Abstract
Background
Prior longitudinal investigations of trajectories of sensory features in Autism Spectrum Development (ASD) have not explored heterogeneity. The present study explores initial levels and trajectories of sensory features in ASD as well as, for comparison, typical development.
Method
Growth mixture modelling was used to explore classes of autistic and typically-developing participants based on caregiver-reported total sensory behaviours on the Short Sensory Profile (SSP) at two time points, when children were aged 2–5 and 4–10 years of age, respectively.
Results
Three classes are described: a mixed class of autistic and typically-developing participants with few problematic sensory behaviours (“Stable Mild”), a mostly-autistic class with more problematic sensory features (“Stable Intense”), and a small class of autistic participants whose sensory features reportedly worsened (“Increasingly Intense”). Autistic participants in the Stable Intense class exhibited high anxiety, while autistic participants in the Increasingly Intense class appeared to obtain high scores on cognitive assessments.
Conclusions
The heterogeneity of sensory features and challenges found in the present study may suggest that practitioners should conduct individualized assessments of sensory features in ASD. Furthermore, practitioners should be aware of links between sensory features and anxiety in ASD, which may imply that sensory accommodations and supports could protect against anxiety. Finally, the worsening of sensory features over time in the Increasingly Intense subgroup may indicate a need for continued monitoring of changes in sensory features, perhaps especially as sensory environments change during periods of transition.
Keywords: Autism, sensory processing, heterogeneity, subgroups, growth mixture model, subtypes
Introduction
The longitudinal development of sensory processing remains under-studied in Autism Spectrum Development (ASD).1 Although evidence of atypical sensory behaviour in ASD has been observed from infancy (e.g., Baranek, 1999; Damiano-Goodwin et al., 2018; Kolesnik et al., 2019) through to adulthood (Billstedt, Gillberg, & Gillberg, 2007; Crane, Goddard, & Pring, 2009), we know relatively little about how these sensory features may change over time. These gaps are unfortunate, given the critical importance of atypical sensory experiences to many autistic people. In a recent qualitative study of the female experience of autism, almost half of the autistic participants volunteered the information that their sensory experiences were the single most debilitating aspects of their lives (Milner, McIntosh, Colvert, & Happé, 2019; but cf. Mottron, 2019).
Developmental Trajectories
It is possible that the manner in which some autistic individuals process or respond to sensory stimuli may change over time. Moreover, another factor that might contribute to developmental changes in sensory processing in ASD could be the surrounding sensory environment (see, e.g., Krieger et al., 2018, 2020; Mostafa, 2008). The sensory inputs to which children are exposed on a day-to-day basis can change as children transition from one environment to another. For example, many young autistic children may transition from environments such as preschool programs or autism early intervention programs, some of which might be delivered at home, to larger mainstream elementary schools. Such transitions could expose children to additional sensory inputs and highlight previously unnoticed challenges.
Some cross-sectional studies have been conducted regarding sensory processing in autism. For example, Kern and colleagues (2006) found a general decrease in severity of sensory features in autism with age from early childhood to adulthood. Furthermore, a meta-analysis indicates that children’s sensory features are most pronounced in ASD relative to Typical Development (TD) between 6 and 9 years of age (Ben-Sasson et al., 2009; Ben-Sasson, Gal, Fluss, Katz-Zetler, & Cermak, 2019).
However, the well-known difficulties associated with cross-sectional designs in studies of developmental change in TD arguably become even more problematic in studies of ASD. Recent autism prevalence data reveal enormous variability in time of diagnosis; in Canada, of those children who would have a diagnosis of autism by the age of 17, only 19% are diagnosed by age 3, 47% by age 5, and 72% by age 10 (Ofner et al. 2018). Age of diagnosis is known to be systematically related to other variables, including the magnitude of autistic features and the presence of co-occurring conditions (Daniels & Mandell, 2014). Thus, cross-sectional studies in ASD could easily confound age with other variables.
Fortunately, recent years have seen the emergence of longitudinal studies exploring scores on sensory measures in ASD (Baranek et al., 2019; Green, Ben-Sasson, Soto, & Carter, 2012; McCormick, Hepburn, Young, & Rogers, 2016; Repetto, Jasmin, Fombonne, Gisel, & Couture, 2017; Wolff et al., 2019). Baranek et al. found that levels of hyporesponsiveness and sensory interests/repetitive behaviours declined over a two-year period in a sample of autistic children, who ranged from 2 – 12 years of age at the first time-point. On the other hand, Wolff et al. found that total sensory features, hyporesponsiveness, and visual modality features increased between 12 and 24 months in infants later diagnosed with autism, while levels of sensory seeking decreased. In the study by McCormick and colleagues, involving three time points at approximately ages 2–3, 4–5, and 8–9 years, sensory features in ASD were stable over time. Sensory features were also stable in the studies by Green et al., which was conducted in toddlers 18–33 months old at Time 1 with Time 2 following after one year, and by Repetto et al., who studied children aged approximately 3–4 years at Time 1 and 5–6 at Time 2. Meanwhile, in TD, McCormick and colleagues found declines in total unusual or problematic sensory behaviours, under-responsiveness and sensation seeking, and visual and auditory sensory sensitivities.2 Wolff et al. found decreases in total sensory features, hyporesponsiveness, sensory seeking, and visual modality features in typically-developing infants, as well as high-risk infants not diagnosed with ASD, between the ages of approximately 12 to 24 months.
However, these studies do not address the variability of sensory processing in ASD. Sensory processing in ASD is extremely heterogeneous (Uljarević et al., 2017), perhaps more so than in TD (see, e.g., Little, Dean, Tomchek, & Dunn, 2017). A number of studies conducted at single time-points (see review by DeBoth & Reynolds, 2017) have defined subgroups of autistic individuals in terms of their sensory features, but these studies cannot provide information about heterogeneity in changes in autistic sensory processing over time.
One promising approach to defining sensory subtypes over longer periods of time involves the use of mixture models. Mixture models estimate classes, representing subcategories of participants, with the effective aim of replicating as closely as possible the pattern of the data. Classes are defined by means and variances, such that each class is essentially a normal distribution. The mixture of these normally-distributed classes should approximate the observed data (Nylund, 2007). The fact that mixture models estimate distributions of classes allows one to obtain indices of the degree to which different models fit their data, which can be used to allow the researcher to select an “optimal” number of classes (Berlin, Williams, & Parra 2014). This is problematic; the problem of determining an “optimal” number of classes or clusters is arguably ill-posed (Fushing & McAssey, 2010), at least when subgroups are overlapping. Notably, if subgroups do not overlap, then the subgroup structure should be visually obvious without use of formal mixture modelling or clustering, suggesting this may be a rare situation in practice. However, fit indices can still be used to reduce the researcher degrees of freedom involved in selecting a solution.
There are different ways that mixture modelling can be applied to longitudinal data. One is latent profile transition analysis (LPTA; see, e.g., Ausderau and colleagues, 2014, 2016), but this does not consider the continuous rate of change in sensory features. In contrast, the growth mixture model (GMM) uses starting points (intercepts) and rates of change (slopes) in levels of a variable to estimate classes (Ram & Grimm, 2009). Thus, use of a GMM could explicitly provide information about how subgroups within the heterogeneous autistic population differ not only in their initial level of sensory behaviours, but also in how trajectories of sensory processing change over time.
Relationship Between Sensory Processing and Other Variables
Prior studies indicate that sensory processing in ASD is linked to anxiety and other affective symptoms (e.g., Ben-Sasson et al., 2008; Uljarević, Lane, Kelly, & Leekam, 2016). Early sensory reactivity has been found to predict later anxiety (Green et al., 2012), suggesting that aversive sensory experiences can exacerbate anxiety in ASD. Furthermore, questionnaire reports of atypical sensory processing in ASD have often been found to be related to lower adaptive functioning scores (e.g., Ausderau et al., 2016; Baker, Lane, Angley, & Young, 2008; Kojovic, Ben Hadid, Franchini, & Schaer, 2019; Tomchek, Little, & Dunn, 2015; K. Williams et al., 2018), which might be seen to further highlight the adverse impacts that atypical sensory processing can have on the daily lives of many autistic individuals. Indeed, atypical sensory processing is related to, or an aspect of, quality of life in ASD (Lin & Huang, 2019; McConachie et al., 2019). It is also possible that atypical sensory processing in ASD might negatively impact acquisition of developmental skills (see, e.g., Baranek et al., 2018; Damiano-Goodwin et al., 2018). This could contribute to these adaptive function effects and might also result in lower measured cognitive abilities. However, as prior research suggests higher parent-estimated developmental skills may be associated with more sensory sensitivities in ASD (Ausderau et al., 2016; cf. Ben-Sasson et al., 2019), higher cognitive ability might enhance autistic individuals’ abilities to convey certain sensory experiences to caregivers.
Present Study
The aim of the present study is to describe longitudinal trajectories of atypical sensory behaviours as indexed by the caregiver-report Short Sensory Profile (SSP) in a sample of young children from the Autism Phenome Project (APP) at the UC Davis Health MIND Institute using a GMM approach. In line with Little et al. (2017), both autistic and typically-developing participants are included in the present analysis. The theoretical rationale for including typically-developing participants is to recognize the reality that there is heterogeneity present both within ASD and within TD and to contextualize sensory processing within both neurotypes by providing information about their degree of overlap. Furthermore, our goal in defining subgroups here is not to assert that there is a truly categorical structure to the longitudinal development of sensory processing in autism (as opposed to a dimensional structure), nor to suggest that any classes generated in this study should be used to classify individuals in community and clinical settings. Instead, we aim to use categorization to illuminate and describe some of the heterogeneity that exists in the longitudinal development of sensory processing within ASD as well as TD.
Although analyses based on mixture models should be considered exploratory, some tentative predictions can be drawn from prior longitudinal studies. First, in line with those studies that have examined sensory processing in ASD longitudinally within age ranges most closely comparable to the present study (i.e., McCormick et al., 2016; Repetto et al., 2017), it is hypothesized that the predominant trend in ASD will be stability of sensory features, represented by the existence of a large class of autistic participants with no overall change in levels of sensory behaviours over time.
Second, and similarly, in line with results obtained by McCormick et al. (2016) and given the relative lack of heterogeneity often found in TD relative to ASD, the present study hypothesizes that most typically-developing participants will be found within a single class characterized by initially mild sensory behaviours as well as a further decrease in “atypical” or problematic sensory behaviours over time.
Thirdly, given the well-established heterogeneity of sensory processing in ASD, it is hypothesized that there will be at least one additional class, largely comprised of autistic participants, differing from the aforementioned ASD-dominated class in the initial level and trajectory of their sensory behaviours.
Finally, we also explored whether classes differed in the variables of anxiety, adaptive functioning, and cognitive ability.
Methods
Participants
The Autism Phenome Project (APP) is a longitudinal investigation of a large sample of autistic and typically-developing children being conducted at the UC Davis Health MIND Institute; the overarching goal of the APP is to define subtypes of ASD. The study was approved by the UC Davis Institutional Review Board and informed consent was obtained from the parent/guardian of each participant. All autistic participants met criteria for a pervasive developmental disorder (based on DSM-IV and Collaborative Programs of Excellence in Autism Network criteria) and passed cut-off scores on the ADOS-G (Lord et al., 2000) and, for either Social or Communication subscales, on the ADI-R (Lord, Rutter, & Le Couteur, 1994). Further details regarding the APP and participant recruitment can be found in previous publications (e.g., Libero et al., 2016; Nordahl et al., 2011). As part of the APP, caregiver-reports of sensory behaviours on the SSP were collected at two time points an average of 2.78 years apart (range 1.15 – 5.31 years). In total, complete SSP forms were available at either time point from 179 autistic (149 male) and 93 typically-developing participants (62 male). Completed forms were available for 160 autistic (131 male) and 85 typically-developing (56 male) participants at the first time point, when participants were 2 – 5 years of age. At the second time point,3 when participants were 4 – 10 years of age, completed forms were available for 87 autistic (68 male) and 55 typically-developing (36 male) participants. Data were available at both time points from 115 participants (68 autistic, 47 typically-developing). Further information regarding participants is given in Table 1.
Table 1.
Descriptive characteristics of the typically-developing and autistic participants regarding whom completed Short Sensory Profiles were returned at each time-point.
| Time 1 (160 ASD, 85 TD participants) | Time 2 (87 ASD, 55 TD participants) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ASD | TD | p Welch’s t | ASD | TD | p (Welch’s t) | |||||
| Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | |||
| Chronological Age (months) | 37.24 (6.08) | 25.00 – 56.00 | 36.12 (7.32) | 24.00 – 56.00 | p = .23 t(146.56) = 1.21 | 68.77 (11.91) | 52.00 – 112.00 | 67.61 (13.64) | 52.00 – 116.00 | p = .60 t(103.53) = 0.52 |
| Cognitive Ability (MSEL DQ at T1; DAS-II GCA at T2) | 62.85 (20.76) | 26.85 – 132.45 | 105.52 (11.60) | 79.89 – 128.62 | p < .0001 t(238.08) = −20.46 | 89.00 (24.43) | 32.00 – 130.00 | 112.80 (10.52) | 85.00 – 146.00 | p < .0001 t(103.43) = −7.46 |
| VABS-II Adaptive Behaviour Composite | 75.67 (11.97) | 51.00 – 111.00 | 110.12 (12.06) | 82.00 – 137.00 | p < .0001 t(156.04) = −20.44 | 78.38 (15.61) | 47.00 – 122.00 | 112.15 (12.05) | 91.00 – 133.00 | p < .0001 t(126.49) = −14.03 |
| ADOS-G Calibrated Severity Score* | 7.76 (168) | 4.00 – 10.00 | N/A | N/A | N/A | 7.65 (1.60) | 4.00 – 10.00 | N/A | N/A | N/A |
See Gotham, Pickles, & Lord (2009) for further details regarding the ADOS calibrated severity score.
Measures
Sensory Behaviours
The Short Sensory Profile (SSP; McIntosh, Miller, & Shyu, 1999), a 38-item caregiver-report questionnaire which has been used in a number of studies to investigate and characterize autistic sensory processing (e.g., Hand, Dennis, & Lane, 2017; Tomchek et al., 2015; Uljarević et al., 2016), was used to investigate sensory behaviours at both time points. The measure was completed at home by the child’s primary caregiver. Higher raw scores reflect relatively typical sensory behaviours, whereas lower raw scores are indicative of more atypical sensory behaviours. In addition to the original seven SSP subscales developed based on a typically-developing sample (McIntosh et al., 1999), two studies have explored SSP factors in samples of autistic children (Tomchek, Huebner, & Dunn, 2014; Z. J. Williams, Failla, Gotham, Woynaroski, & Cascio, 2018). The nine factors included in the most recent solution, developed by Z. J. Williams et al., are described as Low Energy/Weak, Taste/Smell Sensitivity, Hyperactivity/Inattention, Tactile Sensitivity, Movement Sensitivity, Auditory Distractibility, Hyporesponsiveness to Speech, Visual Sensitivity, and Noise Distress. Note that although the Z. J. Williams et al. solution does not include all items in its factors, the total SSP raw scores presented in this paper are based on all items. Missing data were excluded.
Cognitive Ability
The Mullen Scales of Early Learning (MSEL; Mullen, 1995), a standardized measure of cognitive and motor functioning for children under the age of 68 months, was employed to assess cognitive ability at Time 1. Four MSEL subscales were administered: Visual Reception (VR), Fine Motor (FM), Expressive Language (EL), and Receptive Language (RL). A ratio developmental quotient (DQ) was calculated (as mental age/chronological age *100) for full-scale performance.
At Time 2, the Differential Ability Scales, Second Edition (DAS-II; Elliott, 2007) were used to assess cognitive ability. This measure, designed for children aged 2 – 17 years, offers different combinations of subtests for children of different age groups, which were combined to yield a standardized General Conceptual Ability (GCA) score. Although the DAS-II and the MSEL exhibit good convergent validity, as shown by strong correlations between performance on the two measures, DAS-II GCA scores are typically higher than MSEL IQ scores in both ASD and TD (Farmer, Golden, & Thurm, 2018).
Adaptive Behaviour
The parent-report form of the Vineland Adaptive Behavior Scales, Second Edition (VABS-II; Sparrow, Cichetti, & Balla, 2005), a rating scale designed for the assessment of adaptive functioning in developmental disability populations, was completed at home by the child’s primary caregiver at both time points. This form was administered as a questionnaire, not an interview. A standardized composite adaptive behaviour score was calculated.
Anxiety
Finally, the Childhood Behaviour Checklist (CBCL; Achenbach & Rescorla, 2000) was also completed at home by the child’s primary caregiver at both time points. This caregiver-report questionnaire aims to assess problematic internalizing and externalizing behaviours. At Time 1, all forms collected were of the preschool-age version, while at Time 2, the school-age form was collected from 27 participants and the preschool-age version from 120 participants. The CBCL’s DSM-oriented anxiety problems subscale was of particular interest in the present study, given previous reports of relationships between autistic sensory processing and anxiety. This subscale yields both a raw score and a normed T-score; the latter was used in the study.
Latent Growth Models (LGMs)
As a preliminary to the GMM analysis, Mplus version 8.2 (Muthén & Muthén, 1998/2017) was used to estimate latent growth models (LGMs) based on total raw SSP scores from both autistic and typically-developing participants at Times 1 and 2. Models were fitted separately in the ASD and TD groups, as there were not a sufficient number of parameters to estimate a single model with two groups using the Mplus “grouping” option. Furthermore, to minimize the number of estimated parameters, the covariance of intercept and slope was fixed to zero. Linear growth was assumed. (Fit indices for linear and no-growth models are presented in supplementary results.)
Furthermore, an LGM with data from both groups combined together was fitted for the sole purpose of obtaining residual variance estimates. These estimates were subsequently used to fix residual variances in the GMMs, to reduce the need for estimation.
Growth Mixture Model (GMM)
Mplus version 8.2 (Muthén & Muthén, 1998/2017) was also used to estimate GMMs using total raw SSP scores from both autistic and typically-developing participants at Time 1 and Time 2. Due to the existence of only two time points, and the relatively small number of parameters available for model estimation, models were defined in such a way as to minimize the number of parameters. In all models, means of intercepts and slopes were estimated and allowed to vary freely between classes, variances of intercepts and slopes were estimated and held to be equal across classes, and residual variances were fixed to the values obtained in the LGM across both diagnostic groups. Models were run with covariances of intercepts and slopes fixed to zero in all classes and also with covariances of intercepts and slopes estimated and held equal across classes. Models with one to five classes were estimated.
To select the optimal model, the authors drew on a variety of tests and indices: entropy, log-likelihood, Akaike’s Information Criterion (AIC), the Bayesian Information Criterion (BIC), Sample Size-Adjusted BIC (SABIC), the Lo-Mendell-Rubin (LMR) test, and the Bootstrap Likelihood Ratio Test (BLRT). Models that failed to converge normally, or which converged with significantly negative variance or covariance parameters, were discarded.
Results
Reliability
Cronbach’s alpha for the SSP total score was .88 in ASD and .89 in TD at Time 1 and .87 in ASD and .90 in TD at Time 2.
Latent Growth Models (LGMs)
Slopes and intercepts from the separate LGMs estimating initial levels of and changes in SSP raw scores over time separately in each diagnostic group are presented in Table 2. As there were not enough available parameters for a chi-square test of model fit, it was not possible to compare these models to no-growth models. For the same reason, it was not possible to fit a single model containing both diagnostic groups. However, not only did typically-developing participants appear to show fewer atypical sensory behaviours than autistic participants, but the model suggested their sensory behaviour scores increased (i.e., became “more typical”) over time. In the ASD group, participants’ sensory behaviours appeared atypical at Time 1 and declined further by Time 2.
Table 2.
Estimated slopes and intercepts, along with associated standard errors and p-values, from each diagnostic group in the separate LGMs assuming linear growth. P-values reflect the probability of obtaining a mean intercept or slope value equivalently different from zero by chance.
| Diagnostic Group | Intercept | Slope | ||||
|---|---|---|---|---|---|---|
| M | SE | p | M | SE | p | |
| ASD | 138.22 | 1.52 | <.001 | −4.51 | 2.09 | .03 |
| TD | 168.10 | 1.33 | <.001 | 4.62 | 1.57 | .003 |
Selection of Growth Mixture Model (GMM)
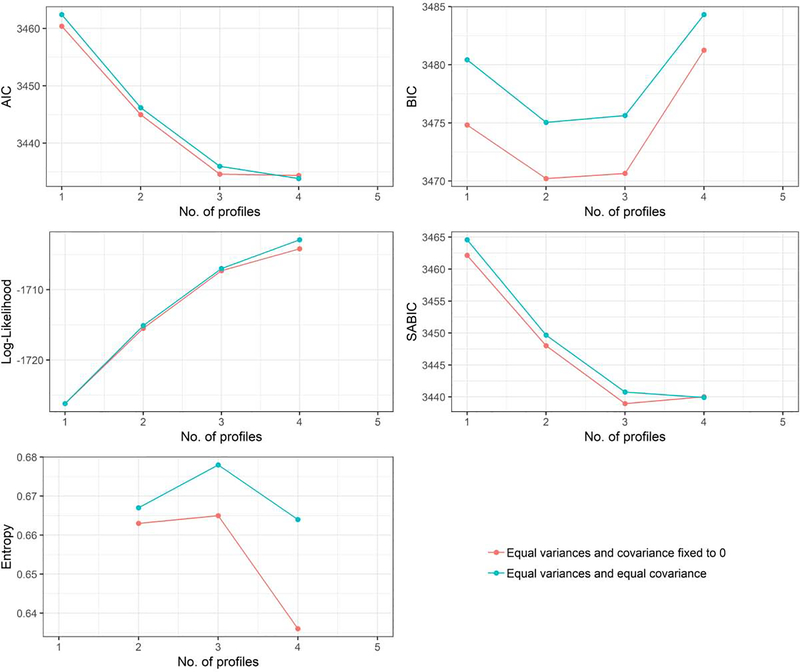
Models with one to four classes, when variances of slope and intercepts were constrained to be equal across all classes, successfully converged. The five-class model was discarded due to a negative variance. Visual inspection of fit indices (Figure 1) for the remaining models appeared to favour a three-class solution. This conclusion was not necessarily supported by the results of the LMR tests. Of those models with estimated rather than fixed covariances, the two-class solution was significantly superior to the one-class solution, p = .003, but the three-class solution was not significantly superior to the two-class solution, p = .10. However, BLRT tests (which simulations suggest may be a high-performing fit index for GMMs; see Nylund, Asparouhov, & Muthén, 2007) indicated that the three-class solution was preferable to the two-class solution, p < .0001, but that a four-class solution offered no further improvement, p = .43. Thus, we selected three as the optimal number of classes. We chose the model for which the covariance of the intercept and slope was estimated, as estimation entails a closer fit of the model to the data.
Figure 1.
Information criteria (AIC, BIC, SABIC), log-likelihood, and entropy corresponding to successfully-fitted models. Low values of AIC, BIC, and SABIC should be interpreted as signs of superior model fit, while higher values of entropy and log-likelihood suggest superior fit. Here, the observed BIC values suggest a two- or three-class solution, entropy suggests a three-class solution, AIC and SABIC imply a three- to four-class solution, and log-likelihood suggests a four-class solution. Thus, the overall pattern appears to favour three classes.
Description of Classes
In the optimal three-class model, the first class was characterized by a pattern of stable, high total raw scores on the SSP (Table 3, Figure 2a, Figure 2b). This reflects a pattern of more typical or less intense sensory behaviours, and the class is accordingly described as a “Stable Mild” class in this paper. The second class, meanwhile, was estimated to have lower scores (i.e., scores reflecting more atypical or intense sensory behaviours) which were also approximately stable over time. This class is described as the “Stable Intense” class. Finally, sensory processing in the third and smallest class was initially relatively typical, as reflected in high total SSP scores, but these scores decreased sharply by Time 2. Thus, this class is termed the “Increasingly Intense” class.
Table 3.
Estimated slopes and intercepts, along with associated standard errors and p-values, from each class in the final model. P-values reflect the probability of obtaining a mean intercept or slope value equivalently different from zero by chance.
| Class | Intercept | Slope | ||||
|---|---|---|---|---|---|---|
| M | SE | p | M | SE | p | |
| 1 (Stable Typical) | 164.07 | 1.86 | <.001 | 2.22 | 2.573 | .39 |
| 2 (Stable Atypical) | 127.32 | 2.27 | <.001 | 1.48 | 2.825 | .60 |
| 3 (Increasingly Intense) | 159.12 | 9.24 | <.001 | −40.24 | 15.94 | .01 |
Figure 2A.
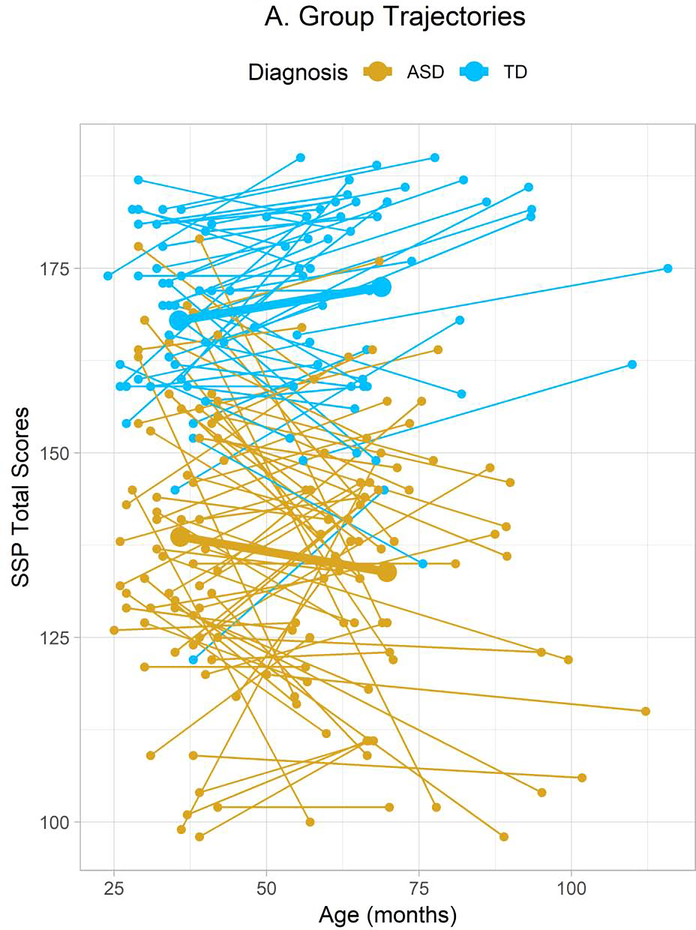
Total SSP score trajectories of individual participants divided by diagnostic group. Small round points represent individual participants’ data points and thin connecting lines represent linear trajectories linking these points. Thicker points and connecting lines represent the intercepts and slopes estimated in the LGMs mapped onto the graph at the average ages of participants in each group at each time point. (Note that time point, but not age, was considered in the LGM and GMM analyses.) SSP data were available from 160 autistic (131 male) and 85 typically-developing (56 male) participants at the first time point and from 87 autistic (68 male) and 55 typically-developing (36 male) participants at the second time point.
Figure 2B.
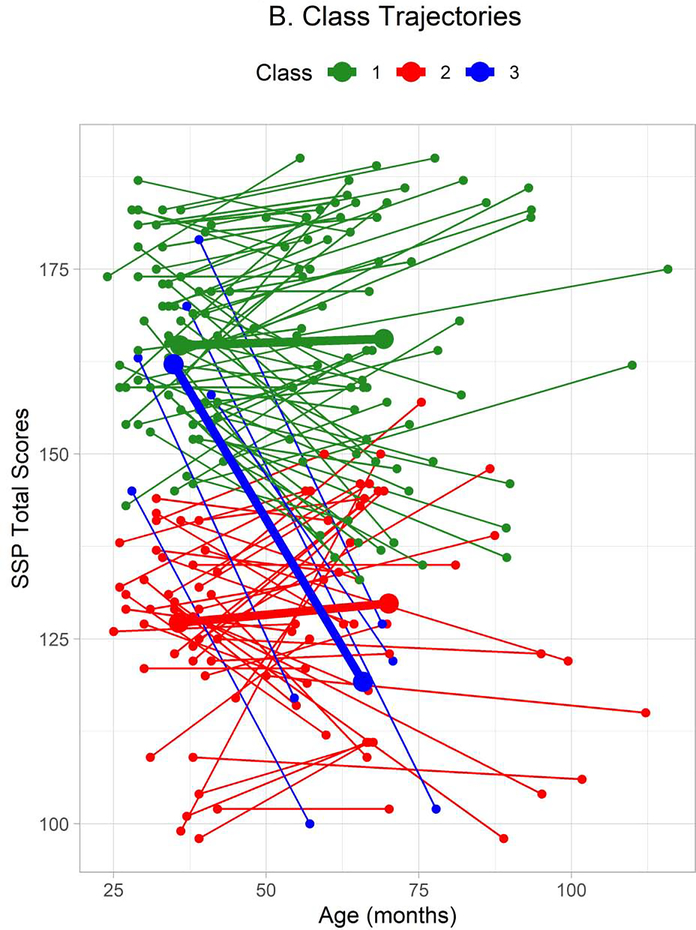
Total SSP score trajectories of individual participants divided by GMM class. Thicker points and connecting lines represent the estimated intercepts and slopes of the classes.
Almost all typically-developing participants were classified in the first, “Stable Mild” class, along with a number of autistic participants. The majority of the autistic participants were classified in the second, “Stable Intense” class, while a small number of autistic participants were classified into the third, “Increasingly Intense” class (Table 4).
Table 4.
Counts and percentages of autistic and typically-developing participants of each sex assigned to each class based on individual likelihood.
| Class 1: Stable Typical | Class 2: Stable Atypical | Class 3: Increasingly Intense | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Male ASD | 52 | 34.90% | 94 | 63.09% | 3 | 2.01% |
| Female ASD | 7 | 23.33% | 20 | 66.67% | 3 | 10.00% |
| Male TD | 60 | 96.77% | 2 | 3.23% | 0 | 0.00% |
| Female TD | 31 | 100.00% | 0 | 0.00% | 0 | 0.00% |
These counts are based on the likelihood of class membership at the individual-participant level, but the model also offers estimated overall counts of participants in each latent class. These counts for the Stable Mild, Stable Intense, and Increasingly Intense groups were 140.87, 112.98, and 18.15, respectively.
To further characterize these classes, scores on each of the SSP factors defined by Z. J. Williams et al. (2018) are presented by class and diagnostic group from only participants with data at both Time 1 and Time 2 (Table 5) along with the results of Wilcoxon signed-rank paired tests comparing scores at each time-point within each class and group. In addition, statistical comparisons across classes at Time 1 (Supplementary Table 3) and Time 2 (Supplementary Table 4) are presented in Supplementary Materials.
Table 5.
Means and standard deviations of Time 1 and Time 2 scores, by class and diagnostic group, on SSP factors from the solution offered by Williams et al. (2018), accompanied by p-values from Wilcoxon signed-rank tests comparing Time 1 and Time 2 scores within each diagnostic group. P-values should be interpreted with caution, as no correction for multiple comparisons has been applied. Only participants with SSP total scores at both time points (68 ASD, 47 TD) are included.
| Class 1, Stable Mild, TD | Class 1, Stable Mild, ASD | Class 2, Stable Intense, ASD | Class 3, Increasingly Intense, ASD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | p | T1 | T2 | p | T1 | T2 | p | T1 | T2 | p | |
| LEW | 29.58 (1.53) | 29.43 (1.09) | .16 | 28.92 (1.92) | 28.19 (3.14) | .40 | 24.21 (6.65) | 21.53 (7.33) | .16 | 26.67 (2.80) | 18.83 (6.24) | .07 |
| TSS | 18.01 (2.68) | 18.15 (3.24) | .81 | 15.70 (4.71) | 14.76 (4.72) | .25 | 10.32 (4.91) | 11.59 (5.13) | .43 | 16.33 (6.25) | 7.50 (4.97) | .06 |
| HYI | 19.78 (3.14) | 21.59 (3.23) | .0007 | 16.70 (3.69) | 16.54 (4.29) | .08 | 12.04 (3.51) | 12.80 (4.49) | .02 | 20.00 (3.90) | 13.50 (3.15) | .04 |
| TS | 18.95 (1.30) | 19.31 (1.36) | .009 | 18.40 (1.56) | 17.88 (2.17) | .57 | 14.75 (3.18) | 16.17 (2.91) | .001 | 17.00 (3.22) | 14.67 (3.33) | .42 |
| MS | 14.11 (1.47) | 14.44 (1.11) | .13 | 13.98 (1.54) | 14.08 (1.79) | .52 | 12.65 (2.55) | 12.50 (2.61) | .94 | 14.67 (0.82) | 13.80 (1.64) | .58 |
| AD | 13.94 (1.12) | 13.69 (1.70) | .97 | 13.11 (1.61) | 11.50 (2.34) | .049 | 10.51 (2.57) | 9.45 (2.63) | .03 | 14.50 (0.84) | 10.00 (2.97) | .04 |
| HRS | 8.29 (1.44) | 8.28 (1.45) | .59 | 5.57 (1.64) | 6.38 (1.44) | .03 | 4.30 (1.43) | 5.35 (1.67) | .002 | 5.83 (2.23) | 4.83 (2.56) | .42 |
| VS | 8.93 (1.19) | 9.04 (1.50) | .55 | 9.17 (1.16) | 9.04 (1.28) | .20 | 6.80 (2.18) | 7.43 (2.18) | .68 | 9.33 (0.82) | 6.67 (3.08) | .14 |
| ND | 8.28 (1.57) | 8.52 (1.83) | .23 | 8.85 (1.32) | 7.23 (1.88) | .01 | 6.62 (2.20) | 6.02 (2.29) | .28 | 9.33 (1.21) | 5.17 (2.14) | .06 |
LEW: Low Energy/Weak, TSS: Taste/Smell Sensitivity, HYI: Hyperactivity/Inattention, TS: Tactile Sensitivity, MS: Movement Sensitivity, AD: Auditory Distractibility, HRS: Hyporesponsiveness to Speech, VS: Visual Sensitivity, ND: Noise Distress
Comparison of Classes in ASD
Chronological Age
In the ASD group, chronological age showed a non-normal distribution, particularly at Time 2. Kruskal-Wallis tests were therefore run to determine whether autistic participants in different classes differed in chronological age at either time point. No effects were found at Time 1, χ2(2) = 2.31, p = .31, or Time 2, χ2(2) = 0.92, p = .63. Furthermore, classes did not differ in interval between time-points, χ2(2) = 0.58, p = .75.
Cognitive Ability
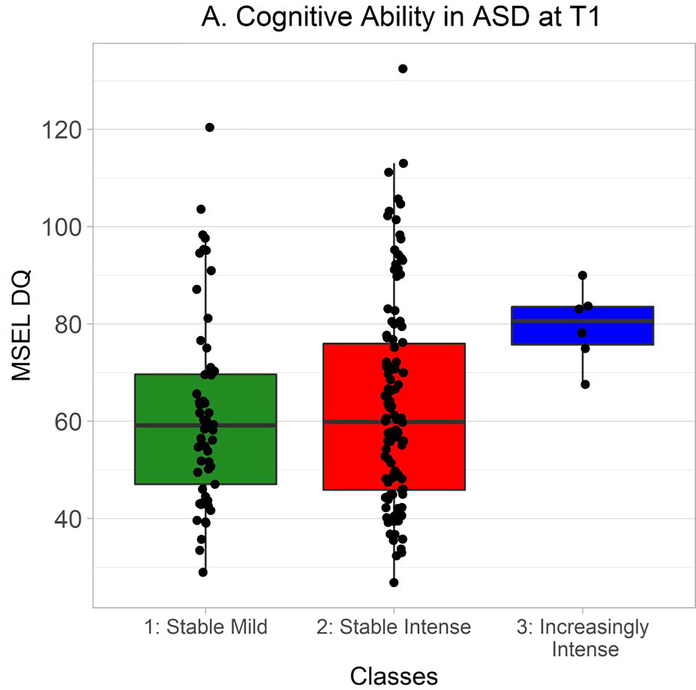
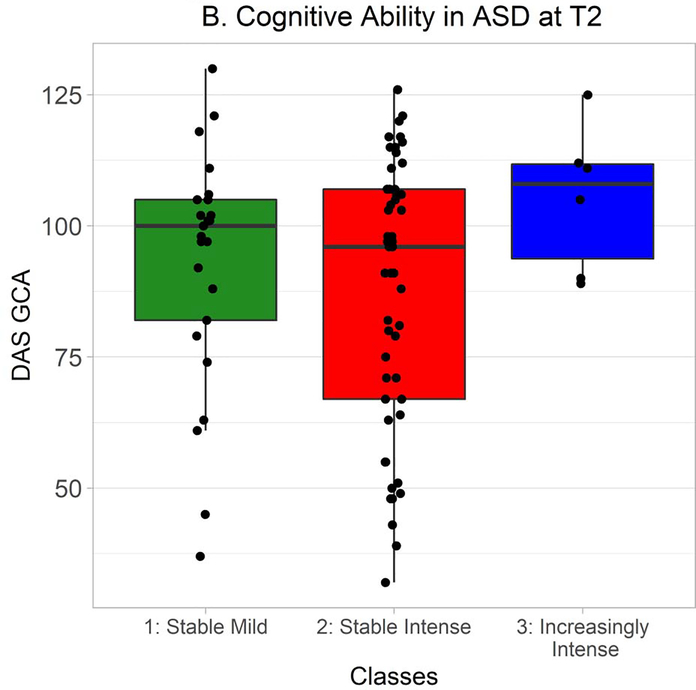
MSEL DQ and DAS GCA were also distributed non-normally in ASD, so Kruskal-Wallis tests were used to explore whether classes differed in cognitive ability. At Time 1, there was a very strong but nonsignificant trend towards an effect of class, χ2(2) = 5.83, p = .05 (Figure 3A). Wilcoxon-Mann-Whitney tests indicated that cognitive ability was greater in the Increasingly Intense class than in the Stable Mild class, W = 65.0, Holm-Bonferroni corrected p = .04, r = −.31, and in the Increasingly Intense class than in the Stable Intense class, W = 152.0, Holm-Bonferroni corrected p = .04, r = −.21. Classes did not differ in cognitive ability at Time 2, χ2(2) = 2.60, p = .27 (Figure 3B).
Figure 3A.
MSEL DQ at Time 1 in autistic participants from each class. Time 1 MSEL data were available from 57 autistic participants in the Stable Mild class, 114 in the Stable Intense class, and 6 in the Increasingly Intense class. Hinges correspond to first and third quartiles (25th and 75th percentiles) and whiskers extend 1.5x the interquartile range outwards from the hinges, or the range of the data (whichever is smaller).
Figure 3B.
DAS GCA at Time 2 in autistic participants from each class. Time 2 DAS data were available from 25 autistic participants in the Stable Mild class, 51 in the Stable Intense class, and 6 in the Increasingly Intense class.
Adaptive Behaviour
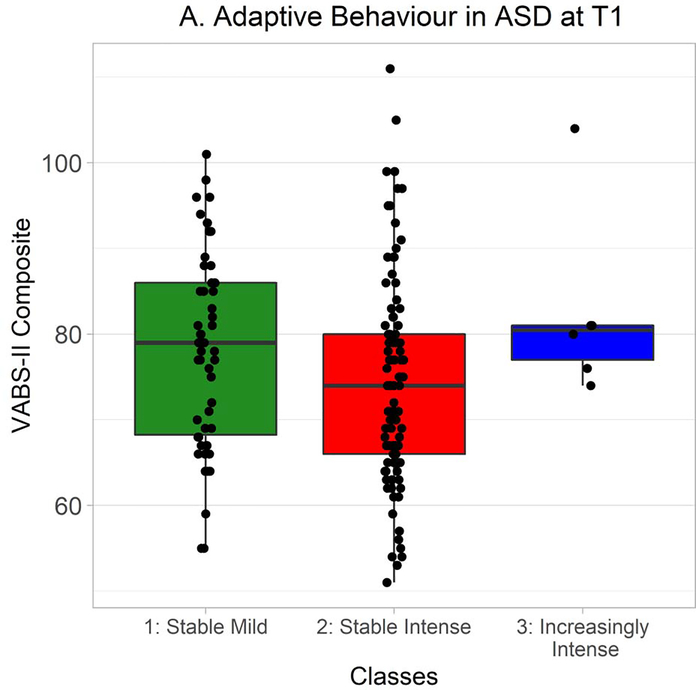
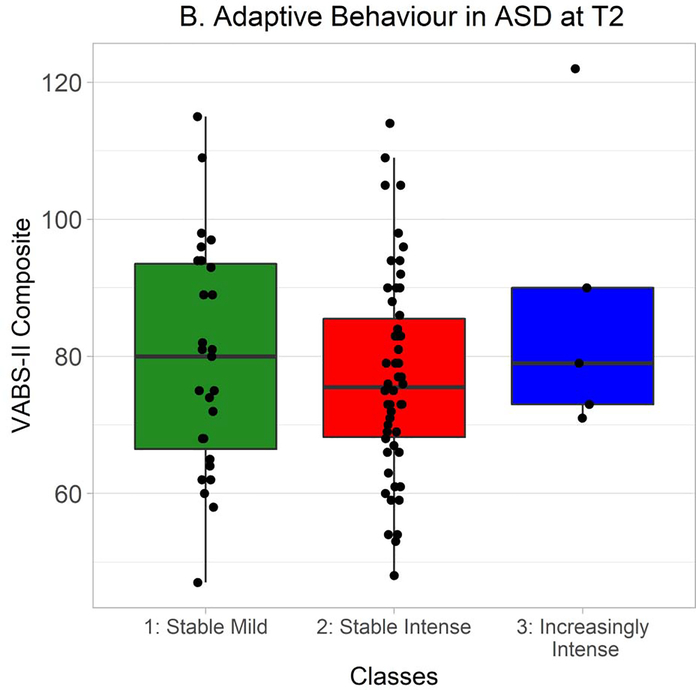
In ASD at Time 1, VABS-II composite scores strongly trended towards being non-normally distributed, so Kruskal-Wallis tests were used to probe differences between classes. Scores significantly differed between classes, χ2(2) = 6.81, p = .03 (Figure 4A). A follow-up Wilcoxon-Mann-Whitney test found a trend, non-significant after correction for multiple comparisons, towards adaptive functioning being greater in the Stable Mild class than the Stable Intense class, W = 3237.0, Holm-Bonferroni corrected p = .09, r = .17. VABS-II composite scores did not differ between classes at Time 2, χ2(2) = 1.10, p = .58 (Figure 4B).
Figure 4A.
VABS-II composite scores at Time 1 in autistic participants from each class. Time 1 VABS-II data were available from 54 autistic participants in the Stable Mild class, 99 in the Stable Intense class, and 6 in the Increasingly Intense class. Hinges correspond to first and third quartiles (25th and 75th percentiles) and whiskers extend 1.5x the interquartile range outwards from the hinges, or the range of the data (whichever is smaller).
Figure 4B.
VABS-II composite scores at Time 2 in autistic participants from each class. Time 2 VABS-II data were available from 27 autistic participants in the Stable Mild class, 54 in the Stable Intense class, and 5 in the Increasingly Intense class.
Anxiety
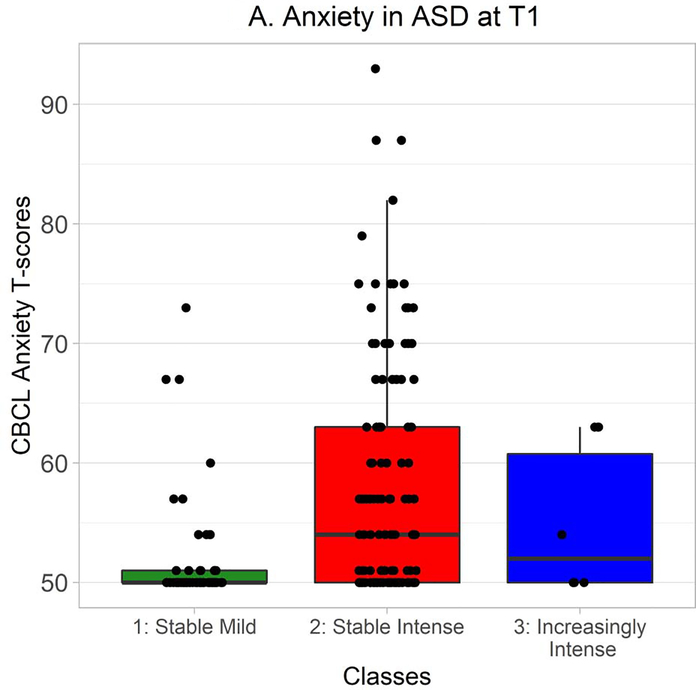
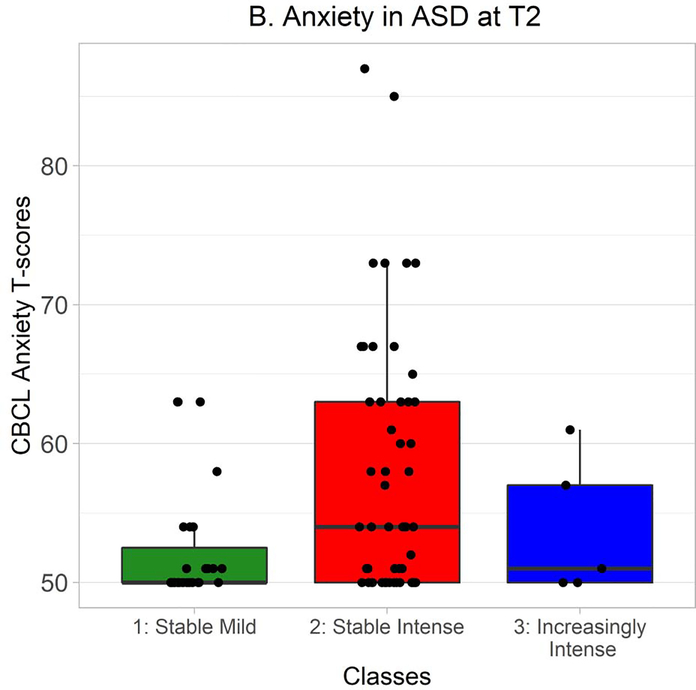
CBCL DSM-oriented anxiety T-scores were distributed non-normally in ASD, so Kruskal-Wallis tests were used to explore whether classes differed in anxiety levels. At Time 1, there was a significant effect of class on anxiety, χ2(2) = 30.13, p < .0001 (Figure 5A). Wilcoxon-Mann-Whitney tests indicated that anxiety was greater in the Stable Intense class than in the Stable Mild class, W = 321.0, Holm-Bonferroni corrected p < .0001, r = −.47. At Time 2, there was also a significant effect of class on anxiety, χ2(2) = 7.71, p = .02 (Figure 5B). Again, Wilcoxon-Mann-Whitney tests indicated that anxiety was greater in the Stable Intense class than in the Stable Mild class, W = 455.5, Holm-Bonferroni corrected p = .02, r = −.30.
Figure 5A.
CBCL DSM-oriented anxiety T-scores at Time 1 in autistic participants from each class. Time 1 CBCL data were available from 57 autistic participants in the Stable Mild class, 111 in the Stable Intense class, and 6 in the Increasingly Intense class. Hinges correspond to first and third quartiles (25th and 75th percentiles) and whiskers extend 1.5x the interquartile range outwards from the hinges, or the range of the data (whichever is smaller).
Figure 5B.
CBCL DSM-oriented anxiety T-scores at Time 2 in autistic participants from each class. Time 2 CBCL data were available from 27 autistic participants in the Stable Mild class, 53 in the Stable Intense class, and 5 in the Increasingly Intense class.
Discussion
The present study found substantial heterogeneity in sensory behaviours, as indexed by SSP scores, in ASD and TD. A total of three classes were defined, varying in the initial level and slope of caregiver-reported sensory behaviours. Furthermore, these classes differed in other variables, suggesting that the classes do not represent random variability or measurement error but that they have real-world importance. While we do not intend to make a strong suggestion that these three classes exist as discrete categorical entities, they appear to illuminate and describe some of the meaningful inter-individual variability in sensory processing that exists in ASD and TD.
The results of the present study were broadly consistent with our hypotheses. As predicted, in the optimal model, the majority of the autistic participants were placed within a single class, the “Stable Intense” class. As the name suggests, this class was characterized by stability of SSP raw scores over time; the slope value estimated for the class was small and nonsignificant. However, these stable sensory features were highly atypical/problematic.
The second hypothesis, regarding typically-developing participants, was also largely sustained. As expected, the majority of typically-developing participants were found within a single class with fewer problematic or unusual sensory behaviours, the “Stable Mild” class; indeed, only two typically-developing participants were placed elsewhere. Unexpectedly, contrary to results from McCormick et al. (2016), total sensory behaviour scores in this class did not show any sign of further increasing between Time 1 and Time 2. However, this lack of a positive slope can be attributed to the presence of a large number of autistic participants in the Stable Mild class. The LGM fitted to the typically-developing participants suggested that SSP scores did modestly increase between the two time-points, reflecting a decrease in sensory behaviours indexed as “atypical” by the SSP in TD. The seemingly-paradoxical idea that sensory features in typically-developing individuals can become more “typical” over time may suggest a need for caution in interpreting SSP results across different age groups in the absence of age-specific norms.
The third hypothesis was also consistent with the obtained pattern of classes. The third hypothesis suggested that there would be at least one additional class, primarily made up of autistic participants, with a different intercept and slope from the autistic-dominated Stable Intense class. In the optimal model, the properties of the “Increasingly Intense” class appeared to be consistent with these predictions. Although only six participants were ultimately placed within this class on the basis of likelihood of class membership, all were autistic. While their initial levels of sensory behaviours appeared relatively mild and “typical,” a sharp decline in SSP scores prior to Time 2 suggested a dramatic increase in the problematic or unusual sensory behaviours. Thus, both the initial level and trajectory of sensory behaviour in the Increasingly Intense class starkly differed from the Stable Intense class.
Increasingly Intense Class
Although only six participants were assigned to the Increasingly Intense class based on individual likelihood, it is worth noting that the overall model estimates ~18 participants should be included in the class. The Increasingly Intense class is defined chiefly by its extreme slope, not its intercept, and not all participants in the present study had data available from both time points. This may account for discrepancies between class assignments and estimated class size.4
However, if one considered only the SSP total scores used in the estimation of the GMM, one might become seriously concerned that the Increasingly Intense class could simply reflect measurement error and regression to the mean. According to this interpretation, the initially-high scores of autistic participants in the Increasingly Intense class, or their later scores at Time 2, might simply have reflected errors or transitory biases on the part of the caregivers completing the forms. However, autistic participants in the Increasingly Intense class appear to have systematically differed from autistic participants in other classes in their performance on another variable. Although the omnibus effect of cognitive ability at Time 1 was not quite significant, Holm-Bonferroni-corrected follow-up comparisons nevertheless suggested that autistic participants in the Increasingly Intense class had higher MSEL DQ scores at Time 1 than autistic participants in the other classes: that is, autistic participants in the Increasingly Intense class appeared to have higher levels of cognitive ability. Furthermore, it should be noted that these results were obtained with a behavioural assessment, whereas the SSP is a caregiver-report questionnaire. This difference in measure type may help to prevent biases affecting performance on one variable from influencing performance on the other. Thus, an interpretation of the Increasingly Intense class as being solely a reflection of measurement error and regression to the mean appears unlikely.
It may be significant that the sensory challenges of participants in the Increasingly Intense class evidently worsened between approximately preschool and school age. The context around autistic people governs the sensory inputs they receive (Mostafa, 2008), so the transition to a school environment, which may be larger, more chaotic, and more overwhelming than the autistic person’s home, might lead to more obvious sensory behaviours. Further research may be needed to fully understand whether this is the case and, if so, why this transition has a stronger impact on a particular subset of autistic children with high cognitive abilities. While we had speculated that cognitive ability might help participants convey their sensory experiences to caregivers, as the cognitive ability effect in the present study was observed at Time 1, before sensory problems in the “Increasingly Intense” class worsened, another possible explanation of the “Increasingly Intense” class could be that high cognitive abilities helped participants to regulate their sensory behaviours at Time 1, although increasing demands might have limited the effectiveness of such regulation by Time 2.
Stable Intense Class
The majority of autistic participants, however, were placed within the “Stable Intense” class. Though sensory processing in this class did not change over time, participants in this class had highly “atypical” sensory features; the class’ intercept value fell within the range of SSP raw scores (141 – 38) that are considered to indicate a “Definite Difference” from normal levels of sensory behaviour. Thus, this class emphasizes the importance of sensory features in the ASD phenotype.
The observed effects of anxiety and adaptive behaviour can perhaps cast light on some of the potential implications of these highly atypical sensory features. At Time 1, adaptive functioning scores of autistic participants differed across classes, and while follow-up comparisons did not attain significance after correction, autistic participants in the Stable Mild class appeared to have higher adaptive functioning scores than autistic participants in the Stable Intense class. Moreover, at both time points, autistic participants in the Stable Mild class had less anxiety than their peers in the Stable Intense class.
There are at least two possible explanations for these findings. First, it should be noted that all three variables – sensory behaviours, anxiety, and adaptive behaviour – discussed in this section were measured by caregiver-report. It is conceivable that the observed effects reflect questionnaire items that do not specifically capture only a single construct, but that are also influenced by other constructs, perhaps due to imperfections in item wordings or perhaps due to halo effects.
Alternatively, the present study may – replicating findings from numerous other studies (e.g., Ausderau et al., 2016; Ben-Sasson et al., 2008; Tomchek et al., 2015; Uljarević et al., 2016) – indicate that these variables are genuinely interrelated. It is not difficult to imagine plausible mechanisms for such relationships. As noted earlier, sensory inputs are determined by the environment around autistic people. Thus, autistic people with atypical sensory processing could have difficulties functioning in everyday environments with problematic sensory inputs, and these difficulties might be reflected in the lower adaptive functioning scores found in the Stable Intense class. Alternatively, sensory differences might impact acquisition of developmental skills, lowering adaptive functioning scores. Anxiety, for its part, might be a natural consequence of negative sensory experiences; if an autistic individual frequently had negative sensory experiences, it might be entirely reasonable for them to anxiously anticipate that, at some point in the future, the problematic sensory inputs that caused their past negative experiences will recur.
Limitations
Although the present study has a number of strengths, including its large and well-characterized longitudinal sample, it also has limitations. Perhaps the greatest weakness of this study is the limited information available for assessment of sensory processing. Multimodal assessment of sensory processing, rather than sole reliance upon single measurement types (such as the caregiver-report questionnaire used in the present study), has been recommended (Uljarević et al., 2017). As noted by Grandin and Panek (2014, pp. 81–83), caregivers do not have direct insight into the minds of their autistic children, and thus, results obtained on caregiver-report questionnaires may not necessarily reflect the true sensory experiences of an individual. It is therefore possible that unusual or problematic behaviours unrelated to sensory processing could be mistaken for sensory-related behaviours. Furthermore, the SSP was not specifically designed for use in autism. Certain SSP items (e.g., “Has difficulty paying attention”) appear likely to tap into constructs unrelated to sensory processing, especially in ASD. Some caution should therefore be exercised in interpreting sensory scores.
Indeed, a second limitation of the present study is its focus on the total sensory score, rather than subscale scores. While it is important to note that seemingly opposite sensory patterns such as hyper- and hypo-sensitivity are actually positively associated with another (Linke et al., 2018), and while supplementary results from the present study suggest that the sensory scores and trajectories of the classes obtained in the present analysis were not driven by particular subscales (see Table 5, Supplementary Tables 3–4), this does not eliminate the possibility that some participants might differ in trajectories of sensory behaviour across the various SSP subscales.
Finally, a third limitation of the present study is its relatively short duration. While it is comparable to previous longitudinal studies of sensory processing in ASD, the present study contains only two measurement points, both in in early childhood, separated by a period of approximately three years. This leaves vast gaps in our knowledge of the development of sensory processing, particularly in older individuals.
Implications
The present study makes a number of contributions to our knowledge regarding the longitudinal development of sensory processing in ASD, as well as regarding TD, which are relevant to professionals and clinicians. First, the division of autistic participants into multiple classes emphasizes the heterogeneous nature of sensory processing in ASD. Even when sensory behaviours from different subscales are collapsed into a total score, it is apparent that there are some autistic individuals with relatively typical sensory features and other individuals with more unusual or problematic sensory behaviours. This supports individualized assessment of sensory features and needs.
Second, the study offers further support to prior research reports suggesting that sensory processing is associated with adaptive functioning and anxiety. Autistic participants who had low scores on the SSP across both time points – indicating stable, high levels of unusual sensory behaviour – were reported to have higher anxiety and (non-significantly, after correction for multiple comparisons) lower adaptive functioning scores than other autistic individuals. This emphasizes the importance of sensory features. Although directionality cannot be inferred from the present study, it is possible that accommodations or supports for sensory features could help individuals to function in their daily environments and could protect against the development of anxiety.
Third, the general stability of autistic participants’ sensory behaviour scores between time points in the Stable Mild and Stable Intense classes appears to support previous studies reporting little longitudinal change in overall levels of sensory features in ASD during early childhood (McCormick et al., 2016; Repetto et al., 2017). This finding is complemented by an apparent slight decrease in “atypical” or problematic sensory behaviours in TD, which is again supported by past research (McCormick et al., 2016).
Finally, the present study points to the existence of a subgroup of autistic individuals who, despite or because of high scores on a measure of cognitive ability, appear to be at risk for an increase in “atypical” or problematic sensory behaviours between preschool- and school-age years. Further research is needed to clearly understand the meaning of this subgroup, it seems quite possible that individuals in this subgroup are particularly sensitive to increasing environmental demands as they enter school environments. Schools can be chaotic and busy, which might put increased stress on an individual’s ability to cope with sensory stimulation. Thus, in times of transition, professionals and caregivers may find it advisable to monitor individuals for signs of increasing sensory challenges.
Supplementary Material
Highlights.
Autistic individuals vary widely in their levels of sensory features and challenges
Sensory features in autism generally remain roughly stable in early childhood
In a small subgroup, sensory features and challenges increase over time
A subgroup with more sensory features and challenges also exhibits elevated anxiety
Acknowledgements
We wish to gratefully acknowledge all of the children and families who generously devoted considerable time and effort to participating in this large longitudinal study, of which collection of Short Sensory Profile was only a small part. We warmly acknowledge the MIND Institute APP implementation and assessment team for their neuropsychological screening work and for coordinating SSP data collection with participants’ families. This study was funded by the UC Davis Health MIND Institute, by the UC Davis Deans’ Distinguished Graduate Fellowship, by the NIH (1R01 MH089626-01), the NIMH (U24MH081810), and by an Autism Center of Excellence grant awarded by the NICHD (P50 HD093079).
Conflict of Interest
None of the authors have any conflict of interest relevant to the present work.
In this paper, we use identity-first language (e.g., “autistic person”) to describe autism in preference to person-first language (e.g., “person with autism”). This partly reflects our recognition that many or most, albeit not all, individuals on the autism spectrum prefer identity-first language (Bury et al., 2020; Kenny et al., 2016). It also reflects concern regarding the potential for person-first language to reflect or even encourage stigma or negative attitudes (see Gernsbacher, 2017; Sinclair, 2013). Similarly, due to community opposition to the terms “disorder” and “condition” (Kenny et al., 2016) and concern that these terms reflect subjective value judgements, we have chosen to use the more neutral term “autism spectrum development” to describe autism.
In the manuscript, the subscale is described as a “visual/auditory filtering” subscale, but it seems reasonable to assume the authors confused the visual/auditory sensitivity subscale with the “auditory filtering” subscale.
There was a further time point between the two time points described in the present study, so the “Time 2” described in the present manuscript may be referred to as “Time 3” in some APP publications; however, no SSP data were collected at this additional time point.
Furthermore, age of diagnosis in ASD varies widely, and individuals with higher cognitive abilities tend to be diagnosed later (Zwaigenbaum et al., 2019). Given the trends towards high levels of cognitive abilities in the Increasingly Intense class, it seems possible that participants in this class are likely to be diagnosed somewhat later than would permit them to participate at Time 1. The idea that participants in the “Increasingly Intense” class might, in the real world, tend to be diagnosed later than other participants is not inconsistent with the present study’s finding that chronological age did not differ across classes. At Time 1, participants are quite young by the standards of community-diagnosed autistic individuals (e.g., see Ofner et al., 2018), raising the possibility that a lack of age differences might reflect something of a floor effect. Individuals who generally tend to be diagnosed later would most likely simply be excluded from the present study. Research with samples of infant siblings followed from birth might allow for investigation of this possibility.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Patrick Dwyer, Center for Mind and Brain, UC Davis, 267 Cousteau Place, Davis, CA, USA 95618.
Clifford D. Saron, Center for Mind and Brain, UC Davis, MIND Institute, UC Davis
Susan M. Rivera, Department of Psychology, UC Davis, Center for Mind and Brain, UC Davis, MIND Institute, UC Davis
References
- Achenbach TM, & Rescorla LA (2000). Manual for the ASEBA preschool forms and profiles. Burlington, VT: University of Vermont. [Google Scholar]
- Ausderau KK, Furlong M, Siderius J, Bulluck J, Little LM, Watson R … Baranek GT (2014). Sensory subtypes in children with autism spectrum disorder: Latent profile transition analysis using a national survey of sensory features. Journal of Child Psychology and Psychiatry, 55(8), 935–944. 10.1111/jcpp.12219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausderau KK, Sideris J, Little LM, Furlong M, Bulluck JC, & Baranek GT (2016). Sensory subtypes and associated outcomes in children with autism spectrum disorders. Autism Research, 9(12), 1316–1327. 10.1002/aur.1626 [DOI] [PubMed] [Google Scholar]
- Baker AEZ, Lane A, Angley MT, & Young RL (2008). The relationship between sensory processing patterns and behavioural responsiveness in autistic disorder: A pilot study. Journal of Autism and Developmental Disorders, 38(5), 867–875. 10.1007/s10803-007-0459-0 [DOI] [PubMed] [Google Scholar]
- Baranek GT (1999). Autism during infancy: A retrospective video analysis of sensory-motor and social behaviors at 9–12 months of age. Journal of Autism and Developmental Disorders, 29(3), 213–224. 10.1023/A:1023080005650 [DOI] [PubMed] [Google Scholar]
- Baranek GT, Carlson M, Sideris J, Kirby AV, Watson LR, Williams KL, & Bulluck J (2019). Longitudinal assessment of stability of sensory features in children with autism spectrum disorder or other developmental disabilities. Autism Research, 12(1), 100–111. 10.1002/aur.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranek GT, Woynaroski TG, Nowell S, Turner-Brown L, DuBay M, Crais ER, & Watson LR (2018). Cascading effects of attention disengagement and sensory seeking on social symptoms in a community sample of infants at-risk for a future diagnosis of autism spectrum disorder. Developmental Cognitive Neuroscience, 29, 30–40. 10.1016/j.dcn.2017.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Sasson A, Cermak SA, Orsmond GI, Tager-Flusberg H, Kadlec MB, & Carter AS (2008). Sensory clusters of toddlers with autism spectrum disorders: Differences in affective symptoms. Journal of Child Psychology and Psychiatry and Allied Disciplines, 49(8), 817–825. 10.1111/j.1469-7610.2008.01899.x [DOI] [PubMed] [Google Scholar]
- Ben-Sasson A, Gal E, Fluss R, Katz-Zetler N, & Cermak SA (2019). Update of a meta-analysis of sensory symptoms in ASD: A new decade of research. Journal of Autism and Developmental Disorders, 49(12), 4974–4996. 10.1007/s10803-019-04180-0 [DOI] [PubMed] [Google Scholar]
- Ben-Sasson A, Hen L, Fluss R, Cermak SA, Engel-Yeger B, & Gal E (2009). A meta-analysis of sensory modulation symptoms in individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders, 39(1), 1–11. 10.1007/s10803-008-0593-3 [DOI] [PubMed] [Google Scholar]
- Berlin KS, Williams NA, & Parra GR (2014). An introduction to latent variable mixture modeling (Part 1): Overview and cross-sectional latent class and latent profile analyses. Journal of Pediatric Psychology, 39(2), 174–187. 10.1093/jpepsy/jst084 [DOI] [PubMed] [Google Scholar]
- Billstedt E, Gillberg IC, & Gillberg C (2007). Autism in adults: Symptom patterns and early childhood predictors. Use of the DISCO in a community sample followed from childhood. Journal of Child Psychology and Psychiatry, 48(11), 1102–1110. 10.1111/j.1469-7610.2007.01774.x [DOI] [PubMed] [Google Scholar]
- Bury SM, Jellett R, Spoor JR, & Hedley D (2020). “It defines who I am” or “it’s something I have”: What language do [autistic] Australian adults [on the autism spectrum] prefer? Journal of Autism and Developmental Disorders. Advance online publication. 10.1007/s10803-020-04425-3 [DOI] [PubMed] [Google Scholar]
- Crane L, Goddard L, & Pring L (2009). Sensory processing in adults with autism spectrum disorders. Autism, 13(3), 215–228. 10.1177/1362361309103794 [DOI] [PubMed] [Google Scholar]
- Damiano-Goodwin CR, Woynaroski TG, Simon DM, Ibañez LV, Murias M, Kirby A, … Cascio CJ (2018). Developmental sequelae and neurophysiologic substrates of sensory seeking in infant siblings of children with autism spectrum disorder. Developmental Cognitive Neuroscience, 29, 41–53. 10.1016/j.dcn.2017.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels AM, & Mandell DS (2014). Explaining differences in age at autism spectrum disorder diagnosis: A critical review. Autism, 18(5), 583–597. 10.1177/1362361313480277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBoth KK, & Reynolds S (2017). A systematic review of sensory-based autism subtypes. Research in Autism Spectrum Disorders, 36, 44–56. 10.1016/j.rasd.2017.01.005 [DOI] [Google Scholar]
- Eliott CD (2007). Differential ability scales (2nd ed.). San Antonio, TX: Harcourt Assessment. [Google Scholar]
- Farmer C, Golden C, & Thurm A (2016). Concurrent validity of the Differential Ability Scales, Second Edition with the Mullen Scales of Early Learning in young children with and without neurodevelopmental disorders. Child Neuropsychology, 22(5), 556–569. 10.1080/09297049.2015.1020775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fushing H, & McAssey MP (2010). Time, temperature, and data cloud geometry. Physical Review, 82: 061110 10.1103/PhysRevE.82.061110 [DOI] [PubMed] [Google Scholar]
- Gernsbacher MA (2017). Editorial Perspective: The use of person-first language in scholarly writing may accentuate stigma. Journal of Child Psychology and Psychiatry and Allied Disciplines, 58(7), 859–861. 10.1111/jcpp.12706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Pickles A, & Lord C (2009). Standardizing ADOS scores for a measure of severity in autism spectrum disorders. Journal of Autism and Developmental Disorders, 39, 693–705. 10.1007/s10803-008-0674-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandin T, & Panek R (2014). The autistic brain: Helping different kinds of minds succeed. New York: Mariner Books. [Google Scholar]
- Green SA, Ben-Sasson A, Soto TW, & Carter AS (2012). Anxiety and sensory over-responsivity in toddlers with autism spectrum disorders: Bidirectional effects across time. Journal of Autism & Developmental Disorders, 42(6), 1112–1119. 10.1007/s10803-011-1361-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand BN, Dennis S, & Lane AE (2017). Latent constructs underlying sensory subtypes in children with autism: A preliminary study. Autism Research, 10(8), 1364–1371. 10.1002/aur.1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny L, Hattersley C, Molins B, Buckley C, Povey C, & Pellicano E (2016). Which terms should be used to describe autism? Perspectives from the UK autism community. Autism, 20(4), 442–462. 10.1177/1362361315588200 [DOI] [PubMed] [Google Scholar]
- Kern JK, Trivedi MH, Garver CR, Grannemann BD, Andrews AA, Savla JS, … Schroeder JL (2006). The pattern of sensory processing abnormalities in autism. Autism, 10(5), 480–494. 10.1177/1362361306066564 [DOI] [PubMed] [Google Scholar]
- Kojovic N, Ben Hadid L, Franchini M, & Schaer M (2019). Sensory processing issues and their association with social difficulties in children with autism spectrum disorders. Journal of Clinical Medicine, 8: 1508 10.3390/jcm8101508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnik A, Ali JB, Gliga T, Guiraud J, Charman T, & Jones EJH (2019). Increased cortical reactivity to repeated tones at 8 months in infants with later ASD. Translational Psychiatry, 9: 46 10.1038/s41398-019-0393-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger B, Piškur B, Schulze C, Beurskens A, & Moser A (2020). Environmental pre-requisites and social interchange: The participation experience of adolescents with autism spectrum disorder in Zurich. Disability and Rehabilitation. Advance online publication. 10.1080/09638288.2020.1753248 [DOI] [PubMed] [Google Scholar]
- Krieger B, Piškur B, Schulze C, Jakobs U, Beurskens A, & Moser A (2018). Supporting and hindering environments for participation of adolescents diagnosed with autism spectrum disorder: A scoping review. PLoS ONE One, 13(8): e0202071 10.1371/journal.pone.0202071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libero LE, Nordahl CW, Li DD, Ferrer E, Rogers SJ, & Amaral DG (2016). Persistence of megalencephaly in a subgroup of young boys with autism spectrum disorder. Autism Research, 9(11), 1169–1182. 10.1002/aur.1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L-Y, & Huang P-C (2019). Quality of life and its related factors for adults with autism spectrum disorder. Disability and Rehabilitation, 41(8), 896–903. 10.1080/09638288.2017.1414887 [DOI] [PubMed] [Google Scholar]
- Linke AC, Jao Keehn RJ, Pueschel EB, Fishman I, & Müller RA (2018). Children with ASD show links between aberrant sound processing, social symptoms, and atypical auditory interhemispheric and thalamocortical functional connectivity. Developmental Cognitive Neuroscience, 29, 117–126. 10.1016/j.dcn.2017.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little LM, Dean E, Tomchek SD, & Dunn W (2017). Classifying sensory profiles of children in the general population. Child: Care, Health and Development, 43(1), 81–88. 10.1111/cch.12391 [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Linda L, Cook EH Jr., Leventhal, Bennett L, DiLavore PC, … Rutter M (2000). The Autism Diagnostic Observation Schedule - Generic: A standard mesure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30(3), 205–223. 10.1023/A:1005592401947 [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, & Le Couteur A (1994). Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24(5), 659–685. 10.1007/BF02172145 [DOI] [PubMed] [Google Scholar]
- McConachie H, Wilson C, Mason D, Garland D, Parr JR, Rattazzi A, … Magiati I (2019). What is important in measuring quality of life? Reflections by autistic adults in four countries. Autism in Adulthood, 2(1), 4–12. 10.1089/aut.2019.0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick C, Hepburn S, Young GS, & Rogers SJ (2016). Sensory symptoms in children with autism spectrum disorder, other developmental disorders and typical development: A longitudinal study. Autism, 20(5), 572–579. 10.1177/1362361315599755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh DN, Miller LJ, & Shyu V (1999). Development and validation of the Short Sensory Profile In Dunn W, Sensory Profile: User’s manual (pp. 59–73). San Antonio, TX: Psychological Corporation. [Google Scholar]
- Milner V, McIntosh H, Colvert E, & Happé F (2019). A qualitative exploration of the female experience of autism spectrum disorder (ASD). Journal of Autism and Developmental Disorders, 49(6), 2389–2402. 10.1007/s10803-019-03906-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafa M (2008). An architecture for autism: Concepts of design intervention for the autistic user. ArchNet IJAR: International Journal of Architectural Research, 2(1), 189–211. [Google Scholar]
- Mottron L (2019). Detrimental “sensitivity” framework misses the positive performance, role and autonomy of autistic perception. Cognitive Neuroscience, 10(3), 168–169. 10.1080/17588928.2019.1596073 [DOI] [PubMed] [Google Scholar]
- Mullen EM (1995). Mullen scales of early learning (AGS ed.). Circle Pines, MN: American Guidance Service. [Google Scholar]
- Muthén LK, & Muthén BO (2017). Mplus user’s guide (8th ed.). Los Angeles, CA: Muthén & Muthén; Original work published 1998 [Google Scholar]
- Nordahl CW, Lange N, Li DD, Barnett LA, Lee A, Buonocore MH, … Amaral DG (2011). Brain enlargement is associated with regression in preschool-age boys with autism spectrum disorders. Proceedings of the National Academy of Sciences, 108(50), 20195–20200. 10.1073/pnas.1107560108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylund KL (2007). Latent transition analysis: Modeling extensions and an application to peer victimization (Doctoral dissertation). Retrieved from https://search.proquest.com/docview/304878179 [Google Scholar]
- Nylund KL, Asparouhov T, & Muthén BO (2007). Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural Equation Modeling, 14(4), 535–569. 10.1080/10705510701575396 [DOI] [Google Scholar]
- Ofner M, Coles A, Decou ML, Do MT, Bienek A, Snider J, & Ugnat A-M (2018). Autism spectrum disorder among children and youth in Canada 2018: A report of the National Autism Spectrum Disorder Surveillance System. Retrieved from https://www.canada.ca/en/public-health/services/publications/diseases-conditions/autism-spectrum-disorder-children-youth-canada-2018.html
- Ram N, & Grimm KJ (2009). Growth mixture models: A method for identifying differences in longitudinal change among unobserved groups. International Journal of Behavioural Development, 33(6), 565–576. 10.1177/0165025409343765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repetto LP, Jasmin E, Fombonne E, Gisel E, & Couture M (2017). Longitudinal study of sensory features in children with autism spectrum disorder. Autism Research and Treatment: 1934701 10.1155/2017/1934701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair J (2013). Why I dislike “Person First” language. Autonomy, the Critical Journal of Interdisciplinary Autism Studies, 1(2), 2–3. Retrieved from http://www.larry-arnold.net/Autonomy/index.php/autonomy/article/view/OP1 [Google Scholar]
- Sparrow SS, Cichetti DV, & Balla DA (2005). Vineland adaptive behavior scales (2nd ed.). Minneapolis, MN: NCS Pearson. [Google Scholar]
- Tomchek SD, Huebner RA, & Dunn W (2014). Patterns of sensory processing in children with an autism spectrum disorder. Research in Autism Spectrum Disorders, 8(9), 1214–1224. 10.1016/j.rasd.2014.06.006 [DOI] [Google Scholar]
- Tomchek SD, Little LM, & Dunn W (2015). Sensory pattern contributions to developmental performance in children with autism spectrum disorder. The American Journal of Occupational Therapy, 69(5): 185040 10.5014/ajot.2015.018044 [DOI] [PubMed] [Google Scholar]
- Uljarević M, Baranek G, Vivanti G, Hedley D, Hudry K, & Lane A (2017). Heterogeneity of sensory features in autism spectrum disorder: Challenges and perspectives for future research. Autism Research, 10(5), 703–710. 10.1002/aur.1747 [DOI] [PubMed] [Google Scholar]
- Uljarević M, Lane A, Kelly A, & Leekam S (2016). Sensory subtypes and anxiety in older children and adolescents with autism spectrum disorder. Autism Research, 9(10), 1073–1078. 10.1002/aur.1602 [DOI] [PubMed] [Google Scholar]
- Williams KL, Kirby AV, Watson LR, Sideris J, Bulluck J, & Baranek GT (2018). Sensory features as predictors of adaptive behaviors: A comparative longitudinal study of children with autism spectrum disorder and other developmental disabilities. Research in Developmental Disabilities, 81, 103–112. 10.1016/j.ridd.2018.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ZJ, Failla MD, Gotham KO, Woynaroski TG, & Cascio C (2018). Psychometric evaluation of the Short Sensory Profile in youth with autism spectrum disorder. Journal of Autism and Developmental Disorders, 48(12), 4231–4249. 10.1007/s10803-018-3678-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Dimian AF, Botteron KN, Dager SR, Elison JT, Estes AM, … IBIS Network. (2019). A longitudinal study of parent-reported sensory responsiveness in toddlers at-risk for autism. Journal of Child Psychology and Psychiatry, 60(3), 314–324. 10.1111/jcpp.12978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Duku E, Fombonne E, Szatmari P, Smith IM, Bryson SE, … Bruno R (2019). Developmental functioning and symptom severity influence age of diagnosis in Canadian preschool children with autism. Paediatrics and Child Health, 24(1), e57–e65. 10.1093/pch/pxy076 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.