Abstract
Background:
The utilization of guideline-directed medical therapy (GDMT) significantly reduces morbidity and mortality in patients with heart failure with reduced ejection fraction (HFrEF). Previous studies have documented the underutilization of GDMT in HFrEF. The present study aimed to determine reasons for underutilization and achievement of target doses of GDMT in patients with de novo diagnosis of HFrEF.
Methods:
Patients presenting with de novo HFrEF at the Veterans Affairs Medical Center were included. Baseline demographic, clinical, and echocardiographic data were collected. The utilization of target doses of GDMT was assessed at the time of discharge and 1-, 3-, 6-, and 12-month follow-up.
Results:
Of the 95 patients who met the criteria for de novo HFrEF, 48 were included in the final analysis. Dose titration of either beta-blocker or angiotensin converting enzyme inhibitors/angiotensin receptor blockers (ACEi/ARB) was attempted in 20 patients (42%) at 1 month, 21 patients (44%) at 3 months, 13 patients (27%) at 6 months, and 14 patients (29%) at 12 months. Nine (19%) patients were on a target dose of beta-blockers and three (6%) patients were on a target dose of an ACEi/ARB at 12 months. The most common reasons for underutilization were patient-level factors, such as hypotension, acute kidney injury/hyperkalemia, and patient noncompliance.
Conclusions:
Utilization and achievement of target doses of GDMT were suboptimal among patients discharged with de novo HFrEF during a 1-year follow-up. Although patient factors may limit the up-titration of therapies, concerted efforts are needed to support primary care physicians in improving adherence to target doses of GDMT in patients with HFrEF.
Keywords: Cardiomyopathy, guideline-directed medical therapy, heart failure
Introduction
Heart failure (HF) is a leading cause of morbidity and mortality in the United States.[1,2] The American Heart Association estimates that, between 2013 and 2016, 6.2 million adults had HF; this number has increased from 5.7 million between 2009 and 2012.[3] It is projected that the prevalence of HF will increase even further, with experts predicting a 46% increase in prevalence between 2012 and 2030.[4] Medical therapy for heart failure with reduced ejection fraction (HFrEF) has led to significant reductions in morbidity and mortality.[5,6,7,8] Consensus treatment guidelines make strong recommendations for the utilization of guideline-directed medical therapy (GDMT), which includes angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEi/ARBs) and beta-blockers in patients with symptomatic heart failure and an EF <40%.[9,10] Utilization of GDMT has been reported, but there are little data specifically addressing the reasons for the underutilization of GDMT in de novo HFrEF.[11] The present study sought to determine the utilization and achievement of target doses of GDMT in patients with de novo HFrEF and to identify reasons for underutilization.
Methods
We conducted a retrospective cohort study at the Veterans Affairs Medical Center. Ethical clearance for this study was approved by the institutional review board at our institution. Inclusion criteria were adults older than age 18 and admission to the inpatient cardiology service with a primary diagnosis of de novo heart failure with a left ventricular ejection fraction of <40%. Exclusion criteria were known diagnosis of HFrEF, an EF >40%, hypertensive urgency/emergency, a life expectancy of less than 1 year, concomitant acute illnesses such as sepsis/acute surgical pathologies, and acute neurological events (stroke/hemorrhage).
Demographics, comorbid conditions, and echocardiographic parameters were recorded at baseline for each participant. The utilization of GDMT was assessed at the time of discharge and during follow-up 1, 3, 6, and 12 months later. Utilization of target doses of beta-blockers (metoprolol succinate 200 mg daily, carvedilol 25–37.5 mg twice daily, and bisoprolol 10 mg daily) and ACEi (Lisinopril 20–40 mg daily, enalapril 10–20 mg twice daily, quinapril 20 mg twice daily, captopril 50 mg three times daily)/ARBs (losartan 100–150 mg daily, candesartan 32 mg daily, and valsartan 160 mg twice daily) was obtained. Since the study dates spanned from 2011–2015, we did not have information on the utilization of angiotensin receptor blockers/neprilysin inhibitors. Reasons for holding therapy or avoiding up-titration of therapy were obtained by review of clinician documentation.
Results
Ninety-five patients with newly diagnosed HFrEF were screened, of which 48 were included in our final analysis [see Figure 1]. Baseline characteristics are presented in Table 1. All patients were males (as the study was conducted at a Veterans Affairs hospital); the mean age was 63 ± 6 years, 79% were Caucasian, and 69% had nonischemic cardiomyopathy. Reasons for underutilization of target doses of GDMT are listed in Table 2. The most common reasons were hypotension, acute kidney injury, and patient noncompliance.
Figure 1.
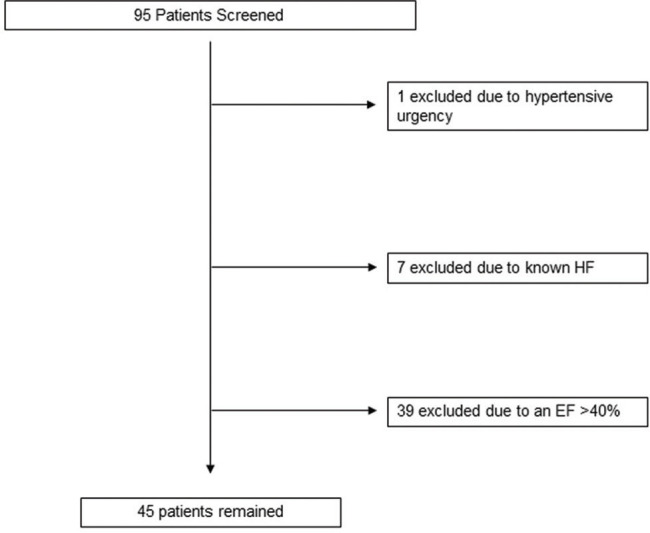
Screening log for selection of de novo heart failure patients
Table 1.
Baseline demographics and characteristics
| Characteristic | Number (percentage) |
|---|---|
| Male | 48 (100%) |
| Mean Age | 63±6 years |
| Ethnicity | |
| White | 38 (79%) |
| African American | 9 (19%) |
| Other race | 1 (2%) |
| Hypertension | 42 (88%) |
| Diabetes Mellitus | 22 (46%) |
| Coronary Artery Disease | 19 (40%) |
| Non-ischemic Cardiomyopathy | 33 (69%) |
| Chronic Kidney Disease | 2 (4%) |
| Chronic Obstructive Pulmonary Disease | 13 (27%) |
| Medication use at baseline: | |
| Beta Blocker | 24 (50%) |
| *ACEi | 18 (38%) |
| †ARB | 1 (2%) |
| *ACEi or †ARB | 19 (40%) |
| Spironolactone | 11 (23%) |
| Hydralazine/Isosorbide Dinitrate | 4 (8%) |
| Loop Diuretics | 20 (42%) |
| Digoxin | 14 (29%) |
| Aspirin | 20 (42%) |
*Denotes angiotensin-converting enzyme inhibitor †Denotes angiotensin receptor blocker
Table 2.
Reasons for underutilization of guideline-directed medical therapy
| Number (percentage) at 1 month | Number (percentage) at 3 months | Number (percentage) at 6 months | Number (percentage) at 12 months | |
|---|---|---|---|---|
| No documentation | 14 (29%) | 13 (27%) | 29 (60%) | 33(69%) |
| Hypotension | 19 (40%) | 22 (46%) | 11 (23%) | 5 (10%) |
| Bradycardia | 3 (6%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Acute Kidney Injury/Hyperkalemia | 4 (8%) | 0 (0%) | 5 (10%) | 5 (10%) |
| Patient Noncompliance | 4 (8%) | 11 (23%) | 0 (0%) | 0 (0%) |
| Drug Allergy | 4 (8%) | 2 (4%) | 0 (0%) | 0 (0%) |
| Other nonspecific Reason | 0 (0%) | 0 (0%) | 3(6%) | 5 (10%) |
Baseline
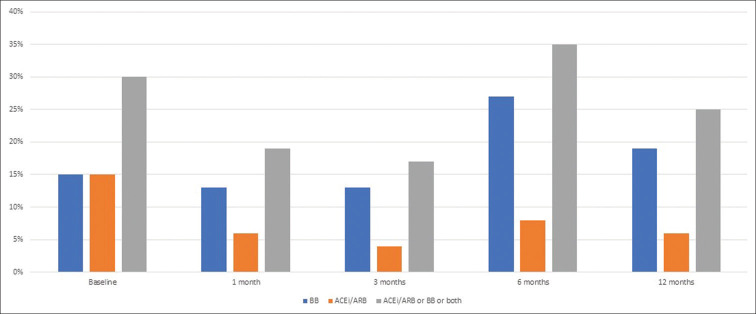
At baseline, 44 (92%) patients were discharged on beta-blockers and 38 (79%) were discharged on an ACEi/ARB, regardless of dose. Seven (15%) patients were on a target dose of a beta-blocker and seven (15%) patients were on a target dose of an ACEi/ARB.
One month
A clinical follow-up appointment with a healthcare provider occurred in 37 (77%) patients at 1 month. Dose titration of either beta-blocker or ACEi/ARB was attempted in 20 (42%) patients at 1 month. Six (13%) patients were on a target dose of a beta-blocker and three (6%) patients were on a target dose of an ACEi/ARB.
Three months
A clinical follow-up appointment with a healthcare provider occurred in 25 (52%) patients at 3 months. Dose titration of either beta-blocker or ACEi/ARB was attempted in 21 (44%) patients at 3 ± 1 month. Six (13%) patients were on a target dose of a beta-blocker and two (4%) patients were on a target dose of an ACEi/ARB.
Six months
A clinical follow-up appointment with a healthcare provider occurred in 26 (54%) patients at 6 months. Dose titration of either beta-blocker or ACEi/ARB was attempted in 13 (27%) patients at 6 ± 1 month. Thirteen (27%) patients were on a target dose of a beta-blocker and four (8%) patients were on a target dose of an ACEi/ARB.
One year
A clinical follow-up appointment with a healthcare provider occurred in 28 (58%) patients at 12 ± 1 month. Dose titration of either beta-blocker or ACEi/ARB was attempted in 14 (29%) patients at 12 months. Nine (19%) patients were on a target dose of a beta-blocker and three (6%) patients were on a target dose of an ACEi/ARB.
The utilization of spironolactone was 23% at baseline, with no attempt to up-titrate the dosage during the follow-up visits. The utilization of isosorbide dinitrate/hydralazine was 8% at baseline, with no attempts to up-titrate the dosage during the follow-up visits. Reasons for failure to up-titrate spironolactone or isosorbide dinitrate/hydralazine were not documented. Rates of the utilization of target doses of ACEi/ARB and beta-blocker over time are depicted in Figure 2.
Figure 2.
Rates of the utilization of target doses of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEi/ARB) and beta-blockers (BB) over time
Discussion
The current study demonstrates that despite a meaningful utilization of basic heart failure drug therapies, such as beta-blockers and renin-angiotensin system blockers, the achievement of target doses, and attempts to up-titrate to target doses of GDMT were subpar. In one study, the prevalence of GDMT use for patients with HFrEF and diabetes mellitus is reported to be relatively high: ACEiARBs (86%) and beta-blockers (83%).[12] Another study comparing patients in the US, with high and low-income Asian countries, identified that rates of utilization of ACEi/ARBs were 77%, 76%, and 69%, respectively; beta-blocker utilization rates were 91%, 87%, and 69%, respectively.[13] However, both of these studies failed to assess the doses of these medications, and the latter study suggests that the utilization of GDMT in HFrEF patients is lower in Asia than in the US, although the difference between rates of utilization of target doses in these regions remains unclear. Our study is unique in that it captures data on the prescription patterns for de novo HFrEF and provides granular data, spread over the first 12 months after diagnosis. It also demonstrates that attempts to up-titrate dosing and documentation of attempts may also be subpar within the Veterans Affairs System, specifically amongst primary care physicians, who assume the majority of the care for these patients after an initial post-hospital visit with cardiovascular physicians.
Heart failure is associated with increased morbidity and mortality. Despite the availability and utilization of GDMT, the mortality rates continue to be unacceptably high in the US and elsewhere. While the reasons for these rates are multifold, underutilization of adequate doses of GDMT is partly to blame. To derive maximal benefits, guidelines recommend that evidence-based HFrEF medications be titrated to the target dose derived from landmark clinical trials, as tolerated.[10,11,14]
While such therapies are readily available with minimal cost-related barriers (especially for traditional HFrEF medical therapies, most of which are available in the generic form), up-titration still poses a challenge to the medical community. Our study provides data from follow-up visits after the initial de novo diagnosis of HFrEF. Our observations are as follows: a) documentation of reasons behind not up-titrating dosing was suboptimal during the follow-up period, and b) renal dysfunction and hypotension were probably the major reasons behind failure to up-titrate ACEi or ARB in this population. Bradycardia and patient noncompliance were limiting factors in the first three months of diagnosis, but not during the subsequent follow-up. Overall, hypotension was the most common reason for the underutilization of GDMT. At any given time during follow-up, 10–45% of patients were not placed on or up-titrated to target doses of GDMT, due to hypotension. These percentages are higher than those seen nationally, where it has been demonstrated that approximately 20% of patients with a systolic blood pressure of less than 110 are receiving target doses of GDMT. In concurrence with our findings that this is a multifactorial issue, even among patients with a systolic blood pressure of greater than 110, approximately 20% of patients were receiving target doses of GDMT.[15] We also noted that for 33 (69%) patients, there was no documented reason for the underutilization of GDMT at 12-month follow-up.
In recently published registry data from the CHAMP-HF study, Greene et al. demonstrated a lack of improvement in the proportions of patients treated with GDMT or maximum doses of GDMT among 2,588 HFrEF patients over 12 months. In addition, they demonstrated that there were no medication changes in more than two-thirds of the patients, despite suboptimal doses.[16,17]
Lack of access to specialized care and lack of education are likely barriers for many primary care providers.[18] Concerted efforts must be made to further improve adherence to target doses of GDMT in patients with HFrEF. Previous data from the IMPROVE-HF registry study suggest that adherence to GDMT and up-titration of drug therapies for HFrEF are better achieved with concerted efforts. Interventions aligned with evidence-based performance measures, clinical decision support tools, structured improvement strategies, and chart audits with feedback resulted in improvements in the utilization of GDMT.[19] A multi-disciplinary approach involving primary care physicians, cardiologists, pharmacists, and other heart failure practitioners can ensure that patients are receiving optimal care while driving the utilization rates higher.[20]
Our study had several limitations. The sample was small and predominantly male (due to being conducted at a Veterans Affairs Medical Center). Since our analysis was performed a few years ago, data on the utilization of Sacubitril/Valsartan, SGLT2 inhibitors, and Ivabradine are not available. Due to the lack of appropriate documentation, it is unknown whether patients had symptomatic or asymptomatic hypotension during follow-up.
Conclusion
Utilization and achievement of target doses of GDMT, specifically beta-blockers, ACEi/ARBs, spironolactone, and isosorbide dinitrate/hydralazine, was suboptimal among patients discharged with newly diagnosed HFrEF, during a 1-year follow-up. Although patient factors, most commonly hypotension and bradycardia, may limit the up-titration of therapies in some patients, concerted efforts are needed to further improve adherence to target doses of GDMT in patients with HFrEF. Given the majority of follow-up visits beyond an initial posthospital visit by a cardiovascular physician are performed by primary care providers, it is prudent to refocus our efforts on improving their ability to achieve target doses of basic heart failure therapies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Bytici I, Bajraktari G. Mortality in heart failure patients. Anatol J Cardiol. 2015;15:63–8. doi: 10.5152/akd.2014.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bui A, Horwich T, Fonarow G. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8:30–41. doi: 10.1038/nrcardio.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin E, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics—2020 update: A report from the American Heart Association. Circulation. 2020;139:e57–e526. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 4.Fang N, Jiang M, Fan Y. Ideal cardiovascular health metrics and risk of cardiovascular disease or mortality: A meta-analysis. Int J Cardiol. 2016;214:279–83. doi: 10.1016/j.ijcard.2016.03.210. [DOI] [PubMed] [Google Scholar]
- 5.Fowler MB. Carvedilol prospective randomized cumulative survival (COPERNICUS) trial: Carvedilol in severe heart failure. Am J Cardiol. 2004;93:35B–9B. doi: 10.1016/j.amjcard.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Fagerberg B. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT-HF) Lancet. 1999;353:2001–7. [PubMed] [Google Scholar]
- 7.The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 8.ATLAS Study Group. Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. Circulation. 1999;100:2312–8. doi: 10.1161/01.cir.100.23.2312. [DOI] [PubMed] [Google Scholar]
- 9.Komajda M, Lapuerta P, Hermans N, Gonzalez-Juanatey JR, van Veldhuisen DJ, Erdmann E, et al. Adherence to guidelines is a predictor of outcome in chronic heart failure: The MAHLER survey. Eur Heart J. 2005;26:1653–9. doi: 10.1093/eurheartj/ehi251. [DOI] [PubMed] [Google Scholar]
- 10.Yancy WC, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2013:e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 11.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776–803. doi: 10.1016/j.jacc.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 12.Bhatt AS, Luo N, Solomon N, Pagidipati NJ, Ambrosio G, Green JB, et al. International variation in characteristics and clinical outcomes of patients with type 2 diabetes and heart failure: Insights from TECOS. Am Heart J. 2019;218:57–65. doi: 10.1016/j.ahj.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 13.Arnold, SV Yap J, Lam CSP, et al. Management of patients with diabetes and heart failure with reduced ejection fraction: an international comparison. Diabetes Obes Metab. 2018;21:261–6. doi: 10.1111/dom.13511. [DOI] [PubMed] [Google Scholar]
- 14.Yancy CW, Januzzi JL, Jr, Allen LA, Butler J, Davis LL, Fonarow GC, et al. 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: Answers to 10 pivotal issues about heart failure with reduced ejection fraction: A report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2018;71:201–30. doi: 10.1016/j.jacc.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 15.Peri-Okonny PA, Mi X, Khariton Y, Patel KK, Thomas L, Fonarow GC, et al. Target doses of heart failure medical therapy and blood pressure: Insights from the CHAMP-HF registry. JACC Heart Fail. 2019;7:350–8. doi: 10.1016/j.jchf.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greene SJ, Fonarow GC, DeVore AD, Sharma PP, Vaduganathan M, Albert NM, et al. Titration of medical therapy for heart failure with reduced ejection fraction: CHAMP-HF registry. J Am Coll Cardiol. 2019;73:2365–83. doi: 10.1016/j.jacc.2019.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, et al. Medical therapy for heart failure with reduced ejection fraction: The CHAMP-HF registry. J Am Coll Cardiol. 2018;72:351–66. doi: 10.1016/j.jacc.2018.04.070. [DOI] [PubMed] [Google Scholar]
- 18.Smeets M, Van Roy S, Aertgeerts B, Vermandere M, Vaes B. Improving care for heart failure patients in primary care, GPs’ perceptions: A qualitative evidence synthesis. BMJ Open. 2016;6:e013459. doi: 10.1136/bmjopen-2016-013459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fonarow GC, Albert NM, Curtis AB, Stough WG, Gheorghiade M, Heywood JT, et al. Improving evidence-based care for heart failure in outpatient cardiology practices: Primary results of the registry to improve the use of evidence-based heart failure therapies in the outpatient setting (IMPROVE HF) Circulation. 2010;122:585–96. doi: 10.1161/CIRCULATIONAHA.109.934471. [DOI] [PubMed] [Google Scholar]
- 20.Morton G, Masters J, Cowburn PJ. Multidisciplinary team approach to heart failure management. Heart. 2018;104:1376–82. doi: 10.1136/heartjnl-2016-310598. [DOI] [PubMed] [Google Scholar]