Abstract
Purpose of Review
Innovative and minimally toxic treatment approaches are sorely needed for the prevention and treatment of hematopoietic acute radiation syndrome (H-ARS). Cell therapies have been increasingly studied for their potential use as countermeasures for accidental and intentional ionizing radiation exposures which can lead to fatal ARS. Mesenchymal stem/stromal cells (MSCs) are a cell therapy that have shown promising results in preclinical studies of ARS, and are being developed in clinical trials specifically for H-ARS. MSCs, MSC-educated macrophages (MEMs) and MSC-exosome educated macrophages (EEMs) all have the potential to be used as adoptive cell therapies for H-ARS. Here we review how MSCs have been reported to mitigate inflammation from radiation injury while also stimulating hematopoiesis during ARS.
Recent findings
We discuss emerging work with immune cell subsets educated by MSCs, including MEMs and EEMs, in promoting hematopoiesis in xenogeneic models of ARS. We also discuss the first placental-derived MSC product to enter phase I trials, PLX-R18, and the challenges faced by bringing MSC and other cell therapies into the clinic for treating ARS.
Summary
Although MSCs, MEMs and EEMs are potential cell therapy candidates in promoting hematopoietic HRS, challenges persist in translational clinical development of these products to the clinic. Whether any of these cellular therapies will be sufficient as stand-alone therapies to mitigate H-ARS or if they will be a bridging therapy that insures survival until a curative allogeneic hematopoietic stem cell transplant can be performed are the key questions that will have to be answered.
Introduction
Ionizing radiation (IR) injury of healthy visceral tissues or organs is an important public health issue that can arise from accidental nuclear or radiological emergencies and terrorist-related improvised nuclear devices, as well as during medical applications such as total body irradiation prior to bone marrow transplantation (BMT) or infusion of medical radioisotopes for cancer treatment. The impact of charged particle-induced radiation injury from solar energetic particle (SEP) and galactic cosmic ray (GCR) exposures is also gaining attention with increased efforts by the National Aeronautics and Space Administration (NASA) to send astronauts back to the Moon and onward to Mars.
IR injuries can be classified into acute and chronic radiation syndromes. The US Centers for Disease Control and Prevention (CDC) defines acute radiation syndrome (ARS) as acute illness caused by IR exposure when: (1) the radiation dose is high [greater ≥~1 Gray (Gy)], (2) the dose is external to the body, (3) the involvement of penetrating ionizing radiations such as high energy X-rays, gamma rays, and neutrons, and (4) the involvement of the entire body receiving the dose. Chronic radiation syndrome is caused by lower radiation doses (< 1 Gy) administered over repeated exposures, and is discussed extensively elsewhere (1–3). IR-induced ARS is a significant problem due to its acute toxicity and mortality; thus, urgent treatment options are needed in acutely-exposed individuals as early as possible after exposure.
The classical disease pathophysiology of ARS includes three major complications: hematopoietic, gastrointestinal (GI) and cardiovascular (CV)/central nervous system (CNS) ARS (4). GI, CV and CNS ARS result from high dose IR exposures in a short time frame with the likely outcome of mortality without clinical intervention. However, hematopoietic ARS (H-ARS) is characterized by the loss of peripheral blood cells due to damage to bone marrow (BM)-derived hematopoietic stem cells, leading to suppression of BM function (5). The secondary neutropenia that results puts the patient at high risk for life-threatening infections. Although allogeneic BMT can be curative for IR-induced BM failure, identifying a suitable matched unrelated donor takes weeks to months. Thus, first-line therapies involve supportive care measures like administration of hematopoietic growth factors to stimulate BM production of leukocytes and help protect patients against infection, and transfusion of red blood cells and platelets for treating anemia and preventing bleeding until an allogeneic BMT can be performed (or the patient’s own BM recovers).
Current Food and Drug Administration (FDA)-approved growth factors such as Neupogen® and Neulasta®, both formulations of filgrastim (recombinant G-CSF), can improve neutrophil recovery but are not curative for H-ARS. Thus, medical countermeasures are sorely needed that can either protect, or even reverse, BM damage associated with H-ARS. Cellular therapies are being increasingly explored due to their favorable toxicity profiles and potential ability to not only modulate the host BM niche but also repair/rejuvenate cells and tissues injured by IR (6–9). Developing cellular therapies as a radiation medical countermeasure (MCM) first requires a detailed understanding of the pathophysiology of H-ARS.
Detrimental changes in the bone marrow after ionizing radiation injury
IR causes cellular damage through both direct and indirect mechanisms. Direct IR effects are mediated by ionizations and excitations induced along particle tracks and if this occurs within cells (and more importantly within cell nuclei), can induce chemical changes of both DNA and/or proteins requiring subsequent repair or removal. Indirect effects are mediated by charged particle-induced water radiolysis and involve the production and local diffusion of multiple free radical species which can similarly alter the chemistries of nearby DNA and proteins (10). Collectively, both direct and indirect IR effects can generate DNA damage that if “mis-repaired” or left unrepaired can generate lethal chromosomal aberrations and subsequent mitotic cell death or the activation of programmed cell death pathways (e.g., apoptosis), depending upon cell lineage. It is also well known that immature stem and progenitor cells undergoing rapid cell division are more radiosensitive than mature non-dividing cells (11). Since the BM is always in a state of active hematopoiesis throughout our lifetime, hematopoietic cells are significantly sensitive targets to IR with exposures causing hypocellularity and increase in BM fat content (12–14).
Hematopoietic stem cells (HSCs) respond to IR through well-defined DNA damage response (DDR) mechanisms (15). If the level of IR-induced DNA damage is sufficient, HSCs undergo apoptosis that is mediated primarily by TP53 and its various downstream effector proteins including Puma. Indeed, it has been shown that HSCs from Puma knockout mice display reduced sensitivity to IR-induced apoptosis (16, 17). IR can also affect HSC differentiation by causing telomere dysfunction and skewing towards lymphoid lineages through the G-CSF/Stat3/BATF-pathway (18). IR also induces senescence of HSCs through other mechanisms including reactive oxygen/nitrogen species (ROS/RNS) (19, 20). Altogether, IR affects the sternness of HSCs by forcing them to undergo apoptosis, senescence and biased differentiation.
Mesenchymal stromal cells (MSCs) are non-hematopoietic stromal/stem cells which are present in the BM niche that support hematopoiesis and bone regeneration (21, 22). Several studies have shown that MSCs are less sensitive to HSCs in the setting of BMT. MSCs derived from BMT recipients are not donor in origin, and thus are always recipient-derived in patients who undergo myeloablative irradiation and chemotherapy for transplant conditioning (23–25). Thus, MSCs survive post-irradiation in BMT recipients, resulting from their greater radioresistance. It has also been demonstrated that although IR affects the proliferation and differentiation capacities of BM MSCs, such effects are transient and MSCs regain their activity after a recovery period (26–29).
MSCs’ reduced sensitivity to IR has been explained by multiple mechanisms. MSCs possess high antioxidant capacity, low levels of pro-apoptotic proteins, and strongly induce DDR pathways which collectively make them survive from radiation injury (30). IR-induced oxidative stress often leads to DNA damage-associated cell death, and MSCs exhibit increased oxidative stress resistance compared to other cell phenotypes which further explains MSCs’ resistance to IR injury (29). Therefore the natural radio-resistant ability of BM MSCs may be utilized to mitigate BM failure and enhance hematopoiesis and an ideal IR MCM strategy. Moreover, the use of macrophages after co-cultivation with MSCs to produce MSC-educated macrophages (MEMs) or the direct use MSC-derived products such as secreted extracellular vesicles and exosomes, are considered promising cell therapy candidates for H-ARS (Fig. 1).
Figure 1. Paradigm of potential of cell therapy candidates to mitigate acute hematopoietic radiation syndrome.
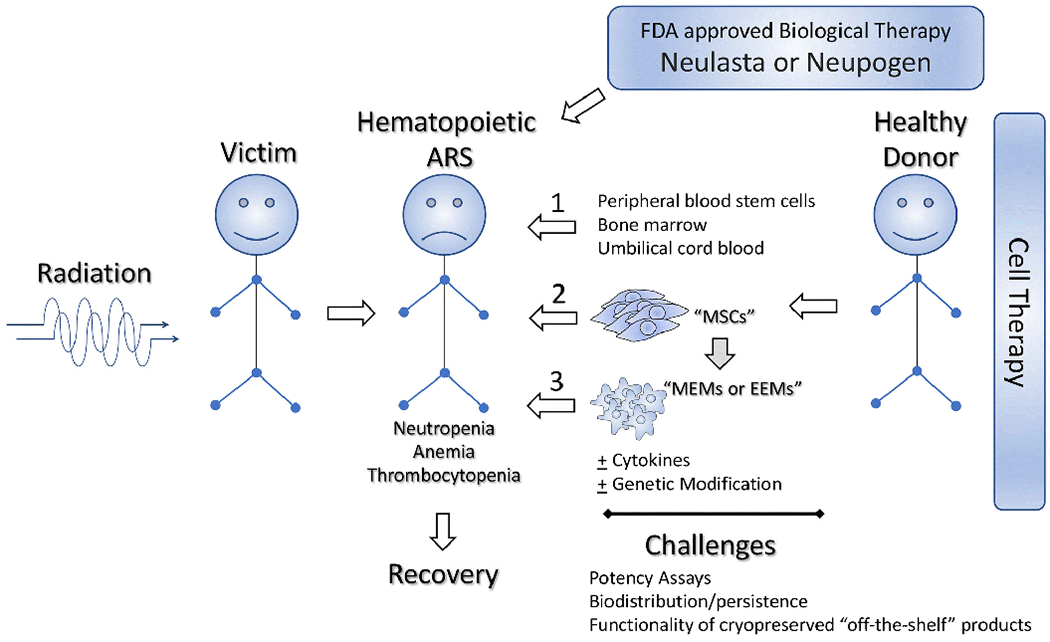
Potential cell therapy candidates: mesenchymal stem/stromal cells (MSCs), MSC-educated macrophages (MEMs), exosome-educated macrophages (EEMs) that mitigate H-ARS is shown along with the challenges in bringing forward their clinical cell therapy application.
Mechanisms underlying MSC-mediated mitigation of H-ARS
MSCs are the leading cell therapy candidate for mitigation of many degenerative and inflammatory disorders, but to date no MSC products are FDA-approved in the U.S. for any indication. However, MSCs are approved for clinical use by regulatory authorities in Europe, Canada, Australia, Japan, and South Korea to treat disorders such as complex perianal fistula from nonactive/mildly active luminal Crohn’s disease and steroid-refractory graft-versus-host-disease (31, 32). Therapeutic MSCs can be isolated from BM, adipose tissue, umbilical cord, cord blood, placenta and dental tissue (33). The International Society for Cell Therapy has provided guidance to define MSCs that are in vitro culture expanded (34–36). MSCs must be adherent, express the surface markers for CD105, CD73 and CD90, lack expression of CD45, CD34, CD14 or CD11b, CD79-alpha or CD19 and HLA-DR and should be able to differentiate to osteoblasts, adipocytes and chondroblasts in vitro (37). Accumulating evidence from preclinical studies demonstrate that infusion of MSCs can protect animals from lethal IR injury (38–48). Several studies have demonstrated that infusion of MSCs protect animals from radiation toxicity by ameliorating damage to the GI tract, CNS and lungs (38–48). However, the potential of MSC’s to mitigate H-ARS is emerging as another promising indication.
BM-derived MSCs endogenously express many genes that support hematopoiesis. In addition, expression of hematopoietic genes remains intact and is not downregulated even in the presence of inflammatory cytokines such as IFNγ (49). Cord blood HSCs co-cultured with BM MSCs in vitro prior to transplantation into patients show faster kinetics of immune reconstitution of neutrophil and platelets compared to cord blood HSCs alone. This accelerated engraftment is believed to be due to MSC-mediated skewing of cord blood HSCs toward progenitor populations committed to megakaryocyte and myeloid lineages (50). Since IR skews HSC differentiation into a lymphoid commitment (18), this characteristic to use MSCs to promote myeloid lineages should be a beneficial attribute as a MCM for H-ARS. In vitro analysis has demonstrated that MSCs can support the expansion of irradiated CD34+ HSCs in the presence of SCF, FLT3 ligand, TPO and IL3 (51). Another study demonstrated that MSCs can rescue CD34+ HSCs from radiation-induced apoptosis and support hematopoietic reconstitution after co-culture (52). Altogether these experimental data suggest that MSCs can support hematopoiesis in patients with H-ARS.
Preclinical in vivo studies also suggest that MSCs can enhance hematopoiesis post-irradiation. While in vivo studies often establish ‘proof of concept’ evidence, not many of the published studies provide mechanistic underpinnings of MSC-mediated mitigation of H-ARS,. Infusion of umbilical cord MSCs modulates the expression of Flt-3L; a growth factor that stimulates the proliferation and differentiation of hematopoietic multipotent progenitors in the BM of irradiated animals and confers radioprotection (53). Administration of MSC-like stromal cells from placenta confers protection of lethally irradiated animals by increasing the number of CD45+/SCA1+ hematopoietic progenitor cells in the BM and the plasma levels of hematopoietic cytokines such as G-CSF, GRO, MCP-1, IL-6 and 1L-8 (53, 54). In addition to hematopoiesis, BM-derived MSC infusion provokes a radioprotective mechanism by dampening inflammatory events, enhancing detoxification and cell cycling, and reducing oxidative stress, which collectively promote hematopoiesis in animals subjected to lethal irradiation (55). Genetic engineering of MSCs is a promising strategy to enhance their potency and functionality, as Gene-modified MSCs (e.g., superoxide dismutase-expressing umbilical cord MSCs) were shown to be superior than naive MSCs in enhancing hematopoietic recovery and conferring protection of sublethally irradiated animals (56). Another approach is the utilization of MSCs in conjunction with HSC to promote hematopoietic recovery and stem cell engraftment. Co-transplantation of MSCs and HSCs enhances the engraftment of CD34+ HSCs in a non-human primate model of HSC transplantation (57). Similarly, co-transplantation of human MSC progenitors and CD34+ HSCs into pre-immune fetal sheep results in early appearance of human donor cells in circulation and boosts cell levels in BM at later time points after transplantation (58). Based on these promising experimental results, some clinical trials have also evaluated the use of MSCs in promoting hematopoietic engraftment. While these trials showed that this approach is safe, unfortunately they were not large enough to statistically demonstrate MSC’s efficacy in promoting hematopoietic engraftment(59). However, a large phase III clinical trial testing MSCs following hematopoietic cell transplantation in steroid-resistant acute graft-versus-host disease model did show anti-GvHD efficacy (60). Since MSCs can promote hematopoiesis and mitigate inflammation and injury in graft-versus-host disease, it stands to reason that their use in combination with HSC transplantation could be a useful approach to rescue H-ARS.
Using MSC or MSC-derived exosomes to educate macrophages for treatment of hematopoietic ARS
One of the major limitations that may prevent random donor allogeneic MSCs from becoming a durable countermeasure for H-ARS is that MSCs persists short term after intravenous infusion in vivo. It is now becoming increasingly appreciated that biologic effects seen after MSC infusion may represent in vivo education of cell subsets like macrophages that have the capacity to replicate and persist in the BM for extended periods of time (61). One strategy that has been developed to recapitulate this observed phenomenon is by using MSC cocultures to educate human macrophages ex vivo. Infusion of these so-called MSC-educated macrophages (MEMs) into immunodeficient mice with lethal ARS (62) was shown to protect against radiation-induced lethality compared to infusion of MSCs themselves (Fig. 1). MEMs showed a phenotype of alternatively-activated macrophages which is distinct from that of classical macrophages isolated from peripheral blood and BM. These anti-inflammatory MEMs are characterized by the high expression of IL-10, transforming growth factor-β1 (TGF-β1), and programmed death ligands (PDL) 1 and 2 (62). TGF-β1 plays a significant role in wound-healing by promoting re-epithelialization, fibroblast proliferation, and angiogenesis, while IL-6 plays an immune-orchestrating role, and IL-10, PDL1 and PDL2 provide substantial immune suppression. All of these pathways work together on MEMs to enable their ability to be radioprotective (62).
One methodology to shorten the biomanufacturing time and utilize an “off the shelf” approach to generate MEMs is to use exosomes from lipopolysaccharide (LPS)-stimulated MSCs to induce macrophages to become educated macrophages (EEMs) that are radioprotective in vivo (63). LPS-EEMs show improved survival and clinical scores for ARS compared to PBS controls, MSCs, uneducated macrophages and EEMs. (63). Moreover, mice treated with of LPS-EEMs show improved hematopoiesis in multiple BM sites as well as the spleen histologically, leading to improved complete blood cell counts in peripheral blood weeks after irradiation. LPS- EEMs exhibit increased gene expression of STAT3, and protein expression of IL-10, IL-15, and FLT3L, which collectively can prevent inflammation, stimulate various immune cell subsets and promote hematopoietic growth. They showed increased phagocytosis, important for the elimination of neutrophils during the healing process and tissue remodeling. In summary, the potential of using MSCs, or derivatives of MSCs like extracellular vesicles, as a means of generating radioprotective cell subsets like macrophages ex vivo, remains an emerging area of research for treating lethal H-ARS.
Current MSC therapies in clinical development
PLX-R18 (Pluristem Therapeutics, Inc) is a placenta-derived, MSC product grown in a current good manufacturing practice (cGMP) 3-dimensional bioreactor that has been granted orphan drug designation by the FDA for the treatment of H-ARS. Preclinical xenogeneic studies in mice showed improved survival when the first dose of PLX-R18 was administered intramuscularly within 48 hours of (7.7 Gy) IR exposure, with lower but still significant improvement 72 hours post-exposure, followed by a second dose 5 days later (54). The mechanism of action is thought to be from secretion of cytokines and growth factors that stimulate hematopoiesis, combined with inhibition of T cell responses as well as inhibition of monocyte differentiation into mature dendritic cells (64). An open label phase I study will evaluate the safety of cryopreserved PLX-R18 thawed for the post-exposure prevention or treatment of H-ARS (NCT03797040), and the safety data combined with the prior preclinical data should inform a biologies license application for FDA approval using the Animal Rule (65). Each subject will receive 2 administrations of PLX-R18 at 4 million cells/kg, 4 days apart, up to a maximum of 400 million cells through intramuscular route of administration. The first administration will preferably be given within 48 hours of suspected exposure . The inclusion criteria include subjects exposed, or suspected to have been exposed, to ionizing radiation of ≥1Gy and is at risk of developing H-ARS, as assessed by the treating physician, based on Radiation Emergency Medical Management guidelines.
Challenges of developing cell therapies for hematopoietic ARS
Despite cell therapies like MSCs being a promising platform for the treatment of H-ARS, there are challenges to achieve sustainable and consistent efficacy. Some of the key factors that confound their widespread application include the source of cells, challenges associated with accurate potency analysis of cell therapy products, delivery issues, biomarker identification of responder and non-responder populations of therapy, and reliability of preclinical animal model studies for accurately predicting human response (66–68).
Although autologous personalized cell therapy is ideal for IR injury, from a feasibility point-of-view, pre-expanded/activated, allogeneic cell therapy products are highly preferred. Practically it is feasible to isolate, expand and cryopreserve cell therapy products in a bank, and then whenever needed, aliquots/doses can be thawed and infused into the patients, and thus bypass lengthy cell manufacturing time required in the setting of autologous cell therapy. This is particularly important in treating H-ARS, since the timing of the therapy is crucial to rescue patients from BM failure as early as possible post-exposure. Although an “off the shelf” cell therapy approach is feasible and perhaps more clinically relevant, the ultimate clinical efficacy of such an approach is yet to be confirmed.
There are two factors that need to be addressed for “off-the-shelf” cell therapy approaches. The first of these is the tissue source from which the MSCs are derived, e.g., BM, adipose tissue, umbilical cord, placenta, etc. (33), since the biology of MSCs, and cells educated by MSCs, could vary based on the source of the cells and choice of the donors from whom these cells are derived. Importantly, in the situation where multiple dosing regimens are needed for efficacy, it is necessary to define if the cells can be derived from a single donor, or if multiple donors will be required for longitudinal administration. Second, the effect of cryopreservation-thawing on the allogeneic cell therapy product needs to be examined to determine whether these processes may impact upon therapeutic efficacy after infusion. Large-scale cryobanking of cellular products, and their infusion into the patients immediately post-thaw, is an attractive option from a clinical perspective. However, an important yet controversial question is whether pre-freeze functionality of cell products like MSCs are equivalent to their freeze/thawed counterparts. Mixed data is emerging on the efficacy of immediately thawed MSCs (69–82). Although freeze-thawing optimization and cell manufacturing technologies may overcome this issue, an important salvage consideration needs to be the culture recovery of cryopreserved cells prior to infusion. Culture recovery is a process where cryopreserved cells can be thawed and cultured in the incubator for a period of 24-48 hours to regain their fitness prior to infusion to preserve viability and hopefully their biologic functionality (76, 82, 83).
Several BMT studies have demonstrated that biodistribution of intravenously infused MSCs is unique as they do not home to BM, unlike HSCs (23, 84–86). It is also demonstrated that MSCs show highly efficient homing to the BM but lose homing ability following culture (87). In contrast, allogeneic BMT in patients with osteogenesis imperfecta had shown that MSCs were detected in the BM, which suggests that physiology of marrow niche and disease status may play an important role in the homing of MSCs (88). Importantly, infused MSCs were not detected in the bone marrow of non-human primates that were subjected to lethal IR (89). Hence the enhancement of hematopoiesis as seen in in the animal model studies could be due to the peripheral modulation of hematopoietic cytokines and growth factors, and thus understanding which of these molecules are stimulated post-infusion is important toward development of potency assays during cGMP production and/or biomarker measurements post-infusion. Dosing and biodistribution of products are also important parameters to consider whenever cellular therapies are infused intravenously. For example, large number of MSCs can be trapped in the lungs post intravenous infusion, and current clinical trials use up to 107 cells/kg body weight in the settings of intravenous infusion (90, 91). Although preclinical animal model studies have demonstrated the efficacy of infusing cells intravenously, intramuscularly, and intraperitoneally (53, 54), and clinical trial with PLX-R18 (NCT03797040) deliver cells intramuscularly innovative approaches are still needed to overcome challenges involved with optimizing dosing by maximizing persistence of cell therapies like MSCs post-infusion.
The United States FDA and European Medicines Agency (EMA) classify in vitro culture-expanded cells as more than minimally manipulated cellular and gene therapy products, which applies to MSCs and macrophages (92, 93). Potency assays are necessary to inform advanced clinical trials and also marketing approval to use these cell therapy products. However, development of potency assays for MSCs, MEMs and EEMs are challenging due to their largely undefined mechanisms of action in humans that predict the recovery of hematopoiesis post-IR injury. Although animal studies provide insight about the potential toxicities and mechanism of action in mitigating H-ARS, discrepancies between animal- vs human-derived MSC populations and their biological properties suggest that animal model studies cannot be entirely translated into human clinical studies (66). MSCs derived from monkey and pig share similarities with human whereas mouse, rat, rabbit, and hamster MSCs are not equivalent to human (94). Hence, caution must be exercised in translating animal studies into clinical potency assay development of MSCs. An ideal approach would be the identification of biomarkers in responders versus non-responders, which could then be used as a measure of potency of the products before infusion. In addition, developing a potency assay that only measures a single cytokine or growth factor from MSCs may not recapitulate the entire mechanism of action and potency of MSCs, especially since the cells are both anti-inflammatory and promote wound healing. Hence a combination approach analyzing components of the secretome, selective transcriptome, and phosphorylation status of critical molecules could collectively be used to predict hematopoietic-repairing potential as a surrogate measure of potency (95, 96). Nonetheless animal studies remain crucial for informing potential toxicities and for fulfilling the Animal Rule, which is a critical pathway for cell therapies to achieve FDA approval for H-ARS.
Conclusion:
MSCs have been successfully developed as a cellular therapy for treating inflammatory disorders and promoting wound healing. In the case of H-ARS, MSCs are also emerging as off-the-shelf cell products with enormous potential, and early clinical testing is underway. Major questions still remain on whether MSCs, or cells educated by MSCs/MSC-exosomes like MEMs and EEMs, can be used as a primary therapy for the prevention or treatment of H-ARS, or whether cellular therapies will have to be combined with currently used approaches like allogeneic hematopoietic stem cell transplantation or FDA-approved growth factors like filgrastim/G-CSF. Increasing support from federal grants as well as continued guidance from regulatory agencies like the FDA and EMA will ensure cellular therapies continue to be developed for this challenging condition.
Acknowledgments:
This work was supported in part by the WES Leukemia Research foundation (R.C.), the Don Anderson GVHD fund and Crystal Carney Fund for Leukemia Research (P.H.), St. Baldrick’s-Stand Up To Cancer Pediatric Dream Team Translational Research Grant SU2C-AACR-DT-27-17, NIH/NCATS UL1TR000427 to the UW ICTR and NIH/NCI P30 CA014520 to the UWCCC (P.H. and C.M.C.), and NIH/NCI R01 CA215461 (C.M.C). Stand Up To Cancer is a division of the Entertainment Industry Foundation. Research grants are administered by the American Association for Cancer Research, the scientific partner of SU2C. The contents of this article do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. None of these funding sources had any input in the study design, analysis, manuscript preparation or decision to submit for publication.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of interest: R.C. is an inventor on a patent application (US Patent 20190099449 A1), and P.H. and C.M.C. have a patent (US Patent 10,166,254 B2), related to this publication. C.M.C. reports honorarium from Nektar Therapeutics, who had no input in the study design, analysis, manuscript preparation or decision to submit for publication. The authors declare that no other relevant financial conflicts of interest exist.
Human and Animal Rights and Informed Consent: This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Reeves GI, Ainsworth EJ. Description of the chronic radiation syndrome in humans irradiated in the former Soviet Union. Radiat Res. 1995;142(2):242–3. [PubMed] [Google Scholar]
- 2.Rybkina VL, Bannikova MV, Adamova GV, Dorr H, Scherthan H, Azizova TV. Immunological Markers of Chronic Occupational Radiation Exposure. Health Phys. 2018;115(1):108–13. [DOI] [PubMed] [Google Scholar]
- 3.Grammaticos P, Giannoula E, Fountos GP. Acute radiation syndrome and chronic radiation syndrome. Hell J Nucl Med. 2013;16(1):56–9. [PubMed] [Google Scholar]
- 4.Stenke L, Lindberg K, Lagergren Lindberg M, Lewensohn R, Valentin J, Powles R, et al. Coordination of Management of the Acute Radiation Syndrome. Radiat Prot Dosimetry. 2018;182(1):80–4. [DOI] [PubMed] [Google Scholar]
- 5.Gorbunov NV, Sharma P. Protracted Oxidative Alterations in the Mechanism of Hematopoietic Acute Radiation Syndrome. Antioxidants (Basel). 2015;4(1):134–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.*DiCarlo AL, Tamarat R, Rios CI, Benderitter M, Czarniecki CW, Allio TC, et al. Cellular Therapies for Treatment of Radiation Injury: Report from a NIH/NIAID and IRSN Workshop. Radiat Res. 2017;188(2):e54–e75. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Important paper which describes the significance and developments of cell therapy for radiation injury.
- 7.Rios C, Jourdain JR, DiCarlo AL. Cellular Therapies for Treatment of Radiation Injury after a Mass Casualty Incident. Radiat Res. 2017;188(2):242–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drouet M, Riccobono D, Francois S. Comments on “Cellular Therapies for Treatment of Radiation Injury after a Mass Casualty Incident” (Radiat Res 2017; 188:242-45). Radiat Res. 2017;188(4):463. [DOI] [PubMed] [Google Scholar]
- 9.Tamarat R, Benderitter M, Jourdain JR, Maidment BW, Macchiarini F, Rios CI, et al. Response to the ‘Comments on “Cellular Therapies for Treatment of Radiation Injury after a Mass Casualty Incident” (Radiat Res 2017; 188:242-45)’ by Drouet et al. (Letters to the Editor, Radiat Res 2017; 188:463). Radiat Res. 2018;189(4):446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stone HB, Coleman CN, Anscher MS, McBride WH. Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol. 2003;4(9):529–36. [DOI] [PubMed] [Google Scholar]
- 11.Bolus NE. Basic Review of Radiation Biology and Terminology. J Nucl Med Technol. 2017;45(4):259–64. [DOI] [PubMed] [Google Scholar]
- 12.Tong XQ, Sugimura H, Kisanuki A, Asato M, Yuki Y, Tamura S, et al. Multiple fractionated and single-dose irradiation of bone marrow. Evaluation by MR and correlation with histopathological findings. Acta Radiol. 1998;39(6):620–4. [DOI] [PubMed] [Google Scholar]
- 13.Hui SK, Sharkey L, Kidder LS, Zhang Y, Fairchild G, Coghill K, et al. The influence of therapeutic radiation on the patterns of bone marrow in ovary-intact and ovariectomized mice. PLoS One. 2012;7(8):e42668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green DE, Rubin CT. Consequences of irradiation on bone and marrow phenotypes, and its relation to disruption of hematopoietic precursors. Bone. 2014;63:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biechonski S, Yassin M, Milyavsky M. DNA-damage response in hematopoietic stem cells: an evolutionary trade-off between blood regeneration and leukemia suppression. Carcinogenesis. 2017;38(4):367–77. [DOI] [PubMed] [Google Scholar]
- 16.Shao L, Sun Y, Zhang Z, Feng W, Gao Y, Cai Z, et al. Deletion of proapoptotic Puma selectively protects hematopoietic stem and progenitor cells against high-dose radiation. Blood. 2010;115(23):4707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu H, Shen H, Yuan Y, XuFeng R, Hu X, Garrison SP, et al. Deletion of Puma protects hematopoietic stem cells and confers long-term survival in response to high-dose gamma-irradiation. Blood. 2010;115(17):3472–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Sun Q, Morita Y, Jiang H, Gross A, Lechel A, et al. A differentiation checkpoint limits hematopoietic stem cell self-renewal in response to DNA damage. Cell. 2012;148(5):1001–14. [DOI] [PubMed] [Google Scholar]
- 19.Meng A, Wang Y, Van Zant G, Zhou D. Ionizing radiation and busulfan induce premature senescence in murine bone marrow hematopoietic cells. Cancer Res. 2003;63(17):5414–9. [PubMed] [Google Scholar]
- 20.Wang Y, Liu L, Pazhanisamy SK, Li H, Meng A, Zhou D. Total body irradiation causes residual bone marrow injury by induction of persistent oxidative stress in murine hematopoietic stem cells. Free Radic Biol Med. 2010;48(2):348–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andrzejewska A, Lukomska B, Janowski M. Concise Review: Mesenchymal Stem Cells: From Roots to Boost. Stem Cells. 2019;37(7):855–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rieger K, Marinets O, Fietz T, Korper S, Sommer D, Mucke C, et al. Mesenchymal stem cells remain of host origin even a long time after allogeneic peripheral blood stem cell or bone marrow transplantation. Exp Hematol. 2005;33(5):605–11. [DOI] [PubMed] [Google Scholar]
- 24.Bartsch K, Al-Ali H, Reinhardt A, Franke C, Hudecek M, Kamprad M, et al. Mesenchymal stem cells remain host-derived independent of the source of the stem-cell graft and conditioning regimen used. Transplantation. 2009;87(2):217–21. [DOI] [PubMed] [Google Scholar]
- 25.Villaron EM, Almeida J, Lopez-Holgado N, Alcoceba M, Sanchez-Abarca LI, Sanchez-Guijo FM, et al. Mesenchymal stem cells are present in peripheral blood and can engraft after allogeneic hematopoietic stem cell transplantation. Haematologica. 2004;89(12):1421–7. [PubMed] [Google Scholar]
- 26.Li J, Kwong DL, Chan GC. The effects of various irradiation doses on the growth and differentiation of marrow-derived human mesenchymal stromal cells. Pediatr Transplant. 2007;11(4):379–87. [DOI] [PubMed] [Google Scholar]
- 27.Singh S, Kloss FR, Brunauer R, Schimke M, Jamnig A, Greiderer-Kleinlercher B, et al. Mesenchymal stem cells show radioresistance in vivo. J Cell Mol Med. 2012;16(4):877–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mussano F, Lee KJ, Zuk P, Tran L, Cacalano NA, Jewett A, et al. Differential effect of ionizing radiation exposure on multipotent and differentiation-restricted bone marrow mesenchymal stem cells. J Cell Biochem. 2010;111(2):322–32. [DOI] [PubMed] [Google Scholar]
- 29.Chen MF, Lin CT, Chen WC, Yang CT, Chen CC, Liao SK, et al. The sensitivity of human mesenchymal stem cells to ionizing radiation. Int J Radiat Oncol Biol Phys. 2006;66(1):244–53. [DOI] [PubMed] [Google Scholar]
- 30.Sugrue T, Lowndes NF, Ceredig R. Mesenchymal stromal cells: radio-resistant members of the bone marrow. Immunol Cell Biol. 2013;91(1):5–11. [DOI] [PubMed] [Google Scholar]
- 31.Cuende N, Rasko JEJ, Koh MBC, Dominici M, Ikonomou L. Cell, tissue and gene products with marketing authorization in 2018 worldwide. Cytotherapy. 2018;20(11):1401–13. [DOI] [PubMed] [Google Scholar]
- 32.Galipeau J, Sensebe L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell. 2018;22(6):824–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le Blanc K, Davies LC. MSCs-cells with many sides. Cytotherapy. 2018;20(3):273–8. [DOI] [PubMed] [Google Scholar]
- 34.Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, et al. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7(5):393–5. [DOI] [PubMed] [Google Scholar]
- 35.Krampera M, Galipeau J, Shi Y, Tarte K, Sensebe L, Therapy MSCCotISfC. Immunological characterization of multipotent mesenchymal stromal cells--The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy. 2013;15(9):1054–61. [DOI] [PubMed] [Google Scholar]
- 36.Galipeau J, Krampera M, Barrett J, Dazzi F, Deans RJ, DeBruijn J, et al. International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy. 2016;18(2):151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7. [DOI] [PubMed] [Google Scholar]
- 38.Bandekar M, Maurya DK, Sharma D, Checker R, Gota V, Mishra N, et al. Xenogeneic transplantation of human WJ-MSCs rescues mice from acute radiation syndrome via Nrf-2-dependent regeneration of damaged tissues. Am J Transplant. 2020. [DOI] [PubMed] [Google Scholar]
- 39.Linard C, Brachet M, L’Homme B, Strup-Perrot C, Busson E, Bonneau M, et al. Long-term effectiveness of local BM-MSCs for skeletal muscle regeneration: a proof of concept obtained on a pig model of severe radiation burn. Stem Cell Res Ther. 2018;9(1):299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim A, Shim S, Kim MJ, Myung JK, Park S. Mesenchymal stem cell-mediated Notch2 activation overcomes radiation-induced injury of the hematopoietic system. Sci Rep. 2018;8(1):9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perez JR, Lee S, Ybarra N, Maria O, Serban M, Jeyaseelan K, et al. A comparative analysis of longitudinal computed tomography and histopathology for evaluating the potential of mesenchymal stem cells in mitigating radiation-induced pulmonary fibrosis. Sci Rep. 2017;7(1):9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee C, Shim S, Jang H, Myung H, Lee J, Bae CH, et al. Human umbilical cord blood-derived mesenchymal stromal cells and small intestinal submucosa hydrogel composite promotes combined radiation-wound healing of mice. Cytotherapy. 2017;19(9):1048–59. [DOI] [PubMed] [Google Scholar]
- 43.Moussa L, Pattappa G, Doix B, Benselama SL, Demarquay C, Benderitter M, et al. A biomaterial-assisted mesenchymal stromal cell therapy alleviates colonic radiation-induced damage. Biomaterials. 2017;115:40–52. [DOI] [PubMed] [Google Scholar]
- 44.Maria OM, Shalaby M, Syme A, Eliopoulos N, Muanza T. Adipose mesenchymal stromal cells minimize and repair radiation-induced oral mucositis. Cytotherapy. 2016;18(9):1129–45. [DOI] [PubMed] [Google Scholar]
- 45.Wang GH, Liu Y, Wu XB, Lu Y, Liu J, Qin YR, et al. Neuroprotective effects of human umbilical cord-derived mesenchymal stromal cells combined with nimodipine against radiation-induced brain injury through inhibition of apoptosis. Cytotherapy. 2016;18(1):53–64. [DOI] [PubMed] [Google Scholar]
- 46.Ono M, Ohnishi S, Honda M, Ishikawa M, Hosono H, Onishi R, et al. Effects of human amnion-derived mesenchymal stromal cell transplantation in rats with radiation proctitis. Cytotherapy. 2015;17(11):1545–59. [DOI] [PubMed] [Google Scholar]
- 47.Jiang X, Jiang X, Qu C, Chang P, Zhang C, Qu Y, et al. Intravenous delivery of adipose-derived mesenchymal stromal cells attenuates acute radiation-induced lung injury in rats. Cytotherapy. 2015;17(5):560–70. [DOI] [PubMed] [Google Scholar]
- 48.Francois M, Birman E, Forner KA, Gaboury L, Galipeau J. Adoptive transfer of mesenchymal stromal cells accelerates intestinal epithelium recovery of irradiated mice in an interleukin-6-dependent manner. Cytotherapy. 2012;14(10):1164–70. [DOI] [PubMed] [Google Scholar]
- 49.Stenger EO, Chinnadurai R, Yuan S, Garcia M, Arafat D, Gibson G, et al. Bone Marrow-Derived Mesenchymal Stromal Cells from Patients with Sickle Cell Disease Display Intact Functionality. Biol Blood Marrow Transplant. 2017;23(5):736–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Lima M, McNiece I, Robinson SN, Munsell M, Eapen M, Horowitz M, et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med. 2012;367(24):2305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mourcin F, Grenier N, Mayol JF, Lataillade JJ, Sotto JJ, Herodin F, et al. Mesenchymal stem cells support expansion of in vitro irradiated CD34(+) cells in the presence of SCF, FLT3 ligand, TPO and IL3: potential application to autologous cell therapy in accidentally irradiated victims. Radiat Res. 2005;164(1):1–9. [DOI] [PubMed] [Google Scholar]
- 52.**Drouet M, Mourcin F, Grenier N, Delaunay C, Mayol JF, Lataillade JJ, et al. Mesenchymal stem cells rescue CD34+ cells from radiation-induced apoptosis and sustain hematopoietic reconstitution after coculture and cografting in lethally irradiated baboons: is autologous stem cell therapy in nuclear accident settings hype or reality? Bone Marrow Transplant. 2005;35(12):1201–9. [DOI] [PubMed] [Google Scholar]; ** Important study that showed Intramuscular injection of MSCs confer radiomitigation.
- 53.Shim S, Lee SB, Lee JG, Jang WS, Lee SJ, Park S, et al. Mitigating effects of hUCB-MSCs on the hematopoietic syndrome resulting from total body irradiation. Exp Hematol. 2013;41(4):346–53 e2. [DOI] [PubMed] [Google Scholar]
- 54.Gaberman E, Pinzur L, Levdansky L, Tsirlin M, Netzer N, Aberman Z, et al. Mitigation of Lethal Radiation Syndrome in Mice by Intramuscular Injection of 3D Cultured Adherent Human Placental Stromal Cells. PLoS One. 2013;8(6):e66549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lange C, Brunswig-Spickenheier B, Cappallo-Obermann H, Eggert K, Gehling UM, Rudolph C, et al. Radiation rescue: mesenchymal stromal cells protect from lethal irradiation. PLoS One. 2011;6(1):e14486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gan J, Meng F, Zhou X, Li C, He Y, Zeng X, et al. Hematopoietic recovery of acute radiation syndrome by human superoxide dismutase-expressing umbilical cord mesenchymal stromal cells. Cytotherapy. 2015;17(4):403–17. [DOI] [PubMed] [Google Scholar]
- 57.Masuda S, Ageyama N, Shibata H, Obara Y, Ikeda T, Takeuchi K, et al. Cotransplantation with MSCs improves engraftment of HSCs after autologous intra-bone marrow transplantation in nonhuman primates. Exp Hematol. 2009;37(10):1250–7 e1. [DOI] [PubMed] [Google Scholar]
- 58.Almeida-Porada G, Porada CD, Tran N, Zanjani ED. Cotransplantation of human stromal cell progenitors into preimmune fetal sheep results in early appearance of human donor cells in circulation and boosts cell levels in bone marrow at later time points after transplantation. Blood. 2000;95(11):3620–7. [PubMed] [Google Scholar]
- 59.Stenger EO, Krishnamurti L, Galipeau J. Mesenchymal stromal cells to modulate immune reconstitution early post-hematopoietic cell transplantation. BMC Immunol. 2015;16:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.**Kurtzberg J, Abdel-Azim H, Carpenter P, Chaudhury S, Horn B, Mahadeo K, et al. A Phase 3, Single-Arm, Prospective Study of Remestemcel-L, Ex Vivo Culture-Expanded Adult Human Mesenchymal Stromal Cells for the Treatment of Pediatric Patients Who Failed to Respond to Steroid Treatment for Acute Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Imoprtant study which showed the efficacy of MSCs in a phase 3 clinical trial involving bone marrow transplanatation.
- 61.Davies LC, Boberg E, Le Blanc K. Commentary: Role of Mesenchymal Stromal Cell-Mediated Crosstalk with Macrophages in Graft-versus-Host Disease and Tissue Repair. Biol Blood Marrow Transplant. 2017;23(6):861–2. [DOI] [PubMed] [Google Scholar]
- 62.**Bouchlaka MN, Moffitt AB, Kim J, Kink JA, Bloom DD, Love C, et al. Human Mesenchymal Stem Cell-Educated Macrophages Are a Distinct High IL-6-Producing Subset that Confer Protection in Graft-versus-Host-Disease and Radiation Injury Models. Biol Blood Marrow Transplant. 2017;23(6):897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** First study showed that MSC educated Macrophages confer radioportection.
- 63.*Kink JA, Forsberg MH, Reshetylo S, Besharat S, Childs CJ, Pederson JD, et al. Macrophages Educated with Exosomes from Primed Mesenchymal Stem Cells Treat Acute Radiation Syndrome by Promoting Hematopoietic Recovery. Biol Blood Marrow Transplant. 2019;25(11):2124–33. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Important study demonstrated that Exosome Educated MSCs confer radioprotection.
- 64.Papait A, Vertua E, Magatti M, Ceccariglia S, De Munari S, Silini AR, et al. Mesenchymal Stromal Cells from Fetal and Maternal Placenta Possess Key Similarities and Differences: Potential Implications for Their Applications in Regenerative Medicine. Cells. 2020;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sher N, Ofir R. Placenta-Derived Adherent Stromal Cell Therapy for Hematopoietic Disorders: A Case Study of PLX-R18. Cell Transplant. 2018;27(1):140–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chinnadurai R, Sands J, Rajan D, Liu X, Arafat D, Das R, et al. Molecular Genetic and Immune Functional Responses Distinguish Bone Marrow Mesenchymal Stromal Cells from Hepatic Stellate Cells. Stem Cells. 2019;37(8):1075–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.**Nolta JA, Galipeau J, Phinney DG. Improving mesenchymal stem/stromal cell potency and survival: Proceedings from the International Society of Cell Therapy (ISCT) MSC preconference held in May 2018, Palais des Congres de Montreal, Organized by the ISCT MSC Scientific Committee. Cytotherapy. 2020;22(3):123–6. [DOI] [PubMed] [Google Scholar]; ** View of International Society for Cell Therapy on improving MSC potency and persistency
- 68.Martin I, Galipeau J, Kessler C, Le Blanc K, Dazzi F. Challenges for mesenchymal stromal cell therapies. Sci Transl Med. 2019;11(480). [DOI] [PubMed] [Google Scholar]
- 69.Francois M, Copland IB, Yuan S, Romieu-Mourez R, Waller EK, Galipeau J. Cryopreserved mesenchymal stromal cells display impaired immunosuppressive properties as a result of heat-shock response and impaired interferon-gamma licensing. Cytotherapy. 2012;14(2):147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoogduijn MJ, de Witte SF, Luk F, van den Hout-van Vroonhoven MC, Ignatowicz L, Catar R, et al. Effects of Freeze-Thawing and Intravenous Infusion on Mesenchymal Stromal Cell Gene Expression. Stem Cells Dev. 2016;25(8):586–97. [DOI] [PubMed] [Google Scholar]
- 71.Kadekar D, Rangole S, Kale V, Limaye L. Conditioned Medium from Placental Mesenchymal Stem Cells Reduces Oxidative Stress during the Cryopreservation of Ex Vivo Expanded Umbilical Cord Blood Cells. PLoS One. 2016;11(10):e0165466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moll G, Alm JJ, Davies LC, von Bahr L, Heldring N, Stenbeck-Funke L, et al. Do cryopreserved mesenchymal stromal cells display impaired immunomodulatory and therapeutic properties? Stem Cells. 2014;32(9):2430–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moll G, Geissler S, Catar R, Ignatowicz L, Hoogduijn MJ, Strunk D, et al. Cryopreserved or Fresh Mesenchymal Stromal Cells: Only a Matter of Taste or Key to Unleash the Full Clinical Potential of MSC Therapy? Adv Exp Med Biol. 2016;951:77–98. [DOI] [PubMed] [Google Scholar]
- 74.Nold P, Hackstein H, Riedlinger T, Kasper C, Neumann A, Mernberger M, et al. Immunosuppressive capabilities of mesenchymal stromal cells are maintained under hypoxic growth conditions and after gamma irradiation. Cytotherapy. 2015;17(2):152–62. [DOI] [PubMed] [Google Scholar]
- 75.Pogozhykh D, Pogozhykh O, Prokopyuk V, Kuleshova L, Goltsev A, Blasczyk R, et al. Influence of temperature fluctuations during cryopreservation on vital parameters, differentiation potential, and transgene expression of placental multipotent stromal cells. Stem Cell Res Ther. 2017;8(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.**Chinnadurai R, Garcia MA, Sakurai Y, Lam WA, Kirk AD, Galipeau J, et al. Actin cytoskeletal disruption following cryopreservation alters the biodistribution of human mesenchymal stromal cells in vivo. Stem Cell Reports. 2014;3(1):60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** First study showed that thawed MSCs from cryopreservation display defective biodistribution
- 77.Barcia RN, Santos JM, Teixeira M, Filipe M, Pereira ARS, Ministro A, et al. Umbilical cord tissue-derived mesenchymal stromal cells maintain immunomodulatory and angiogenic potencies after cryopreservation and subsequent thawing. Cytotherapy. 2017;19(3):360–70. [DOI] [PubMed] [Google Scholar]
- 78.Burand AJ, Gramlich OW, Brown AJ, Ankrum JA. Function of Cryopreserved Mesenchymal Stromal Cells With and Without Interferon-gamma Prelicensing is Context Dependent. Stem Cells. 2017;35(5):1437–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cruz FF, Borg ZD, Goodwin M, Sokocevic D, Wagner D, McKenna DH, et al. Freshly thawed and continuously cultured human bone marrow-derived mesenchymal stromal cells comparably ameliorate allergic airways inflammation in immunocompetent mice. Stem Cells Transl Med. 2015;4(6):615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gramlich OW, Burand AJ, Brown AJ, Deutsch RJ, Kuehn MH, Ankrum JA. Cryopreserved Mesenchymal Stromal Cells Maintain Potency in a Retinal Ischemia/Reperfusion Injury Model: Toward an off-the-shelf Therapy. Sci Rep. 2016;6:26463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luetzkendorf J, Nerger K, Hering J, Moegel A, Hoffmann K, Hoefers C, et al. Cryopreservation does not alter main characteristics of Good Manufacturing Process-grade human multipotent mesenchymal stromal cells including immunomodulating potential and lack of malignant transformation. Cytotherapy. 2015;17(2):186–98. [DOI] [PubMed] [Google Scholar]
- 82.Oja S, Kaartinen T, Ahti M, Korhonen M, Laitinen A, Nystedt J. The Utilization of Freezing Steps in Mesenchymal Stromal Cell (MSC) Manufacturing: Potential Impact on Quality and Cell Functionality Attributes. Front Immunol. 2019;10:1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chinnadurai R, Copland IB, Garcia MA, Petersen CT, Lewis CN, Waller EK, et al. Cryopreserved Mesenchymal Stromal Cells Are Susceptible to T-Cell Mediated Apoptosis Which Is Partly Rescued by IFNgamma Licensing. Stem Cells. 2016;34(9):2429–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Simmons PJ, Przepiorka D, Thomas ED, Torok-Storb B. Host origin of marrow stromal cells following allogeneic bone marrow transplantation. Nature. 1987;328(6129):429–32. [DOI] [PubMed] [Google Scholar]
- 85.Cilloni D, Carlo-Stella C, Falzetti F, Sammarelli G, Regazzi E, Colla S, et al. Limited engraftment capacity of bone marrow-derived mesenchymal cells following T-cell-depleted hematopoietic stem cell transplantation. Blood. 2000;96(10):3637–43. [PubMed] [Google Scholar]
- 86.Leibacher J, Henschler R. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res Ther. 2016;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rombouts WJ, Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 2003;17(1):160–70. [DOI] [PubMed] [Google Scholar]
- 88.Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5(3):309–13. [DOI] [PubMed] [Google Scholar]
- 89.Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101(8):2999–3001. [DOI] [PubMed] [Google Scholar]
- 90.Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 2012;7(10):e47559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Krueger TEG, Thorek DLJ, Denmeade SR, Isaacs JT, Brennen WN. Concise Review: Mesenchymal Stem Cell-Based Drug Delivery: The Good, the Bad, the Ugly, and the Promise. Stem Cells Transl Med. 2018;7(9):651–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mendicino M, Fan Y, Griffin D, Gunter KC, Nichols K. Current state of U.S. Food and Drug Administration regulation for cellular and gene therapy products: potential cures on the horizon. Cytotherapy. 2019;21(7):699–724. [DOI] [PubMed] [Google Scholar]
- 93.Mendicino M, Bailey AM, Wonnacott K, Puri RK, Bauer SR. MSC-based product characterization for clinical trials: an FDA perspective. Cell Stem Cell. 2014;14(2):141–5. [DOI] [PubMed] [Google Scholar]
- 94.Su J, Chen X, Huang Y, Li W, Li J, Cao K, et al. Phylogenetic distinction of iNOS and IDO function in mesenchymal stem cell-mediated immunosuppression in mammalian species. Cell Death Differ. 2014;21(3):388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chinnadurai R, Rajakumar A, Schneider AJ, Bushman WA, Hematti P, Galipeau J. Potency Analysis of Mesenchymal Stromal Cells Using a Phospho-STAT Matrix Loop Analytical Approach. Stem Cells. 2019;37(8):1119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chinnadurai R, Rajan D, Qayed M, Arafat D, Garcia M, Liu Y, et al. Potency Analysis of Mesenchymal Stromal Cells Using a Combinatorial Assay Matrix Approach. Cell Rep. 2018;22(9):2504–17. [DOI] [PMC free article] [PubMed] [Google Scholar]


