Abstract
Background
The distraction-based growth-friendly technique has become a mainstay of treatment for young children with long-spanned congenital scoliosis. However, in patients who are 9 years to 11 years old, the choice is much less clear, and posterior spinal fusion is also a potential option.
Questions/purposes
Comparing growth-friendly scoliosis surgery and posterior spinal fusion, which technique (1) provides greater correction of spinal deformity, (2) is associated with more surgical complications, and (3) results in greater improvement in pulmonary function tests, health-related quality of life scores, other patient-reported outcomes?
Methods
Between 2009 and 2017, one spinal center performed 212 spinal interventions for scoliosis in patients aged between 9 years and 11 years old and who had open triradiate cartilage, including 40 patients with growth-friendly approaches (34 with growing-rod technique and six with a vertical expandable prosthetic titanium rib) and 172 with one-stage posterior spinal fusion, respectively. During this period, our general indications for using growth-friendly surgery were patients with open triradiate cartilage, major curve higher than 40°, and upper and lower end vertebrae involving at least eight segments. Twelve patients with a median (range) age of 9.3 years (9 to 11) treated with growth-friendly surgery met the following inclusion criteria: (1) had at least two lengthening procedures before definitive spinal fusion along with 2 years of follow-up after definitive spinal fusion; (2) had been followed until skeletal maturity (Risser grade ≥ 4); and (3) with complete radiographic and clinical data (health-related quality of life (HRQoL) and pulmonary function test results) preoperatively and at the latest follow-up. A group of patients between 9 years and 11 years old and underwent one-stage posterior spinal fusion was selected from our database of patients with congenital scoliosis. Our general indications for using one-stage posterior spinal fusion were patients with a major curve greater than 40°, and with thoracic height higher than 18 cm. Sixty-two patients who had open triradiate cartilage and had been followed until skeletal maturity (Risser grade ≥ 4) were accounted for. In this retrospective, controlled study, we matched patients in the posterior spinal fusion group to those 12 patients who had growth-friendly surgery by age, sex, pathologic findings, major curve size, and location of the apex of the major curve (2:1 matching provided 24 patients in the control group). The median (range) age was 9.8 years (9 to 11). We then compared the groups in terms of magnitude of correction and postoperative complications. Surgical complications, including infection, implant-related complications, and alignment-related complications were evaluated and classified using the surgical complications grading system. Pulmonary function tests and HRQoL were also compared between groups. Pulmonary function tests were performed at the same center with a spirometer. HRQoL were assessed by questionnaire, including the 24-item Early-onset Scoliosis questionnaire for parent-reported outcomes and the Scoliosis Research Society-22 questionnaire for patient-reported outcomes. All patients involved in this study gave their informed consent.
Results
The posterior spinal fusion group achieved a greater correction magnitude at the latest follow-up (median [range] 46% [28 to 70] versus median 34% [9 to 58], difference of medians = 11%; p < 0.001) than the growth-friendly group. A higher proportion of patients in the growth-friendly group had complications than in the posterior spinal fusion group (7 of 12 versus 4 of 24; p = 0.03). There were no between-group differences in terms of pulmonary function tests. Few differences were found between the groups in terms of 24-item Early-onset Scoliosis parental impact (median [range] 60 [44 to 83] for the growth-friendly group versus median 71 [55 to 87] for the posterior spinal fusion group, difference of medians = 13; p = 0.001), financial burden (median 44 [30 to 55] for the growth-friendly group versus median 62 [53 to 75] for the posterior spinal fusion group, difference of medians = 16; p < 0.001) and the Scoliosis Research Society-22 self-image scores (median 3.8 [3.2 to 4.3] for the growth-friendly group versus median 4.4 [4.1 to 4.6] for the posterior spinal fusion group, difference of medians = 0.5; p = 0.006) at the latest follow-up, and those differences that were observed all favored the posterior spinal fusion group.
Conclusions
In light of the superior deformity correction and fewer observed complications with posterior spinal fusion, and the absence of important differences in validated outcomes scores or pulmonary function tests, posterior spinal fusion might be a better choice for 9- to 11-year-old children with long-spanned congenital scoliosis and limited growth potential in the intended instrumentation area.
Level of Evidence
Level III, therapeutic study.
Introduction
For young patients with congenital scoliosis secondary to a solitary hemivertebra, one-stage posterior hemivertebra resection with short pedicle screw fixation has gradually become the mainstay of treatment [5, 16, 24]. But in patients with a long-spanned, rigid kyphoscoliosis curve, definitive spinal fusion at a young age may result in a short trunk, pulmonary dysfunction, and even iatrogenic thoracic insufficiency syndrome [14, 15, 19]. In past decades, the distraction-based growth-friendly technique has been widely suggested for young children because of its effectiveness in controlling spinal deformities and permitting respiratory system development [9, 11, 21]. However, the ability to facilitate ongoing spinal growth comes at a cost during growth-friendly treatment, with more distraction procedures and higher complication rates compared with fusion surgery [8, 12, 13, 20]. A multicenter study from the Growing Spine Study Group reported that the overall complication rate per procedure was 19%, and the authors revealed that 58% of patients had at least one complication during the distraction period [2].
Generally, pediatric surgeons would accept (and recommend that their patients and families accept) the greater complication risk and choose growth-friendly treatment for children younger than 8 years, when the chest wall and pulmonary alveoli are developing rapidly. But in patients who are 9 years to 11 years old, the choice is much less clear, and posterior spinal fusion is a potential option. In idiopathic scoliosis, one case-matched study compared the radiographic outcomes of growing rod and one-stage spinal fusion; it found that growing rod treatment did not benefit 9- to 11-year-old children in terms of major curve correction or spinal height elongation [18]. However, the etiology in their study was limited to idiopathic scoliosis, and they did not evaluate health-related quality of life (HRQoL) and pulmonary function tests. To the best of our knowledge, surgical outcomes including major curve correction, surgical complications pulmonary function tests and HRQoL outcomes between growth-friendly treatment and posterior spinal fusion in 9- to 11-year-old patients with long-spanned congenital scoliosis have not yet been compared.
We therefore compared growth-friendly scoliosis surgery and posterior spinal fusion, and asked which technique (1) provides greater correction of spinal deformity, (2) is associated with more surgical complications, and (3) results in greater improvement in pulmonary function tests, HRQoL scores, and other patient-reported outcomes?
Patients and Methods
This was a single-center retrospective study that was approved by the institutional review board of our hospital. Patients with long-spanned congenital scoliosis (with upper- and lower-end vertebrae involving at least eight segments) who underwent the distraction-based growth-friendly technique and posterior spinal fusion between January 2009 and July 2017 were considered potentially eligible for inclusion.
Treatment Algorithm and Surgical Procedures
During the study period, our general indications for using distraction-based growth-friendly surgery, including growing rod and vertical expandable prosthetic titanium rib (VEPTR), were patients with open triradiate cartilage, a major curve greater than 40°, and with a long-spanned curve. Growing rods were preferentially used, but VEPTR was selected for those patients with thoracic dysplasia. Growth-friendly treatment included instrumentation implantation at the index surgery and regular lengthening procedures during follow-up. During the index surgical procedure, each patient in the growth-friendly group underwent placement of instrumentation, without any other interventions that sought to address the congenital vertebral anomalies. Subsequent lengthening procedures at intervals approximately 6 to 10 months apart. After several lengthening procedures, definitive spinal fusion was recommended for patients with a Risser grade ≥ 4 or those with limited growth remaining. During the final fusion procedure, rib cradles in VEPTR systems were exchanged with a pedicle screw or hook fixation at the spine. In most patients, the proximal and distal foundation sites during the distraction period were maintained for fusion in situ. However, for patients with alignment-related complications or a relatively stiff spine, the fusion levels were extended, and multilevel Ponte osteotomies were performed to achieve spinal balance.
For patients with open triradiate cartilage and a long-spanned curve, growth-friendly treatment was recommended. One-stage posterior spinal fusion was chosen for patients whose parents did not allow their child to undergo growth-friendly treatment. Our general indications for using one-stage posterior spinal fusion were patients with a major curve greater than 40° and with thoracic height (T1-T12 height) higher than 18 cm. All these patients underwent segmental spinal instrumentation and fusion via a one-stage, posterior-only approach. We chose the uppermost instrumented vertebra at the upper end of the vertebrae or the supraadjacent level and the lowest instrumented vertebra at the stable vertebra or last touching vertebra by the central sacral vertical line. Pedicle screws were preferably placed at anchoring sites, but hooks were used otherwise. During this period, we also used multilevel Ponte osteotomies at the apex and at the superior and inferior adjacent levels. We generally used these approaches when vertebral anomalies with asymmetric growth potential located around the apex.
Participants and Demographics
Between 2009 and 2017, one spinal center performed 212 spinal interventions in patients with scoliosis who were between 9 years and 11 years old and who had open triradiate cartilage; this included 40 patients with growth-friendly approaches and 172 with one-stage posterior spinal fusion. Twelve patients (this included seven males and five females, median [range] age 9 years [9 to 11] at the time of the index surgery; nine with a growing rod and three with VEPTR) treated with growth-friendly surgery met the following inclusion criteria: (1) had at least two lengthening procedures before definitive spinal fusion along with 2 years of follow-up after definitive spinal fusion; (2) had been followed until skeletal maturity [Risser grade ≥ 4]); and (3) with complete radiographic and clinical data (HRQoL and pulmonary function test results) preoperatively and at the latest follow-up. According to the classification of congenital spinal anomalies [17], six patients had Type 1 (formation failures), one had Type 2 (segmentation failures), and five had Type 3 (mixed type, formation, and segmentation failures).
A group of patients whose parents did not allow their child to undergo the growth-friendly treatment and underwent one-stage spinal fusion were selected from our database of patients with congenital scoliosis. Sixty-two patients who had open triradiate cartilage and had been followed until skeletal maturity (Risser grade ≥ 4) with complete radiographic and clinical data were accounted for. In this retrospective, controlled study, we then matched patients in the posterior spinal fusion group to those 12 patients who had growth-friendly surgery in a 2:1 ratio based on age (within 10 months), sex, pathologic findings (including the type of vertebral anomalies), major curve size (within 10°), and location of the major curve’s apex (within two vertebral bodies). Eventually, 24 patients (14 males and 10 females; (median [range] age 10 years [9 to 11]; median follow-up duration 7 years [5 to 8 years]) were recruited and constituted the posterior spinal fusion group (Fig. 1).
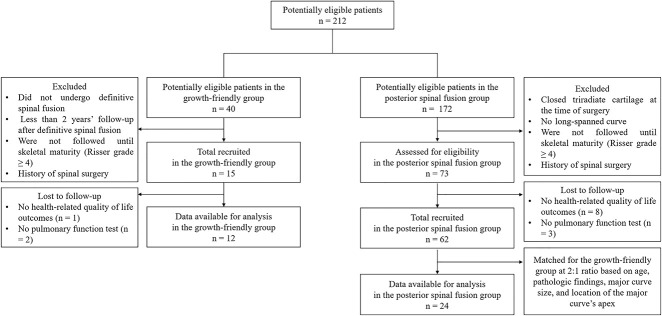
Fig. 1.
This flowchart shows the inclusion process of the current study.
The growth-friendly and posterior spinal fusion groups were well-matched for age, sex, open triradiate cartilage, and pathologic findings (Table 1). In the growth-friendly group, the median (range) number of lengthening procedures was 4 (3 to 6) and the median age at the time of definitive spinal fusion was 13 years (11 to 15). There was no difference in follow-up interval from the initial surgery to the latest follow-up evaluation between the two groups (median [range] 7.2 years [5 to 9] for the growth-friendly group versus median 6.8 years [5 to 8] for the posterior spinal fusion group, difference of medians = 0.5; p = 0.14). The fusion levels in the growth-friendly group were longer than those in the posterior spinal fusion group (median 14.7 [12 to 16] versus median 12.5 [10 to 15], difference of medians = 1.9; p = 0.003).
Table 1.
Demographic data of patients in the growth-friendly and posterior spinal fusion groups
| Parameters | Growth-friendly group (n = 12) | Posterior spinal fusion group (n = 24) | p value | |
| Vertebral anomalies | I: formation failures | 6 | 12 | |
| II: segmentation failures | 1 | 2 | ||
| III: mixed types | 5 | 10 | ||
| Age (years) | At index surgery | 9.3 (9 to 11) | 9.8 (9 to 11) | 0.61 |
| At latest follow-up interval | 16.9 (15 to 18) | 16.4 (14 to 18) | 0.33 | |
| Follow-up (years) | Index surgery to definitive spinal fusion | 4.6 (2 to 6) | ||
| Definitive spinal fusion to latest follow-up | 2.8 (2 to 4) | |||
| Index surgery to follow-up | 7.2 (5 to 9) | 6.8 (5 to 8) | 0.14 | |
| Fusion and spanned levels | Distraction periods | 13.8 (11 to 15) | ||
| Definitive spinal fusion | 14.7 (12 to 16) | 12.5 (10 to 15) | 0.003 | |
Data are presented as the median (range).
Primary and Secondary Study Outcomes
Our primary study outcomes were the magnitude of correction and postoperative complications. Magnitude of correction was measured on standing posteroanterior and lateral radiographs, including the major curve and its correction percentage. Postoperative correction percentage was calculated with (preoperative major curve –postoperative major curve) * 100%/ Preoperative major curve, and correction percentage at most recent follow-up was calculated with (preoperative major curve –major curve at most recent follow-up) * 100%/ Preoperative major curve. Additionally, we evaluated the following parameters: (1) trunk shift, which was the perpendicular distance from the sacrum’s center to the plumb line drawn from the midpoint of the C7 vertebra body; (2) T1-S1 height, which was the vertical distance between the midpoints of the superior endplates of T1 and S1; (3) T1-T12 height, which was the vertical distance between the midpoint of the superior endplate of T1 and the inferior endplate of T12; (4) space available for the lung, which was the ratio of the height of the concave hemithorax (the distance from the middle of the most-cephalad rib down to the center of the hemidiaphragm) to that of the convex hemithorax [4]; (5) global kyphosis, which was defined as the angle between the superior endplate of the most tilted vertebra cranially and the inferior endplate of the most tilted vertebra caudally; and (6) lumbar lordosis. In this study, all radiographs were measured twice at an interval of 1 week by two well-trained senior residents who were not involved in surgery, and the mean of both measurements were adopted for analysis in this study. Intraclass correlation coefficient method was calculated for inter- and intraobserver variability. Intra- and interobserver correlation coefficients for estimating the spinal parameters were 0.82 and 0.81, suggesting high reliability of these measurements.
Surgical complications, including infection, implant-related complications, and alignment-related complications, were recorded based on radiographs by an individual resident who was not directly involved in patient care. Implant-related complications included rod fracture and failure of anchor fixation. With regard to alignment-related complications, we mainly focused on coronal imbalance, trunk shift (defined as the horizontal distance between the C7 plumb line and the central sacral vertical line greater than 20 mm); adding-on, which was defined as an increase in the number of vertebrae in the measured curve either proximally or distally, combined with a curve increase of more than 6° from the first postoperative radiograph; proximal junctional kyphosis (defined as a proximal or distal junctional angle greater than 10° and an angle at least 10° greater than the preoperative measurement). The complications were also evaluated using the surgical complications grading system proposed by Smith et al. [22], where severity refers to the level of care and urgency needed to treat the complication, and can be classified as follows: (1) Grade 1—does not result in unplanned surgery and can be corrected at the next scheduled surgery; (2) Grade 2—results in an unplanned surgery, with 2A involving a single additional operation and 2B involving multiple additional operations for resolution; (3) Grade 3—alters the planned course of treatment; and (4) Grade 4—death.
Our secondary study outcomes were pulmonary function tests and health-related quality of life scores. Pulmonary function tests were performed at the same center with a spirometer (Jaeger, Welzburg, Germany). The percentage of the predicted value for each parameter was calculated using Knudsen prediction equations. Arm span was used instead of height to calculate body size-adjusted predicted values because of the decreased spinal height in patients with scoliosis. Here, forced vital capacity, forced expired volume in 1 second, and forced expired volume in 1 second/forced vital capacity were recorded, and these values were further denoted by the percentage ratio of the actual value to the predicted value. All participants had complete pulmonary function test records before the index surgery and at the latest follow-up examination. HRQoL was assessed by questionnaire, including the 24-item Early-onset Scoliosis questionnaire [6] for parent-reported outcomes and the Scoliosis Research Society-22 questionnaire [7] for patient-reported outcomes. The 24-item Early-onset Scoliosis questionnaire was completed by the child’s parents before the index surgery and during follow-up assessments. In contrast, SRS-22 questionnaire was completed by the patients independently at the latest follow-up examination. The evaluated scale scores range between 0 and 100 (poor to excellent) for each domain in 24-item Early-onset Scoliosis questionnaires and between 0 to 5 (poor to excellent) for each domain in SRS-22 questionnaires.
Statistical Analyses
Statistical analyses were performed using SPSS software (SPSS 22.0, SPSS Inc, Chicago IL, USA). The statistical data are presented as the median and range. Statistical data were compared between the groups by Mann-Whitney U tests and within groups by a Wilcoxon Signed Rank test, respectively. Chi-square tests were performed to compare categorical variables between the two groups. p values < 0.05 were considered statistically significant.
Results
Spinal Deformity Correction
The posterior spinal fusion group achieved a greater correction magnitude after the initial surgery (median [range] 54% [38 to 72] versus median 40% [14 to 69], difference of medians =15%; p = 0.005) as well as at latest follow-up (median 46% [28 to 70] versus median 34% [9 to 58], difference of medians = 11%; p < 0.001) than the growth-friendly group (Fig. 2). In the sagittal plane, there was no difference between two groups in terms of global kyphosis after the initial surgery and at the latest follow-up (Table 2). Additionally, the average spinal and thoracic height gain at the latest follow-up were not different between the two groups (Table 3).
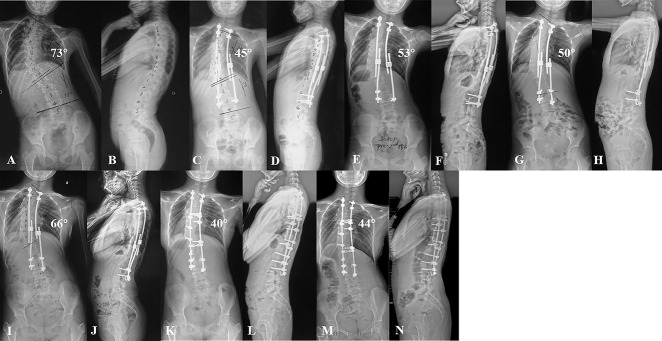
Fig. 2 A-N.
(A-B) These radiographs are from a 9-year-old girl with congenital scoliosis in the growth-friendly group. (C-D) After the index surgery, the major curve notably improved; however, (E, F) pedicle screw (L3) dislodgement occurred before the second lengthening procedure and (G-H) the screw was replaced during the lengthening procedure. (I-J) During 5 years of follow-up, the patient underwent four lengthening procedures. The major curve increased during the distraction period. (K-L) This patient underwent definitive spinal fusion at 14 years old; the major curve improved and (M-N) remained steady after 3 years of follow-up.
Table 2.
Comparison of radiographic features between the growth-friendly and posterior spinal fusion groups
| Parameters | Growth-friendly group (n = 12) | Posterior spinal fusion group (n = 24) | p value | |
| Major curve (°) | Before index procedure | 89 (64 to 122) | 94 (63 to 125) | 0.94 |
| After index procedure | 55 (38 to 78) | 41 (24 to 71) | 0.01 | |
| Correction percentage (%) | 40 (14 to 69) | 54 (38 to 72) | 0.005 | |
| Before definitive spinal fusion | 63(57 to 86) | |||
| After definitive spinal fusion | 54 (40 to 82) | |||
| Latest follow-up | 58 (44 to 82) | 47 (26 to 70) | 0.007 | |
| Correction percentage (%) | 34 (9 to 58) | 46 (28 to 70) | < 0.001 | |
| Trunk shift (mm) | Before index procedure | 8 (-7 to 25) | 11 (-7 to 17) | 0.71 |
| After index procedure | 5 (-12 to 16) | 8 (-10 to 18) | 0.11 | |
| Before definitive spinal fusion | 12 (3 to 28) | |||
| After definitive spinal fusion | 15 (7 to 25) | |||
| Latest follow-up | 14 (4 to 28) | 8 (-5 to 15) | 0.16 | |
| Global kyphosis (°) | Before index procedure | 55 (26 to 92) | 61 (21 to 97) | 0.55 |
| After index procedure | 43 (21 to 69) | 36 (21 to 68) | 0.33 | |
| Before definitive spinal fusion | 41 (26 to 63) | |||
| After definitive spinal fusion | 37 (21 to 61) | |||
| Latest follow-up | 43 (24 to 67) | 39 (19 to 64) | 0.14 | |
| Lumbar lordosis (°) | Before index procedure | 42 (29 to 57) | 47 (28 to 76) | 0.58 |
| After index procedure | 38 (31 to 61) | 37 (21 to 64) | 0.21 | |
| Before definitive spinal fusion | 41 (26 to 45) | |||
| After definitive spinal fusion | 36 (23 to 41) | |||
| Latest follow-up | 39 (21 to 54) | 40 (23 to 72) | 0.67 | |
Data are presented as the median (range); postoperative correction percentage was calculated with (preoperative major curve – postoperative major curve) * 100%/ Preoperative major curve; Correction percentage at most recent follow-up was calculated with (preoperative major curve –major curve at most recent follow-up) * 100%/ Preoperative major curve; global kyphosis, the angle between the superior endplate of the most tilted vertebra cranially and the inferior endplate of the most tilted vertebra caudally.
Table 3.
Comparison of spinal and thoracic growth between the growth-friendly and posterior spinal fusion
| Parameters | Growth-friendly group (n = 12) | Posterior spinal fusion group (n = 24) | p value | |
| Space available for the lung | Before index procedure | 0.79 (0.64 to 0.90) | 0.80 (0.67 to 0.89) | 0.42 |
| After index procedure | 0.82 (0.79 to 0.94) | 0.83 (0.76 to 0.94) | 0.16 | |
| Before definitive spinal fusion | 0.81 (0.76 to 0.92) | |||
| After definitive spinal fusion | 0.82 (0.78 to 0.93) | |||
| Latest follow-up | 0.82 (0.77 to 0.93) | 0.83 (0.74 to 0.92) | 0.47 | |
| T1-S1 height (cm) | Before index procedure | 28 (26 to 32) | 29 (26 to 32) | 0.34 |
| After index procedure | 31 (28 to 33) | 33 (29 to 35) | 0.001 | |
| Before definitive spinal fusion | 36 (30 to 39) | |||
| After definitive spinal fusion | 37 (31 to 40) | |||
| Latest follow-up | 38 (32 to 42) | 36 (32 to 40) | 0.27 | |
| T1-S1 gain (%) | 25 (19 to 31) | 22 (13 to 27) | 0.15 | |
| T1-T12 height (cm) | Before index procedure | 19 (17 to 21) | 20 (18 to 22) | 0.52 |
| After index procedure | 21 (19 to 22) | 23 (22 to 25) | 0.004 | |
| Before definitive spinal fusion | 24 (22 to 25) | |||
| After definitive spinal fusion | 24 (23 to 26) | |||
| Latest follow-up | 25 (24 to 27) | 24 (22 to 27) | 0.136 | |
| T1-T12 gain (%) | 22 (16 to 25) | 19 (12 to 30) | 0.101 | |
Data are presented as the median (range); T1-S1 height gain was calculated with (T1-S1 value at the latest follow-up – T1-S1 value before surgery) * 100%/ T1-S1 value at the latest follow-up; T1-T12 height gain was calculated with (T1-T12 value at the latest follow-up – T1-T12 value before surgery) * 100%/ T1-T12 value at the latest follow-up.
Complications
A higher proportion of patients in the growth-friendly group had complications than in the spinal fusion group (7 of 12 versus 4 of 24; p = 0.03) (Fig. 3). In the growth-friendly group, seven unplanned revision surgical procedures were performed for seven implant-related complications, including one patient with rib cradle migration during VEPTR treatment. In contrast, none of patients in the posterior spinal fusion group underwent additional surgery for postoperative complications. According to the surgical complications grading system we used [22], 13 complications in the growth-friendly group and six in the posterior spinal fusion group were evaluated as Grade 1. In addition, seven complications in the growth-friendly group were considered Grade 2A. No complication events reached Grade 3 or 4 (Table 4).
Fig. 3 A-F.
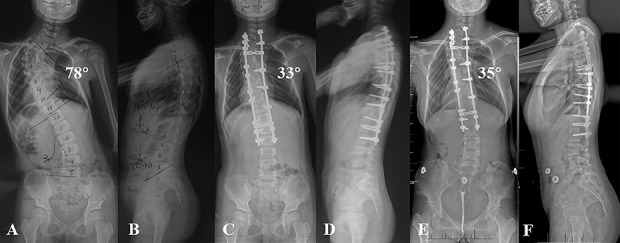
(A-B) These radiographs are from a 9-year-old girl with congenital scoliosis in the posterior spinal fusion group. (C-D) After the index surgery, the major curve notably improved. (E-F) On radiographs taken at 8 years after the procedure, the major curve appeared steady and no complications were noted.
Table 4.
Comparison of postoperative complications between the growth-friendly and posterior spinal fusion groups
| Type of complications | Growth-friendly group (n = 12) | Posterior spinal fusion group (n = 24) | |
| Distraction periods | Definitive spinal fusion | ||
| Rod fracture | 4 (2 of 12) | 0 | 0 |
| Pedicle screw, hook, or rod dislodgment | 2 (2 of 12) | 1 (1 of 12) | 0 |
| Proximal junctional kyphosis | 4 (3 of 12) | 0 | 2 (2 of 24) |
| Coronal imbalance | 3 (3 of 12) | 3 (3 of 12) | 2 (1 of 24) |
| Adding-on | 1 (1 of 12) | 0 | 2 (2 of 24) |
| Superficial infection | 2 (2 of 12) | 0 | 0 |
| Total adverse events | 20 (7 of 12) | 6 (4 of 24) | |
Data are presented as the number of complications (number of patients with at least one complication, proportion of patients with at least one complication); adding-on was defined as an increase in the number of vertebrae in the measured curve either proximally or distally, combined with a curve increase of more than 6° from the first postoperative radiograph.
Pulmonary Function Tests and HRQoL Scores
There were no between-group differences in the severity of the baseline pulmonary function or the final outcomes (Table 5). At the latest follow-up, few differences were noted between the groups in terms of 24-item Early-onset Scoliosis parental impact (median [range] 60 [44 to 83] for the growth-friendly group versus median 71 [55 to 87] for the posterior spinal fusion group, difference of medians = 13; p = 0.001), financial burden (median 44 [30 to 55] for the growth-friendly group versus median 62 [53 to 75] for the posterior spinal fusion group, difference of medians = 16; p < 0.001) and the Scoliosis Research Society-22 self-image scores (median 3.8 [3.2 to 4.3] for the growth-friendly group versus median 4.4 [4.1 to 4.6] for the posterior spinal fusion group, difference of medians = 0.5; p = 0.006), and those differences that were observed all favored the posterior spinal fusion group (Table 6).
Table 5.
Comparison of pulmonary functional test results between the growth-friendly and posterior spinal fusion groups
| Parameters | Growth-friendly group (n = 12) | Posterior spinal fusion group (n = 24) | p value | |
| Preoperative (%) | FEV1 | 64 (49 to 79) | 66 (48 to 82) | 0.48 |
| FVC | 67 (51 to 82) | 69 (52 to 79) | 0.31 | |
| FEV1/FVC | 80 (74 to 89) | 79 (72 to 88) | 0.43 | |
| Latest follow-up (%) | FEV1 | 72 (57 to 82) | 73 (58 to 86) | 0.57 |
| FVC | 75 (58 to 88) | 70 (56 to 87) | 0.61 | |
| FEV1/FVC | 82 (75 to 93) | 81 (76 to 90) | 0.44 | |
Data are presented as the median (range). FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity.
Table 6.
Comparison of healthy-related quality of life between the growth-friendly and posterior spinal fusion groups
| Parameters | Growth-friendly group (n = 12) | Posterior spinal fusion group (n = 24) | p value | |
| EOSQ-24 scores before surgery | General health | 57 (42 to 68) | 55 (47 to 71) | 0.23 |
| Pain/discomfort | 61 (48 to 78) | 63 (51 to 84) | 0.33 | |
| Daily living | 53 (44 to 66) | 57 (47 to 73) | 0.25 | |
| Fatigue/energy level | 57 (42 to 69) | 61 (48 to 79) | 0.34 | |
| Transfer | 64 (48 to 79) | 62 (49 to 77) | 0.81 | |
| Satisfaction | 61 (52 to 74) | 57 (50 to 61) | 0.43 | |
| Emotion | 69 (58 to 81) | 66 (53 to 82) | 0.37 | |
| Parental impact | 45 (37 to 59) | 47 (36 to 61) | 0.39 | |
| Physical function | 64 (55 to 79) | 61 (51 to 77) | 0.41 | |
| Pulmonary function | 57 (47 to 70) | 64 (49 to 76) | 0.58 | |
| Financial burden | 48 (36 to 59) | 52 (38 to 63) | 0.29 | |
| EOSQ-24 scores at the latest follow-up examination | General health | 62 (52 to 76) | 68 (55 to 83) | 0.27 |
| Pain/discomfort | 67 (56 to 82) | 70 (59 to 84) | 0.51 | |
| Daily living | 70 (55 to 81) | 76 (61 to 89) | 0.31 | |
| Fatigue/energy level | 72 (62 to 86) | 74 (58 to 87) | 0.57 | |
| Transfer | 69 (57 to 81) | 67 (55 to 81) | 0.72 | |
| Satisfaction | 69 (54 to 83) | 71 (59 to 85) | 0.42 | |
| Emotion | 76 (63 to 87) | 75 (63 to 85) | 0.71 | |
| Parental impact | 60 (44 to 83) | 71 (55 to 87) | 0.001 | |
| Physical function | 76 (65 to 87) | 73 (61 to 86) | 0.34 | |
| Pulmonary function | 72 (68 to 80) | 74 (64 to 82) | 0.45 | |
| Financial burden | 44 (30 to 55) | 62 (53 to 75) | < 0.001 | |
| SRS-22 scores at the latest follow-up | Function | 4.2 (3.8 to 4.9) | 4.6 (4.0 to 4.8) | 0.13 |
| Pain | 4.4 (4.0 to 4.8) | 4.7 (4.3 to 4.8) | 0.34 | |
| Mental health | 4.1 (3.9 to 4.5) | 4.3 (4.0 to 4.7) | 0.15 | |
| Self-image | 3.8 (3.2 to 4.3) | 4.4 (4.1 to 4.6) | 0.006 | |
| Satisfaction | 4.3 (3.8 to 4.7) | 4.5 (3.7 to 4.9) | 0.38 | |
Data are presented as the median (range); EOSQ-24 = 24-item Early-onset Scoliosis questionnaires; SRS-22 = Scoliosis Research Society-22 questionnaires.
Discussion
Growth-friendly treatment for young children with long-spanned congenital scoliosis involves repetitive surgical distraction across the unfused portion of a deformed spine and final fusion when the spine is skeletally mature [10]. Pediatric orthopaedic surgeons generally accept the greater complication risk associated with the additional procedures used in a growth-friendly approach and choose the growth-friendly treatment for children younger than 8 years. But in patients who are 9 years to 11 years old, posterior spinal fusion is an alternative, since some research suggests that spinal fusion offers greater curve correction and involves fewer surgical interventions than growth-friendly treatment in 9- to-11-year-old patients with idiopathic scoliosis [18]. However, to our knowledge, no study has compared these approaches for 9- to 11-year-old patients with long-spanned congenital scoliosis. Therefore, we compared growth-friendly scoliosis surgery and posterior spinal fusion and asked which technique (1) provides greater correction of spinal deformity, (2) is associated with more surgical complications, and (3) results in greater improvement in pulmonary function tests, HRQoL scores, other patient-reported outcomes? We found that patients who underwent posterior spinal fusion did not differ in terms of spinal growth and pulmonary functional test outcomes compared with those who underwent the growth-friendly treatment but had better spinal deformity correction.
Limitations
There are several limitations to this study. First, this was a retrospective study, which raises the possibility of selection bias, assessment bias, and transfer bias; however, the two groups were well matched in terms of demographics and radiographic characteristics, and both groups were followed until Risser grade ≥ 4. The sample size was small, but it was large enough to provide sufficient power to detect differences in correction between two surgical strategies. Second, surgical strategies for the growth-friendly group included growing rods and VEPTR. In our spine center, VEPTR were only selected for patients with thoracic dysplasia. The two distraction-based growth-friendly strategies have been shown to have similar distraction principle during distraction periods. Hence, we did not exclude patients who underwent VEPTR in the growth-friendly group. Additionally, due to the relatively small sample size in patients who underwent VEPTR, the current study could not compare growing rods and VEPTR. Third, the indications of posterior spinal fusion in patients in this age group with congenital scoliosis are difficult to generalize to other age groups. Therefore, we caution readers to consider this when interpreting our findings. Finally, noninvasive lengthening using magnetic controlled growing rods has been used in many spine centers. As reported previously, they may decrease the risk of some complications, such as intraoperative anesthesia-related complications and postoperative wound-related complications [21]. Recently, some centers have reported complications and limitations of this technique [1]. However, this technique has not been used in our spine center. Therefore, the current study did not compare surgical outcomes between magnetic controlled growing rod and posterior spinal fusion.
Correction of Deformity
We found that posterior spinal fusion resulted in greater correction of deformity than did growth-friendly techniques. The limited correction in the growth-friendly group may be related to the characteristics of growth-friendly instrumentation and postoperative complications. Because the growth-friendly system, either growing rods or VEPTR, simply combines upper and lower instrumentation with rods, a lack of apical control may lead to asymmetrical growth of congenital anomalies and great correction loss during distraction periods [23, 25]. In the current study, the magnitude of the major curve in the growth-friendly group increased after the index surgery, even when lengthening procedures were performed regularly. Furthermore, autofusion of the spinal segments spanned by instrumentation may also influence deformity correction at the definitive spinal fusion. Studies have suggested that a spine previously instrumented with a solid device is stiffer than a previously unoperated vertebral column [9, 10, 20]. In a retrospective study of evaluating the spinal mobility among 58 patients who underwent lengthening procedures, 81% (47 of 58) patients were found to have some areas of autofusion and even a completely fused spine [9]. Autofusion and rigid segments may lead to limited correction of a spinal deformity during definitive spinal fusion.
Complications
We saw fewer major complications in children treated with posterior spinal fusion than those treated with growth-friendly techniques. These complications in the growth-friendly group, including rod fracture, pedicle screw dislodgement, and alignment-related complications, mainly occurred during the distraction period. It should be noted that coronal imbalance and the crankshaft phenomenon observed during the distraction period could only minimally be improved because of rigid spinal segments, even if instrumented levels were extended and multilevel Ponte osteotomies were performed during the final fusion. In addition, repeated lengthening procedures have been reported to increase postoperative wound complications [1]. Our finding was consistent with results reported in the that study. In a retrospective study that compared young children who underwent either growing rod treatment and posterior spinal fusion, the authors reported that growing rod treatment was an independent risk factor of instrumentation-related complication [26]. They also suggested that early definitive fusion may be preferable in patients aged 9 years or older. Considering our results and those of these other studies [1, 26], parents of patients older than 9 years should be informed that growth-friendly treatment may be associated with an increased risk of complications.
Pulmonary Function Tests and HRQoL Scores
We found no differences in pulmonary function test results, and the few differences we observed in HRQoL outcomes scores, including the 24-item Early-onset Scoliosis parental impact, financial burden, and the Scoliosis Research Society-22 self-image scores, generally favored the posterior spinal fusion group. Pulmonary function development among young children may be associated with a growth of thoracic height. Spinal fusion across the thoracic spine at a young age may result in a relatively short trunk [4, 14]. It has been reported that thoracic growth in children who underwent early spinal fusion before the age of 5 years was only half of that of those who did not have early surgery [3]. In contrast, for young children with idiopathic scoliosis aged 9 years to 11 years, no differences in spinal height gain were found between those who underwent posterior spinal fusion compared with the growth-friendly treatment [18]. Similarly, the current study also noted that 9- to 11-year old patients with congenital scoliosis who underwent posterior spinal fusion treatment had only marginally less spinal height gain compared with those who underwent the growth-friendly treatment. This may have accounted for the absence of differences in pulmonary function test results between two groups. Prior research suggests that patients who undergo multiple surgical procedures were found to have more psychosocial stress and a poor quality of life [1]. In the current study, comparison of HRQoL generally favored the posterior spinal fusion group. The relatively poor outcomes in HRQoL scores in the growth-friendly group may be associated with multiple surgical procedures during distraction periods.
Conclusions
Considering the superior deformity correction and fewer observed complications with posterior spinal fusion, and the absence of important differences in validated outcomes scores or pulmonary function tests, we suggest that posterior spinal fusion might be a better choice for 9- to 11-year-old children with long-spanned congenital scoliosis and limited growth potential in the intended instrumentation area. Regardless of the technique chosen, we believe it is essential to evaluate each patient’s preoperative condition, including nutrition, spinal height, and pulmonary function, and to consider (and discuss with families) the risks and benefits of each strategy. For patients with thoracic dysplasia syndrome, based on ample prior evidence [15, 25]. we believe the growth-friendly treatment is a better option to maintain spinal growth and allow the child’s pulmonary system to develop to the greatest extent possible. In contrast, for 9- to 11-year-old patients with long-spanned congenital scoliosis and limited growth potential in the intended instrumentation area, our study suggests that posterior spinal fusion treatment seems to be the better choice.
Footnotes
Each author certifies that neither he, nor any member of his immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Bauer JM, Yorgova P, Neiss G, Rogers K, Sturm PF, Sponseller PD, Luhmann S, Pawelek JB, Shah SA. Early Onset Scoliosis: Is there an Improvement in Quality of Life With Conversion From Traditional Growing Rods to Magnetically Controlled Growing Rods? J Pediatr Orthop. 2019;39:e284-e288. [DOI] [PubMed] [Google Scholar]
- 2.Bess S, Akbarnia BA, Thompson GH, Sponseller PD, Shah SA, El Sebaie H, Boachie-Adjei O, Karlin LI, Canale S, Poe-Kochert C, Skaggs DL. Complications of growing-rod treatment for early-onset scoliosis: analysis of one hundred and forty patients. J Bone Joint Surg Am. 2010;92:2533-2543. [DOI] [PubMed] [Google Scholar]
- 3.Bowen RE, Scaduto AA, Banuelos S. Does early thoracic fusion exacerbate preexisting restrictive lung disease in congenital scoliosis patients? J Pediatr Orthop. 2008;28:506-511. [DOI] [PubMed] [Google Scholar]
- 4.Campbell RM, Jr., Smith MD, Mayes TC, Mangos JA, Willey-Courand DB, Kose N, Pinero RF, Alder ME, Duong HL, Surber JL. The characteristics of thoracic insufficiency syndrome associated with fused ribs and congenital scoliosis. J Bone Joint Surg Am. 2003;85:399-408. [DOI] [PubMed] [Google Scholar]
- 5.Chen Z, Qiu Y, Zhu Z, Li S, Chen X, Xu L, Sun X. Posterior-only Hemivertebra Resection for Congenital Cervicothoracic Scoliosis: Correcting Neck Tilt and Balancing the Shoulders. Spine (Phila Pa 1976). 2018;43:394-401. [DOI] [PubMed] [Google Scholar]
- 6.Cheung JP, Cheung PW, Wong CK, Samartzis D, Luk KD, Lam CL, Cheung KM. Psychometric Validation of the Traditional Chinese Version of the Early Onset Scoliosis-24 Item Questionnaire (EOSQ-24). Spine (Phila Pa 1976). 2016;41:E1460-e1469. [DOI] [PubMed] [Google Scholar]
- 7.Cheung KM, Senkoylu A, Alanay A, Genc Y, Lau S, Luk KD. Reliability and concurrent validity of the adapted Chinese version of Scoliosis Research Society-22 (SRS-22) questionnaire. Spine (Phila Pa 1976). 2007;32:1141-1145. [DOI] [PubMed] [Google Scholar]
- 8.Dede O, Motoyama EK, Yang CI, Mutich RL, Walczak SA, Bowles AJ, Deeney VF. Pulmonary and Radiographic Outcomes of VEPTR (Vertical Expandable Prosthetic Titanium Rib) Treatment in Early-Onset Scoliosis. J Bone Joint Surg Am. 2014;96:1295-1302. [DOI] [PubMed] [Google Scholar]
- 9.Flynn JM, Tomlinson LA, Pawelek J, Thompson GH, McCarthy R, Akbarnia BA. Growing-rod graduates: lessons learned from ninety-nine patients who completed lengthening. J Bone Joint Surg Am. 2013;95:1745-1750. [DOI] [PubMed] [Google Scholar]
- 10.Hasler CC. Early-onset Scoliosis: Contemporary Decision-making and Treatment Options. J Pediatr Orthop. 2018;(38 Suppl 1):S13-s20. [DOI] [PubMed] [Google Scholar]
- 11.Helenius IJ, Oksanen HM, McClung A, Pawelek JB, Yazici M, Sponseller PD, Emans JB, Sanchez Perez-Grueso FJ, Thompson GH, Johnston C, Shah SA, Akbarnia BA. Outcomes of growing rod surgery for severe compared with moderate early-onset scoliosis: a matched comparative study. Bone Joint J. 2018;100:772-779. [DOI] [PubMed] [Google Scholar]
- 12.Johnston CE, Tran DP, McClung A. Functional and Radiographic Outcomes Following Growth-Sparing Management of Early-Onset Scoliosis. J Bone Joint Surg Am. 2017;99:1036-1042. [DOI] [PubMed] [Google Scholar]
- 13.Kabirian N, Akbarnia BA, Pawelek JB, Alam M, Mundis GM, Jr., Acacio R, Thompson GH, Marks DS, Gardner A, Sponseller PD, Skaggs DL. Deep Surgical Site Infection Following 2344 Growing-Rod Procedures for Early-Onset Scoliosis: Risk Factors and Clinical Consequences. J Bone Joint Surg Am. 2014;96:e128. [DOI] [PubMed] [Google Scholar]
- 14.Karol LA. The Natural History of Early-onset Scoliosis. J Pediatr Orthop. 2019;39:S38-s43. [DOI] [PubMed] [Google Scholar]
- 15.Karol LA, Johnston C, Mladenov K, Schochet P, Walters P, Browne RH. Pulmonary function following early thoracic fusion in non-neuromuscular scoliosis. J Bone Joint Surg Am. 2008;90:1272-1281. [DOI] [PubMed] [Google Scholar]
- 16.Li S, Chen ZH, Qiu Y, Xu L, Chen X, Du CZ, Zhu ZZ, Sun X. Coronal Decompensation After Posterior-only Thoracolumbar Hemivertebra Resection and Short Fusion in Young Children With Congenital Scoliosis. Spine (Phila Pa 1976). 2018;43:654-660. [DOI] [PubMed] [Google Scholar]
- 17.McMaster MJ, Ohtsuka K. The natural history of congenital scoliosis. A study of two hundred and fifty-one patients. J Bone Joint Surg Am. 1982;64:1128-1147. [PubMed] [Google Scholar]
- 18.Pawelek JB, Yaszay B, Nguyen S, Newton PO, Mundis GM, Akbarnia BA. Case-Matched Comparison of Spinal Fusion Versus Growing Rods for Progressive Idiopathic Scoliosis in Skeletally Immature Patients. Spine (Phila Pa 1976). 2016;41:234-238. [DOI] [PubMed] [Google Scholar]
- 19.Phillips JH, Knapp DR, Jr., Herrera-Soto J. Mortality and morbidity in early-onset scoliosis surgery. Spine (Phila Pa 1976). 2013;38:324-327. [DOI] [PubMed] [Google Scholar]
- 20.Poe-Kochert C, Shannon C, Pawelek JB, Thompson GH, Hardesty CK, Marks DS, Akbarnia BA, McCarthy RE, Emans JB. Final Fusion After Growing-Rod Treatment for Early Onset Scoliosis: Is It Really Final? J Bone Joint Surg Am. 2016;98:1913-1917. [DOI] [PubMed] [Google Scholar]
- 21.Skov ST, Wijdicks SPJ, Bunger C, Castelein RM, Li H, Kruyt MC. Treatment of early-onset scoliosis with a hybrid of a concave magnetic driver (magnetic controlled growth rod) and a contralateral passive sliding rod construct with apical control: preliminary report on 17 cases. Spine J. 2018;18:122-129. [DOI] [PubMed] [Google Scholar]
- 22.Smith JT, Johnston C, Skaggs D, Flynn J, Vitale M. A New Classification System to Report Complications in Growing Spine Surgery: A Multicenter Consensus Study. J Pediatr Orthop. 2015;35:798-803. [DOI] [PubMed] [Google Scholar]
- 23.Sun X, Xu L, Chen Z, Shi B, Chen X, Li S, Du C, Zhou Q, Qiu Y, Zhu Z. Hybrid Growing Rod Technique of Osteotomy With Short Fusion and Spinal Distraction: An Alternative Solution for Long-Spanned Congenital Scoliosis. Spine (Phila Pa 1976). 2019;44:707-714. [DOI] [PubMed] [Google Scholar]
- 24.Wang S, Lin G, Yang Y, Cai S, Zhuang Q, Tian Y, Zhang J. Outcomes of 360 degrees Osteotomy in the Cervicothoracic Spine (C7-T1) for Congenital Cervicothoracic Kyphoscoliosis in Children. J Bone Joint Surg Am. 2019;101:1357-1365. [DOI] [PubMed] [Google Scholar]
- 25.Xu L, Qiu Y, Chen Z, Shi B, Chen X, Li S, Du C, Zhu Z, Sun X. A re-evaluation of the effects of dual growing rods on apical vertebral rotation in patients with early-onset scoliosis and a minimum of two lengthening procedures: a CT-based study. J Neurosurg Pediatr. 2018;22:306-312. [DOI] [PubMed] [Google Scholar]
- 26.Yao Z, Li H, Zhang X, Li C, Qi X. Incidence and Risk Factors for Instrumentation-related Complications After Scoliosis Surgery in Pediatric Patients With NF-1. Spine (Phila Pa 1976). 2018;43:1719-1724. [DOI] [PubMed] [Google Scholar]