Activating KRAS mutations are present in 25% of human cancer. Although oncogenic Ras was deemed “undruggable” in the past, recent efforts led to the development of pharmacological inhibitors targeting the KRASG12C mutant, which have shown promise in early clinical trials. The development of allele-specific K-RasG12C inhibitors marked a new chapter in targeting oncogenic KRAS mutant in cancer. However, drug resistance against these new drugs will likely limit their efficacy in the clinic. Genome-wide approaches have been used to interrogate the mechanisms of resistance to K-RasG12C inhibitors, which would facilitate the development of therapeutics overcoming drug resistance. This article reviews the latest progress in resistance to K-RasG12C-targeted therapies and aims to provide insight in future research targeting drug resistance in cancer.
Keywords: oncogene, KRAS inhibitor, targeted therapy, drug resistance
Graphical Abstract

Public Summary
-
•
Clinical grade K-RasG12C inhibitor marks a new chapter in targeted drug discovery
-
•
Resistance to K-RasG12C inhibitors is driven by intrinsic or acquired mechanisms
-
•
Co-targeting vertical Ras signaling overcomes resistance to K-RasG12C inhibition
-
•
Standard-of-care chemo- and immunotherapies synergize with K-RasG12C inhibition
Main Text
Introduction
KRAS (Kirsten rat sarcoma 2 viral oncogene homolog) is one of the most prevalent oncogenes in a variety of human cancers.1,2 Although there is compelling evidence that oncogenic KRAS drives tumorigenesis, efforts to target mutant KRAS have been stalled for many years. Recently, progress has been made in the development of therapeutics against KRASG12C mutation,3, 4, 5, 6, 7 which is present in ~15% of lung adenocarcinoma and 0%–8% of other cancers (Table 1).8,9 Results from early-stage clinical trials indicate that many cancer patients in this subgroup could significantly benefit from these novel therapeutics.6,7 However, therapeutic resistance to K-RasG12C inhibition is not only noted in preclinical tumor models,10, 11, 12 but also in the clinic. Tumor relapses were commonly observed among patients who initially responded well to these therapies.6,13,14 Recently, several studies elegantly elaborated the mechanisms underlying resistance to K-RasG12C inhibition,10, 11, 12 and there were pioneering efforts using combinatorial treatments to overcome drug resistance.6,7,10, 11, 12,15 Here, we will briefly summarize KRAS activating mutation in cancer and revisit recent strategies targeting mutant K-Ras activation. We will then mainly focus on mechanisms of resistance to K-Ras inhibitors and recent progress in overcoming resistance to K-Ras inhibition.
Table 1.
Frequencies of KRASG12C Mutation among Different Types of Cancers
| Cancer Type | KRASG12C Mutation Frequency (%) | Data Source |
|---|---|---|
| Lung adenocarcinoma | ~15 | The Tumor Sequencing Project |
| Colorectal carcinoma | ~8 | Memorial Sloan Kettering Cancer Center |
| Pancreatic adenocarcinoma | ~4 | University of Texas Southwestern Medical Center |
| Urothelial carcinoma | ~2 | Beijing Genomics Institute (2013) |
| Stomach cancer | ~1 | Tianjin Medical University Cancer Institute and Hospital (2015) |
| Mesothelioma | 0–1 | The Cancer Genome Atlas |
K-Ras Signaling Pathway in Cancer
In humans, three RAS genes (HRAS [Harvey rat sarcoma viral oncogene homolog], NRAS [neuroblastoma RAS viral oncogene homolog], and KRAS) encode four 21-kDa small GTPases (H-Ras, N-Ras, K-Ras4A, and K-Ras4B) with high homogeneity.16,17 Ras protein structures consist of a GDP/GTP binding domain (G domain) and a C terminus that is responsible for membrane targeting.18,19 By binding to either guanosine 5′-diphosphate (GDP) or guanosine-5′-triphosphate (GTP), Ras proteins are maintained in inactive or active states, respectively.20,21 The ratio between active, GTP-bound Ras protein and inactive, GDP-bound Ras is regulated by several regulatory proteins that integrate inputs from multiple cellular signaling pathways.22 Upon growth factor stimulation, Ras interacts with guanine nucleotide exchange factors (GEFs), which dissociate Ras-bound GDP.23 Due to the excess of intracellular GTP compared with GDP, the free Ras proteins preferentially bind GTP and become activated.24 On the other hand, Ras GTPase-activating proteins (GAPs) inactivate Ras by promoting the hydrolysis of Ras-bound GTP to GDP.23
In a normal cell, Ras activation status is intricately regulated by the subcellular translocation of GEFs and GAPs.23 For example, the activation of receptor tyrosine kinases (RTKs) recruit SOS Ras/Rac guanine nucleotide exchange factor 1 (SOS1), a Ras GEF, to activate Ras on the plasma membrane.25,26 The activation of Ras promotes cell growth and survival through the canonical mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinases (ERK), and phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin complex 1 (mTORC1) signaling pathways, as has been extensively reviewed elsewhere (Figure 1).27,28 Ras signaling pathways are tightly regulated in normal cells and malignant transformation occurs when Ras proteins are constitutively activated.1,29,30 In most cases, the mutations of RAS genes in tumor cells confers resistance to GAPs-mediated GTP hydrolysis, locking Ras protein in its active state that favors tumor growth.30,31 Despite RTK signaling independence, RAS mutant cancer cells require the Src homology 2-containing phosphotyrosine phosphatase (SHP2) for cell proliferation in vivo, as well as in growth factor-limited in vitro conditions.32 It is not fully understood how SHP2 activates mutant Ras under these conditions, although its interaction with Ras GEF SOS1 is presumably involved.33 Currently, RAS mutation testing using allele-specific PCR, real-time PCR, or nucleic acid sequencing is applied in the clinic to determine optimal anticancer treatments for cancer patients, which also provides invaluable data about the frequency of each RAS subfamily gene mutation in cancer.34,35 Among different RAS gene mutations, KRAS mutation is the most frequently observed, accounting for 83% of all RAS mutations in human cancers.8,36 Glycine codon 12 (G12) and G13 mutations represent 81.5% and 14% of all KRAS mutations, respectively, while the mutations in other positions of KRAS gene are rare.8,36 Mechanistically, the small side chain of glycine at codons 12 and 13 makes wild-type K-Ras accessible to the arginine finger of most GAPs for triggering GTP hydrolysis.37,38 Upon G12 and G13 mutation, GAPs-mediated K-Ras inactivation is hampered, resulting in the accumulation of GTP-bound active K-Ras.24,39
Figure 1.
Ras Signaling Pathway.
In normal physiological conditions, the binding of receptor tyrosine kinases (RTKs) with growth factors activate SHC-GRB2-SOS1 complex, which induces the formation of active GTP-bound Ras proteins. Downstream signals of Ras GTP include the activation of MAPK/ERK and PI3K/AKT/mTORC1 signaling pathways, which promotes cell growth. GEF, guanine nucleotide exchange factor; GAP, GTPase-activating protein.
Drugs Targeting Mutant K-Ras
Pioneering Efforts on K-Ras Inhibition
In the past three decades, there has been intense interest in the development of therapeutics targeting oncogenic K-Ras. However, most attempts directly targeting the K-Ras protein have been unsuccessful for two important reasons.40, 41, 42, 43 First, K-Ras protein has a relatively smooth surface, with few well-characterized hydrophobic pockets where compounds could bind.41,44 Second, the efforts of trying to design small molecules blocking the GTP binding site on K-Ras have been challenging, given the extremely high affinity of K-Ras for GTP.41,45 These failures have led to the perception that K-Ras is not druggable. Nonetheless, several pioneering studies have identified a series of small-molecule compounds that could directly bind to K-Ras protein. For example, SCH-54292 (6) was designed to suppress the formation of active GTP-bound Ras by binding to a cleft close to switch II on Ras protein.46 4,6-Dichloro-2-methyl-3-aminoethyl-indole (DCAI) is another group of compounds that bind to a unique pocket adjacent to the switch I/II region of Ras and interfere with the interaction between Ras and SOS1.47 Unfortunately, these compounds unselectively target wild-type as well as mutant K-Ras proteins. Although exciting progress has been made using these approaches, the pharmacological targeting of mutant K-Ras is still considered preferable, since the direct targeting of mutant K-Ras protein will help to avoid adverse effects resulting from the inhibition of wild-type Ras signaling in non-malignant cells. Apart from these direct K-Ras targeting strategies, several indirect approaches have been proposed for inhibiting K-Ras signaling, which have been extensively reviewed elsewhere.40,41,48
Direct K-RasG12C Inhibitors
Recently, the heroic effort to target oncogenic Ras mutants has resulted in the development of several promising small-molecule cysteine-reactive inhibitors, such as compound 12, that covalently modified the mutant K-RasG12C protein.3 The crystallographic structure of K-RasG12C covalently bound by cysteine-reactive compounds revealed a special allosteric switch II pocket that is not apparent in naive K-RasG12C protein.3 The binding of these compounds to K-RasG12C induces structurally disordered switch I/II, which converts the GTP preference of naive K-RasG12C to GDP. Consequently, a large fraction of K-RasG12C protein is trapped in the inactive GDP-bound state, which impairs its interaction with downstream effectors, such as rapidly accelerated fibrosarcoma kinase (RAF).3,4,49 The identification of the novel allosteric site inspires additional search for K-RasG12C inhibitors applicable in the clinic. ARS-853 was firstly selected as a potent inhibitor that suppresses K-RasG12C-dependent cell proliferation.4,49 However, it failed to demonstrate in vivo efficacy due to undesirable pharmacokinetics.4,5,49 Other efforts led to the development of a series of next-generation K-RasG12C inhibitors that are highly potent and active in vivo, including ARS-1620, AMG-510, and MRTX849.5, 6, 7 ARS-1620 was designed based on the structure of ARS-853 through scaffold optimization, and was the first proof-of-concept drug demonstrating the feasibility of targeting K-RasG12C in vivo.5 AMG-510 and MRTX849 are structural derivatives of ARS-1620 with improved sensitivity of K-RasG12C recognition. They were obtained by screening His95 groove-binding molecules and optimizing for favorable drug-like properties, respectively (Figure 2).6,7 In 2019, phase I/II clinical trials of both AMG-510 and MRTX849 demonstrated safety and therapeutic activity, notably in non-small cell lung cancer (NSCLC), where the frequency of KRASG12C mutation approaches 15% (Table 2).6,50,51 Remarkably, AMG-510 or MRTX849 treatment resulted in tumor regression in approximately 50% of NSCLC patients with K-RasG12C mutation.6,50
Figure 2.
Chemical Structures of K-RasG12C Inhibitors.
Table 2.
A Brief Summary of Current K-RasG12C Inhibitors
| K-RasG12C Inhibitor | Developer/Year | IC50 for H358 Cells In Vitro | Cmax at 100 mg/kg p.o. | Significance | Stage | Recommended Phase 2 Dose | Overall Response Rate | Reference |
|---|---|---|---|---|---|---|---|---|
| Compound 12 | University of California, San Francisco (2013) | 10 μM | NA | first proof-of-concept K-RasG12C inhibitor | NA | NA | NA | Ostrem et al.3 |
| ARS-853 | Wellspring Biosciences (2016) | 2.5 μM | NA | robust cell-active K-RasG12C inhibitor | NA | NA | NA | Patricelli et al.4 |
| ARS-1620 | Wellspring Biosciences (2018) | 120 nM | 1.3 μM | first proof-of-concept compound for in vivo K-RasG12C inhibition | preclinical | NA | NA | Janes et al.5 |
| AMG-510 | Amgen Inc (2019) | 10 nM | 1.2 μM | first potent and selective K-RasG12C inhibitor in clinic, first proof-of-concept compound for K-RasG12C inhibition-driven antitumor immunity | phase 1/2 | 960 mg, daily | 48% in KRASG12C NSCLC, 6% in other KRASG12C mutant cancers | Canon et al.6 |
| MRTX1257 | Mirati Therapeutics, Inc (2019) | NA | NA | highly sensitive K-RasG12C inhibitor | preclinical | NA | NA | Marx et al.52 |
| MRTX849 | Mirati Therapeutics, Inc (2019) | 100 nM | 1.4 μM | potent and highly selective K-RasG12C inhibitor in clinic | phase 1/2 | 600 mg twice daily | 50% in KRASG12C NSCLC, 14% in other KRASG12C mutant cancers | Hallin et al.7 |
| JNJ-74699157 (ARS-3248) | Johnson & Johnson/Wellspring Biosciences (2019) | NA | NA | potent and highly selective clinical K-RasG12C inhibitor | phase 1 | NA | NA | NCT04006301 at ClinicalTrials.gov |
| LY3499446 | Eli Lilly | NA | NA | potent and highly selective clinical K-RasG12C inhibitor | phase 1/2 | NA | NA | NCT04165031 at ClinicalTrials.gov |
Besides the well-studied approach exploiting the allosteric switch II pocket, Lim et al.53 proposed an alternative strategy for K-RasG12C inhibition by targeting the catalytic site on K-Ras protein. SML-10-70-1, a GDP-derived analog that covalently modifies cysteine 12 of K-RasG12C, was identified with anti-proliferative activity in KRASG12C mutant models.53 However, SML-10-70-1 functioned as a nonspecific K-RasG12C inhibitor, as K-RasG12S-bearing cells also responded to this compound.53
Targeting KRAS Mutations Other Than G12C
Currently, KRASG12C is the only targetable form of KRAS mutation because the thiol group of cysteine 12 provides a unique handle that can be covalently modified by thiol-reacting small-molecule inhibitors.3 Nonetheless, emerging evidence suggests that other KRAS mutants might also be druggable. For example, by screening phage-displayed peptide libraries against K-RasG12D versus wide type K-Ras, peptide KRpep-2 (Ac-RRCPLYISYDPVCRR-NH2), and the optimized sequence, KRpep-2d (Ac-RRRRCPLYISYDPVCRRRR-NH2), were identified as capable of selectively inhibiting K-RasG12D and downstream ERK activation.54 In addition, both Mirati Therapeutics and Revolution Medicines have launched preclinical programs for the development of allele-specific K-RasG12D inhibitors.55,56 Above all, such pioneering studies may eventually contribute to the development of drugs targeting all types of KRAS mutations.
Mechanisms Underlying Resistance to K-RasG12C Inhibitors
Although results from early-stage clinical trials have shown promise, it should be cautioned that ~50% of KRASG12C mutant NSCLC failed to respond to K-RasG12C-targeted therapies. In preclinical studies, cancer relapse is common even among animals that respond well to these drugs in the beginning.6,50 This is not surprising, since decades of research on targeted cancer therapy have shown that the majority of patients who respond well to initial treatment develop resistance.57, 58, 59, 60 Although long-term efficacy of K-RasG12C-targeted therapies in patients is still unknown,6,50 adaptive resistance to both AMG-510 and MRTX849 has been observed in preclinical animal models.7,11
Intrinsic Resistance to K-RasG12C Inhibitors
Resistance to anticancer drugs can be either intrinsic or acquired.57,60 Intrinsic resistance pre-exists before treatment, whereas acquired resistance is developed among patients initially responsive to the treatment.57,60 Given that only 50% of patients with K-RasG12C mutant NSCLC would benefit from K-RasG12C-targeted therapies,6,50 it would be plausible that certain subgroups of patients are intrinsically resistant to K-RasG12C inhibitors. Preclinically, among 12 American Type Culture Collection-derived KRASG12C mutant cell lines, 11 exhibited heterogeneous inhibition of cell growth after AMG-510 treatment, whereas 1 failed to respond.6 In addition, the in vivo responses to MRTX849 in 25 KRASG12C mutant human cell line-derived xenografts were individually represented as sensitive, partially sensitive, or refractory.7 These preclinical findings strongly imply that intrinsic resistance might be accountable for the heterogeneous responses to K-RasG12C inhibition in the clinic.4, 5, 6, 7,49 Mechanistically, low dependency on K-Ras signaling could confer intrinsic resistance to K-RasG12C inhibitors.12,58,61 Singh et al.62 found that K-Ras signaling dependency varies across cell models harboring mutant KRAS, implicating that KRAS mutant cancer might not always be addicted to K-Ras signaling. In some cases, even complete ablation of KRAS gene using CRISPR-Cas9 technology does not affect cell viability in KRAS mutant models.63 By comparing the viability of cells treated with either ARS-1620 or mitogen-activated protein kinase (MEK) inhibitor trametinib, Misale et al.12 concluded that a fraction of KRASG12C mutant cell lines are refractory to both drugs because viability in these cells was not primarily controlled by ERK, the major K-Ras downstream effector. In general, tumor cell growth is primarily controlled by the canonical MAPK/ERK and PI3K/AKT/mTORC1 signaling pathways, despite the dependency on either pathway varying across models.64,65 Although active Ras protein could interact with PI3K p110 subunit for AKT activation,66,67 PI3K activation is not solely controlled by Ras.68,69 In most KRASG12C mutant models, the phosphorylation status of AKT and mTORC1-effector ribosomal protein S6 is barely affected by K-RasG12C inhibition.6,7,12,49 Instead, K-RasG12C inhibitors act primarily through targeting MAPK/ERK pathway for growth inhibition.4, 5, 6, 7,49 Therefore, the redundancy of parallel growth signals could bypass the necessity of mutant K-Ras in promoting cell proliferation and underlies the inherent resistance to K-RasG12C-targeted therapies (Figure 3A–3C).12,63 Besides the weak dependence on K-Ras signaling for proliferation, intrinsic resistance could be attributable to concurrent genetic alterations that impede the activity of K-RasG12C inhibitors.57,58 For example, secondary KRAS mutations would confer intrinsic resistance to ARS-853 in KRASG12C mutant models by either potentiating nucleotide exchange (secondary mutation: Y40A, N116H, or A146V) or impairing inherent GTPase activity (secondary mutation: A59G, Q61L, or Y64A) (Figure 3D).49 Notably, the mutation status of KRAS gene could be heterogeneous in the same patient, which leads to the heterogeneous responses to K-RasG12C inhibition across individuals, as well as in different tumors of the same patient (Figure 3E).70,71
Figure 3.
Intrinsic Resistance to Drugs Targeting K-RasG12C.
(A) K-RasG12C inhibitors function mainly through suppressing the MAPK/ERK signaling pathway and variably affecting other growth-promoting signaling pathways, including the PI3K/AKT/mTORC1 pathway.
(B and C) However, the extent to which cell proliferation depends on the MAPK/ERK pathway varies across models. KRASG12C mutant cells that heavily depend upon MAPK/ERK signaling for proliferation are highly sensitive to K-RasG12C inhibitors (B). Otherwise, cells will be refractory to K-RasG12C inhibition (C).
(D) Secondary KRAS mutations conferring either increased GEF activity or decreased GAP activity to K-Ras protein will result in resistance to K-RasG12C inhibitors.
(E) The mutation status of KRASG12C may be heterogeneous among primary tumor and metastases, as well as across individual intratumor cancer cells, which leads to inconsistent responses to K-RasG12C inhibitors among patients.
Acquired Resistance to K-RasG12C Inhibitors
It has been long recognized that acquired resistance will invariably occur after prolonged treatment with targeted drugs, including those against Ras downstream effectors, such as RAF and MEK.72, 73, 74 Although the adaptive responses to Ras downstream inhibition would differ from Ras targeting, lessons learnt from the well-studied RAF and MEK inhibitors has provided key insights into the mechanism of resistance to K-RasG12C inhibition.10,11 For example, prolonged treatment of either RAF or MEK inhibitors results in rebounded ERK activation due to the amplification of upstream drivers, such as RTKs and Ras.72,74 Interestingly, the restoration of flux through the ERK pathway was similarly observed after ARS-1620 treatment, and the feedback activation of Ras upstream RTKs has been raised as one of the key mechanisms of resistance to ARS-1620.12,15 Recently, Xue et al.10 described an exquisite mechanism of the rapid adaption of cancer cells to K-RasG12C inhibitor and demonstrated that subpopulations of isogenic KRASG12C mutant cells responded to K-RasG12C inhibition in a heterogeneous manner. It is found that a subpopulation of cells rapidly generates acquired resistance to ARS-1620 by regaining K-Ras activity, while others would either be quiescent or undergo apoptosis.10 In this study, the adaption of cells to K-RasG12C inhibition was dependent on new K-RasG12C synthesis, rather than the activity of other wild-type Ras isoforms.10 Due to the increased dependence on epidermal growth factor receptor (EGFR) and SHP2 signal in surviving cells, newly synthesized K-RasG12C was maintained in its GTP-bound state to promote cell proliferation.10 Furthermore, aurora kinase A (AURKA) was found to facilitate the escape of initial drug-induced quiescence through its interaction with K-RasG12C and downstream protein c-Raf.10 In another study, Ryan et al.11 elaborated a similar acquired resistance pathway to K-RasG12C inhibition. They found that Ras downstream effectors, including MEK, ERK, and 40S ribosomal protein S6 kinase were reactivated 24–48 h after ARS-1620 and AMG-510 treatment, despite a rapid inactivation of these molecules within 4 h.11 However, in direct contrast to findings made by Xue et al.,10 increased GTP-bound wild-type Ras (N-Ras and H-Ras) proteins were responsible for restoring MAPK activation after drug treatments, whereas K-Ras is maintained in its inactive state.11 This discrepancy could not be solely attributed to diverse experimental systems adopted between research groups since both of them used cell lines, such as H358, H23, and H1792 in ARS-1620 study, and GTP-bound K-Ras was further analyzed using similar assays.10,11 Despite apparent discrepancy on the role of wild-type Ras activation, both studies revealed that the restoration of overall Ras activity was due to increased RTK-SHP2 activation (Figure 4).10,11 These acquired resistance pathways must be overcome to achieve complete and durable therapeutic responses by K-RasG12C inhibition in clinic.
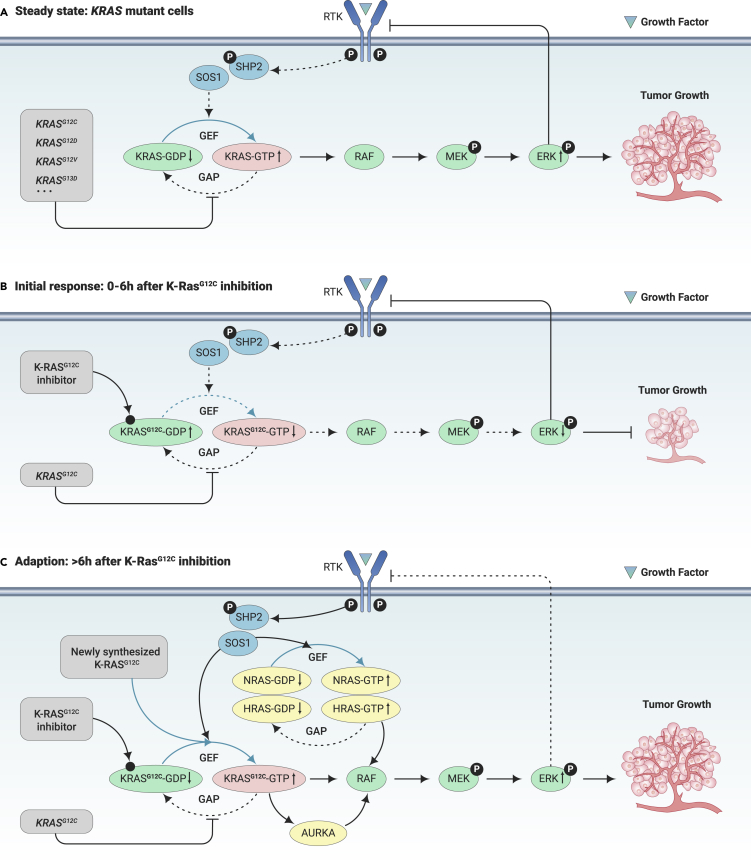
Figure 4.
Acquired Resistance to Drugs Targeting K-RasG12C.
(A) Steady state of KRAS mutant cells. Activating mutations of KRAS genes confer resistance to GAP-mediated K-Ras GTP hydrolysis, leading to uncontrolled activation of K-Ras downstream signaling and tumor growth.
(B) Initial response to K-RasG12C inhibition. K-RasG12C inhibitors function by locking K-RasG12C in its GDP-binding state and hence suppress MAPK/ERK signaling and tumor growth.
(C) Adaptive resistance to K-RasG12C inhibition. After initial response, ERK-mediated feedback inhibition of vertical RTKs/SHP2 pathway is lifted, which induces the activation of N-Ras, H-Ras, and K-RasG12C. Given that RTKs/SHP2 signaling pathway is hyperactive in this stage, newly synthesized K-RasG12C immediately binds with GTP, potentiating the feedback adaptive resistance. In addition, AURKA operates by interacting with GTP-bound K-RasG12C and promotes downstream RAF activation. Consequently, ERK is reactivated and tumor growth is resumed.
Resistance to K-RasG12C Inhibitors in Patients
In the clinic, intrinsic and acquired resistance may co-exist and intertwine in the same patient treated with K-RasG12C-targeted therapies.57,61 Genomic heterogeneity arising from a variety of co-occurring mutations may contribute to the heterogeneous extent of adaptive resistance across KRAS mutant cancers.57,58 For example, the expression of select HER (human epidermal growth factor receptor) family members of RTKs and genes controlling early cell-cycle transition exhibited a correlation with the degree of therapeutic response to MRTX849,7 although individual genetic alterations, including TP53 (tumor protein p53), CDKN2A (cyclin-dependent kinase inhibitor 2A), STK11 (serine/threonine kinase 11), and KRAS mutant allele frequency failed to predict adaptive resistance.6,7,11 Therefore, therapeutic schemes for K-RasG12C inhibition should be adjusted based on the genomic analysis of co-existing genetic alterations, such as RTK mutations and secondary RAS mutations to achieve ideal therapeutic activity for these ground-breaking medications.7,10,49
Overcoming Resistance to Mutant K-Ras Inhibitors
Due to the redundancy of signals that control tumor growth, drug combinations targeting multiple key nodes in growth-promoting signaling pathways has led to increasing Food and Drug Administration (FDA) approvals.75,76 Mechanistically, regimens that combine different anticancer drugs target pro-growth or pro-survival signaling pathways in either a synergistic or an additive manner, which reduces drug resistance.77,78 To overcome the resistance to targeted K-RasG12C inhibition, pioneering efforts have been made on combinatorial K-Ras-targeted therapies, which have shown promising preclinical results. In general, these combinatorial K-RasG12C inhibition strategies could be categorized as vertical Ras pathway co-targeting, as well as drug combinations involving chemotherapeutics or immunotherapeutics (summarized in Table 3).
Table 3.
Overcoming Resistance to K-RasG12C Inhibition through Combinatorial Therapies
| Combinatorial Strategy | Combination | Drug Combo | Reference | Mechanism of Action | Significance | Stage |
|---|---|---|---|---|---|---|
| RTK co-inhibition | K-RasG12C inhibitor + EGFR inhibitor | ARS-853 + Erlotinib ARS-853 + Gefitinib ARS-1620 + Erlotinib AMG-510 + Erlotinib |
Patricelli et al.4 Lito et al.49 Ryan et al.11 Canon et al.6 Misale et al.12 |
targeting EGFR-mediated feedback resistance | highly effective in models that heavily depend on individual RTKs for feedback resistance, yet not universally effective across different KRASG12C mutant models | preclinical |
| K-RasG12C inhibitor + HER kinases inhibitor | ARS-853 + Afatinib ARS-1620 + Afatinib AMG-510 + Afatinib MRTX849 + Afatinib |
Patricelli et al.4 Lito et al.49 Ryan et al.11 Canon et al.6 Hallin et al.7 |
targeting HER kinases-mediated feedback resistance | |||
| K-RasG12C inhibitor + c-MET inhibitor | ARS-853 + Crizotinib ARS-1620 + Crizotinib |
Lito et al.49 Ryan et al.11 |
targeting c-MET-mediated feedback resistance | |||
| K-RasG12C inhibitor + SRC/ABL inhibitor | ARS-853 + Saracatinib | Lito et al.49 | targeting SRC/ABL-mediated feedback resistance | |||
| K-RasG12C inhibitor + FGFR inhibitor | ARS-853 + PD173974 ARS-1620 + BGJ398 |
Lito et al.49 Ryan et al.11 Misale et al.12 |
targeting FGFR-mediated feedback resistance | |||
| SHP2 co-inhibition | K-RasG12C inhibitor + SHP2 inhibitor | ARS-1620 + SHP099 ARS-1620 + RMC-4550 AMG-510 + SHP099 AMG-510 + RMC-4550 MRTX849 + RMC-4550 |
Ryan et al.11 Xue et al.10 Canon et al.6 Hallin et al.7 |
overcoming K-RasG12C inhibition-mediated adaptive feedback resistance by co-targeting SHP2, which integrates signaling from multiple RTKs to Ras | a feasible strategy to improve the clinical efficacy of K-RasG12C inhibition across different models | phase 1 in preparation |
| SOS1 co-inhibition | K-RasG12C inhibitor + SOS1 inhibitor | ARS-853 + BAY-293 | Hillig et al.79 | blocking K-RasG12C reactivation by disrupting RAS-SOS1 interaction | first combinatorial strategy co-targeting the guanine nucleotide exchange reaction of Ras GTPase | preclinical |
| AURKA co-inhibition | K-RasG12C inhibitor + AURKA inhibitor | ARS-1620 + Alisertib | Xue et al.10 | overcoming K-RasG12C reactivation and the escape of quiescence by disrupting Ras-Raf interaction | a proof-of-concept strategy co-targeting the reciprocal AURKA signaling pathway | preclinical |
| MAPK/ERK pathway co-inhibition | K-RasG12C inhibitor + MEK1/2 inhibitor | ARS-853 + Trametinib ARS-1620 + Trametinib AMG-510 + Trametinib |
Patricelli et al.4 Lito et al.49 Misale et al.12 Canon et al.6 |
eliminating bypass or residual MEK1/2 signaling that induces resistance | effective in models that heavily depend on MAPK/ERK pathway for proliferation | preclinical |
| K-RasG12C inhibitor + ERK inhibitor | ARS-853 + SCH984 | Lito et al.49 | inhibiting the feedback reactivation of ERK signaling | limited combinatorial effects | ||
| PI3K/AKT pathway co-inhibition | K-RasG12C inhibitor + PI3K inhibitor | ARS-853 + BAY806946 ARS-1620 + GDC0941 AMG-510 + AMG-511 |
Lito et al.49 Misale et al.12 Canon et al.6 |
overcoming resistance to K-RasG12C inhibition by either decreasing PIP3-bound-GABs that promote ERK reactivation, or inducing concomitant shut-down of both MAPK/ERK and PI3K/AKT pathways | highly effective in KRASG12C mutant models that take advantage of PI3K/AKT signaling for cell growth | preclinical |
| K-RasG12C inhibitor + AKT inhibitor | ARS-853 + MK2206 ARS-1620 + MK2206 AMG-510 + AZD-5363 |
Lito et al.49 Misale et al.12 Canon et al.6 |
inducing concomitant shut-down of AKT/mTOR singling pathway, which promotes cell proliferation | |||
| K-RasG12C inhibitor + mTOR inhibitor | ARS-853 + AZD8255 ARS-1620 + AZD8055 MRTX849 + Vistusertib |
Lito et al.49 Misale et al.12 Hallin et al.7 |
||||
| RTK and mTOR co-inhibition | K-RasG12C inhibitor + IGF1R inhibitor + mTOR inhibitor | ARS-1620 + Linstinib + Everolimus | Molina-Arcas et al.15 | co-targeting IGF1R and mTOR/AKT-mediated intrinsic and adaptive resistance | effective in KRAS mutant cells showing reduced KRAS dependency for growth | preclinical |
| Chemotherapy co-treatment | K-RasG12C inhibitor + chemotherapeutic | AMG-510 + Carboplatin | Canon et al.6 | potentiating tumoricidal effects of K-RasG12C inhibitor by causing DNA damage | combining targeted K-RasG12C inhibition with a standard-of-care chemotherapeutic | preclinical |
| K-RasG12C inhibitor + CDK4/6 inhibitor | MRTX849 + Palbociclib | Hallin et al.7 | Blocking RB/E2F-dependent cell proliferation in CDKN2A-null models through K-RasG12C-CDK4/6 co-inhibition | effective in MRTX849-refractory models with CDKN2A homozygous deletion | preclinical | |
| Immunotherapy co-treatment | K-RasG12C inhibitor + immune checkpoint inhibitor | AMG-510 + anti-PD-1 MRTX849 + anti-PD-1 |
Canon et al.6 Briere et al.13 |
potentiating K-RasG12C inhibition-driven antitumor immunity and immunological memory | unraveling a long-overlooked pairing between targeted therapy and immunotherapy in cancer treatment | phase 1 in preparation |
Co-targeting Vertical Ras Signaling Pathway
a. Co-targeting K-RasG12C and RTKs
Upon K-Ras inhibition, the relief of ERK-mediated negative feedback promotes the expression of RTKs, which in turn reactivates Ras signaling to confer therapeutic resistance.80,81 Since acquired resistance primarily occurs via adaptive RTK-SHP2-induced reactivation of Ras and its downstream effectors,10,11 combining K-RasG12C inhibitor with drugs targeting RTK signaling might be beneficial. The caveat with combination therapies is that using two or more inhibitors would significantly increase toxicity if they non-selectively act on normal cells and cancer cells.76,82 However, since K-RasG12C inhibitors are highly specific for the mutant protein, drug combinations involving K-RasG12C inhibitors would likely be better-tolerated in patients.83 RTK inhibitors were among the first sets of combinatorial drugs that were tested to potentiate the therapeutic efficacy of K-RasG12C inhibitors. In 2016, two research groups set out to investigate RTK inhibitors used in combination with ARS-853.4,49 Patricelli et al.4 demonstrated that the pretreatment of either EGFR inhibitor erlotinib or HER kinases inhibitor afatinib primed H358 cells for ARS-853-induced K-Ras inhibition. Lito et al.49 reported that the effect of ARS-853 was potentiated by EGFR inhibition in H2122 and Calu-1 cells. However, in H1792 and H2030 cells, concurrent inhibitions of tyrosine-protein kinase Met (c-MET), proto-oncogene tyrosine-protein kinase Src (SRC) or fibroblast growth factor receptor (FGFR) were found to be more effective, implicating heterogeneous involvement of different RTKs in acquired resistance to K-RasG12C targeting.49 To assess the role of individual RTKs involved in Ras reactivation, Ryan et al.11 carried out a phospho-RTK array analysis. Their results indicated that after ARS-1620 treatment, the phosphorylation of multiple RTKs increased with high heterogeneity among different KRASG12C mutant models. Using high-throughput drug screening, Misale et al.12 identified several RTK inhibitors exhibiting strong synergies with ARS-1620. Unfortunately, synergistic effects of these combinations were not consistent across different cell models. Together, these studies suggest that strategies targeting a single RTK in combination with K-RasG12C inhibition might not be universally effective in cancer therapy (Table 3).
b. Co-targeting K-RasG12C and RTK-to-Ras Common Nodes
Given the individual-specific involvement of RTK, targeting common nodes downstream of multiple RTK signaling pathways may represent a feasible strategy to overcome adaptive Ras reactivation across heterogeneous KRAS mutant cancers.11,84 RTK-associated phosphatase SHP2 has emerged as a potentially targetable RTK signaling node and has attracted extensive research interests.7,10,11 In normal cells, RTKs activates Ras by recruiting the SHC-GRB2-SOS1 complex independent of SHP2.85 However, in KRAS mutant cancer cells, SHP2 inhibition trigger a senescence response in vivo and in growth factor-limited conditions.32 Previous experiences with trametinib, a clinical MEK inhibitor have taught us that targeted inhibition of Ras downstream effectors, including MEK would inevitably trigger the relief of ERK-mediated feedback inhibition of RTK signaling, which results in adaptive Ras reactivation that heavily depends on SHP2.86 In these regards, SHP2 was purposed as a combinatorial target in overcoming the resistance to MEK inhibition and presumably for drugs targeting K-RasG12C.32,86,87 Although how SHP2 activates Ras is still an open question, studies made by Ryan et al.,11 Xue et al.,10 and their colleagues showed that concurrent inhibition of SHP2 and K-RasG12C by small-molecule inhibitors (e.g., SHP099 and ARS-1620) enhanced the antiproliferative effects of KRASG12C inhibitors in both in vitro and in vivo models. In another study, the combination of MRTX849 and RMC-4550, a more clinically relevant SHP2 inhibitor, was found to further inhibit K-Ras signaling and exhibit increased antitumor responses in MRTX849-sensitive and -refractory models.7 These compelling preclinical evidences supporting the synergy between K-RasG12C and SHP2 inhibition provided the foundation for early-phase clinical trials of combination therapy by both Amgen and Mirati Therapeutics in KRASG12C-mutant cancers.14 In addition to SHP2, efforts have also been made co-targeting another common node for RTK-induced Ras activation, SOS1, the guanine nucleotide exchange factor that activates K-Ras.88 BAY-293, a potent SOS1 inhibitor, was recently found to synergize with ARS-853 to inhibit Ras activation and cell proliferation by disrupting Ras-SOS1 interaction (Table 3).79
c. Co-targeting K-RasG12C and Its Downstream Effectors
Because K-Ras is a key signaling protein controlling multiple pathways critical for cell proliferation and survival, co-targeting K-Ras upstream was hypothesized to be superior to co-targeting downstream, as it would not only induce more potent inhibition of one specific downstream pathway, but also avoid the activation of another parallel signaling pathway that eventually lead to tumor growth.84 This hypothesis was supported by recent preclinical studies on the RAF inhibitor as well as its combinations in BFAFV600E mutant cancer, and implicated a similar resistant mechanism followed by K-RasG12C inhibition and co-inhibitions involving K-RasG12C. To be specific, although combinatorial BRAF/MEK targeting has led to successful FDA approval for BFAFV600E mutant melanoma, recent research raises concerns about the release of upstream RTK and Ras signals that activate reciprocal growth-promoting signaling pathways, such as the PI3K/AKT/mTORC1 pathway in preclinical melanoma models.89 In early-phase clinical trials for BFAFV600E mutant colorectal cancer where EGFR is the dominant RTK driving tumor growth, co-inhibition of EGFR and RAF, with or without downstream MEK inhibition, are required to induce durable complete tumor regression.83 In line with the these findings, inconsistent results were observed co-treating KRASG12C mutant cancer cells with K-RasG12C inhibitors plus either MEK or ERK inhibitors, limiting the use of these drug combinations in the clinic.6,12,49 Recently, several publications have shown that co-inhibition of K-RasG12C together with a common node of reciprocal pathway might also be promising. Using unbiased combinatorial drug screening approaches, it was found that EGFR, FGFR, the Src family kinases, and PI3K inhibitors were synergistic with K-RasG12C inhibition in some preclinical models, although only PI3K inhibitors exhibited consistent synergistic effects across different models.12 Furthermore, co-targeting K-RasG12C with PI3K effectors, such as ATK and mTOR has also been shown to be effective in a series of preclinical studies.6,7,10,15,49 The broad efficacy of K-RasG12C/PI3K co-inhibition could be due to decreased phosphatidylinositol (3,4,5)-trisphosphate (PIP3)-bound GABs that promote ERK reactivation, or the concomitant shut-down of ERK and PI3K pathways inducing maximum growth inhibition.12 In another study, Xue et al.10 discovered a novel target, AURKA, by combining the results of single-cell RNA sequencing with CRISPR-Cas9 screening. Significant synergy was observed between ARS-1620 and alisertib, a specific AURKA inhibitor in treating ARS-1620-refractory KRASG12C mutant cancers.10 Mechanistically, AURKA inhibition functions by disrupting the stable interaction between K-Ras and proto-oncogene c-RAF (c-Raf), resulting in sustained ERK suppression and quiescence (Table 3).10
Combining K-RasG12C Inhibition with Standard-of-Care Chemotherapeutics
As cancer patients routinely receive antitumor treatments, such as chemotherapy,90,91 it would be interesting to test if the combinatory use of standard-of-care chemotherapeutics with K-RasG12C inhibitors could achieve enhanced efficacy. Indeed, AMG-510 and MRTX849, two clinical K-RasG12C inhibitors, have been recently tested in combination with carboplatin and palbociclib, respectively, in KRASG12C mutant lung cancers.6,7 Carboplatin is a one of the most common platinum-based chemotherapeutics.92 Although single treatment with either AMG-510 or carboplatin inhibited tumor growth, combination treatments with both drugs resulted in significantly enhanced tumor regression in xenograft mouse models.6 Similarly, co-treatment of MRTX849 with palbociclib, a recently FDA-approved CDK4/6 inhibitor, induced more comprehensive tumor regression in several MRTX849-refractory models by profoundly suppressing the retinoblastoma protein (Rb)/E2F transcription factor (E2F) pathway.7 These studies together provide rationales for combining chemotherapy with K-RasG12C inhibition for cancer management in clinic (Table 3).
Combining K-RasG12C Inhibition with Immunotherapies
KRAS mutant cancers are immunosuppressive in nature because oncogenic K-Ras signaling induces the expression of immune modulatory factors, such as interleukin-10 and transforming growth factor β to promote an immunosuppressive tumor microenvironment.93 Therefore, K-Ras inhibition might be able to covert the immunosuppressive microenvironment to one that favors antitumor immune response. Canon et al.6 reported that the clinical K-RasG12C inhibitor AMG-510 could drive antitumor immunity. Treatment with AMG-510 at high dose resulted in durable tumor regression in immunocompetent mice, whereas in immunocompromised mice, tumors rapidly returned after a short response.6 The durable drug response observed in immunocompetent mice could be, at least in part, attributable to the increased tumor cell expression of major histocompatibility complex class I-presented antigens and T cell-recruiting chemokines, leading to enhanced T cell priming and infiltration required for long-term antitumor responses.6 Consistently, MRTX849, another clinical K-RasG12C inhibitor, has been shown to promote tumor antigen presentation and recondition tumor microenvironments in CT-26 KRASG12C tumor models.13 These studies, demonstrating the immunoregulatory effects of K-RasG12C inhibitors, brought a new horizon in the clinical application of targeted therapies to improve antitumor immunity.
Over the past decade, immunotherapy has permanently changed cancer treatment landscape and is considered the “fifth pillar” of cancer therapy.94, 95, 96 The importance of cancer immunotherapy has been acknowledged in 2018 by the Nobel prize awarded for the discoveries made on immune checkpoints, including cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1), which are exploited by tumor cells for immune evasion.97 Disruption of this axis using checkpoint inhibitors induces tumor remission and has led to a series of FDA approvals for hematologic and solid cancers.97,98 Notably, Canon et al.,6 Briere et al.,13 and their colleagues were the first to explore the long-overlooked pairing between targeted therapy and immunotherapy, and described how two novel clinical K-RasG12C inhibitors, AMG-510 and MRTX849, could potentiate antitumor immunity in combination with checkpoint inhibitors. When co-administered with an anti-PD-1 antibody, both AMG-510 and MRTX849 were shown to induce durable complete tumor regression in the majority of mice bearing CT-26 KRASG12C tumors.6,13 The synergy between K-RasG12C inhibition and immunotherapy could be due to further-boosted tumoricidal-activity of T cells induced by the blockade of immune-suppressive PD-1/PD-L1 signaling.6 Aside from sustained primary KRASG12C tumor regression, combination treatment provided significant protection against subsequent re-challenges with homogeneous KRASG12D tumors, which implicated the establishment of antigen-specific memory responses.6 Given that intratumoral KRAS mutation status could be heterogeneous between primary tumor and metastases in the same patient,70 immunological memory induced by combinatory K-RasG12C and checkpoint inhibition would be beneficial for patients across all stages of cancer (Table 3).
Conclusions and Future Perspectives
The heroic effort in the research of targeted therapies for mutant K-Ras, long considered “undruggable,” has recently resulted in the development of several clinical-grade K-RasG12C inhibitors.4, 5, 6, 7,49 The third-generation K-RasG12C inhibitors, such as AMG-510 and MRTX849, allowed a first-of-its-kind targeting of mutant K-Ras in the clinic, achieving ~50% overall responses in a relatively small cohort of KRASG12C-mutant NSCLC patients.6,50 Although early-phase clinical trial results among NSCLC patients are promising, responses from colorectal cancer patients treated with either AMG-510 or MRTX849 were far less impressive, which raises the question of whether the overall efficacy of K-RasG12C inhibitors might be cancer type dependent.51 In depth investigation of the intrinsic resistance mechanisms to K-RasG12C inhibition should be conducted in future clinical trials that involve large patient cohorts with different types of cancer. Importantly, long-term follow-up study of patients receiving K-RasG12C inhibitors will provide new insights on the occurrence and mechanism of acquired resistance to these targeted drugs.
Complete and durable responses to targeted cancer therapies are rare in patients with advanced-stage cancers.61 Adaptive Ras reactivation through RTK-SHP2 pathway is one of the well-characterized mechanisms accountable for acquired resistance to K-RasG12C inhibition.10,11 Despite concerns on the emergence of alternative resistance limiting the efficacy of vertical pathway co-targeting, early-phase clinical trials combining K-RasG12C inhibitors with drugs targeting SHP2 will be carried out in the near future.14 Data from the clinical trials of combination therapies will be crucial to demonstrate whether this co-targeting strategy could benefit KRASG12C cancer patients. At this moment, it is still debatable whether the adaptive activation of downstream Ras signaling is mediated by wild-type Ras (N-Ras and H-Ras) or K-RasG12C.10,11 Further researches clarifying this discrepancy will not only deepen our understanding about the mechanism of adaptive response to K-RasG12C inhibition, but also provide a basis for combination therapy targeting both wild-type (N/H-) and mutant (K-) Ras subfamily members. In these regards, one potential combinatorial drug candidate would be BI 1701963, a clinical pan-Ras inhibitor provided that wild-type Ras proteins are involved in the resistance to K-RasG12C inhibition.99 Furthermore, co-targeting K-RasG12C with SOS1 or GRB2 could also be promising in clinic given that interactions between SOS1 and K-Ras, as well as SOS1 with its adaptor GRB2 are required for RTK-mediated adaptive Ras reactivation.25,26,88 Currently, drugs targeting GRB2-SOS1 interaction are under investigation.100 Synergistic antiproliferative activity has been reported upon co-inhibition of K-RasG12C and SOS1, although these findings need to be validated in in vivo studies.79 In addition, future studies are expected to evaluate the combinatorial effects of K-RasG12C and GRB2 inhibition.
Over the past decades, the combination of targeted therapies and immunotherapy has largely been under-investigated in cancer managements.6,101 The dual roles of mutant K-Ras in cell proliferation and immunosuppression indicate that K-RasG12C inhibitors would be perfect candidate drugs combining with immunotherapeutic in cancer therapy. Interestingly, the combination of AMG-510 with anti-PD-1 induced an effective memory immune response that protected against subsequent tumor re-challenge, providing a rationale for combinatorial K-Ras/checkpoint inhibition as a neo-adjuvant therapy in the future.6 Although there is strong preclinical evidence, the future application of combined immunotherapy and targeted therapy, including K-RasG12C inhibition should be carefully examined in clinic to avoid potential adverse reactions,102 as cautioned by the life-threatening interstitial pneumonitis happened in NSCLC patients who received EGFR inhibitor AZD9291 in combination with MEDI4736, an immune checkpoint blocker.103 At this moment, it is still unknown if the combination between K-RasG12C inhibition and radiotherapies would also be beneficial to patients, and future research is expected to illustrate the efficacy of these combinations.
The genomic-scale understanding of KRASG12C mutant cancer is promising with regard to overcoming resistance to K-RasG12C inhibition.104 Although no individual genetic alteration at baseline seems to correlate with the therapeutic efficacy of K-RasG12C inhibitors, pre-existing clustered genetic variations, including the overexpression of selected HER family genes could provide some clues on the therapeutic response to MRTX849.7 In addition, trajectory analysis of ARS-1620-treated cells revealed heterogeneous adaptations to K-RasG12C inhibition.10 These findings together point out the necessity of systematic tumor profiling both at baseline and longitudinally during drug treatment, as it will provide not only genetic signatures predicting intrinsic resistance among patients, but also secondary medications targeting the adaptive tumor selection imposed by K-RasG12C inhibition. Notably, genetic alterations associated with the response to combinatorial therapies remain to be characterized in the clinic. For example, KEAP1 (Kelch-like ECH-associated protein 1)/NFE2L2 (nuclear factor erythroid 2-related factor 2) mutation, frequently observed in KRAS mutant lung cancers, accounts for poor response to checkpoint inhibition.105 In this case, it would be interesting to examine if combinatorial K-RasG12C and checkpoint inhibitions remain to be superior to solely K-RasG12C inhibition in treating KRAS mutant NSCLC with concurrent KEAP1/NFE2L2 mutations.
Acknowledgments
This manuscript is supported by R01 CA233844 from the NIH.
Declaration of interests
The authors declare no competing interests.
References
- 1.Jančík S., Drábek J., Radzioch D., Hajdúch M. Clinical relevance of KRAS in human cancers. J. Biomed. Biotechnol. 2010;2010 doi: 10.1155/2010/150960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bos J.L. Ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 3.Ostrem J.M., Peters U., Sos M.L., Wells J.A., Shokat K.M. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503:548–551. doi: 10.1038/nature12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patricelli M.P., Janes M.R., Li L.-S., Hansen R., Peters U., Kessler L.V., Chen Y., Kucharski J.M., Feng J., Ely T., et al. Selective inhibition of oncogenic KRAS output with small molecules targeting the inactive state. Cancer Discov. 2016;6:316–329. doi: 10.1158/2159-8290.CD-15-1105. [DOI] [PubMed] [Google Scholar]
- 5.Janes M.R., Zhang J., Li L.-S., Hansen R., Peters U., Guo X., Chen Y., Babbar A., Firdaus S.J., Darjania L., et al. Targeting KRAS mutant cancers with a covalent G12C-specific inhibitor. Cell. 2018;172:578–589.e17. doi: 10.1016/j.cell.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Canon J., Rex K., Saiki A.Y., Mohr C., Cooke K., Bagal D., Gaida K., Holt T., Knutson C.G., Koppada N., et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575:217–223. doi: 10.1038/s41586-019-1694-1. [DOI] [PubMed] [Google Scholar]
- 7.Hallin J., Engstrom L.D., Hargis L., Calinisan A., Aranda R., Briere D.M., Sudhakar N., Bowcut V., Baer B.R., Ballard J.A., et al. The KRASG12C inhibitor MRTX849 provides insight toward therapeutic susceptibility of KRAS-mutant cancers in mouse models and patients. Cancer Discov. 2020;10:54–71. doi: 10.1158/2159-8290.CD-19-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forbes S.A., Beare D., Boutselakis H., Bamford S., Bindal N., Tate J., Cole C.G., Ward S., Dawson E., Ponting L., et al. COSMIC: somatic cancer genetics at high-resolution. Nucleic Acids Res. 2017;45:D777–D783. doi: 10.1093/nar/gkw1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang H., Liang S.-Q., Schmid R.A., Peng R.-W. New horizons in KRAS-mutant lung cancer: dawn after darkness. Front. Oncol. 2019;9:953. doi: 10.3389/fonc.2019.00953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xue J.Y., Zhao Y., Aronowitz J., Mai T.T., Vides A., Qeriqi B., Kim D., Li C., de Stanchina E., Mazutis L., et al. Rapid non-uniform adaptation to conformation-specific KRAS(G12C) inhibition. Nature. 2020;577:421–425. doi: 10.1038/s41586-019-1884-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryan M.B., Fece de la Cruz F., Phat S., Myers D.T., Wong E., Shahzade H.A., Hong C.B., Corcoran R.B. Vertical pathway inhibition overcomes adaptive feedback resistance to KRASG12C inhibition. Clin. Cancer Res. 2020;26:1633–1643. doi: 10.1158/1078-0432.CCR-19-3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Misale S., Fatherree J.P., Cortez E., Li C., Bilton S., Timonina D., Myers D.T., Lee D., Gomez-Caraballo M., Greenberg M., et al. KRAS G12C NSCLC models are sensitive to direct targeting of KRAS in combination with PI3K inhibition. Clin. Cancer Res. 2019;25:796–807. doi: 10.1158/1078-0432.CCR-18-0368. [DOI] [PubMed] [Google Scholar]
- 13.Briere D.M., Calinisan A., Aranda R., Sudhakar N., Hargis L., Gatto S., Fernandez-Banet J., Pavlicek A., Engstrom L.D., Hallin J., et al. Abstract LB-C09: the KRASG12C inhibitor MRTX849 reconditions the tumor immune microenvironment and leads to durable complete responses in combination with anti-PD-1 therapy in a syngeneic mouse model. Mol. Cancer Ther. 2019;18 LB-LB-C09. [Google Scholar]
- 14.Hata A.N., Shaw A.T. Resistance looms for KRAS G12C inhibitors. Nat. Med. 2020;26:169–170. doi: 10.1038/s41591-020-0765-z. [DOI] [PubMed] [Google Scholar]
- 15.Molina-Arcas M., Moore C., Rana S., Maldegem F.van, Mugarza E., Romero-Clavijo P., Herbert E., Horswell S., Li L.-S., Janes M.R., et al. Development of combination therapies to maximize the impact of KRAS-G12C inhibitors in lung cancer. Sci. Transl. Med. 2019;11:eaaw7999. doi: 10.1126/scitranslmed.aaw7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karnoub A.E., Weinberg R.A. Ras oncogenes: split personalities. Nat. Rev. Mol. Cell Biol. 2008;9:517–531. doi: 10.1038/nrm2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colicelli J. Human RAS superfamily proteins and related GTPases. Sci. STKE. 2004;2004:RE13. doi: 10.1126/stke.2502004re13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trahey M., McCormick F. A cytoplasmic protein stimulates normal N-ras p21 GTPase, but does not affect oncogenic mutants. Science. 1987;238:542–545. doi: 10.1126/science.2821624. [DOI] [PubMed] [Google Scholar]
- 19.Willumsen B.M., Christensen A., Hubbert N.L., Papageorge A.G., Lowy D.R. The p21 ras C-terminus is required for transformation and membrane association. Nature. 1984;310:583–586. doi: 10.1038/310583a0. [DOI] [PubMed] [Google Scholar]
- 20.Castellano E., Santos E. Functional specificity of Ras isoforms. Genes Cancer. 2011;2:216–231. doi: 10.1177/1947601911408081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Omerovic J., Laude A.J., Prior I.A. Ras proteins: paradigms for compartmentalised and isoform specific signalling. Cell Mol. Life Sci. 2007;64:2575–2589. doi: 10.1007/s00018-007-7133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simanshu D.K., Nissley D.V., McCormick F. RAS proteins and their regulators in human disease. Cell. 2017;170:17–33. doi: 10.1016/j.cell.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vigil D., Cherfils J., Rossman K.L., Der C.J. Ras superfamily GEFs and GAPs: validated and tractable targets for cancer therapy? Nat. Rev. Cancer. 2010;10:842–857. doi: 10.1038/nrc2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Traut T.W. Physiological concentrations of purines and pyrimidines. Mol. Cell Biochem. 1994;140:1–22. doi: 10.1007/BF00928361. [DOI] [PubMed] [Google Scholar]
- 25.Batzer A.G., Rotin D., Ureña J.M., Skolnik E.Y., Schlessinger J. Hierarchy of binding sites for Grb2 and Shc on the epidermal growth factor receptor. Mol. Cell. Biol. 1994;14:5192–5201. doi: 10.1128/mcb.14.8.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qian X., Esteban L., Vass W.C., Upadhyaya C., Papageorge A.G., Yienger K., Ward J.M., Lowy D.R., Santos E. The Sos1 and Sos2 Ras-specific exchange factors: differences in placental expression and signaling properties. EMBO J. 2000;19:642–654. doi: 10.1093/emboj/19.4.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajalingam K., Schreck R., Rapp U.R., Albert Š. Ras oncogenes and their downstream targets. Biochim. Biophys. Acta Mol. Cell Res. 2007;1773:1177–1195. doi: 10.1016/j.bbamcr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Young A., Lyons J., Miller A.L., Phan V.T., Alarcón I.R., McCormick F. Chapter 1 Ras signaling and therapies. Adv. Cancer Res. 2009;102:1–17. doi: 10.1016/S0065-230X(09)02001-6. [DOI] [PubMed] [Google Scholar]
- 29.Hobbs G.A., Der C.J., Rossman K.L. RAS isoforms and mutations in cancer at a glance. J. Cell Sci. 2016;129:1287–1292. doi: 10.1242/jcs.182873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prior I.A., Lewis P.D., Mattos C. A comprehensive survey of Ras mutations in cancer. Cancer Res. 2012;72:2457–2467. doi: 10.1158/0008-5472.CAN-11-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bos J.L., Rehmann H., Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 32.Mainardi S., Mulero-Sánchez A., Prahallad A., Germano G., Bosma A., Krimpenfort P., Lieftink C., Steinberg J.D., de Wit N., Gonçalves-Ribeiro S., et al. SHP2 is required for growth of KRAS-mutant non-small-cell lung cancer in vivo. Nat. Med. 2018;24:961–967. doi: 10.1038/s41591-018-0023-9. [DOI] [PubMed] [Google Scholar]
- 33.Nichols R.J., Haderk F., Stahlhut C., Schulze C.J., Hemmati G., Wildes D., Tzitzilonis C., Mordec K., Marquez A., Romero J., et al. RAS nucleotide cycling underlies the SHP2 phosphatase dependence of mutant BRAF-, NF1- and RAS-driven cancers. Nat. Cell Biol. 2018;20:1064–1073. doi: 10.1038/s41556-018-0169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cree I.A. Diagnostic RAS mutation analysis by polymerase chain reaction (PCR) Biomol. Detect. Quantif. 2016;8:29–32. doi: 10.1016/j.bdq.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Krieken J.H.J., Rouleau E., Ligtenberg M.J.L., Normanno N., Patterson S.D., Jung A. RAS testing in metastatic colorectal cancer: advances in Europe. Virchows Arch. 2016;468:383–396. doi: 10.1007/s00428-015-1876-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Bryan J.P. Pharmacological targeting of RAS: recent success with direct inhibitors. Pharmacol. Res. 2019;139:503–511. doi: 10.1016/j.phrs.2018.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmadian M.R., Stege P., Scheffzek K., Wittinghofer A. Confirmation of the arginine-finger hypothesis for the GAP-stimulated GTP-hydrolysis reaction of Ras. Nat. Struct. Biol. 1997;4:686–689. doi: 10.1038/nsb0997-686. [DOI] [PubMed] [Google Scholar]
- 38.Gremer L., Gilsbach B., Ahmadian M.R., Wittinghofer A. Fluoride complexes of oncogenic Ras mutants to study the Ras-RasGap interaction. Biol. Chem. 2008;389:1163–1171. doi: 10.1515/BC.2008.132. [DOI] [PubMed] [Google Scholar]
- 39.Moghadamchargari Z., Huddleston J., Shirzadeh M., Zheng X., Clemmer D.E., Raushel F.M., Russell D.H., Laganowsky A. Intrinsic GTPase activity of K-RAS monitored by native mass spectrometry. Biochemistry. 2019;58:3396–3405. doi: 10.1021/acs.biochem.9b00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu P., Wang Y., Li X. Targeting the untargetable KRAS in cancer therapy. Acta Pharm. Sin. B. 2019;9:871–879. doi: 10.1016/j.apsb.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cox A.D., Fesik S.W., Kimmelman A.C., Luo J., Der C.J. Drugging the undruggable Ras: mission possible? Nat. Rev. Drug Discov. 2014;13:828–851. doi: 10.1038/nrd4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dang C.V., Reddy E.P., Shokat K.M., Soucek L. Drugging the “undruggable” cancer targets. Nat. Rev. Cancer. 2017;17:502–508. doi: 10.1038/nrc.2017.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCormick F. Targeting KRAS directly. Annu. Rev. Cancer Biol. 2018;2:81–90. [Google Scholar]
- 44.Nussinov R., Tsai C.-J., Mattos C. “Pathway drug cocktail”: targeting Ras signaling based on structural pathways. Trends Mol. Med. 2013;19:695–704. doi: 10.1016/j.molmed.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y., Larraufie M.-H., Musavi L., Akkiraju H., Brown L.M., Stockwell B. Design of small molecules that compete with nucleotide binding to an engineered oncogenic KRAS allele. Biochemistry. 2018;57:1380–1389. doi: 10.1021/acs.biochem.7b01113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ganguly A.K., Pramanik B.N., Huang E.C., Liberles S., Heimark L., Liu Y.H., Tsarbopoulos A., Doll R.J., Taveras A.G., Remiszewski S., et al. Detection and structural characterization of ras oncoprotein-inhibitors complexes by electrospray mass spectrometry. Bioorg. Med. Chem. 1997;5:817–820. doi: 10.1016/s0968-0896(97)00021-7. [DOI] [PubMed] [Google Scholar]
- 47.Maurer T., Garrenton L.S., Oh A., Pitts K., Anderson D.J., Skelton N.J., Fauber B.P., Pan B., Malek S., Stokoe D., et al. Small-molecule ligands bind to a distinct pocket in Ras and inhibit SOS-mediated nucleotide exchange activity. Proc. Natl. Acad. Sci. U S A. 2012;109:5299–5304. doi: 10.1073/pnas.1116510109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Porru M., Pompili L., Caruso C., Biroccio A., Leonetti C. Targeting KRAS in metastatic colorectal cancer: current strategies and emerging opportunities. J. Exp. Clin. Cancer Res. 2018;37:57. doi: 10.1186/s13046-018-0719-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lito P., Solomon M., Li L.-S., Hansen R., Rosen N. Allele-specific inhibitors inactivate mutant KRAS G12C by a trapping mechanism. Science. 2016;351:604–608. doi: 10.1126/science.aad6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pasi J., Kyri P., Ignatius O., Igor R., Melissa J. A phase 1 clinical trial evaluating the pharmacokinetics (PK), safety, and clinical activity of MRTX849, a mutant-selective small molecule KRAS G12C inhibitor, in advanced solid tumors. 2019. https://www.mirati.com/wp-content/uploads/2019/10/AACR-NCI-EORTC-Clinical-Data-Presentation_Janne_October-2019-1-1.pdf
- 51.Klempner S.J., Hata A.N. Can the help match the hype? KRASG12C-specific inhibitors and beyond. Cancer Discov. 2020;10:20–22. doi: 10.1158/2159-8290.CD-19-1255. [DOI] [PubMed] [Google Scholar]
- 52.Marx M.A., Baer B.R., Ballard J., Blake J.F., Bouhana K., Briere D.M., Burgess L.E., Burkhard M.R., Chiang H., Chicarelli M.J., et al. Abstract B30: structure-based drug discovery of MRTX1257, a selective, covalent KRAS G12C inhibitor with oral activity in animal models of cancer. Mol. Cancer Res. 2020;18:B30. [Google Scholar]
- 53.Lim S.M., Westover K.D., Ficarro S.B., Harrison R.A., Choi H.G., Pacold M.E., Carrasco M., Hunter J., Kim N.D., Xie T., et al. Therapeutic targeting of oncogenic K-Ras by a covalent catalytic site inhibitor. Angew. Chem. Int. Ed. 2014;53:199–204. doi: 10.1002/anie.201307387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sakamoto K., Kamada Y., Sameshima T., Yaguchi M., Niida A., Sasaki S., Miwa M., Ohkubo S., Sakamoto J.-I., Kamaura M., et al. K-Ras(G12D)-selective inhibitory peptides generated by random peptide T7 phage display technology. Biochem. Biophys. Res. Commun. 2017;484:605–611. doi: 10.1016/j.bbrc.2017.01.147. [DOI] [PubMed] [Google Scholar]
- 55.Armstrong M. Asco 2019—KRAS chase heats up with Amgen data. 2019. https://www.evaluate.com/vantage/articles/events/conferences/asco-2019-kras-chase-heats-amgen-data
- 56.Mirati Therapeutics Targeting the genetic and immunological drivers of cancer. 2020. https://s23.q4cdn.com/174398288/files/doc_presentations/2020/02/MRTX-Corporate-Presentation_23February2020.pdf
- 57.Holohan C., Van Schaeybroeck S., Longley D.B., Johnston P.G. Cancer drug resistance: an evolving paradigm. Nat. Rev. Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 58.Garraway L.A., Jänne P.A. Circumventing cancer drug resistance in the era of personalized medicine. Cancer Discov. 2012;2:214–226. doi: 10.1158/2159-8290.CD-12-0012. [DOI] [PubMed] [Google Scholar]
- 59.Brown R., Curry E., Magnani L., Wilhelm-Benartzi C.S., Borley J. Poised epigenetic states and acquired drug resistance in cancer. Nat. Rev. Cancer. 2014;14:747–753. doi: 10.1038/nrc3819. [DOI] [PubMed] [Google Scholar]
- 60.Gottesman M.M. Mechanisms of cancer drug resistance. Annu. Rev. Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 61.Vasan N., Baselga J., Hyman D.M. A view on drug resistance in cancer. Nature. 2019;575:299–309. doi: 10.1038/s41586-019-1730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singh A., Greninger P., Rhodes D., Koopman L., Violette S., Bardeesy N., Settleman J. A gene expression signature associated with “K-Ras addiction” reveals regulators of EMT and tumor cell survival. Cancer Cell. 2009;15:489–500. doi: 10.1016/j.ccr.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Muzumdar M.D., Chen P.-Y., Dorans K.J., Chung K.M., Bhutkar A., Hong E., Noll E.M., Sprick M.R., Trumpp A., Jacks T. Survival of pancreatic cancer cells lacking KRAS function. Nat. Commun. 2017;8:1090. doi: 10.1038/s41467-017-00942-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sever R., Brugge J.S. Signal transduction in cancer. Cold Spring Harb. Perspect. Med. 2015;5:a006098. doi: 10.1101/cshperspect.a006098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martin G.S. Cell signaling and cancer. Cancer Cell. 2003;4:167–174. doi: 10.1016/s1535-6108(03)00216-2. [DOI] [PubMed] [Google Scholar]
- 66.Castellano E., Downward J. RAS interaction with PI3K. Genes Cancer. 2011;2:261–274. doi: 10.1177/1947601911408079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rodriguez-Viciana P., Warne P.H., Dhand R., Vanhaesebroeck B., Gout I., Fry M.J., Waterfield M.D., Downward J. Phosphatidylinositol-3-OH kinase direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 68.Janku F., Yap T.A., Meric-Bernstam F. Targeting the PI3K pathway in cancer: are we making headway? Nat. Rev. Clin. Oncol. 2018;15:273–291. doi: 10.1038/nrclinonc.2018.28. [DOI] [PubMed] [Google Scholar]
- 69.Fruman D.A., Chiu H., Hopkins B.D., Bagrodia S., Cantley L.C., Abraham R.T. The PI3K pathway in human disease. Cell. 2017;170:605–635. doi: 10.1016/j.cell.2017.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kordiak J., Szemraj J., Grabska-Kobylecka I., Bialasiewicz P., Braun M., Kordek R., Nowak D. Intratumor heterogeneity and tissue distribution of KRAS mutation in non-small cell lung cancer: implications for detection of mutated KRAS oncogene in exhaled breath condensate. J. Cancer Res. Clin. Oncol. 2019;145:241–251. doi: 10.1007/s00432-018-2779-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Richman S.D., Chambers P., Seymour M.T., Daly C., Grant S., Hemmings G., Quirke P. Intra-tumoral heterogeneity of KRAS and BRAF mutation status in patients with advanced colorectal cancer (aCRC) and cost-effectiveness of multiple sample testing. Anal. Cell Pathol. (Amst) 2011;34:61–66. doi: 10.3233/ACP-2011-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Luebker S.A., Koepsell S.A. Diverse mechanisms of BRAF inhibitor resistance in melanoma identified in clinical and preclinical studies. Front. Oncol. 2019;9:268. doi: 10.3389/fonc.2019.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kakadia S., Yarlagadda N., Awad R., Kundranda M., Niu J., Naraev B., Mina L., Dragovich T., Gimbel M., Mahmoud F. Mechanisms of resistance to BRAF and MEK inhibitors and clinical update of US Food and Drug Administration-approved targeted therapy in advanced melanoma. Onco Targets Ther. 2018;11:7095–7107. doi: 10.2147/OTT.S182721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Poulikakos P.I., Solit D.B. Resistance to MEK inhibitors: should we co-target upstream? Sci. Signal. 2011;4:pe16. doi: 10.1126/scisignal.2001948. [DOI] [PubMed] [Google Scholar]
- 75.Lopez J.S., Banerji U. Combine and conquer: challenges for targeted therapy combinations in early phase trials. Nat. Rev. Clin. Oncol. 2017;14:57–66. doi: 10.1038/nrclinonc.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mokhtari R.B., Homayouni T.S., Baluch N., Morgatskaya E., Kumar S., Das B., Yeger H. Combination therapy in combating cancer. Oncotarget. 2017;8:38022–38043. doi: 10.18632/oncotarget.16723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Robert C., Grob J.J., Stroyakovskiy D., Karaszewska B., Hauschild A., Levchenko E., Chiarion Sileni V., Schachter J., Garbe C., Bondarenko I., et al. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N. Engl. J. Med. 2019;381:626–636. doi: 10.1056/NEJMoa1904059. [DOI] [PubMed] [Google Scholar]
- 78.Planchard D., Besse B., Groen H.J.M., Souquet P.-J., Quoix E., Baik C.S., Barlesi F., Kim T.M., Mazieres J., Novello S., et al. An open-label phase 2 trial of dabrafenib plus trametinib in patients with previously treated BRAF V600E–mutant metastatic non-small cell lung cancer. Lancet Oncol. 2016;17:984–993. doi: 10.1016/S1470-2045(16)30146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hillig R.C., Sautier B., Schroeder J., Moosmayer D., Hilpmann A., Stegmann C.M., Werbeck N.D., Briem H., Boemer U., Weiske J., et al. Discovery of potent SOS1 inhibitors that block RAS activation via disruption of the RAS–SOS1 interaction. Proc. Natl. Acad. Sci. U S A. 2019;116:2551–2560. doi: 10.1073/pnas.1812963116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Duncan J.S., Whittle M.C., Nakamura K., Abell A.N., Midland A.A., Zawistowski J.S., Johnson N.L., Granger D.A., Jordan N.V., Darr D.B., et al. Dynamic reprogramming of the kinome in response to targeted MEK inhibition in triple-negative breast cancer. Cell. 2012;149:307–321. doi: 10.1016/j.cell.2012.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun C., Bernards R. Feedback and redundancy in receptor tyrosine kinase signaling: relevance to cancer therapies. Trends Biochem. Sci. 2014;39:465–474. doi: 10.1016/j.tibs.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 82.Olson E. Combination therapies in advanced, hormone receptor-positive breast cancer. J. Adv. Pract. Oncol. 2018;9:43–54. [PMC free article] [PubMed] [Google Scholar]
- 83.Corcoran R.B., André T., Atreya C.E., Schellens J.H.M., Yoshino T., Bendell J.C., Hollebecque A., McRee A.J., Siena S., Middleton G., et al. Combined BRAF, EGFR, and MEK inhibition in patients with BRAFV600E-mutant colorectal cancer. Cancer Discov. 2018;8:428–443. doi: 10.1158/2159-8290.CD-17-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yaeger R., Solit D.B. Overcoming adaptive resistance to KRAS inhibitors through vertical pathway targeting. Clin. Cancer Res. 2020;26:1538–1540. doi: 10.1158/1078-0432.CCR-19-4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Torres-Ayuso P., Brognard J. Shipping out MEK inhibitor resistance with SHP2 inhibitors. Cancer Discov. 2018;8:1210–1212. doi: 10.1158/2159-8290.CD-18-0915. [DOI] [PubMed] [Google Scholar]
- 86.Wong G.S., Zhou J., Liu J.B., Wu Z., Xu X., Li T., Xu D., Schumacher S.E., Puschhof J., McFarland J., et al. Targeting wild-type KRAS-amplified gastroesophageal cancer through combined MEK and SHP2 inhibition. Nat. Med. 2018;24:968–977. doi: 10.1038/s41591-018-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ruess D.A., Heynen G.J., Ciecielski K.J., Ai J., Berninger A., Kabacaoglu D., Görgülü K., Dantes Z., Wörmann S.M., Diakopoulos K.N., et al. Mutant KRAS -driven cancers depend on PTPN11/SHP2 phosphatase. Nat. Med. 2018;24:954–960. doi: 10.1038/s41591-018-0024-8. [DOI] [PubMed] [Google Scholar]
- 88.Boriack-Sjodin P.A., Margarit S.M., Bar-Sagi D., Kuriyan J. The structural basis of the activation of Ras by Sos. Nature. 1998;394:337–343. doi: 10.1038/28548. [DOI] [PubMed] [Google Scholar]
- 89.Lito P., Pratilas C.A., Joseph E.W., Tadi M., Halilovic E., Zubrowski M., Huang A., Wong W.L., Callahan M.K., Merghoub T., et al. Relief of profound feedback inhibition of mitogenic signaling by RAF inhibitors attenuates their activity in BRAFV600E melanomas. Cancer Cell. 2012;22:668–682. doi: 10.1016/j.ccr.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Miller K.D., Nogueira L., Mariotto A.B., Rowland J.H., Yabroff K.R., Alfano C.M., Jemal A., Kramer J.L., Siegel R.L. Cancer treatment and survivorship statistics, 2019. Cancer J. Clin. 2019;69:363–385. doi: 10.3322/caac.21565. [DOI] [PubMed] [Google Scholar]
- 91.Falzone L., Salomone S., Libra M. Evolution of cancer pharmacological treatments at the turn of the third millennium. Front. Pharmacol. 2018;9:1300. doi: 10.3389/fphar.2018.01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang, C.-Y., Ju, D.-T., Chang, C.-F., Muralidhar Reddy, P., and Velmurugan, B.K. A review on the effects of current chemotherapy drugs and natural agents in treating non–small cell lung cancer. Biomedicine (Taipei) 7.23 [DOI] [PMC free article] [PubMed]
- 93.Cullis J., Das S., Bar-Sagi D. Kras and tumor immunity: friend or foe? Cold Spring Harb. Perspect. Med. 2018;8:a031849. doi: 10.1101/cshperspect.a031849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McLaughlin K. The promise of immunotherapy. Cancertoday. 2014 https://www.cancertodaymag.org:443/Pages/Spring2014/Editors-Letter-Kevin-McLaughlin-Immunotherapy-Immune-System.aspx [Google Scholar]
- 95.Kruger S., Ilmer M., Kobold S., Cadilha B.L., Endres S., Ormanns S., Schuebbe G., Renz B.W., D’Haese J.G., Schloesser H., et al. Advances in cancer immunotherapy 2019—latest trends. J. Exp. Clin. Cancer Res. 2019;38:268. doi: 10.1186/s13046-019-1266-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dobosz P., Dzieciątkowski T. The intriguing history of cancer immunotherapy. Front. Immunol. 2019;10:2965. doi: 10.3389/fimmu.2019.02965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wei S.C., Duffy C.R., Allison J.P. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069–1086. doi: 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- 98.Hargadon K.M., Johnson C.E., Williams C.J. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int. Immunopharmacol. 2018;62:29–39. doi: 10.1016/j.intimp.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 99.Gort E., Johnson M.L., Hwang J.J., Pant S., Dünzinger U., Riemann K., Kitzing T., Janne P.A. A phase I, open-label, dose-escalation trial of BI 1701963 as monotherapy and in combination with trametinib in patients with KRAS mutated advanced or metastatic solid tumors. JCO. 2020;38:TPS3651. [Google Scholar]
- 100.Yu Y., Nie Y., Feng Q., Qu J., Wang R., Bian L., Xia J. Targeted covalent inhibition of Grb2-Sos1 interaction through proximity-induced conjugation in breast cancer cells. Mol. Pharm. 2017;14:1548–1557. doi: 10.1021/acs.molpharmaceut.6b00952. [DOI] [PubMed] [Google Scholar]
- 101.van Maldegem F., Downward J. Mutant KRAS at the heart of tumor immune evasion. Immunity. 2020;52:14–16. doi: 10.1016/j.immuni.2019.12.013. [DOI] [PubMed] [Google Scholar]
- 102.Kennedy L.B., Salama A.K.S. A review of cancer immunotherapy toxicity. CA: A Cancer J. Clin. 2020;70:86–104. doi: 10.3322/caac.21596. [DOI] [PubMed] [Google Scholar]
- 103.Oxnard G.R., Ramalingam S.S., Ahn M.-J., Kim S.-W., Yu H.A., Saka H., Horn L., Goto K., Ohe Y., Cantarini M., et al. Preliminary results of TATTON, a multi-arm phase Ib trial of AZD9291 combined with MEDI4736, AZD6094 or selumetinib in EGFR-mutant lung cancer. JCO. 2015;33:2509. [Google Scholar]
- 104.Ashley E.A. Towards precision medicine. Nat. Rev. Genet. 2016;17:507–522. doi: 10.1038/nrg.2016.86. [DOI] [PubMed] [Google Scholar]
- 105.Arbour K.C., Jordan E., Kim H.R., Dienstag J., Yu H.A., Sanchez-Vega F., Lito P., Berger M., Solit D.B., Hellmann M., et al. Effects of co-occurring genomic alterations on outcomes in patients with KRAS-mutant non-small cell lung cancer. Clin. Cancer Res. 2018;24:334–340. doi: 10.1158/1078-0432.CCR-17-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]