Abstract
Background:
Mucosal melanomas are rare, poorly understood neoplasms without a consensus standard of care.
Objective:
We sought to define mucosal melanoma tumor characteristics and the racial/ethnic attributes of patients with mucosal melanomas.
Methods:
We analyzed 130,920 cutaneous melanomas and 1919 mucosal melanomas recorded in the population-based California Cancer Registry from 1988 to 2013.
Results:
Although only 1% of melanomas occurring in nonHispanic whites were mucosal, other racial/ethnic groups had a higher proportion of mucosal melanomas (15% for Asian/Pacific Islanders, 9% for nonHispanic blacks, and 4% for Hispanics). Anorectal mucosal melanomas were most common in female Asian/Pacific Islanders, whereas genitourinary mucosal melanomas were highest in non-Hispanic whites, and head and neck tumors were most common among Hispanics. Stage at presentation was not uniform among racial/ethnic groups, with Asian/Pacific Islanders having the highest rates of metastasis.
Limitations:
The lack of a standardized staging system for mucosal melanomas confounds classification and knowledge regarding metastasis. Small sample size limits comparative analysis across race, stage, site, and depth.
Conclusion:
Mucosal melanomas differ by race/ethnicity with regard to anatomic site, stage, and depth. Because early detection offers the best chance of increased survival, greater awareness will aid clinicians who care for patients at risk for these aggressive tumors.
Keywords: California, extracutaneous melanoma, melanoma, melanoma detection, mucosal melanoma, population-based database, race/ethnicity, screening
Mucosal melanomas arise from melanocytes located in mucosal membranes lining the respiratory, gastrointestinal, and urogenital tract. They are rare and represent only about 1.4% of all melanomas,1 but they are known to behave more aggressively and have a less favorable prognosis compared with other melanoma subtypes. Although mucosal melanomas share similar histologic characteristics with cutaneous melanomas, they confer a worse prognosis. Few population-based studies (eg, from geographic regions or national databases) have described the tumor characteristics and the characteristics of patients with mucosal melanomas, and even less is known about the incidence of mucosal melanomas among various racial/ethnic groups.
Although population-based studies found that the incidence of mucosal melanomas is higher among nonHispanic white than nonHispanic black individuals,1,2 data on other racial/ethnic groups are more limited. Detailed tumor characteristics for mucosal melanomas were provided by Koomen et al,3 who presented differing anatomic site distributions of mucosal melanomas, but only occurring in Holland’s white population. Previous reports have suggested that nonHispanic black and Hispanic individuals are given a diagnosis of mucosal melanomas more often than cutaneous, ocular, or unknown primary melanomas.4 From 1988 to 2010, the rate of mucosal melanomas showed no significant difference between nonHispanic white, Hispanic, nonHispanic black, and Asian/Pacific Islander groups, but the number of cases was too small to reliably analyze racial differences between specific mucosal locations.2
California has among the highest rates of melanoma in the world,5,6 and has a variety of racial/ethnic groups, all of whom get melanoma. Recently, the Hispanic population became the majority ethnic group in California,7 and Asian/Pacific Islander subpopulations currently comprise 15% of California’s population.8 As a result, the occurrence and characteristics of melanoma, especially mucosal melanomas, in all racial/ethnic groups other than nonHispanic whites is of great interest. The objective of our study was to contribute to the very limited information on a population level regarding the incidence, anatomic site, stage at diagnosis, and thickness of mucosal melanomas in racially diverse populations. We present data on the occurrence of mucosal melanoma in nonHispanic white, nonHispanic black, Hispanic, and Asian/Pacific Islander populations in California from 1988 to 2013, focusing on patient and tumor characteristics, to provide clues for targeted cancer control efforts.
METHODS
Source of data
Data were obtained from the California Cancer Registry (CCR) (www.ccrcal.org). Since 1988, statewide cancer data have been reported in a uniform way. This population-based cancer surveillance system represents a cooperative relationship among hospitals and other cancer diagnostic or treatment facilities, regional registries, and the California Department of Health Services. It comprises 10 regional registries that report cancer incidence data to the Cancer Surveillance Section of the California Department of Health Services.
Cancer incidence data were based on new cases of cancer that were first diagnosed among California residents from January 1, 1988, to December 31, 2012, and were reported to the CCR as of November 2013. Data on histologic type and tumor thickness were abstracted from the patient’s medical records and pathology reports. Coding of histologic type and tumor thickness were completed according to the International Classification of Diseases.
Tumor characteristics
We categorized melanomas into cutaneous, mucosal, and ocular, following the methods of McLaughlin et al.1 Ocular melanomas are not presented in these results, but are used in the calculation of proportions of mucosal melanomas among all melanomas. Only invasive melanomas were considered here, and were classified by their Breslow depth (thickness in millimeters), anatomic site, and histologic type. Thickness was categorized in the same groups typically found in survival analyses and representing the levels most commonly used to describe the changing incidence of melanoma (<1 mm, 1-<2 mm, 2-<4 mm, and ≥4 mm). Morphology was restricted to codes 8720 through 8790 from the International Classification of Diseases for Oncology, Third Edition.
Anatomic site was identified from International Classification of Diseases for Oncology, Second Edition topography codes, in which the skin of the anus and perianal skin are considered to be cutaneous. Cutaneous melanomas (International Classification of Diseases for Oncology, Second Edition code C44.0-C44.9) excluded the skin of the vulva, penis, and scrotum, which are instead included with mucosal melanomas. Melanomas with unknown primary site were considered to be cutaneous.
We adopted the staging system of the Surveillance, Epidemiology and End Results (SEER) program, distinguishing between localized (to the tumor boundary), regional spread (with lymph node or direct extension involvement only), and distant metastatic involvement.
Demographic characteristics
Race/ethnicity.
Race/ethnicity was grouped into the mutually exclusive categories of nonHispanic whites, nonHispanic blacks, Hispanics, and Asian/ Pacific Islanders, according to the race/ethnicity reported in medical records. Persons in any category with a last name on the 1980 US Census list of 12,497 Hispanic surnames were also categorized as Hispanic. Maiden name, when present, was used instead of last name to identify Hispanic women by surname. The use of surname to identify persons of Hispanic ethnicity was adopted by the CCR because of the recognized underreporting of Hispanic ethnicity on the medical record and death certificate. Overall statewide cancer incidence rates for Hispanics, based on this definition, are about 14% higher than those based on medical record and death certificate alone, and rates for non-Hispanic whites are about 1.4% lower. This definition of Hispanic has been widely used in reports of CCR data, including our own previous work on melanoma.9
Denominator data.
The CCR annual population estimates from 1988 to 2012 by age, sex, and race/ethnicity were used for the calculation of rates. They are based on data from the 1990, 2000, and 2010 US population censuses, with linear interpolation for the intercensal years and extrapolation for 1988 to 1989 and 2011 to 2012.
RESULTS
Between 1988 and 2013, a total of 146,845 new diagnoses of melanoma were registered in California. During this time period, 140,263 new diagnoses of cutaneous melanoma, 4639 new diagnoses of ocular melanoma, and 1943 new diagnoses of mucosal melanoma were registered. A total of 137,332 melanomas presented in the four main race/ethnicity groups used in this study (ie, nonHispanic white, Hispanic, nonHispanic black, and Asian/Pacific Islander). Of the 1919 mucosal melanomas classified into the four main race/ethnicity groups, 72.6% were diagnosed in nonHispanic whites (N = 1392), 2.6% were in nonHispanic blacks (N = 49), 15.2% were in Hispanics (N = 292), and 9.7% were in Asian/Pacific Islanders (N = 186). With regard to histology, 76% of mucosal melanomas were not otherwise specified, 7% were nodular, 6.9% were superficial spreading (the majority of which were genitourinary mucosal melanomas), 1.9% were amelanotic, and smaller percentages were other histologic subtypes.
Overall distribution of mucosal melanomas by sex and race/ethnicity
In contrast to cutaneous melanoma, where men were 20% more likely to be affected than women, mucosal melanoma was more common in females than males (Table I), occurring almost twice as frequently in females than males (68.2% vs 31.8%). The pattern of increased occurrence of mucosal melanomas in females was similar across racial/ethnic groups, but was most striking among Asians, where 70% of mucosal melanomas occurred in females, compared with an almost equal distribution by sex in cutaneous melanoma. Although only 1% of melanomas occurring in whites were mucosal melanomas, other racial/ethnic groups had a higher proportion of mucosal melanomas (9% for nonHispanic blacks, 4% for Hispanics, and 15% for Asians).
Table I.
Type of melanoma (cutaneous vs mucosal) by race and sex, California Cancer Registry, 1988 to 2013 (N = 137,332), with cutaneous and mucosal melanoma AAIR per million with 95% confidence interval with Tiwari et al16 (2006) modification
| Cutaneous |
Mucosal |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Males, N (%) | Males AAIR | Females, N (%) | Females AAIR | Total cutaneous melanoma |
Total cutaneous melanoma AAIR |
Males, N (%) | Males AAIR | Females, N (%) | Females AAIR | Total mucosal melanoma | Total AAIR | Mucosal melanomas as a proportion of all melanomas |
|
| NonHispanic white | 73,393 (59.74%) | 33.4 (33.1-33.6) | 49,467 (40.25%) | 20.5 (20.4-20.7) | 122,860 | 26.0 (25.9-26.2) | 438 (31.47%) | 0.2 (0.2-0.2) | 954 (68.53%) | 0.3 (0.3-0.4) | 1392 | 0.3 (0.3-0.3) | 1.1% |
| NonHispanic black | 212 (48.18%) | 1.1 (0.9-1.3) | 228 (51.82%) | 0.9 (0.8-1.1) | 440 | 1.0 (0.9-1.1) | 16 (32.65%) | 0.1 (0.1-0.2) | 33 (67.35%) | 0.1 (0.1-0.2) | 49 | 0.1 (0.1-0.2) | 9.4% |
| Hispanic | 2803 (42.34%) | 4.5 (4.3-4.7) | 3820 (57.68%) | 4.4 (4.2-4.5) | 6623 | 4.3 (4.2-4.5) | 98 (33.56%) | 0.2 (0.1-0.2) | 194 (66.44%) | 0.3 (0.2-0.3) | 292 | 0.2 (0.2-0.3) | 4.0% |
| Asian/Pacific Islander | 493 (49.45%) | 1.3 (1.1-1.4) | 504 (50.55%) | 1.0 (0.9-1.1) | 997 | 1.1 (1.0-1.2) | 55 (29.57%) | 0.1 (0.1-0.2) | 131 (70.43%) | 0.3 (0.2-0.3) | 186 | 0.2 (0.2-0.3) | 14.8% |
| Other | 4971 (53.21%) | 4372 (46.79%) | 9343 | 11 (45.83%) | 13 (54.17%) | 24 | 0.3% | ||||||
| Total | 81,872 | 23.7 (23.5-23.8) | 58,391 | 14.1 (14.0-14.2) | 140,263 | 18.1 (18.0-18.2) | 618 (31.8%) | 0.2 (0.2-0.2) | 1325 (68.2%) | 0.3 (0.3-0.3) | 1943 | 0.3 (0.2-0.3) | 1.3% |
AAIR, Age-adjusted incidence rates.
Distribution of mucosal melanomas by anatomic site
Among the 1919 mucosal melanomas studied, the most common sites were genitourinary (39.1%, N = 751), nasal/sinus (23.8%, N = 456), anorectal (18.2%, N = 350), oral cavity (9.5%, N = 183), and other (9.3%, N = 179). Although anorectal tumors accounted for only 16% of mucosal melanomas among whites, for all other racial/ethnic groups, anorectal cancers accounted for a quarter of all mucosal melanomas (Table II). Tumors occurring in the oral cavity and nasal/sinus were distributed similarly across racial/ethnic groups. NonHispanic whites had the highest proportion of genitourinary mucosal melanomas (42%), whereas Hispanics had only 28% of their mucosal melanomas arising in genitourinary sites.
Table II.
Mucosal melanoma by anatomic site, race/ethnicity, and sex, California Cancer Registry 1988 to 2013 (N = 137,332)
| NonHispanic white male, N (%) |
NonHispanic white female, N (%) |
NonHispanic white total, N (%) |
NonHispanic black male, N (%) |
NonHispanic black female, N (%) |
NonHispanic black total, N (%) |
Hispanic males, N (%) |
Hispanic female, N (%) |
Hispanic total, N (%) |
Asian/Pacific Islander male, N (%) |
Asian/Pacific Islander female, N (%) |
Asian/Pacific Islander total, N (%) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oral cavity | 61 (53.51%) | 53 (46.49%) | 114 (8.2%) | 4 (66.67%) | 2 (33.33%) | 6 (12.2%) | 18 (42.86%) | 24 (57.14%) | 42 (14.4%) | 9 (42.86%) | 12 (57.14%) | 21 (11.3%) |
| Anorectal | 95 (43.38%) | 124 (56.62%) | 219 (15.7%) | 4 (36.36%) | 7 (63.64%) | 11 (22.4%) | 30 (40.54%) | 44 (59.46%) | 74 (25.3%) | 13 (28.26%) | 33 (71.74%) | 46 (24.7%) |
| Nasal/sinus | 155 (46.97%) | 175 (53.03%) | 330 (23.7%) | 8 (72.73%) | 3 (27.27%) | 11 (22.4%) | 31 (41.89%) | 43 (58.11%) | 75 (25.3%) | 16 (39.02%) | 25 (60.98%) | 41 (22.0%) |
| Genitourinary | 37 (6.34%) | 547 (93.66%) | 584 (42.0%) | 0 | 18 (100%) | 18 (36.7%) | 8 (9.64%) | 75 (90.36%) | 83 (28.4%) | 9 (13.64%) | 57 (86.36%) | 66 (35.5%) |
| Other | 90 (62.07%) | 55 (37.93%) | 145 (10.4%) | 0 | 3 (100%) | 3 (6.1%) | 11 (57.89%) | 8 (42.11%) | 19 (6.5%) | 8 (66.67%) | 4 (33.33%) | 12 (6.5%) |
| Total | 438 | 954 | 1392 (100%) | 16 | 33 | 49 (100%) | 98 | 194 | 292 (100%) | 55 | 186 (100%) |
“Other” race/ethnicity is not present in this table.
Among nonHispanic whites and nonHispanic blacks, oral cavity and nasal/sinus tumors were more common among males than females, whereas among Hispanics and Asian/Pacific Islanders, oral cavity tumors were more common among females (Table II). Females had more anorectal tumors than males, especially among Asian/Pacific Islanders, where more than 70% of anorectal tumors occurred in females (Table II).
Distribution of mucosal melanomas by stage at diagnosis
Mucosal melanomas presented at substantially later stages than cutaneous melanomas, with 46% involving regional or remote spread (compared with only 12% among cutaneous melanomas), and 20% involving remote spread, compared with only 4% among cutaneous melanomas (Table III). Although 10% of mucosal melanomas were unstageable, only 5% of cutaneous melanomas were unable to be staged, the most common reason being an inability to identify the primary tumor. Among racial/ethnic groups, stage of presentation of mucosal melanomas was not uniform, with Asian/Pacific Islanders having 55% of their tumors with regional or remote involvement, compared with 49% of Hispanics, 47% of nonHispanic blacks, and only 45% of nonHispanic whites. The distribution of stage by race/ethnicity did not vary by sex.
Table III.
Mucosal melanoma by stage at diagnosis by race/ethnicity, California Cancer Registry, 1988 to 2013 (N = 137,332)
| Cutaneous |
Mucosal |
|||||
|---|---|---|---|---|---|---|
| Stage | Total N (%) |
NonHispanic white N (%) |
NonHispanic black N (%) |
Hispanic N (%) |
Asian/Pacific Islander N (%) |
Total N (%) |
| Unknown | 6473 (4.9) | 137 (9.8) | 9 (18.4) | 28 (9.6) | 21 (11.3) | 195 (10.2) |
| Localized | 108,976 (83.2) | 635 (45.6) | 17 (34.7) | 120 (41.1) | 62 (33.3) | 834 (43.5) |
| Regional | 9692 (7.4) | 361 (25.9) | 13 (26.5) | 89 (30.5) | 53 (28.5) | 516 (26.9) |
| Remote | 5779 (4.4) | 259 (18.6) | 10 (20.4) | 55 (18.8) | 50 (26.9) | 374 (19.5) |
| Total | 130,920 | 1392 | 49 | 292 | 186 | 1919 |
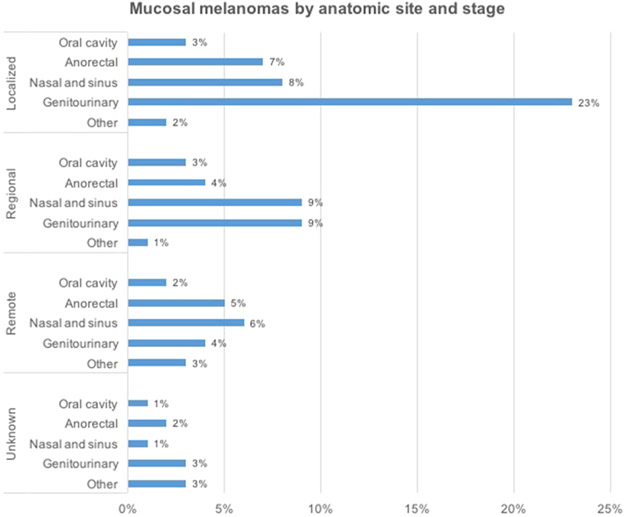
Localized mucosal melanomas were more likely to occur in genitourinary sites than any other (Fig 1), whereas for mucosal melanomas with regional involvement, nasal/sinus and genitourinary tumors were roughly equally likely to occur. Anorectal mucosal melanomas tended to present uniformly across all stages of disease, whereas both nasal/sinus and genitourinary tumors were more likely to present at an earlier stage (Fig 1).
Fig 1.
Distribution of mucosal melanomas by anatomic site and stage at diagnosis, California Cancer Registry, 1988-2003, (N = 137,332).
Distribution of mucosal melanomas by tumor thickness and race/ethnicity
Table IV shows the percentages of mucosal melanomas by tumor thickness and race/ethnicity, with percentages representing the number of melanomas with unknown tumor thickness excluded. About 11% of cutaneous melanoma were of unknown thickness, whereas 36% of mucosal melanomas were of unknown thickness. In contrast to cutaneous melanomas, mucosal melanomas presented as thicker lesions, with between 61% (for nonHispanic whites and Hispanics) and 54% (for nonHispanic blacks and Asian/Pacific Islanders) of tumors greater than 1 mm, whereas in cutaneous lesions, only 40% were greater than 1 mm at diagnosis. The majority of thicker mucosal melanomas (43%) fell within the range of 2 mm to less than 4 mm, a depth that accounted for between 39% and 46% of mucosal melanomas depending on racial/ethnic group. Hispanics had the highest proportion of mucosal melanomas greater than 4-mm thick (14%), more than 3 times the proportion of cutaneous melanomas exceeding 4-mm thick.
Table IV.
Mucosal melanoma by tumor thickness (Breslow depth) at diagnosis, race/ethnicity, California Cancer Registry, 1988 to 2013 (N = 137,332)
| Cutaneous |
Mucosal |
|||||
|---|---|---|---|---|---|---|
| Depth | Total, N (%) | NonHispanic white, N (%) |
NonHispanic black, N (%) |
Hispanic, N (%) |
Asian/Pacific Islander, N (%) |
Total, N (%) |
| <1 mm | 70,665 (60.5) | 350 (38.9) | 12 (46.2) | 70 (38.5) | 49 (39.5) | 481 (39.0) |
| 1-<2 mm | 19,076 (16.3) | 103 (11.4) | 1 (3.9) | 6 (3.3) | 10 (8.1) | 120 (9.7) |
| 2-<4 mm | 19,626 (16.8) | 383 (42.5) | 10 (38.5) | 80 (44.0) | 57 (46.0) | 530 (43.0) |
| ≥4 mm | 7399 (6.3) | 65 (7.2) | 3 (11.5) | 26 (14.3) | 8 (6.5) | 102 (8.3) |
| Total without unknowns | 116,766 | 901 | 26 | 182 | 124 | 1233 |
| Unknown | 14,153 (10.8) | 491 (35.3) | 23 (46.9) | 110 (37.7) | 62 (33.3) | 686 (55.6) |
| Total with unknowns | 130,919 (100) | 1392 | 49 | 292 | 186 | 1919 (100) |
DISCUSSION
This report documents the relative percentages of cutaneous and mucosal melanomas reported to the CCR during the 25-year time period from 1988 to 2013. To our knowledge, this represents one of the largest databases comprising patient characteristics and staging for mucosal melanomas among patients from a variety of races/ethnicities.
In comparison with nonHispanic white populations, a larger proportion of melanomas occurring in Hispanic, Asian/Pacific Islander, and nonHispanic black populations were mucosal rather than cutaneous. The stage of presentation varied with respect to race/ethnicity, and some of the most prominent differences were observed in Asian/Pacific Islanders. This population also had the most advanced stage of mucosal melanoma at diagnosis and had the lowest frequencies of presenting with localized disease. Interestingly, Asian/Pacific Islanders had the smallest proportion of mucosal melanomas presenting at a greater than 4-mm thickness. There was no unusual preponderance of any type of anatomic site in Asian/Pacific Islanders, compared with other racial/ethnic groups. The increased incidence of aggressive mucosal melanomas among Asian/Pacific Islanders begs for further elucidation of the genetic or environmental differences causing this finding. Increased screening efforts may be undertaken in Asian/Pacific Islander patients because they are the most likely to have advanced disease.
As one would expect, mucosal melanomas occurred more frequently in females than in males, across all racial/ethnic groups, because of lesions arising in the female genital tract. Considering that nonHispanic whites had the highest proportions of genitourinary mucosal melanomas, as has been shown in previous population-based studies,1,2 we can stress the importance of maintaining a high degree of suspicion regarding melanocytic lesions on genital mucosa in nonHispanic white patients. A pigmented vulvovaginal melanoma may present as a friable brown-black macule or patch with irregular borders, associated with pruritus or bleeding. The differential diagnosis for a pigmented lesion on the mucosal vulva or vagina includes physiological hyperpigmentation, postinflammatory hyperpigmentation, vulvar melanosis/lentiginosis, melanocytic nevi, pigmented condylomas, and vulvar intraepithelial neoplasia. Amelanotic vulvovaginal melanomas, which were rare in our data set, can present as a skin-colored, pink, reddish nodule on the vulva and should be biopsied to be differentiated from other neoplasms and pyogenic granulomas.
Because Hispanics had the highest proportion of oral cavity lesions, clinicians may consider having a lower threshold for performing biopsies on oral melanocytic lesions. In the oral cavity, mucosal melanoma most commonly presents on the palate or gingiva as an asymptomatic, slow-growing brown or black patch with asymmetric borders, or as a rapidly enlarging nodule associated with ulceration, bleeding, and pain.10 The differential diagnosis would include pigmented nevi, melanotic macules, and oral melanoacanthomas.11 It can be difficult to differentiate clinically between a nevus and an early lesion of mucosal melanoma, so it is recommended that that these lesions be excised and submitted for histopathologic evaluation.
Consistent with previous reports,4 mucosal melanomas in our study presented at a uniformly late stage, with almost 20% involving remote metastasis, compared with less than 5% of cutaneous melanomas. This is likely because mucosal melanomas lack early presenting signs, arise in occult anatomic sites, and may be biologically more aggressive than cutaneous melanomas.12 Stage at diagnosis also varied by anatomic site. Localized mucosal melanomas were more likely to occur in genitourinary sites than any other, whereas for mucosal melanomas with regional involvement, nasal/sinus and genitourinary tumors were roughly equally likely to occur. Anorectal mucosal melanomas tended to present uniformly across all stages of disease, whereas both nasal/sinus and genitourinary tumors were more likely to present at an earlier stage.
Although the majority of individuals given a diagnosis of mucosal melanomas from all racial/ethnic groups (ranging from 61%-54%) had thicker mucosal melanoma tumors than cutaneous melanomas, Hispanics had the highest proportion of mucosal melanomas greater than 4-mm thick, more than twice the proportion of cutaneous melanomas exceeding 4-mm thick. Consistent with previous population-based data regarding mucosal melanoma thickness,12-14 mucosal melanomas uniformly presented as thicker lesions. It is possible that the growth pattern of thick, primary mucosal melanomas causes them to behave in a manner similar to thick, ulcerated nodular cutaneous melanomas, accounting for the majority of patients with mucosal melanomas presenting with regional or remote disease.15
Our analysis has several important limitations. The lack of a standardized staging system for mucosal melanomas may have resulted in potential misclassification of the extent of spread of some mucosal melanomas. Although this is one of the largest studies of mucosal melanoma occurring in minor races/ethnicities, because of small sample size we could not compare race, stage, site, and thickness simultaneously. Because the database used did not have data on the Fitzpatrick skin type or genetic characteristics of the cases or their tumors, it is difficult to determine whether the differences observed by race/ethnicity are a result of genetics or the environment. However, this lack of genetic data is offset by the fact that we used a large, reliable, and up-to-date population-based registry, rather than data from a single clinical institution, in which patient participation could represent a biased and not easily generalizable selection of mucosal melanomas.
Conclusion
We present data on the occurrence of mucosal melanoma in nonHispanic white, nonHispanic black, Hispanic, and Asian/Pacific Islander populations in California from 1988 to 2013. We found that mucosal melanomas differ by race/ethnicity with regard to anatomic site, stage, and depth. Because early detection offers the best chance of increased survival, future efforts should focus on increasing awareness and screening efforts of mucosal melanoma, while simultaneously researching the genetic changes underlying mucosal melanomas in hopes of developing systemic therapies for these aggressive tumors.
CAPSULE SUMMARY.
Population-based data regarding mucosal melanoma in racial/ethnic groups other than nonHispanic whites and blacks are sparse.
The incidence of mucosal melanomas varies by anatomic site and race/ethnicity.
Clinicians should have a low threshold for performing biopsies on suspicious mucosal lesions, to reduce the burden of late mucosal melanoma diagnoses.
Acknowledgments
Dr Cockburn was supported in part by the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement U58DP003862-01 awarded to the California Department of Public Health.
Footnotes
Conflicts of interest: None declared.
REFERENCES
- 1.McLaughlin CC, Wu XC, Jemal A, Martin HJ, Roche LM, Chen VW. Incidence of noncutaneous melanomas in the U.S. Cancer. 2005;103(5):1000–1007. [DOI] [PubMed] [Google Scholar]
- 2.Bishop KD, Olszewski AJ. Epidemiology and survival outcomes of ocular and mucosal melanomas: a population-based analysis. Int J Cancer. 2014;134:2961–2971. [DOI] [PubMed] [Google Scholar]
- 3.Koomen ER, de Vries E, van Kempen LC, et al. Epidemiology of extracutaneous melanoma in The Netherlands. Cancer Epidemiol Biomarkers Prev. 2010;19(6):1453–1459. [DOI] [PubMed] [Google Scholar]
- 4.Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998;83(8):1664–1678. [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. [DOI] [PubMed] [Google Scholar]
- 6.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. [DOI] [PubMed] [Google Scholar]
- 7.State of California, Department of Finance. Report P-1 (Race): State and County Population Projections by Race/Ethnicity, 2010-2060, Sacramento, California, January 2013. [Google Scholar]
- 8.United States Census Bureau. Overview of Race and Hispanic Origin: 2010, Washington, District of Columbia, March 2011. [Google Scholar]
- 9.Cockburn MG, Zadnick J, Deapen D. Developing epidemic of melanoma in the Hispanic population of California. Cancer. 2006;106(5):1162–1168. [DOI] [PubMed] [Google Scholar]
- 10.Hicks MJ, Flaitz CM. Oral mucosal melanoma: epidemiology and pathobiology. Oral Oncol. 2000;36(2):152–169. [DOI] [PubMed] [Google Scholar]
- 11.Mihajlovic M, Vlajkovic S, Jovanovic P, Stefanovic V. Primary mucosal melanomas: a comprehensive review. Int J Clin Exp Pathol. 2012;5(8):739–753. [PMC free article] [PubMed] [Google Scholar]
- 12.Thoelke A, Willrodt S, Hauschild A, Schadendorf D. Primary extracutaneous malignant melanoma: a comprehensive review with emphasis on treatment. Onkologie. 2004;27(5):492–499. [DOI] [PubMed] [Google Scholar]
- 13.Moreno MA, Hanna EY. Management of mucosal melanomas of the head and neck: did we make any progress? Curr Opin Otolaryngol Head Neck Surg. 2010;18(2):101–106. [DOI] [PubMed] [Google Scholar]
- 14.Mücke T, Hölzle F, Kesting MR, et al. Tumor size and depth in primary malignant melanoma in the oral cavity influences survival. J Oral Maxillofac Surg. 2009;67(7):1409–1415. [DOI] [PubMed] [Google Scholar]
- 15.Patrick RJ, Fenske NA, Messina JL. Primary mucosal melanoma. J Am Acad Dermatol. 2007;56(5):828–834. [DOI] [PubMed] [Google Scholar]
- 16.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006;15(6):547–569 [DOI] [PubMed] [Google Scholar]