Abstract
Objective:
To assess the prevalence rates of impaired glucose tolerance and gestational diabetes among expectant mothers, as well as the prevalence rate of undiagnosed diabetes mellitus in walk-in patients at selected health centres in North Central Trinidad.
Design:
A cross-sectional study over the period January 2012 to December 2016.
Setting:
Primary health care centres.
Sample Population:
Pregnant women aged 18–45 years who were within their second and third trimester of pregnancy, and for undiagnosed diabetes mellitus the sample population consisted of males and females over the age of 18.
Methodology:
Medical records of 90 pregnant women and 174 walk-in patients who received care at the selected health centres during the period January 2012 to December 2016 were examined and the following were recorded: age, ethnicity, parity, gravidity, past medical/surgical history, past obstetric history, oral glucose tolerance test results, random blood glucose results, HbA1c results, and family history of diabetes mellitus (DM).
Results:
The sample population was 90 expectant mothers and 174 walk-in patients. However, valid results were available for 50 expectant mothers and 78 walk-in patients. Of the 50 valid results for expectant mothers, 1 mother had a confirmed diagnosis recorded for gestational diabetes mellitus (GDM) yielding a prevalence of 2% for GDM. Age was positively correlated with the diagnosis of impaired glucose tolerance (IGT). (P = 0.028). Of the 141 valid entries for walk-in patients, 25 had a confirmed diagnosis of DM yielding a prevalence of ~ 18% for undiagnosed DM. A family history of diabetes was positively correlated with a subsequent diagnosis of DM among previously undiagnosed diabetes.
Conclusion:
The prevalence rate for GDM was found to be 2% and the prevalence rate for undiagnosed DM in walk-in patients was 18%.
Keywords: Gestational diabetes, prevalence, primary care, undiagnosed diabetes mellitus
Introduction
Impaired glucose tolerance (IGT) is the transition state between normal glycaemia and diabetes mellitus (DM). It increases an individual's risk for cardiovascular disease and the development of type 2 diabetes mellitus (T2DM). Gestational diabetes mellitus (GDM) occurs with onset or first recognition during pregnancy. During pregnancy, the placenta produces a variety of hormones, which impair the action of insulin on body cells, deranging carbohydrate metabolism thus increase the blood glucose levels.[1] GDM usually develops during the second half of the pregnancy and sometimes as early as the 20th week.[2] Moreover, some pregnant women have previously undiagnosed DM. It is therefore important that all pregnant women have to be tested in early pregnancy, as well as in the second and third trimesters to detect GDM. In 2019 there were an estimated 223 million women (20–79 years) living with diabetes. This number is projected to increase to 343 million by 2045. A total of 20 million or 16% of live births had some form of hyperglycaemia in pregnancy. An estimated 84% were due to GDM and 1 in 6 births was affected by GDM. The vast majority of cases of hyperglycaemia in pregnancy were in low-income and middle-income countries, where access to maternal care is often limited.[3]
In Trinidad and Tobago, the prevalence of impaired glucose tolerance and gestational diabetes amongst expectant mothers has not been extensively researched. A previous study done exclusively on patients at the Mt. Hope Women's Hospital in 2009 found the prevalence rate of GDM to be steadily rising; 1.67%, 4.58% and 6.67% during the years 2005, 2006 and 2007 respectively. This study expanded the sample size and area to further the understanding of GDM in Trinidad & Tobago. It revealed that some risk factors of GDM are unavoidable, but a few are related to poor lifestyle habits such as being overweight, gaining excessive weight during pregnancy and having unmanaged hypertension. It also proposed that additional studies be carried out so the incidence of GDM in Trinidad and Tobago can be further ascertained.[4] The prevalence of obesity is also increasing in the region as part of the global pandemic.[5] This transition toward non communicable diseases and its risk factors dictates that the primary care approach be shifted toward health promotion and disease prevention especially as it relates to DM and its risk factors.
In Trinidad and Tobago, the prevalence of impaired glucose tolerance and GDM among expectant mothers has not been extensively researched. A previous study done exclusively on patients at the Mt. Hope Women's Hospital in 2009 found the prevalence rate of GDM to be steadily rising; 1.67%, 4.58%, and 6.67% during the years 2005, 2006, and 2007, respectively. This study expanded the sample size and area to further the understanding of GDM in Trinidad and Tobago. It revealed that some risk factors of GDM are unavoidable, but a few are related to poor lifestyle habits such as being overweight, gaining excessive weight during pregnancy and having unmanaged hypertension. It also proposed that additional studies be carried out so the incidence of GDM in Trinidad and Tobago can be further as certained.[4]
The effects of diabetes on women's health and by extension the general population have far reaching consequences as it relates to adverse health outcomes including premature death and disease thereby compounding health care costs.
Background
The World Health Organization's Global Report on diabetes (2016) revealed that the prevalence of DM is steadily increasing over the years.[6] In the US the Center for disease control estimated a prevalence rate of 13% among US adults and a 6% prevalence rate of GDM.[7]
Previously, diabetes was more prevalent mainly in high-income countries but epidemiological transition has accounted for an increased prevalence in lower and middle-income nations. The lack of proper healthcare and education about the disease in some of these nations exacerbates the issue. Women who were previously diagnosed with DM or have a family history of DM or who are obese, have an increased risk of developing GDM.[1] Infants born to mothers with GDM have an increased risk of mortality and morbidity due to the following conditions: respiratory distress, growth abnormalities (large for gestational age (LGA), small for gestational age (SGA)), hyperviscosity secondary to polycythaemia, hypoglycaemia, congenital malformations, hypocalcaemia, hypomagnesemia, and iron abnormalities.[8] Regionally, Boyne reported that the prevalence rate of diabetes was between 11% and 18% of the population for several countries in the Caribbean region. He referred diabetes as an epidemic and stated that a sedentary lifestyle and increase in the prevalence of diabetes are the factors that fuelled same.[5]
The global burden of diabetes has increased tremendously over the past few decades, and its prevalence has risen greatly in lower and middle-income countries compared with high-income countries.[8] It is well known that the longer a person lives undiagnosed and untreated with diabetes, the worse their health outcomes are likely to be. GDM increases the risk of birth complications, infant birth weight, and likelihood of developing DM type 2 later in life for both mother and infant. In a study of GDM among expectant mothers admitted to the Mount Hope Women's Hospital, Trinidad, prevalence rates were reported to be 1.67%, 4.58%, and 6.67% each year between January 2005 and December 2007, respectively, and were projected to increase in the coming years. This retrospective study examined the clinical data of patients who were admitted and followed through to delivery, while examining variables and identifying any possible associations with the occurrence of GDM. The prevalence of GDM was reported as 3% with the greatest proportion of affected women belonging to the age group 35–39 years. The characteristic that conferred the greatest risk of GDM was a history of GDM in a previous pregnancy (41 times increased risk). As such, the importance of proper history taking and screening by primary care physicians is critical in the antenatal care and management of this condition.[4] A systematic review and meta-analysis of the prevalence and risk factors of GDM yielded a pooled prevalence of GDM of 11.5% (95% CI 10.9–12.1) in Asia.[9] In India, Nasreen Noor et al. conducted a cross-sectional study of pregnant women and reported a much higher proportion of ~40%for GDM, whereas a significant portion was at risk for GDM, particularly middle aged women from urban areas.[10] In Qatar Al-Hamaq et al. reported a GDM prevalence rate of 16.3%.[11] The study also revealed that some of the risk factors for developing GDM was obesity, advanced maternal age, low monthly income, and a family history of diabetes. Of those risk factors, obesity was the most significant. It also concluded that maternal complications such as pregnancy-induced hypertension (19.1% vs 10.3%; P < 0.001), pre-eclampsia (7.3% vs 3.8%; P = 0.012), antepartum haemorrhage (19.2% vs 14.6%; P = 0.05), and caesarean section (27.9% vs 12.4%; P < 0.001) were significantly higher in GDM women. As for the offspring of mothers with GDM, this study showed that they were at increased risk of preterm birth (12.6% vs 8.3%; P = 0.03), macrosomia (10.3% vs 5.9%; P = 0.01), and birth trauma (8% vs 3%; P < 0.001). In the USA, Dabelea et al. discovered that the prevalence of GDM doubled between 1994 and 2002 among women of various ethnic/racial backgrounds living in Colorado from 2.1% in 1994 to 4.1% in 2002.[12]
The issue of undiagnosed DM should be of great concern to primary care practitioners as it reveals gaps in screening and failure to identify a sometimes large hidden burden of disease. In India, close to half of the people with diabetes are not aware of their disease status and a large subset of these people are at risk for poor detection despite having access to healthcare.[13] Trinidad and Tobago, although a much smaller nation has a large Indo-population that may similarly be at risk. The estimation of the magnitude of the undiagnosed can encourage healthcare workers to enhance the provision of preventative services and hopefully the adoption of healthier lifestyles by patients.
Setting
This project was conducted at health centres in North Central Trinidad, namely the Arouca, Tunapuna, and the St. Joseph enhanced health centre. Those health centres provide antenatal services and perform routine screening and blood testing of pregnant women thereby facilitating collection of data relevant to this study. Those health centres also offer walk-in clinics available to patients, from which data related to random blood glucose tests can be accessed.
Ethical approval
Ethical approval was granted from the University of West Indies on May 11, 2017 by the Campus Ethics Committee. Further approval was granted from the Chief Executive Officer of the North Central Regional Health Authority on May 26, 2017.
Methodology
Data were collected retrospectively by examination of the medical records of antenatal clinic patients and walk-in patients who accessed healthcare services at three health centres.
During the period, January 2012 to December 2016, random sampling was performed to obtain data from the antenatal and walk-in log books at the clinics. The process for random selection involved the following: First, an nth number was calculated:
nth number = Total number of patients ÷ sample size
A random number generator was used to generate a random number as the starting number which would be the first patient file. Following this, the nth number of files were skipped and the subsequent file being the second patient file. Pregnant woman between 18 and 45 years, who were diagnosed with GDM by their attending physician based on the criteria provided by the WHO were selected for this study. With respect to undiagnosed DM, the sample population consisted of walk-in patients over the age of 18 years with previously undiagnosed DM in accordance with the WHO diagnostic criteria for DM.
The sample sizes were then calculated and found to be minimum 45 cases (3% prevalence rate[4]) for GDM and 174 cases (13% prevalence rate[14]) for undiagnosed DM. Both were calculated at 5% precision. The minimum 45 cases for GDM were however increased to 90 files in order to increase the power of the study. The following formula was used to calculate the sample sizes needed[15]:

Sample size calculation for GDM study:

Sample size calculation for walk-in study:

Inclusion criteria used for the GDM prevalence study were as follows:
Pregnant woman diagnosed as per the WHO criteria for GDM
Age group 18–45 years Inclusion criteria used for the prevalence of undiagnosed DM was as follows:
Patients not previously diagnosed with DM as per the WHO criteria for DM
Patients with medical records indicating subsequent diagnosis of DM as per WHO guidelines
Patients over 18 years
Excluded were:
Patients who were previously diagnosed with DM
Expectant mothers who were out of the age group 18–45 years
Miscarriages that occurred before delivery
The following demographics were recorded: age, ethnicity, parity, gravida, body mass index (BMI), family history of DM, history of GDM, past medical/surgical history, past obstetrical history, OGTT, HbA1c, and fasting blood glucose values.
Data were recorded using a standardized collection template and further analyzed using statistical package for the social sciences (SPSS) version 18.
Standard WHO diagnostic criteria were applied for diagnosis of IG, GDM, and DM.[8,14]
Results
The data for the investigation on prevalence of IGT and GDM among expectant mothers was marred by missing entries, particularly those for the selected diagnostic tests—the oral glucose tolerance test (OGTT) and the random blood glucose test. Of the 90 sample files that were obtained 20 had valid entries for the OGTT (≈22%) and 40 had valid entries for the random blood glucose test (≈44%) [Figure 1].
Figure 1.

Valid vs missing responses for diagnostic test
In analysis of the data, variables were assigned categorically into “normal” and “abnormal” based on the results of the OGTT and fasting blood glucose test [Figure 2].
Figure 2.
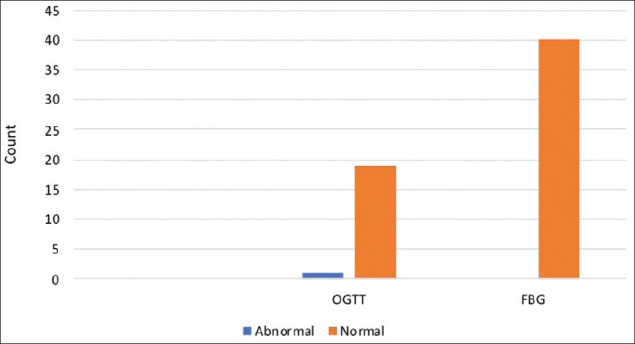
Results of oral glucose tolerance and fasting blood glucose tests
One case out of the sample population showed an abnormal reading for the OGTT and none showed abnormal for the fasting blood glucose test. The data sample population yielded a prevalence rate of 2%.
Further analysis was done to determine what factors contribute to hyperglycaemia and would thus increase the risk of impaired glucose tolerance and gestational diabetes. Age was found to be positively correlated with impaired glucose tolerance (P = 0.028). ANOVA testing of other characteristics revealed no other significant correlation with IGT tests.
Analysis revealed that there was no significant correlation between obstetric history and blood glucose levels in the sample population (P > 0.05) [Figures 3 and 4].
Figure 3.
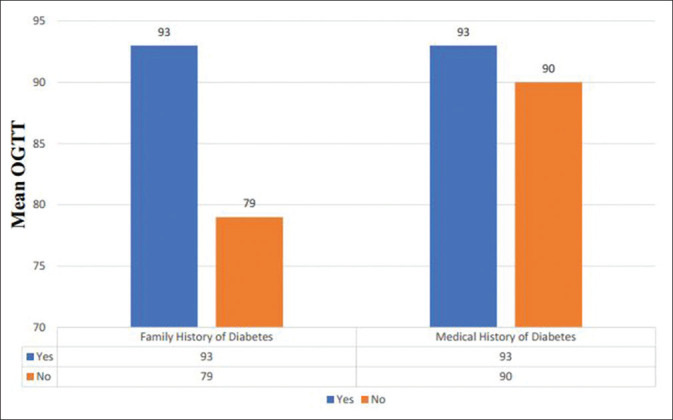
Mean OGTT of expectant mother in relation to family and medical history of DM
Figure 4.
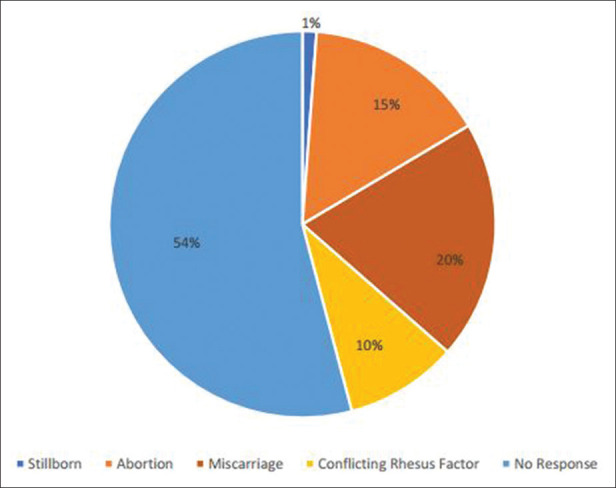
Obstetric history of the sample population
The data for the investigation on undiagnosed diabetes in walk-in patients also contained missing entries particularly those for the selected diagnostic tests—the oral glucose tolerance test (85%), fasting blood glucose (84%), random blood glucose (20%), and glycated haemoglobin (44%). Out of the 174 patient files reviewed, 33 had missing entries thereby yielding a total of 141 valid entries for analysis [Figure 5].
Figure 5.
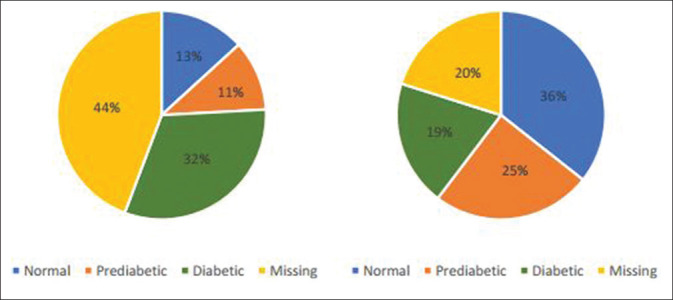
HbA1c and random blood glucose test results
To calculate the prevalence of undiagnosed diabetes in the sample population, “undiagnosed diabetes” was first defined as those persons who had no previously documented history of diabetes but their test values were in the range for diagnosis of diabetes. All the diagnostic tests were used to give a total number of persons in the sample population who had undiagnosed diabetes resulting in 25 out of the 141valid entries yielding a prevalence rate of ~18%.
Many of these cases were women who had medical histories of hypertension (56%) and were between 58 and 69 years. Most of the cases with undiagnosed diabetes also had a family history of diabetes (78%) [Figure 6]. Chi-squared testing revealed a statistically significant correlation between a positive family history of diabetes and the subsequent diagnosis of DM (P < 0.05).
Figure 6.
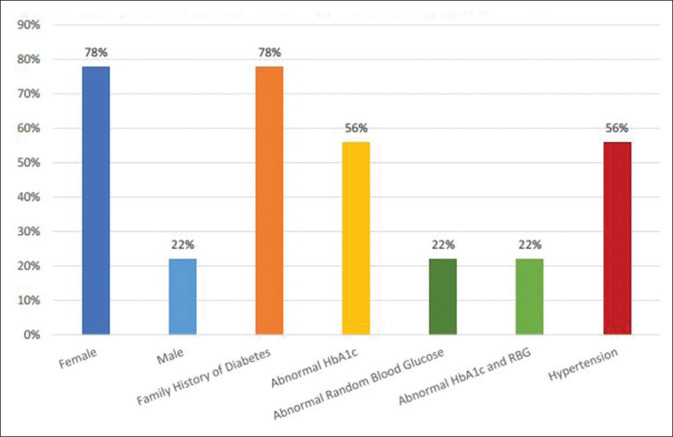
Demographics and characteristics of the undiagnosed diabetic patients
Regression analysis was performed but failed to identify any factor that was correlated with undiagnosed DM.
Discussion
Our study demonstrated a prevalence rate of 2% for gestational diabetes mellitus (GDM) at the NCRHA health centres studied. This rate compares favourably with M. Clapperton et al. who found a prevalence rate of 1.67% in 2005 at the Mt. Hope Women's Hospital, Trinidad (MHWH).[4] The MHWM is the main tertiary health institution serving the same region as the NCRHA health centres involved in our study, as such, similar demographics were involved. However, M. Clapperton et al. also predicted the prevalence rate of GDM to rise to between 6.67% and 9.31% by 2008. Our study was conducted at primary healthcare-based facilities and therefore is unable to confirm the predicted upward trend. However, the 2% prevalence rate for GDM in our setting coupled with a national prevalence of 14.5% suggests that the prevalence of GDM at the MHWH may be much higher today and warrants further investigation. Regionally, double digit prevalence rates for GDM have been documented by L. Guariguata et al. (2014), where the estimated prevalence rate of GDM for the North American and Caribbean regions was 10.4%.[16] The variance observed may be accounted for by data available at the MHWM vs health centres, as the health centres are primary care oriented, often with referral of high risk pregnancy cases to the MHWM. The inadequate documentation and loss to follow-up as evidenced by missing entries o may contribute to an under-estimation of the prevalence rates for GDM in Trinidad and Tobago.
Notwithstanding, age was strongly correlated to elevated blood glucose levels in pregnancy (P = 0.028). No other statistically significant associations were found with respect to medical history of diabetes mellitus, family history or past obstetric history.
Advanced maternal age is a well-documented risk factor for GDM. During the second and early third trimesters of pregnancy, insulin resistance is greatly increased. In normal women, their pancreas maintains the ability to meet the demands of this physiological insulin resistance. However, women with GDM experience hyperglycaemia unless otherwise treated.[17] At the Summary and Recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus 2005, researchers suggested various factors which may contribute toward GDM, including autoimmune, genetic abnormalities, and chronic β-cell defects.[18] Over years, as women get older, insulin secretion decreases and with the further metabolic stresses placed by pregnancy, hyperglycaemia may develop in susceptible individuals.
The prevalence rate of 18% for undiagnosed diabetes mellitus amongst walk-in patients is higher than the International Diabetes Federation estimate of 14.5%.[19] This variance may be accounted for by factors such as the relatively small sample size and demographics of the urbanized populations accessing care at these health centres. This urbanized population may have been more like to consume unhealthy diets and lead sedentary lifestyles as compared with more rural areas of Trinidad and Tobago. These factors coupled with a rising global prevalence of diabetes mellitus due to obesity[20] may account for the increased rate of 18% found in our study. It was observed that 78% of patients with a family history of diabetes mellitus subsequently went on to develop DM themselves (P < 0.05). This may be explained by genetics which plays a major role in type 1 and type 2 diabetes. Several genes contribute to a patient's genetic predisposition for either type 1 or type 2 diabetes. Genetics coupled with other factors such as climate, bacterial/viral infections and lifestyle choices tend to trigger the onset of DM.[21]
Undiagnosed DM poses great risks to individuals as it can be a clinically silent disease often associated with other cardiovascular risk factors which may be present for many years before clinical symptoms occur. Several long-term complications such as cardiovascular disease, diabetic retinopathy, diabetic neuropathy, and diabetic nephropathy can occur if DM is left untreated. GDM also presents risks to both mother and baby such as women being more susceptible to later developing Type 2 DM, while the baby may experience growth abnormalities. With a rise in both DM and GDM predicted worldwide, these health concerns should be of importance to everyone. Proper screening, history taking, diagnosis, and management of both DM and GDM should be emphasized to primary care physicians to further protect maternal and child health and by extension public health.
Limitations
Missing files and data within files.
Loss to follow-up as some patients were referred to other tertiary health facilities.
Limited generalizability of our findings.
Conclusion
In conclusion, the prevalence rate of gestational diabetes mellitus was found to be 2% and the prevalence rate of undiagnosed diabetes in walk-in patients was found to be 18%. For GDM, the research also showed that there was a strong correlation between age and increased blood glucose level (P < 0.05). Older women exhibited higher blood glucose levels than younger women. For undiagnosed DM, it was observed that 78% of persons who had a family history of diabetes mellitus had increased random blood glucose values. Second, women with hypertension aged 58–69 years also had greater than normal random blood glucose values. One of the major limitations of this study was missing data thereby reducing its internal and external validity.
Recommendations
The correlation of increased age with IGT levels in expectant mothers suggests that focused screening for GDM be offered to older mothers to better diagnose GDM, identify other antenatal risk factors and ultimately improve maternal and child health.
The prevalence levels found in the study suggest that it may also be prudent to offer appropriate, universal screening for GDM and DM at all antenatal and walk-in clinics due to the high prevalence nationally and the propensity for adverse health outcomes.
The computerization of medical records should be implemented to reduce the number of missing files, data, and poor documentation thereby enhancing data collection.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Caribbean Public Health Agency. Maternal care in pregnancy-Guidelines for the Caribbean CARPHA. 2014. Available from: http://carphaorg/guidelines/pregnancyguidelinespdf .
- 2.Mayo Clinic. Gestational Diabetes. Available from: http://wwwmayoclinicorg/diseasesconditions/gestational-diabe tes/home/ovc-20317173 .
- 3.International Diabetes Federation. IDF Diabetes Atlas. 9th ed. Brussels, Belgium: International Diabetes Federation; 2019. [PubMed] [Google Scholar]
- 4.Clapperton M, Jarvis J, Mungrue K. Is gestational diabetes mellitus an important contributor to metabolic disorders in Trinidad and Tobago? Obstet Gynecol Int. 2009;2009:289329. doi: 10.1155/2009/289329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyne M. Diabetes in the Caribbean: Trouble in paradise. Insulin. 2009;4:94–105. [Google Scholar]
- 6.Roglic G. WHO Global report on diabetes: A summary. Int J Non-Commun Dis. 2016;1:3–8. [Google Scholar]
- 7.U. S. Department of Health and Human Services Centers for Disease Control and Prevention National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States. 2020 [Google Scholar]
- 8.World Health Organization. Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy. WHO; 2013. Available from: http://appswhoint/iris/bitstream/10665/85975/1/WHO_NMH_MND_132_engpdf . [PubMed] [Google Scholar]
- 9.Lee KW, Ching SM, Ramachandran V, Yee A, Hoo FK, Chia YC, et al. Prevalence and risk factors of gestational diabetes mellitus in Asia: Asystematic review and meta-analysis. BMC Pregnancy Childbirth. 2018;18:494. doi: 10.1186/s12884-018-2131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noor N, Ahmed A, Baig MS, Shah MI, Ali HN, Rajput AH, et al. Cross sectional analysis of rate of presentation of patients with gestational diabetes. Indo Am J Pharm Sci. 2017;4:1924–8. [Google Scholar]
- 11.Bener A, Saleh NM, Al-Hamaq A. Prevalence of gestational diabetes and associated maternal and neonatal complications in a fast-developing community: Global comparisons. 2011;3:367–73. doi: 10.2147/IJWH.S26094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dabelea D, Snell-Bergeon JK, Hartsfield CL, Bischoff KJ, Hamman RF, McDuffie RS, et al. Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort. Diabetes Care. 2005;28:579–84. doi: 10.2337/diacare.28.3.579. [DOI] [PubMed] [Google Scholar]
- 13.Claypool KT, Chung MK, Deonarine A, Gregg EW, Patel CJ. Characteristics of undiagnosed diabetes in men and women under the age of 50 years in the Indian subcontinent: The national family health survey (NFHS-4)/Demographic health survey 2015-2016. BMJ Open Diabetes Res Care. 2020:8. doi: 10.1136/bmjdrc-2019-000965. e000965doi: 101136/bmjdrc-2019-000965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia WHO/IDF. 2006. Available from: http://wwwwhoint/diabetes/publications/Definition%20and%20diagnosis%20of%20diabetes_newpdf .
- 15.Charan J, Biswas T. How to calculate sample size for different study designs in medicalresearch? Indian J Psychol Med. 2013;35:121–6. doi: 10.4103/0253-7176.116232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guariguata L, Linnenkamp U, Beagley J, Whiting DR, Cho NH. Global estimates of the prevalence of hyperglycaemia in pregnancy. DiabetesRes Clin Pract. 2014;103:176–85. doi: 10.1016/j.diabres.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association. Standards of medical care in diabetes 2017 ADA. 2017. Availablefrom: http://carediabetesjournalsorg/content/diacare/suppl/2016/12/15/40 Supplement_1DC1/DC_40_S1_finalpdf .
- 18.Metzger BE, Buchanan TA, Coustan DR, de Leiva A, Dunger DB, Hadden DR, et al. Summary and Recommendations of the Fifth International Workshop Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(Suppl 2):S251–60. doi: 10.2337/dc07-s225. [DOI] [PubMed] [Google Scholar]
- 19.International Diabetes Federation. IDF NAC region IDF. 2017. [Last accessed on 2017 Aug 05]. Available from: https://wwwidforg/our-netw ork/regions-members/north-america-and-caribbean/members/73-trinidad-and-tobagohtml .
- 20.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes. Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. Genetics and Diabetes WHO. [Last accessed on 2017 Jul 04]. Available from: http://wwwwhoint/genomics/about/Diab etis-finpdf .


