Abstract
Introduction:
Periodontitis is associated with many chronic health conditions including diabetes, cardiovascular disease, and rheumatoid arthritis (RA). RA and periodontitis have similarities in inflammatory mechanism, morphology, and histopathology. Osteoarthritis (OA) is a chronic, multifactorial degenerative disease characterized by the deterioration of cartilage in joints.
Objective:
The aim of this study was to evaluate the prevalence and severity of periodontal disease in patients with established RA and OA.
Materials and Methods:
A total of 200 patients reporting to the Department of Orthopaedics, KIMSDU, Karad were included in the study. Patients were divided into two groups: Group 1 that included 100 patients with established RA diagnosed according to American College of Rheumatology (ACR) classification 1987 criteria and Group 2 that comprised 100 patients diagnosed with OA. Demographic profile, medical and dental history, oral hygiene practices, and smoking status of study participants were recorded. Periodontal status of the patients were evaluated using the simplified oral hygiene index (OHI-S), Loe and Silness gingival index (GI), probing pocket depth (PPD), and clinical attachment level (CAL). On the basis of the CAL score periodontitis severity was defined as slight, moderate, and severe. Rheumatoid Factor (RF) and C-reactive protein (CRP) were considered as a serological marker in RA. Serological tests were performed to measure RF and CRP. Periodontal parameters and serological tests were correlated.
Results:
This study reported 45% severe periodontitis prevalence in RA compared to OA group, which was 33%. Severity of periodontitis is significantly greater in RF positive RA group with mean CAL 5.38 mm compared to RF negative RA group with mean CAL 2.81 mm (P = 0.001). There was moderate positive correlation found between RF titer and severity of periodontitis (r = 0.311).
Conclusion:
The severity of periodontitis was significantly higher among the patients with established RA as compared to patients with OA. RF positive patients had higher periodontal disease compared to RF negative patients. There was an increase in the mean clinical attachment loss with increase in RF titer.
Keywords: Osteoarthritis, periodontitis, rheumatoid arthritis, rheumatoid factor
Introduction
Rheumatoid arthritis (RA) is a chronic auto-immune inflammatory condition of the bone. RA mainly affects wrist joints, results in swelling of synovial, progressive destruction of articular cartilage, bone erosion, and ultimately leads to loss of joint integrity. The prevalence of RA has been estimated to be 0.7% in India.[1] The occurrence of RA increases with the age and higher in female than male (3:1).[2]
Osteoarthritis (OA) is a chronic, degenerative disease with a multifactorial etiology. OA is characterized by the deterioration of cartilage in joints which results in stiffness, pain, and impaired movement.[3] Prevalence of OA ranges from 17% to 60.6% in India.[4]
The concept that RA and periodontal disease (PD) share a common inflammatory mechanism was first reported by Snyderman and McCarty.[5] RA and PD are self-sustaining inflammations that cause joint damage and alveolar bone loss, respectively.[6,7,8,9]
Porphyromonas gingivalis (Pg) is a key microrganism involved in the development and progression of PD. Pg contains Peptidylarginine deiminase (PAD) enzyme capable of citrullination of peptides, which is an important etiopathologic event in RA.[10] This has driven interest in additional research on citrullination as a mechanistic link between the RA and PD.
Contradictory results have been observed on the relationship between RA and PD.[11,12,13,14,15,16] Tang et al.[17] reported good evidence to support an association between RA and PD based on clinical parameters and moderate evidence for serological parameters. Similar prevalence of PD has been observed in patients with OA and RA.[18]
The scientific literature relating the association between PD, OA and RA are scarce. Therefore, this study was conducted to evaluate and compare the prevalence and severity of PD in patients with RA and OA.
Materials and Methods
This cross-sectional study was carried out in the in the Department of Orthopaedics, Krishna Institute of Medical Sciences, Karad. Ethical clearance was obtained from the ethical committee of Krishna Institute of Medical Sciences Deemed University (Ref. no. KIMSDU/IEC/02/2018). The nature and purpose of the study was explained to the subjects and a written consent was obtained before commencing the study. A total of 200 patients reporting to the Department of Orthopaedics, Krishna Institute of Medical Sciences, Karad were included in the study. Patients were divided into two groups, Group 1 included 100 patients with established RA according to American College of Rheumatology (ACR) classification 1987 criteria.[19] Group 2 comprised 100 patients suffering from OA diagnosed as per clinical and radiographic findings. This study was conducted during the period from February 2018 to September 2019.
The inclusion criteria and exclusion criteria were as follows. The patients with RA and OA with age ≥30 years and having more than 8 teeth present were included in the study. The periodontitis-associated inflammatory burden decreases with decreasing number of teeth, hence the criteria of eight remaining teeth was chosen.11 Patients with other systemic diseases or conditions (e.g. diabetes mellitus) that are known as risk factors for periodontitis were excluded. Patients suffering from infections or conditions which may have rheumatoid factor (RF) present such as systemic lupus erythematosus, Sjögren's syndrome, hepatitis, syphilis, infectious mononucleosis, liver disease, and sarcoidosis were excluded. Patients with history of periodontal therapy and use of any medications influencing periodontal tissues within six months were not considered. Recruitment and allocation of patients is shown in Figure 1.
Figure 1.
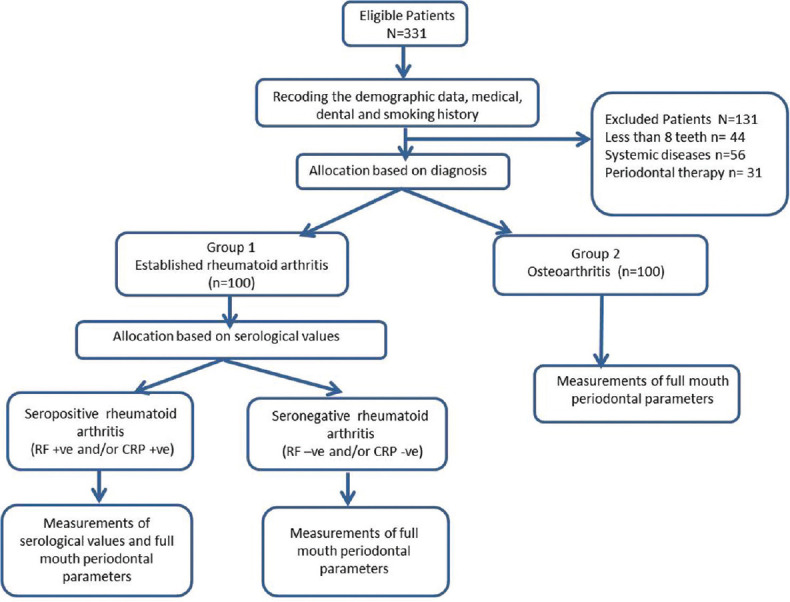
Recruitment and allocation of patients
Demographic profile, medical and dental history, oral hygiene practices, and smoking status of study participants were recorded. Smokers were classified into current smokers, former smokers and nonsmokers according to the Centers for Disease Control and Prevention (CDC). Periodontal status of the patients was evaluated using the simplified oral hygiene index (OHI-S), Loe and Silness gingival index (GI), probing pocket depth (PPD), and clinical attachment level (CAL). PPD and CAL were examined on six sites per tooth using a graduated Williams's periodontal probe. On the basis of the CAL score periodontitis severity was defined as slight (CAL 1–2 mm), moderate (CAL 3–4 mm), and severe (CAL ≥5 mm) according to American Academy of Periodontology classification (1999).[20]
RF and C-reactive protein (CRP) were considered as a serological marker in patients with RA. Two mL of antecubital venous blood sample was collected by routine method maintaining all the aseptic precautions in plain vacutainers. The collected blood was centrifuged at 2500 revolutions/minute for 15 min to separate the serum. The serum samples were immediately analyzed for RF and CRP with a quantitative latex agglutination test (Rhelax RF and Relax CRP Immunological Kit, Tulip Diagnostics, Goa, India).
All the collected data were statistically analyzed using Statistical Package for the Social Sciences (SPSS) software program, version 20. Results were expressed as mean ± standard deviation, or number (percentage). The continuous variables between the groups, and between subgroups of patients with RA (RF positive and negative) were compared using Student's t test. The Chi-square test for the categorical variables was performed when appropriate. The significance of differences in mean values was tested by one way analysis of variance (ANOVA) test. A value of P < 0.05 was considered statistical significant. The correlations between periodontal parameters and RA disease activity/characteristics were analyzed by Pearson correlation test.
Results
Hundred patients with RA and 100 patients with OA were included in this study. Of 100 patients enrolled in Group 1, 59 were females and 41 were males with mean age ranging from 48.63 ± 14.18 years. Group 2 consisted of 58 females and 42 males with mean age 56.75 ± 11.75 years. There were 17 current smokers, 16 former smokers and 67 nonsmokers in group 1. Group 2 comprised 15 current smokers, 15 former smokers and 70 non-smokers. Group 1 included 58 RF positive and 42 RF negative patients whereas 54 were CRP positive and 46 were CRP negative [Table 1].
Table 1.
Demographic features, smoking status, frequency of RF, and CRP in patients with RA and OA
| Variable | Category | Mean±SD/ n (%) | |
|---|---|---|---|
| RA group | OA group | ||
| Age | – | 48.63±14.18 | 56.75±11.75 |
| Gender | Male | 41 (41.0) | 42 (42.0) |
| Female | 59 (59.0) | 58 (58.0) | |
| Smoking status | Current | 17 (17.0) | 15 (15.0) |
| Former | 16 (16.0) | 15 (15.0) | |
| Nonsmokers | 67 (67.0) | 70 (70.0) | |
| RF status | Positive | 58 (58.0) | – |
| Negative | 42 (42.0) | – | |
| CRP | Positive | 54 (54.0) | – |
| Negative | 46 (46.0) | – | |
The mean OHI-S, GI, PPD, and CAL in patients with RA and OA are given in Table 2. No statistically significant differences were found between OHI-S, GI, and PPD in Group 1 and Group 2 patients. A significant difference was found in mean CAL in Group 1 and Group 2 patients with P < 0.05.
Table 2.
Mean OHI-S, GI, PPD, and CAL of the study subjects
| Variable | Groups | n | Mean | Mean difference | t | P |
|---|---|---|---|---|---|---|
| OHIS | RA | 100 | 2.376±0.95 | 0.004 | 0.029 | 0.977 (NS) |
| OA | 100 | 2.372±0.98 | ||||
| GI | RA | 100 | 1.537±0.50 | –0.138 | –1.904 | 0.058 (NS) |
| OA | 100 | 1.675±0.52 | ||||
| PPD | RA | 100 | 3.682±1.77 | –0.065 | –0.269 | 0.788 (NS) |
| OA | 100 | 3.747±1.64 | ||||
| CAL | RA | 100 | 4.301±2.08 | 0.684 | 2.434 | 0.016* |
| OA | 100 | 3.617±1.89 |
NS=nonsignificant Independent t test; * indicates significant at P≤0.05; PPD and CAL are expressed in mm
The prevalence of gingivitis and periodontitis in patients with RA and OA is given in Table 3. No statistically significant difference was found in the prevalence of gingivitis (P = 0.473) and periodontitis (P = 0.379) in both the groups.
Table 3.
Prevalence of gingivitis and periodontitis among both the study groups
| Variable | Categories | RA n (%) | OA n (%) | Chi-square value | P |
|---|---|---|---|---|---|
| Gingivitis | Mild | 25 (25%) | 18 (18%) | 1.497 | 0.473 (NS) |
| Moderate | 56 (56%) | 60 (60%) | |||
| Severe | 19 (19%) | 22 (22%) | |||
| Periodontitis | Slight | 22 (22%) | 29 (29%) | 3.083 | 0.379 (NS) |
| Moderate | 33 (33%) | 38 (38%) | |||
| Severe | 45 (45%) | 33 (33%) |
NS=nonsignificant. Chi-square test
The correlation of CAL in both RA and OA groups with respect to age is shown in Table 4. There was low positive correlation observed between CAL and age in Group 1, whereas moderate positive correlation was observed in Group 2. CAL scores in Groups 1 and 2, showed an increase with increasing age (r = 0.311 and 0.555, respectively) [Table 4].
Table 4.
Correlation of age with CAL in the RA and OA groups
| Groups | Mean age (in years) | Mean CAL (in mm) | Pearson correlation coefficient (r) |
|---|---|---|---|
| RA | 48.63±14.18 | 4.301±2.08 | 0.311 (Low positive correlation) |
| OA | 56.75±11.75 | 3.617±1.89 | 0.555 (Moderate positive correlation) |
Pearson correlation test
The correlation of CAL in both RA and OA groups with respect to gender and smoking status is represented in Table 5. No statistically significant difference was found in mean CAL according to the gender and smoking status in both the groups.
Table 5.
Comparison of mean CAL with gender and smoking status in the RA and OA groups
| Groups | Variables | n | CAL Mean | P |
|---|---|---|---|---|
| RA | Female | 59 | 4.185±2.08 | 0.506a (NS) |
| Male | 41 | 4.468±2.10 | ||
| OA | Female | 58 | 3.562±1.94 | 0.734a (NS) |
| Male | 42 | 3.693±1.86 | ||
| RA | Current smokers | 17 | 4.535±2.13 | 0.107b (NS) |
| Former smokers | 16 | 5.213±2.06 | ||
| Non-smokers | 67 | 4.024±2.04 | ||
| OA | Current smokers | 15 | 3.607±1.62 | 0.121b (NS) |
| Former smokers | 15 | 4.527±1.97 | ||
| Non-smokers | 70 | 3.424±1.89 |
NS=nonsignificant. CAL is expressed in mm. aIndependent t test . bOne-way ANOVA test
Table 6 depicts the difference in mean values of OHI-S, GI, PPD, and CAL according to the RF and CRP status in Group 1 patients. The values of OHI-S, GI, PPD, and CAL were found to be significantly higher in RF positive and CRP positive patients (P < 0.05).
Table 6.
Difference in mean values of OHI-S, GI, PPD, and CAL according to the RF and CRP status in patients with RA
| Variables | Status | n | Mean | P | ||
|---|---|---|---|---|---|---|
| RF | OHIS | Positive | 58 | 2.567±1.03 | 0.017* | |
| Negative | 42 | 2.112±0.76 | ||||
| GI | Positive | 58 | 1.729±0.41 | 0.001* | ||
| Negative | 42 | 1.271±0.50 | ||||
| PPD | Positive | 58 | 4.605±1.18 | 0.001* | ||
| Negative | 42 | 2.407±1.66 | ||||
| CAL | Positive | 58 | 5.381±1.70 | 0.001* | ||
| Negative | 42 | 2.810±1.59 | ||||
| CRP | OHIS | Positive | 55 | 2.559±0.97 | 0.035* | |
| Negative | 46 | 2.161±0.88 | ||||
| GI | Positive | 54 | 1.696±0.46 | 0.001* | ||
| Negative | 46 | 1.350±0.49 | ||||
| PPD | Positive | 54 | 4.174±1.51 | 0.003* | ||
| Negative | 46 | 3.104±1.89 | ||||
| CAL | Positive | 54 | 5.067±2.07 | 0.001* | ||
| Negative | 46 | 3.402±1.73 |
Independent t test; * indicates significant at P≤0.05; PPD and CAL are expressed in mm
Table 7 represents the prevalence of gingivitis and periodontitis in Group 1 patients, according to the RF and CRP status. Higher prevalence of gingivitis and periodontitis was found in RF positive and CRP positive patients.
Table 7.
Frequency and percentage of gingivitis and periodontitis in patients with RA according to the RF and CRP status
| Variable | Category | RF Status | CRP Status | ||
|---|---|---|---|---|---|
| Positive n (%) | Negative n (%) | Positive n (%) | Negative n (%) | ||
| Gingivitis | Mild | 3 (5.2) | 22 (52.4) | 6 (11.1) | 19 (41.3) |
| Moderate | 41 (70.7) | 15 (35.7) | 35 (64.8) | 21 (45.7) | |
| Severe | 14 (24.1) | 5 (11.9) | 13 (24.1) | 6 (13.0) | |
| Total | 58 (100) | 42 (100) | 54 (100) | 46 (100) | |
| P | 0.001* | 0.002* | |||
| Periodontitis | Slight | 3 (5.1) | 22 (52.3) | 5 (9.2) | 18 (39.2) |
| Moderate | 15 (25.9) | 13 (31.0) | 14 (25.9) | 16 (34.8) | |
| Severe | 40 (69.0) | 7 (16.6) | 35 (64.9) | 12 (26.0) | |
| Total | 58 (100) | 42 (100) | 54 (100) | 46 (100) | |
| P | 0.001* | 0.001* | |||
Chi-square test; * indicates significant at P≤0.05
The correlation of mean CAL with the RF status is shown in Graph 1. There was moderate positive correlation between RF titer (r = 0.311) and mean CAL. A statistically significant difference was found in mean CAL values among different RF titer (P = 0.001). Our study results suggested that mean CAL values varied with varying RF titer.
Graph 1.
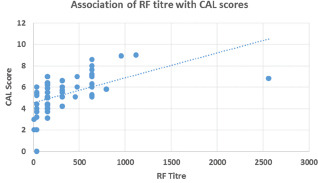
Cluster analysis of mean CAL with respect to RF titer
The correlation of mean CAL with the CRP is shown in Graph 2. There was weak positive correlation between CRP titer and mean CAL (r = 0.141). There was an increase in CAL with an increase in CRP titer but the difference was not statistically significant (P = 0.216).
Graph 2.
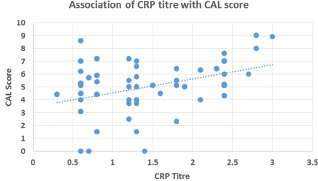
Cluster analysis of mean CAL with respect to CRP titer
Discussion
Primary care includes disease prevention, health promotion, and maintenance by patient counseling and education. It deals with diagnosis and treatment of variety acute and chronic illnesses which provides patient activism in the health-care system. However, primary health-care physicians should accomplish a cost-effective care by coordination of health care services.[21]
The dental profession shares the same common goal by prevention, diagnosis, and treatment of diseases affecting the oral cavity to improve the health and quality of life of patients. Lack of good oral health can increase the risk of certain systemic diseases and complications. Whether causal or coincidental, in older persons both oral cavity diseases and musculoskeletal disorders are common and cause potentially serious morbidity. Some musculoskeletal diseases, including arthritic disorders osteoporosis, and Paget's disease, may directly affect the oral cavity and adjacent structures.[22]
Chronic periodontitis is presently considered as a risk factor for RA. Both PD and RA were linked together with a gram-negative bacteria i.e. P. gingivalis. The enzyme peptidylarginine deiminase (PAD) characterized by P. gingivalis contributes to catalyzing citrullination. PAD-mediated citrullination is a posttranslational modification playing a central role in the production of anticyclic citrullinated peptide antibodies (ACPAs).[23,24] A positive RF had a major impact on mortality rate in patients with RA.[25] The presence of RF and ACPA is most important for the diagnosis and prognosis of the disease. A recent clinical trial has confirmed that RF was associated with disease remission, but ACPA positivity was not associated with sustained remission.[26] Hence, we have evaluated the prevalence and severity of PD in patients with established RA and OA in this study. Furthermore, whether the contribution RF and CRP alters the disease activity of periodontitis was assessed.
This study did not find a significant difference in prevalence of gingivitis and periodontitis in RA and OA group. This study reported 45% severe periodontitis prevalence in RA compared to OA group which was 33%. The prevalence of moderate to severe periodontitis was similar among arthritis groups (78% in RA; 71% in OA). These findings were in accordance with the previous studies which reported no significant differences were found in periodontitis prevalence between RA and OA.[12,18] On the contrary, higher prevalence of severe periodontitis in patients with RA (44.92%) compared to OA (12.1%) was reported by Rodríguez-Lozano et al.[27]
In this study, the differences between the mean OHI-S, GI, scores and PPD values among the RA and OA group were found to be statistically nonsignificant. Furthermore, patients with RA showed statistically significant increase in mean CAL compared to the patients with OA. The similar results were found in study conducted by Rodríguez-Lozano et al.[28] In a case-control study, Zhao et al.[29] reported significantly worse PD severity in Chinese patients with RA. In the multivariate analysis, earlier literature had found association between periodontitis severity, RA, age, female gender, socioeconomic status and osteoporosis.[27,28] This study reported a weak positive correlation between mean CAL value and age in patients with RA, whereas a moderate positive correlation present among patients with OA. The similar results for positive correlation of age with periodontal severity in patients with RA was observed in a recent study.[29] These results are in disagreement with a study by Marotte et al.[30] who found no association between periodontitis severity (i.e. mean CAL) and age. It has been reported that females have a more aggressive RA disease with increased activity of the disease and disability.[31] Owing to these findings, periodontitis severity in different gender among the RA and OA groups was compared in this study. No significant difference was found between the mean CAL values in males and females of both the groups. Furthermore, smoking is a common risk factor for both periodontitis and RA.[6,32] While comparing the smoking status with periodontal severity, this study did not find significant difference in periodontal severity of current smokers compared to former and non-smokers. This might be attributed to the low percentage of current and former smokers in the study.
This study reported significantly higher prevalence of severe periodontitis in RF positive patients (69%) than RF negative patients (16.6%) (P = 0.001). A moderate positive correlation was observed between the presence of periodontitis and RF. Similar prevalence of moderate to severe periodontitis in RF positive and RF negative patients have been reported by Dissick et al.[33] Findings of this study are in agreement with most evidence suggesting a possible association between presence of RF and the presence of periodontitis.[34,35,36] Goh et al.[36] observed positive association of clinical measures of PD (probing depth, attachment loss, and bleeding) with RF seropositivity. These results are in contrast with the studies who found no association between presence of RF and the presence of periodontitis.[27,37,38] Ziebolz et al.[37] stated the reason for these contradictory results could be the influence of the anti-rheumatic medication on the inflammatory parameters.
This is the first study as per author's knowledge where correlation between PD severity and RF titer was explored. Quantitative analysis of RF titer revealed significant increase in mean CAL with increase in RF titer. Both periodontitis and RA are chronic inflammatory diseases which are known to raise the markers of systemic inflammation such as serum CRP. This study reported the increased prevalence of gingivitis and periodontitis in CRP positive patients with RA. Furthermore, while correlating mean CAL values with CRP titer we observed weak positive correlation. The mean CAL values were increased with increase in CRP titer. This study did not find statistical significant association between periodontitis and the presence of CRP in this study. On the contrary, Susanto et al.[12] observed higher hsCRP levels in patients with RA and severe periodontitis. On the basis of the important biochemical, cellular, genetic, microbiological, and clinical studies, Eezammuddeen et al.[39] reported that there is a good evidence available now to support a relationship between RA and periodontitis.
The strength of this study rests in several facts, including its focus to diagnose the patients with RA with revised 1987 ACR criteria and association of periodontitis severity with quantitative RF and CRP status. This particular criteria was chosen because, in population-based studies the incidence of RA and mostly seronegative RA may be underestimated when diagnosed according to 2010 ACR/EULAR criteria.[40] However, few limitations of this study include lesser sample size, non-consideration of disease extent in chronic periodontitis patients. This may results in failure to assess influence of PD extent on RA and OA. Also, severity of OA was not assessed; hence, its association with severity of periodontitis could not be established.
As the impact of oral health on systemic health is proven, primary care physicians (PCPs) can play a significant role in maintaining and improving their patients’ oral health. However, a lack of proper training and education on oral health issues among PCPs may limit their role in oral health promotion. By understanding the basics of periodontitis, the PCPs can educate patients and appropriately refer patients with signs and symptoms of PD to dentists/periodontists.
Conclusion
The severity of periodontitis was significantly higher among the patients with established RA as compared to patients with OA. RF positive patients had higher PD compared to RF negative patients. The mean clinical attachment loss increased with increasing RF titer.
Recommendations and Future Perspective
Periodontists should highlight the importance of PD treatment which will prevent worsening of RA.
PCPs/rheumatologists should recommend routine periodontal examination for all patients with RA. Closer attention to oral hygiene and timely intervention for treatment of periodontitis will improve quality of life in patients with RA.
Future research should focus on contribution of periodontitis treatment on inflammatory status of patients with RA.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient (s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–61. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 2.Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part I. Arthritis Rheum. 2008;58:15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 3.Arya RK, Jain V. Osteoarthritis of the knee joint: An overview. JIACM. 2013;14:154–62. [Google Scholar]
- 4.Sharma MK, Swami HM, Bhatia V, Verma A, Bhatia SP, Kaur G. An epidemiological study of correlates of osteoarthritis in geriatric population of UT Chandigarh. Indian J Community Med. 2007;32:77–8. [Google Scholar]
- 5.Snyderman R, McCarty GA. Analogous mechanisms of tissue destruction in rheumatoid arthritis and periodontal disease. In: Genco RJ, Mergenhagen SE, editors. Host-parasite interaction in periodontal diseases. Washington: American Society of Microbiology; 1982. pp. 354–62. [Google Scholar]
- 6.Joseph R, Jose Raj MG, Sundareswaran S, Kaushik PC, Nagrale AV, Jose S, et al. Does a biological link exist between periodontitis and rheumatoid arthritis? World J Rheumatol. 2014;4:80–7. [Google Scholar]
- 7.Koziel J, Mydel P, Potempa J. The link between periodontal disease and rheumatoid arthritis: An updated review. Curr Rheumatol Rep. 2014;16:408. doi: 10.1007/s11926-014-0408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batool H, Afzal N, Shahzad F, Kashif M. Relationship between rheumatoid arthritis and chronic periodontitis. J Med Radiol Pathol Surg. 2016;2:11–4. [Google Scholar]
- 9.Payne JB, Gloub LM, Thiele GM, Mikuls TR. The link between periodontitis and rheumatoid arthritis: A periodontist's perspective. Curr Oral Health Rep. 2015;2:20–9. doi: 10.1007/s40496-014-0040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGraw WT, Potempa J, Farley D, Travis J. Purification, characterization, and sequence analysis of a potential virulence factor from Porphyromonas gingivalis, peptidylarginine deiminase. Infect Immun. 1999;67:3248–56. doi: 10.1128/iai.67.7.3248-3256.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roopa DA, Agrawal N, Johari S, Tripathi A, Gopal S. Prevalence of periodontitis among rheumatoid arthritis patients: An epidemiological study. Rama Univ J Dent Sci. 2015;2:2–8. [Google Scholar]
- 12.Susanto H, Nesse W, Kertia N, Soeroso J, van Reenen YH, Hoedemaker E, et al. Prevalence and severity of periodontitis in indonesian patients with rheumatoid arthritis. J Periodontol. 2013;84:1067–74. doi: 10.1902/jop.2012.110321. [DOI] [PubMed] [Google Scholar]
- 13.Sjöström L, Laurell L, Hugoson A, Håkansson JP. Periodontal conditions in adults with rheumatoid arthritis. Community Dent Oral Epidemiol. 1989;17:234–6. doi: 10.1111/j.1600-0528.1989.tb00623.x. [DOI] [PubMed] [Google Scholar]
- 14.Kässer UR, Gleissner C, Dehne F, Michel A, Willershausen-Zönnchen B, Bolten WW. Risk for periodontal disease in patients with longstanding rheumatoid arthritis. Arthritis Rheum. 1997;40:2248–51. doi: 10.1002/art.1780401221. [DOI] [PubMed] [Google Scholar]
- 15.Albandar JM. Some predictors of radiographic alveolar bone height reduction over 6 years. J Periodontal Res. 1990;25:186–92. doi: 10.1111/j.1600-0765.1990.tb01042.x. [DOI] [PubMed] [Google Scholar]
- 16.Tolo K, Jorkjend L. Serum antibodies and loss of periodontal bone in patients with rheumatoid arthritis. J Clin Periodontol. 1990;17:288–91. doi: 10.1111/j.1600-051x.1990.tb01091.x. [DOI] [PubMed] [Google Scholar]
- 17.Tang Q, Fu H, Qin B, Hu Z, Liu Y, Liang Y, et al. A possible link between rheumatoid arthritis and periodontitis: A systematic review and meta-analysis. Int J Periodontics Restorative Dent. 2017;37:79–86. doi: 10.11607/prd.2656. [DOI] [PubMed] [Google Scholar]
- 18.Coburn BW, Sayles HR, Payne JB, Redman RS, Markt JC, Beatty MW, et al. Performance of self-reported measures for periodontitis in rheumatoid arthritis and osteoarthritis. J Periodontol. 2015;86:16–26. doi: 10.1902/jop.2014.140339. [DOI] [PubMed] [Google Scholar]
- 19.Arnett FC, Edworthy SM, Bloch DA, Mcshane DJ, Fries JF, Cooper NS, et al. The american rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 20.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 21.Wright B, Nice AJ. Variation in local health department primary care services as a function of health center availability. J Public Health Manag Pract. 2015;21:E1–E9. doi: 10.1097/PHH.0000000000000112. [DOI] [PubMed] [Google Scholar]
- 22.Gambhir RS. Primary care in dentistry-an untapped potential. J Family Med Prim Care. 2015;4:13–8. doi: 10.4103/2249-4863.152239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harvey GP, Fitzsimmons TR, Dhamarpatni AA, Marchant C, Haynes DR, Bartold PM. Expression of peptidylarginine deiminase-2 and -4, citrullinated proteins and anti-citrullinated protein antibodies in human gingiva. J Periodont Res. 2013;48:252–61. doi: 10.1111/jre.12002. [DOI] [PubMed] [Google Scholar]
- 24.Valesini G, Gerardi MC, Iannuccelli C, Pacucci VA, Pendolino M, Shoenfeld Y. Citrullination and autoimmunity. Autoimmun Rev. 2015;14:490–7. doi: 10.1016/j.autrev.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez A, Icen M, Kremers HM, Crowson CS, Davis JM, Therneau TM, et al. Mortality trends in rheumatoid arthritis: The role of rheumatoid factor. J Rheumatol. 2008;35:1009–14. [PMC free article] [PubMed] [Google Scholar]
- 26.Paulshus Sundlisæter N, Olsen IC, Aga AB, Hammer HB, Uhlig T, van der Heijde D, et al. Predictors of sustained remission in patients with early rheumatoid arthritis treated according to an aggressive treat-to-target protocol. Rheumatology. 2018;57:2022–31. doi: 10.1093/rheumatology/key202. [DOI] [PubMed] [Google Scholar]
- 27.Rodríguez-Lozano B, González-Febles J, Garnier-Rodríguez JL, Dadlani S, Bustabad-Reyes S, Sanz M, et al. Association between severity of periodontitis and clinical activity in rheumatoid arthritis patients: A case-control study. Arthritis Res Ther. 2019;21:27. doi: 10.1186/s13075-019-1808-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demmer RT, Molitor JA, Jacobs DR, Michalowicz BS. Periodontal disease, tooth loss and incident rheumatoid arthritis: Results from the first national health and nutrition examination survey and its epidemiologic follow-up study. J Clin Periodontol. 2011;38:998–1006. doi: 10.1111/j.1600-051X.2011.01776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao R, Gu C, Zhang Q, Zhou W, Feng G, Feng X, et al. Periodontal disease in Chinese patients with rheumatoid arthritis: A case-control study. Oral Dis. 2019;25:2003–9. doi: 10.1111/odi.13176. [DOI] [PubMed] [Google Scholar]
- 30.Marotte H, Farge P, Gaudin P, Alexandre C, Mougin B, Miossec P. The association between periodontal disease and joint destruction in rheumatoid arthritis extends the link between the HLA-DR shared epitope and severity of bone destruction. Ann Rheum Dis. 2006;65:905–9. doi: 10.1136/ard.2005.036913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Intriago M, Maldonado G, Cárdenas J, Ríos C. Clinical characteristics in patients with rheumatoid arthritis: Differences between genders. Sci World J. 2019;2019 doi: 10.1155/2019/8103812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kallberg H, Ding B, Padyukov L, Bengtsson C, Rönnelid J, Klareskog L, et al. Smoking is a major preventable risk factor for rheumatoid arthritis: Estimations of risks after various exposures to cigarette smoke. Ann Rheum Dis. 2011;70:508–11. doi: 10.1136/ard.2009.120899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dissick A, Redman RS, Jones M, Rangan BV, Reimold A, Griffiths GR, et al. Association of periodontitis with rheumatoid arthritis: A pilot study. J Periodontol. 2010;81:223–30. doi: 10.1902/jop.2009.090309. [DOI] [PubMed] [Google Scholar]
- 34.Mikuls TR, Payne JB, Yu F, Thiele GM, Reynolds RJ, Cannon GW, et al. Periodontitis and Porphyromonas gingivalis in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66:1090–100. doi: 10.1002/art.38348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goh CE, Kopp J, Papapanou PN, Molitor JA, Demmer RT. Association between serum antibodies to periodontal bacteria and rheumatoid factor in the third national health and nutrition examination survey. Arthritis Rheumatol. 2016;68:2384–93. doi: 10.1002/art.39724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalez SM, Payne JB, Yu F, Thiele GM, Erickson AR, Johnson PG, et al. Alveolar bone loss is associated with circulating anti-citrullinated protein antibody (ACPA) in patients with rheumatoid arthritis. J Periodontol. 2015;86:222–31. doi: 10.1902/jop.2014.140425. [DOI] [PubMed] [Google Scholar]
- 37.Ziebolz D, Pabel SO, Lange K, Krohn-Grimberghe B, Hornecker E, Mausberg RF. Clinical periodontal and microbiologic parameters in patients with rheumatoid arthritis. J Periodontol. 2011;82:1424–32. doi: 10.1902/jop.2011.100481. [DOI] [PubMed] [Google Scholar]
- 38.Pischon N, Pischon T, Kroger J, Gulmez E, Kleber BM, Bernimoulin JP, et al. Association among rheumatoid arthritis, oral hygiene, and periodontitis. J Periodontol. 2008;79:979–86. doi: 10.1902/jop.2008.070501. [DOI] [PubMed] [Google Scholar]
- 39.Eezammuddeen NN, Vaithilingam RD, Hassan NHM, Bartold PM. Association Between Rheumatoid Arthritis and Periodontitis: Recent Progress. Curr Oral Health Rep. 2020 [Google Scholar]
- 40.Myasoedova E, Davis J, Matteson E, Crowson C. Performance of 2010 ACR/EULAR and 1987 ACR criteria for classification of rheumatoid arthritis in a population-based incidence cohort, 2010-2014 [abstract] Arthritis Rheumatol. 2019;71(Suppl 10) [Google Scholar]


