Abstract
Background:
The phase II J003 (N = 169) and phase III RECOURSE (N = 800) trials demonstrated a significant improvement in survival with trifluridine (FTD)/tipiracil (TPI) versus placebo in patients with refractory metastatic colorectal cancer. This post hoc analysis investigated pharmacokinetic data of FTD/TPI exposure and pharmacodynamic markers, such as chemotherapy-induced neutropenia (CIN) and clinical outcomes.
Patients and methods:
A total of 210 patients from RECOURSE were enrolled in this substudy. A limited sampling approach was used, with three pharmacokinetic samples drawn on day 12 of cycle 1. Patients were categorized as being above or below the median area under the plasma concentration–time curve (AUC) for FTD and TPI. We conducted a post hoc analysis using the entire RECOURSE population to determine the correlations between CIN and clinical outcome. We then carried out a similar analysis on the J003 trial to validate the results.
Results:
In the RECOURSE subset, patients in the high FTD AUC group had a significantly increased CIN risk. Analyses of the entire population demonstrated that FTD/TPI-treated patients with CIN of any grade in cycles 1 and 2 had significantly longer median overall survival (OS) and progression-free survival (PFS) than patients who did not develop CIN and patients in the placebo group. Patients who required an FTD/TPI treatment delay had increased OS and PFS versus those in the placebo group and those who did not develop CIN. Similar results were obtained in the J003 cohort.
Conclusions:
In RECOURSE, patients with higher FTD drug exposure had an increased CIN risk. FTD/TPI-treated patients who developed CIN had improved OS and PFS versus those in the placebo group and those who did not develop CIN. Similar findings were reported in the J003 cohort, thus validating the RECOURSE results. The occurrence of CIN may be a useful predictor of treatment outcomes for FTD/TPI-treated patients.
ClinicalTrials.gov identifier:
NCT01607957 (RECOURSE).
Japan Pharmaceutical Information Center number:
JapicCTI-090880 (J003).
Keywords: chemotherapy-induced neutropenia, FTD/TPI, J003, metastatic colorectal cancer, RECOURSE
INTRODUCTION
Trifluridine (FTD)/tipiracil (TPI) is a novel oral therapy comprising an antineoplastic thymidine-based nucleoside analog, FTD, and a thymidine phosphorylase inhibitor, TPI. TPI improves the bioavailability of FTD by inhibiting its catabolism by thymidine phosphorylase, resulting in a 37-fold increase in FTD area under the curve (AUC).1,2
Two trials, J003 (JapicCTI-090880) and RECOURSE (NCT01607957), demonstrated that FTD/TPI improved survival in refractory metastatic colorectal cancer (mCRC). The J003 trial, a phase II, randomized, placebo-controlled study in 169 refractory mCRC patients from Japan, demonstrated a 3.4-month overall survival (OS) benefit (9.0 versus 6.6 months) for FTD/TPI-treated patients compared with those receiving placebo.3 Similarly, the phase III, placebo-controlled RECOURSE trial of 800 patients with refractory mCRC demonstrated a significant improvement in median OS [7.1 versus 5.3 months; hazard ratio (HR), 0.68; P < 0.001] and progression-free survival (PFS) (2.0 versus 1.7 months; HR, 0.48; P < 0.001).4 The most frequently reported adverse event was chemotherapy-induced neutropenia (CIN), with 67% of patients experiencing at least grade 1 CIN.4 Interestingly, a number of groups have recently reported that the onset of CIN is an indication of better treatment outcomes in mCRC patients treated with FTD/TPI,5–7 with the development of higher-grade CIN (grade ≥3) at any time of FTD/TPI treatment being associated with longer PFS and OS.8
To investigate the relationship between FTD/TPI exposure, efficacy, and safety, we carried out a pharmacokinetic (PK) and pharmacodynamic (PD) substudy of the RECOURSE trial. We then conducted a post hoc analysis using data from the entire RECOURSE trial to further characterize the relationship between CIN and the clinical efficacy of FTD/TPI. Following this, we carried out a similar analysis on the J003 trial to validate these results.
METHODS
Study designs
The study designs and FTD/TPI dosing in RECOURSE and J003 were similar and have been described previously.3,4 Patients received placebo or FTD/TPI 35 mg/m2 orally twice daily on days 1–5 and 8–12 of every 28-day cycle.
Assessments
CIN grades were classified according to National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.9 For patients who enrolled in the optional RECOURSE PK/PD substudy, blood samples were collected at steady state on day 12 of cycle 1 at 1, 3, and 6 hours after the morning dose of FTD/TPI. Daily AUC values were estimated using a non-linear mixed-effect modeling program (NONMEM® version 7.2.0; ICON plc, Dublin, Ireland) for both FTD and TPI. All data summaries and listings were produced using SAS version 9.1.3 (SAS Institute Inc., Carey, NC). Patients were divided into high versus low AUC groups for both components based on the median daily AUC values of FTD and TPI.
Statistical analysis
Analyses of OS and PFS according to PK parameters and onset of CIN were carried out using a non-stratified Cox regression to estimate HRs. Survival distribution was estimated using the Kaplan–Meier method.
RESULTS
RECOURSE patient characteristics
Overall PK/PD population.
Of the 800 patients enrolled in RECOURSE, 210 participated in this substudy (FTD/TPI n = 138; placebo n = 72). Patient demographics and baseline characteristics were mostly comparable (supplementary Table S1, available at Annals of Oncology online) and generally representative of the overall study.
AUC subgroups.
The median (range) FTD AUC was 43.51 (15.2–84.6) μg·h/ml and the median (range) TPI AUC was 0.65 (0.2–2.9) μg·h/ml.The mean age (standard deviation) in the high FTD AUC group was 62.1 (±10.8) versus 60.6 (±9.9) years in the low FTD AUC group (supplementary Table S1, available at Annals of Oncology online). Approximately twice as many patients in the high FTD and TPI AUC groups had mild (creatinine clearance 60–89 ml/min) and moderate (creatinine clearance 30–59 ml/min) renal impairment at baseline (high FTD: 39.1% mild, 17.4% moderate; high TPI: 37.7% mild, 18.8% moderate) versus the low FTD and TPI AUC groups (low FTD: 15.9% mild, 5.8% moderate; low TPI: 17.4% mild, 4.3% moderate). These differences were statistically significant (P < 0.0001), and this association is currently being investigated in an ongoing phase I study (NCT02301117).
FTD/TPI efficacy in the PK/PD population
OS and PFS.
In the RECOURSE PK/PD subset, patients treated with FTD/TPI also had improved median OS and PFS compared with patients treated with placebo [HR, 0.58; 95% confidence interval (CI), 0.42–0.80 and HR, 0.34; 95% CI, 0.24–0.49, respectively] (Table 1; supplementary Table S2, available at Annals of Oncology online).
Table 1.
Median OS, PFS, and time to ECOG PS ≥2 in the PK/PD population of RECOURSE
| FTD |
TPI |
Overall PK/PD population |
||||||
|---|---|---|---|---|---|---|---|---|
| High FTD AUC (n = 69) |
Low FTD AUC (n = 69) |
Placebo (n = 72) |
High TPI AUC (n = 69) |
Low TPI AUC (n = 69) |
Placebo (n = 72) |
FTD/TPI (n = 138) |
Placebo (n = 72) |
|
| Median OS, months (95% CI) | 9.2 (7.6–10.7) | 7.2 (5.0–9.7) | 5.6 (4.0–7.3) | 7.8 (6.1–10.7) | 9.2 (7.2–10.2) | 5.6 (4.0–7.3) | 8.9 (7.2–9.9) | 5.6 (4.0–7.3) |
| Median PFS, months (95% CI) | 3.7 (2.1–3.9) | 2.0 (1.9–3.9) | 1.8 (1.6–1.8) | 2.0 (1.9–3.7) | 3.7 (2.1–4.3) | 1.8 (1.6–1.8) | 3.3 (1.9–3.8) | 1.8 (1.6–1.8) |
| Median time to ECOG PS ≥2, months (95% CI) | 7.8 (6.7–9.9) | 5.6 (4.4–9.5) | 6.3 (5.0–8.8) | 7.8 (6.1–9.6) | ||||
AUC, area under the curve; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; FTD, trifluridine; OS, overall survival; PD, pharmacodynamic; PFS, progression-free survival; PK, pharmacokinetic; TPI, tipiracil.
Median OS tended to be longer in the high versus low FTD AUC groups [9.2 versus 7.2 months, respectively (HR, 0.72; 95% CI, 0.46–1.11)], but did not reach statistical significance (Figure 1A; Table 1; supplementary Table S2, available at Annals of Oncology online).There was no significant difference in median PFS in the high versus low FTD AUC groups (HR, 0.82; 95% CI, 0.57–1.18) (Figure 1C; Table 1; supplementary Table S2, available at Annals of Oncology online).
Figure 1.
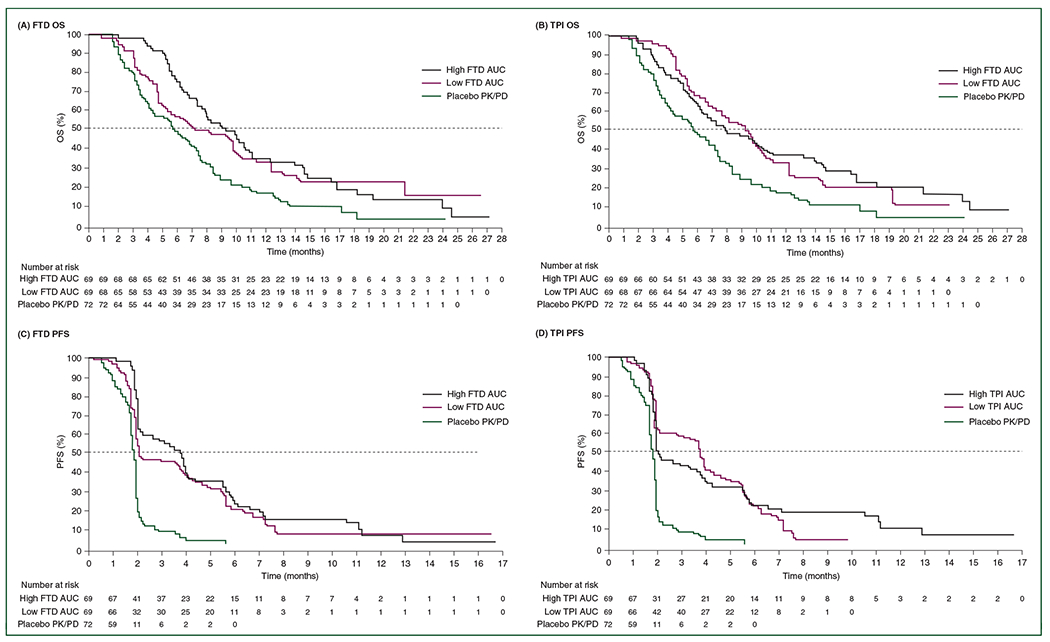
RECOURSE: Kaplan–Meier estimates of overall survival (OS) and progression-free survival (PFS) in the pharmacokinetic/pharmacodynamic (PK/PD) population according to high and low area under the curve (AUC) of trifluridine (FTD) (OS: A; PFS: C) or tipiracil (TPI) (OS: B; PFS: D) or placebo treatment.
Other efficacy measures.
The high FTD AUC group demonstrated a significantly longer time to progression to Eastern Cooperative Oncology Group performance status (ECOG PS) ≥2 than the low AUC group (HR, 0.64; 95% CI, 0.44–0.93) (supplementary Table S2, available at Annals of Oncology online). There was a statistically significant trend (P < 0.05) towards longer duration of treatment with FTD/TPI in the high versus low FTD AUC groups, with a median total duration of 13.9 versus 6.1 weeks, respectively.
FTD/TPI safety in the AUC subgroups.
As expected, the safety profile for FTD/TPI-treated patients within the PK/PD population was similar to that for the overall RECOURSE population (supplementary Table S3, available at Annals of Oncology online).
There was a greater risk of any-grade and grade ≥3 CIN in the high versus low FTD AUC groups (supplementary Table S3, available at Annals of Oncology online). Patients with higher FTD AUC were also more likely to experience grade ≥1 CIN in cycles 1 and 2 (supplementary Tables S4 and S5, available at Annals of Oncology online). Any-grade adverse events of interest according to maximum CIN grade in cycles 1 and 2 are listed in supplementary Table S6, available at Annals of Oncology online. In the PK/PD cohort, FTD AUC and maximum plasma concentration tended to be numerically higher among those who developed any-grade CIN versus those who did not (supplementary Table S7, available at Annals of Oncology online), but was not statistically significant.
Analysis of CIN and dose delays in RECOURSE
Neutropenia.
In the entire RECOURSE study population, of the patients treated with FTD/TPI, 353 (66%) experienced any-grade CIN and 175 (33%) did not experience CIN (supplementary Table S8, available at Annals of Oncology online). The first onset of CIN was generally observed during the first two cycles of study drug treatment (supplementary Table S8, available at Annals of Oncology online). Median OS, PFS, and time to ECOG PS ≥2 of FTD/TPI-treated patients with CIN of any grade in cycles 1 and 2 (n = 329) were significantly longer than of those without CIN (n = 205) [OS: 9.3 versus 4.4 months (HR, 0.40; P < 0.0001); PFS: 3.5 versus 1.8 months (HR, 0.50; P < 0.0001); time to ECOG PS ≥2: 7.4 versus 3.3 months (HR, 0.39; P < 0.0001)] (Table 2). This was particularly true for those with grade ≥3 CIN [OS: 9.8 versus 4.4 months (HR, 0.38; P < 0.0001); PFS: 3.7 versus 1.8 months (HR, 0.45; P < 0.0001); time to ECOG PS ≥2: 7.2 versus 3.3 months (HR, 0.39; P < 0.0001)] (Table 2). A multivariate analysis carried out was also consistent with these results (supplementary Table S9, available at Annals of Oncology online). Those treated with FTD/TPI who experienced first CIN of any grade in cycle ≥2 also had statistically significantly improved median OS versus placebo (9.1 versus 6.3 months; P < 0.05), PFS versus placebo (3.5 versus 1.8 months; P < 0.05), and time to ECOG PS ≥2 versus placebo (8.1 versus 5.5 months; P < 0.05) (supplementary Table S10, available at Annals of Oncology online). Patients who developed grade ≥3 CIN during cycle 1 had a nearly twofold increase in median OS compared with patients receiving placebo (10.1 versus 5.3 months; P < 0.05) (supplementary Table S11, available at Annals of Oncology online).
Table 2.
Clinical end points in FTD/TPI-treated patients according to CIN grade during cycles 1 and 2 in RECOURSE
| Treatment end pointsa | CIN grade |
Comparison | Univariate comparison, HR (95% CI)* |
|||||
|---|---|---|---|---|---|---|---|---|
| No CIN (n = 205) |
Grade ≥1 (n = 329) |
Grade 1–2 (n = 168) |
Grade ≥3 (n = 161) |
Grade ≥1 | Grade 1–2 | Grade ≥3 | ||
| Median OS, months | 4.4 | 9.3 | 9.1 | 9.8 | Versus placebo Versus no CIN |
0.51 (0.43–0.61) 0.40 (0.33–0.48) |
0.57 (0.46–0.70) 0.45 (0.36–0.57) |
0.48 (0.39–0.60) 0.38 (0.30–0.48) |
| Median PFS, months | 1.8 | 3.5 | 2.1 | 3.7 | Versus placebo Versus no CIN |
0.36 (0.30–0.43) 0.50 (0.41–0.61) |
0.43 (0.34–0.53) 0.58 (0.46–0.73) |
0.32 (0.25–0.40) 0.45 (0.35–0.57) |
| Median time to ECOG PS ≥2, months | 3.3 | 7.4 | 7.5 | 7.2 | Versus placebo Versus no CIN |
0.47 (0.39–0.57) 0.39 (0.31–0.48) |
0.47 (0.37–0.59) 0.40 (0.31–0.52) |
0.47 (0.37–0.61) 0.39 (0.30–0.51) |
CI, confidence interval; CIN, chemotherapy-induced neutropenia; ECOG PS, Eastern Cooperative Oncology Group performance status; FTD, trifluridine; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; TPI, tipiracil.
Kaplan–Meier estimates.
All comparisons are significant (P < 0.0001). Stratified log-rank test (stratification factors: KRAS status, time since diagnosis of first metastasis, region).
Onset of grade ≥3 CIN, regardless of timing, indicated significant improvements in OS versus patients receiving placebo and those who did not develop CIN (Figure 2; supplementary Figures S1, S2, S3, and S4; supplementary Table S11, available at Annals of Oncology online).
Figure 2.
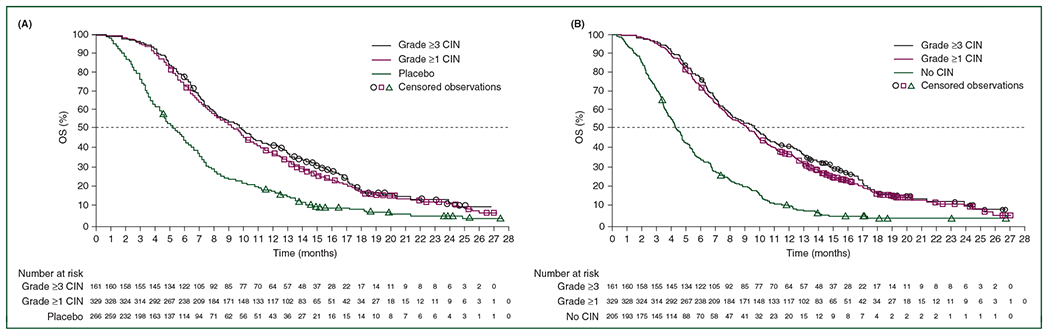
RECOURSE: Kaplan–Meier estimates of overall survival (OS) in cycles 1 and 2 according to chemotherapy-induced neutropenia (CIN) grade versus placebo (A) and no CIN (B).
Treatment delays.
Patients who required an FTD/TPI treatment delay had a statistically significant increase in OS and PFS compared with those receiving placebo and those who did not have an FTD/TPI treatment delay (both comparisons P < 0.05).
Compared with FTD/TPI-treated patients with no treatment delay, the OS HR was 0.18 for a delay of ≥8 days and 0.31 for a delay of 4–7 days (P < 0.05) (Table 3). These findings suggest an association between longer dose delays due to CIN and longer improved outcomes.
Table 3.
Survival and incidence of any-grade CIN in FTD/TPI-treated patients who experienced treatment delays in RECOURSEa
| n (%)b | Median OS, months | OS HR versus placebo (95% CI) | OS HR versus no delay (95% CI) | Median PFS, months | PFS HR versus placebo (95% CI) | PFS HR versus no delay (95% CI) | Patients with CIN, n (%)c | CIN RRd (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|
| ≥8 days (n = 533) | 108 (20.3) | 14.4 | 0.31 (0.23–0.40)* | 0.18 (0.13–0.25)* | 5.8 | 0.16 (0.12–0.22)* | 0.16 (0.12–0.22)* | 96 (88.9) | 1.88 (1.64–2.16)* |
| ≥4 and <8 days (n = 533) | 137 (25.7) | 9.7 | 0.51 (0.41–0.64)* | 0.31 (0.23–0.40)* | 3.7 | 0.33 (0.26–0.41)* | 0.33 (0.26–0.42)* | 121 (88.3) | 1.87 (1.63–2.14)* |
| None (n = 533) | 288 (54.0) | 4.9 | 1.21 (1.01–1.44)** | — | 1.8 | 0.94 (0.78–1.12) | — | 136 (47.2) | — |
| Placebo (n = 265)e | 265 (100) | 5.3 | — | — | 1.7 | — | — | 2 (0.8) | — |
CI, confidence interval; CIN, chemotherapy-induced neutropenia; FTD, trifluridine; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; RR, relative risk; TPI, tipiracil.
Across all cycles, 289 patients (54.2%) in the FTD/TPI group had adverse events that resulted in interruptions in dosing, dose delays, and/or dose reductions compared with 36 patients (13.6%) in the placebo group.
Percentage of as-treated population in the specific treatment group.
Treatment delays may or may not be related to a specific neutropenic event.
Relative risk versus no delay.
Includes 14 placebo patients (5.3%) who experienced some cycle delay of ≥4 days.
Indicates significant (P < 0.05) improvement in survival for the extent of the dose delay group versus the placebo group.
Indicates significant (P < 0.05) improvement in survival for the placebo group versus the extent of the dose delay group.
Validation of correlation between CIN and survival in J003
For the final survival and CIN analysis of J003, 112 patients were included.3 The median follow-up was 57.5 months, with 167 OS events (98.8% of the total) compared with 123 in the primary analysis.3 Median OS remained unchanged from the primary analysis (FTD/TPI: 9.0 months; placebo: 6.6 months) (supplementary Figure S5, available at Annals of Oncology online).
Similar to RECOURSE, the first onset of CIN in J003 was generally observed during the first two treatment cycles (supplementary Table S8, available at Annals of Oncology online). FTD/TPI-treated patients with CIN of any grade in cycles 1 and 2 had significantly longer OS, PFS, and time to ECOG PS ≥2 than those receiving placebo and those who did not develop CIN (Figure 3; supplementary Table S12; supplementary Figure S6, available at Annals of Oncology online). This was particularly true for those with grade ≥3CIN.
Figure 3.
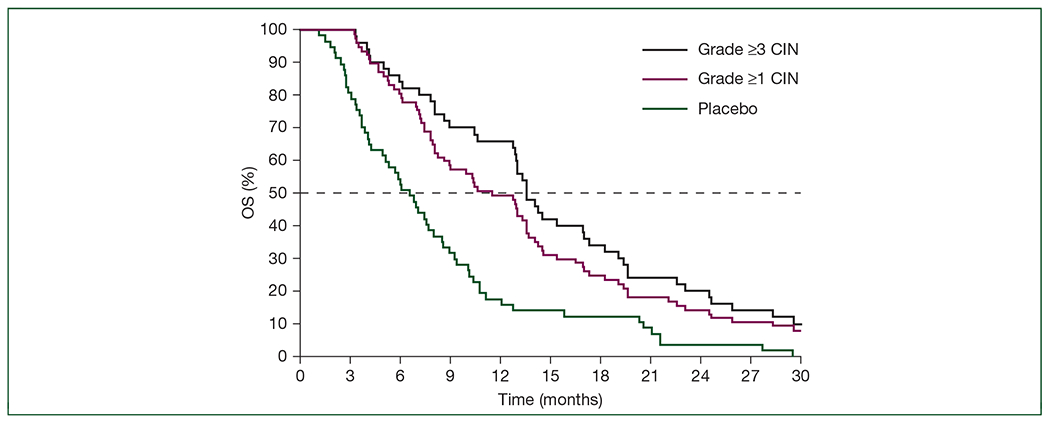
J003: Kaplan–Meier estimates of overall survival (OS) in cycles 1 and 2 according to chemotherapy-induced neutropenia (CIN) grade.
DISCUSSION
In RECOURSE, patients who were treated with FTD/TPI and demonstrated higher FTD exposure, as determined by AUC, showed an increased risk of CIN. CIN appears to be primarily a surrogate of effective dosing, but is also associated with improved outcomes over those with lower FTD exposure. Although improvement in OS fell short of statistical significance in the high versus low FTD AUC groups, the time to ECOG PS ≥2 was significantly longer. Taken together, this suggests that relatively high FTD levels in plasma may be associated with better clinical outcomes.
Analysis of RECOURSE PK/PD suggests a dose–response relationship between FTD exposure and CIN, in agreement with dose-escalation studies finding that a higher rate of CIN at higher doses of FTD/TPI leads to greater efficacy of the drug.10,11 Notably, no such dose–response correlations were observed between FTD exposure and other frequently reported adverse events associated with FTD/TPI, including thrombocytopenia, anemia, and diarrhea. The same association is not thought to be found with TPI alone. TPI is a PK modulator which inhibits thymidine phosphorylase to enhance the systemic exposure to FTD by preventing both gastrointestinal and hepatic metabolism of FTD. The total dose of orally administered TPI will contribute to the inhibition of gastrointestinal metabolism of FTD, whereas only the absorbed fraction of TPI can inhibit hepatic metabolism. This has led to the observation that higher exposure to TPI was not associated with longer OS due to the poor absorption of TPI into the systemic circulation.
The onset of any-grade CIN during cycles 1 and 2 represented an independent predictive marker of significantly longer OS, PFS, and time to ECOG PS ≥2. Grade ≥3 CIN was most strongly correlated with the observed improvement in treatment outcomes for all end points regardless of timing of onset. Longer treatment delays were associated with longer OS and PFS compared with patients receiving placebo and those who did not experience treatment delays, which could suggest that dose density is less important than dose exposure itself.
Further, the post hoc analysis of J003 demonstrated that CIN was associated with better OS, PFS, and time to ECOG PS worsening in FTD/TPI-treated patients compared with those receiving placebo and those who did not develop CIN. Thus, these analyses of J003 validated the results from RECOURSE, indicating that CIN during the early stages of treatment may act as a surrogate marker for FTD/TPI efficacy in patients with mCRC.
Although severe decreases in neutrophil count pose a risk to patients, CIN can be managed and the rate of febrile neutropenia in RECOURSE was relatively low (4%).4 The observations in this trial suggest that the possibility of maintaining higher doses of FTD/TPI could be beneficial to patients. While the RECOURSE and J003 trials were not designed to evaluate this question, it is reasonable that clinicians try to avoid unnecessary FTD/TPI dose reductions. Instead of dose reduction, potential strategies that can maintain the dosages of FTD/TPI include treatment delays (without impact on survival, as demonstrated by our data) and, if CIN does not improve, the appropriate use of granulocyte colony-stimulating factor in a reactive manner.
While this paper supports previous findings of a similar nature,5,12,13 the PK/PD analysis and comparison with placebo are new data. Overall, the PK/PD analysis was limited by the small number of patients in the AUC subgroups; sample sizes may have been too small to detect meaningful differences and may well contribute to a false negative due to inadequate power. Additionally, these are not cause-and-effect analyses and are subject to all potential biases of retrospective patient selection not based on baseline characteristics and not consistent with the randomization algorithm. This is a hypothesis-generating study and future analysis should be carried out to expand on these findings.
Conclusions
This PK/PD analysis of RECOURSE suggests that mCRC patients who experience proper dosing of FTD/TPI and therefore achieve higher plasma levels of FTD may experience improved efficacy outcomes. FTD/TPI-treated patients who developed CIN have a survival advantage over patients receiving placebo and those who did not develop CIN during RECOURSE and J003. CIN, irrespective of the timing of onset, is associated with higher FTD AUC and appears to correlate with improved outcomes when compared with lower FTD AUC, with the most pronounced effect seen in those with grade ≥3 CIN. The presence or absence of CIN may be a surrogate marker of the clinical response to FTD/TPI.
Supplementary Material
ACKNOWLEDGEMENTS
The authors were responsible for the content and editorial decisions related to the development of this manuscript and received no honoraria or compensation associated with it. Writing assistance in the preparation of this manuscript was provided by Phase Five Communications and Complete HealthVizion, supported by Taiho Oncology, Inc. and Taiho Pharmaceutical Co., Ltd.
FUNDING
This study was supported by Taiho Pharmaceutical Co., Ltd., Tokyo, Japan and Taiho Oncology, Inc., Princeton, NJ, USA. No grant numbers are applicable.
DISCLOSURE
TY has received research funding from Chugai Pharmaceutical Co., Ltd., Sanofi K.K., Sumitomo Dainippon Pharma Co., Ltd., and MSD. JMC has received research funding from Merck and Tesaro, has had a consulting or advisory role with Bristol-Myers Squibb, and has received travel funding from Bristol-Myers Squibb, Agios, and Roche. EVC has had a consulting or advisory role with AstraZeneca, Bayer, Bristol-Myers Squibb, Celgene, Lilly, Roche, Servier, MSD, Merck KGaA, and Novartis. EVC’s institution has received research funding from Amgen, Bayer, Boehringer Ingelheim, Lilly, Novartis, Roche, Celgene, Ipsen, Merck, Merck KGaA, Servier, and Bristol-Myers Squibb. AO has been employed by Celgene. AO has also received honoraria from Ono Pharmaceutical Co., Ltd., Bristol-Myers Squibb, and Chugai Pharmaceutical Co., Ltd., and research funding from Bristol-Myers Squibb. ES has received honoraria from Taiho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Merck Serono Co., Ltd., Yakult, Eli Lilly, and Sanofi. AF has received honoraria from Amgen, Bayer, Merck, Roche, Servier, and Eli Lilly. AF has also had a consulting or advisory role with Amgen, Bayer, Merck, Bristol-Myers Squibb, Roche, Sanofi, Servier, and Eli Lilly. AF’s institution has received research funding from Amgen, Bayer, Roche, Servier, and MSD. KY has received honoraria from Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Yakult Honsha, Takeda, Bayer, Merck Serono, Bristol-Myers Squibb, Taiho Pharmaceutical Co., Ltd., Eli Lilly, Sanofi, and Ono Pharmaceutical Co., Ltd. KY has also received research funding from Taiho Pharmaceutical Co., Ltd. TN has received honoraria from Taiho Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Yakult, Bayer, Eli Lilly, Takeda Pharmaceutical Co., Ltd., and Merck Serono. RG-C and an immediate family member have received honoraria from Roche, Merck, Sanofi, Bayer, Novartis, Ipsen, AAA, Pfizer, Bristol-Myers Squibb, MSH, Boehringer Ingelheim, Eli Lilly, PharmaMar, and Servier. RG-C has also received research funding from Pfizer and fees for travel, accommodation, and expenses from Roche, Merck, Novartis, Ipsen, and Pfizer. YK has received honoraria from Taiho Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Sanofi, Eli Lilly, Yakult, Nipro, Merck, Daiichi-Sankyo Co., Ltd., Takeda, and Bayer. YK has also received research funding from Taiho Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Sanofi, Eli Lilly, Yakult, Daiichi-Sankyo Co., Ltd., Takeda, Bayer, and Shinogi. HB has received honoraria from Taiho Pharmaceutical Co., Ltd., Eli Lilly, and Ono Pharmaceutical Co., Ltd. HB has also received research funding from Taiho Pharmaceutical Co., Ltd., Merck Serono Co., Ltd., Toyama Chemical Co., Ltd., Novartis, Yakult Honsha Co., Ltd., and Johnson & Johnson K.K. GA has had a consulting or advisory role with Bristol-Myers Squibb, Roche, Amgen, Sanofi-Aventis, and Servier. GA has also received fees for travel, accommodation, and expenses from Roche, Servier, and Amgen. AT has received honoraria from Taiho Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Merck Serono, Takeda Pharmaceutical Co., Ltd., and Bristol-Myers Squibb. AT has also participated in speaker bureaus for Taiho Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., and Merck Serono. AS has received honoraria from, has had a consulting or advisory role with, and has participated in speaker bureaus for, Servier. KY has had a consulting or advisory role with Daiichi Sankyo Co., Ltd. and Bristol-Myers Squibb. KY has also participated in speaker bureaus for Taiho Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Merck Serono, Bayer, Yakult Honsha, Eli Lilly, Ono Pharmaceutical Co., Ltd., and Sanofi, and received research funding from Ono Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Daiichi Sankyo Co., Ltd., Eli Lilly, Gilead, Yakult Honsha, and MSD. MP has received research funding and honoraria from Taiho Pharmaceutical Co., Ltd. and Servier. KM has received honoraria from Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Yakult, Eli Lilly, and Merck Serono. AZ has participated in speaker bureaus for Amgen, Servier, Bayer, and Sanofi. YS has received honoraria from Taiho Pharmaceutical Co., Ltd., Eli Lilly Japan, Chugai Pharmaceutical Co., Ltd., and Takeda. YS has also received research funding from Taiho Pharmaceutical Co., Ltd., Eli Lilly Japan, Merck Serono, MSD Oncology, and Yakult Honsha. YT has received honoraria from Merck Serono, Eli Lilly Japan, Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Kyowa Kirin, Yakult Honsha, Eisai Co., Ltd., and Medicon. HSH has had a consulting or advisory role with Bayer, Genentech, Amgen, and Merck. TM has participated in speaker bureaus for Taiho Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Merck Serono, Yakult Honsha, Takeda Pharmaceutical Co., Ltd., Novel Pharma, Sanofi-Aventis, and Eli Lilly Japan. TM has also received research funding from Takeda Pharmaceutical Co., Ltd., Yakult Honsha, and MSD. BT has received research funding from Amgen, Astellas, AstraZeneca, Bayer, Janssen-Cilag, Pfizer, Bristol-Myers Squibb, Ipsen, and Servier. BT has also received honoraria from Amgen, Astellas, Bayer, Bristol-Myers Squibb, Janssen-Cilag, MSD, Novartis, Sanofi, Tolmar, and Ipsen. BT's institution has received research funding from Amgen, Novartis, Aslan, GSK, Janssen-Cilag, MSD, Taiho, Akeso, MedImmune, AstraZeneca, Aptevo, and Servier. TE has received honoraria from Chugai Pharmaceutical Co., Ltd., Eli Lilly, Taiho Pharmaceutical Co., Ltd., Merck Serono, Ono Pharmaceutical Co., Ltd., Nihon Kayaku, and Eisai Co., Ltd. TE has also received research funding from Eli Lilly, Taiho Pharmaceutical Co., Ltd., Novartis, Daiichi Sankyo Co., Ltd., DS Pharma Biomedical Co., Ltd., AstraZeneca, Merck Serono, Ono Pharmaceutical Co., Ltd., Boehringer Ingelheim, and MSD. CH has had a consulting or advisory role with Taiho Pharmaceutical Co., Ltd. TT holds stocks or other ownership interest with Otsuka Holdings. FB is an employee of Taiho Oncology, Inc. LM is an employee of Stathmi, Inc. and has had a consulting or advisory role with Taiho Pharmaceutical Co., Ltd. FY is an employee of Taiho Oncology, Inc. FY has also received fees for travel, accommodation, and expenses from Taiho Oncology, Inc. All remaining authors have declared no conflicts of interest.
REFERENCES
- 1.Emura T, Suzuki N, Fujioka A, et al. Potentiation of the antitumor activity of α, α, α-trifluorothymidine by the co-administration of an inhibitor of thymidine phosphorylase at a suitable molar ratio in vivo. Int J Oncol. 2005;27:449–455. [PubMed] [Google Scholar]
- 2.Cleary JM, Rosen LS, Yoshida K, et al. A phase 1 study of the pharmacokinetics of nucleoside analog trifluridine and thymidine phosphorylase inhibitor tipiracil (components of TAS-102) vs trifluridine alone. Invest New Drugs. 2017;35:189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshino T, Mizunuma N, Yamazaki K, et al. TAS-102 monotherapy for pretreated metastatic colorectal cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2012;13:993–1001. [DOI] [PubMed] [Google Scholar]
- 4.Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909–1919. [DOI] [PubMed] [Google Scholar]
- 5.Hamauchi S, Yamazaki K, Masuishi T, et al. Neutropenia as a predictive factor in metastatic colorectal cancer treated with TAS-102. Clin Colorectal Cancer. 2017;16:51–57. [DOI] [PubMed] [Google Scholar]
- 6.Kasi PM, Kotani D, Cecchini M, et al. Chemotherapy induced neutropenia at 1-month mark is a predictor of overall survival in patients receiving TAS-102 for refractory metastatic colorectal cancer: a cohort study. BMC Cancer. 2016;16:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishina T, Yoshino T, Shinozaki E, et al. Onset of neutropenia as an indicator of treatment response in the randomized phase II of TAS-102 vs placebo in Japanese patients with metastatic colorectal cancer (Study J003-10040030). J Clin Oncol. 2016;34(15 Suppl):Abstract no. 3557. [Google Scholar]
- 8.Cremolini C, Rossini D, Martinelli E, et al. Trifluridine/tipiracil (TAS-102) in refractory metastatic colorectal cancer: a multicenter register in the frame of the Italian compassionate use program. Oncologist. 2018;23:1178–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Cancer Institute. Common Terminology Criteria for Adverse Events v4.03 (CTCAE), 2010. Available at https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/. Accessed November 1, 2018.
- 10.Doi T, Ohtsu A, Yoshino T, et al. Phase I study of TAS-102 treatment in Japanese patients with advanced solid tumours. Br J Cancer. 2012;107:429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Overman MJ, Kopetz S, Varadhachary G, et al. Phase I clinical study of three times a day oral administration of TAS-102 in patients with solid tumors. Cancer Invest. 2008;26:794–799. [DOI] [PubMed] [Google Scholar]
- 12.Tan X, Wen Q, Wang R, Chen Z. Chemotherapy-induced neutropenia and the prognosis of colorectal cancer: a meta-analysis of cohort studies. Expert Rev Anticancer Ther. 2017;17:1077–1085. [DOI] [PubMed] [Google Scholar]
- 13.Kimura M, Usami E, Iwai M, et al. Severe neutropenia: a prognosticator in patients with advanced/recurrent colorectal cancer under oral trifluridine-tipiracil (TAS-102) chemotherapy. Pharmazie. 2017;72:49–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


