The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has emerged in China at the end of 2019 and has rapidly spread to Asia, Oceania, Europe, and America causing the coronavirus disease-19 (COVID-19) pandemic [1] and more than 700,000 deaths worldwide as of August 6, 2020. Epidemiology analyses have been showing higher mortality due to COVID-19 in Europe than in China [2]. In Table 1, we report the number of fatalities due to COVID-19 both in relation to the number of total cases (i.e., mortality) and the entire population of the indicated country (i.e., death/1 million population) as of August 5, 2020. The two parameters are differently influenced by several variables (e.g., the number and type of tests each country has used to confirm the clinical diagnosis, the access to hospitalization, or the parameters used to ascribe death to COVID-19). Considering that the mortality parameter strictly depends on the number of test performed in each country, and that testing has neither been homogeneously performed in the different countries [3] nor the entire populations of these countries have been screened, we focused on the death/1 million population. As reported in Table 1, COVID-19-related deaths are much less in China than in Europe. Furthermore, deaths related to COVID-19 are not equally distributed in Europe. Northern European countries, for example, Denmark, Germany, and Norway, have experienced rates of COVID-19-related deaths closer to China than Southern European countries like Italy, Spain, or France. There are several exceptions to this apparent rule. For example, the Belgian National Health Institute has been counting even suspected cases of COVID-19-related deaths, regardless of whether the deceased person was tested. Northern European countries like the UK and Sweden did not impose a lockdown, thus diverging from the politics of social containment to face the pandemic adopted by several other European nations. These considerations may apply to other countries worldwide.
Table 1.
Summary of population median age, COVID-19*-related mortality, and ACE polymorphisms in the different countries under evaluation.
| Nation | Population median age** | Mortality, total deaths/total cases (%)** | Death/1M population** | ACE polymorphisms | ||||
|---|---|---|---|---|---|---|---|---|
| I/I | I/D | D/D | Studied population age | Reference | ||||
| China | 38.4 | 5.58 | 3 | 0.39 | 0.43 | 0.18 | < 70 | He Q et al. (2013) PLoS One 8: e75870 |
| Austria | 43.5 | 3.34 | 80 | 0.21 | 0.50 | 0.29 | < 75 | Sunder-Plassmann G et al. (2002) Crit Care Med 30: 2236-2241 |
| Belgium | 41.9 | 14.0 | 850 | 0.17 | 0.48 | 0.35 | < 83 | Tournoy KG et al. (1996) Clin Chim Acta 255: 39-55 |
| Bosnia and Herzegovina | 43.1 | 2.90 | 114 | 0.18 | 0.52 | 0.30 | < 55 | Klupka-Saric I et al. (2011) Genet Test Mol Biomarkers 15: 835-838 |
| Croatia | 44.3 | 2.89 | 38 | 0.28 | 0.50 | 0.22 | < 60 | Lovrecic L et al. (2006) Acta Neurol Scand 114: 374-377 |
| Czechia | 43.2 | 2.21 | 36 | 0.19 | 0.48 | 0.33 | < 55 | Hladikova M et al. (2011) J Neurol Sci 303: 31-34 |
| Denmark | 42.3 | 4.38 | 106 | 0.23 | 0.51 | 0.26 | < 55 | Panza F et al. (2003) Exp Gerontol 38: 1015-1020 |
| Finland | 43.1 | 7.33 | 60 | 0.24 | 0.49 | 0.27 | NR | Pietinalho A et al. (1999) Eur Respir J 13: 723-726 |
| France | 42.3 | 15.84 | 464 | 0.18 | 0.46 | 0.36 | < 55 | Rigat B et al. (1990) J Clin Invest 86: 1343-1346 |
| Germany | 45.7 | 4.35 | 110 | 0.21 | 0.52 | 0.27 | < 90 | Hucl T et al. (2009) Eur J Gastroenterol Hepatol 21: 1032-1035 |
| Greece | 45.6 | 4.3 | 20 | 0.17 | 0.48 | 0.35 | < 80 | Karagiannis A et al. (2004) Eur Neurol 51: 148-152 |
| Hungary | 43.3 | 13.12 | 62 | 0.28 | 0.49 | 0.23 | < 80 | Szolnoki Z et al. (2001) J Neurol 248: 756-761 |
| Northern Ireland | 38.2 | 6.72 | 357 | 0.24 | 0.49 | 0.27 | < 65 | Kee F et al. (2000) Eur J Clin Invest 30: 1076-1082 |
| Italy | 47.3 | 14.17 | 582 | 0.13 | 0.47 | 0.40 | < 70 | Panza F et al. (2003) Exp Gerontol 38: 1015-1020 |
| Italy | 0.12 | 0.42 | 0.46 | ≥ 100 | Panza F et al. (2003) Exp Gerontol 38: 1015-1020 | |||
| Lithuania | 45.1 | 3.74 | 29 | 0.26 | 0.46 | 0.28 | < 75 | Kupcinskas J (2011) BMC Med Genet 12: 112 |
| Montenegro | 39.8 | 1.58 | 84 | 0.19 | 0.53 | 0.28 | NR | Kostic M et al. (2004) Pediatr Nephrol 19: 853-857 |
| Netherlands | 43.3 | 10.99 | 359 | 0.24 | 0.50 | 0.26 | < 65 | van der Sman-de Beer F et al. (2005) Kidney Int 68: 2237-2243 |
| Norway | 39.8 | 2.74 | 47 | 0.23 | 0.51 | 0.26 | < 70 | Tronvik E, et al. (2008) BMC Neurol 8: 4 |
| Poland | 41.7 | 3.59 | 46 | 0.35 | 0.43 | 0.22 | < 50 | Zak I et al. (2003) Acta Biochim Pol 50: 527-534 |
| Portugal | 46.2 | 3.36 | 171 | 0.17 | 0.47 | 0.36 | < 70 | Pereira da Silva A et al. (2019) Mol Cell Biochem 455: 61-71 |
| Russia | 39.6 | 1.67 | 99 | 0.25 | 0.46 | 0.29 | < 92 | Farrer LA et al. (2000) Arch Neurol 57: 210-214 |
| Serbia | 41.6 | 2.26 | 69 | 0.17 | 0.53 | 0.29 | < 65 | Zivkovic M et al. (2016) J Neurol Sci 363: 29-32 |
| Slovenia | 44.5 | 5.61 | 60 | 0.26 | 0.53 | 0.21 | < 59 | Salobir B et al. (2007) Med Sci Monit 13: CR538-542 |
| Spain | 44.9 | 8.14 | 609 | 0.14 | 0.51 | 0.35 | NR | Martinez E et al. (2000) J Hum Hypertens 14: 131-135 |
| Sweden | 41.1 | 7.08 | 569 | 0.22 | 0.51 | 0.27 | > 18 | Kurland L et al. (2001) J Hypertens 19: 1783-1787 |
| United Kingdom | 40.5 | 15.12 | 682 | 0.24 | 0.50 | 0.26 | < 55 | Marshall RP et al. Am J Respir Crit Care Med 166: 646-650 |
*COVID-19 coronavirus disease 19, NR not reported, Ref reference, 1M one million, I insertion, D deletion
**Data source: https://www.worldometers.info/coronavirus/ updated to August 5, 2020.
Median age of the population (Table 1), social behaviors that are more distinctive of Southern European countries (e.g., intense social life in crowded places, warm greetings, apartments shared by youngsters and elders), or even air pollution [4–6] are additional factors that have been implicated in COVID-19-related mortality. As for the European countries analyzed in Table 1, median age of inhabitants does not appear to have a relevant impact on COVID-19-related mortality (Fig. 1a).
Fig. 1.
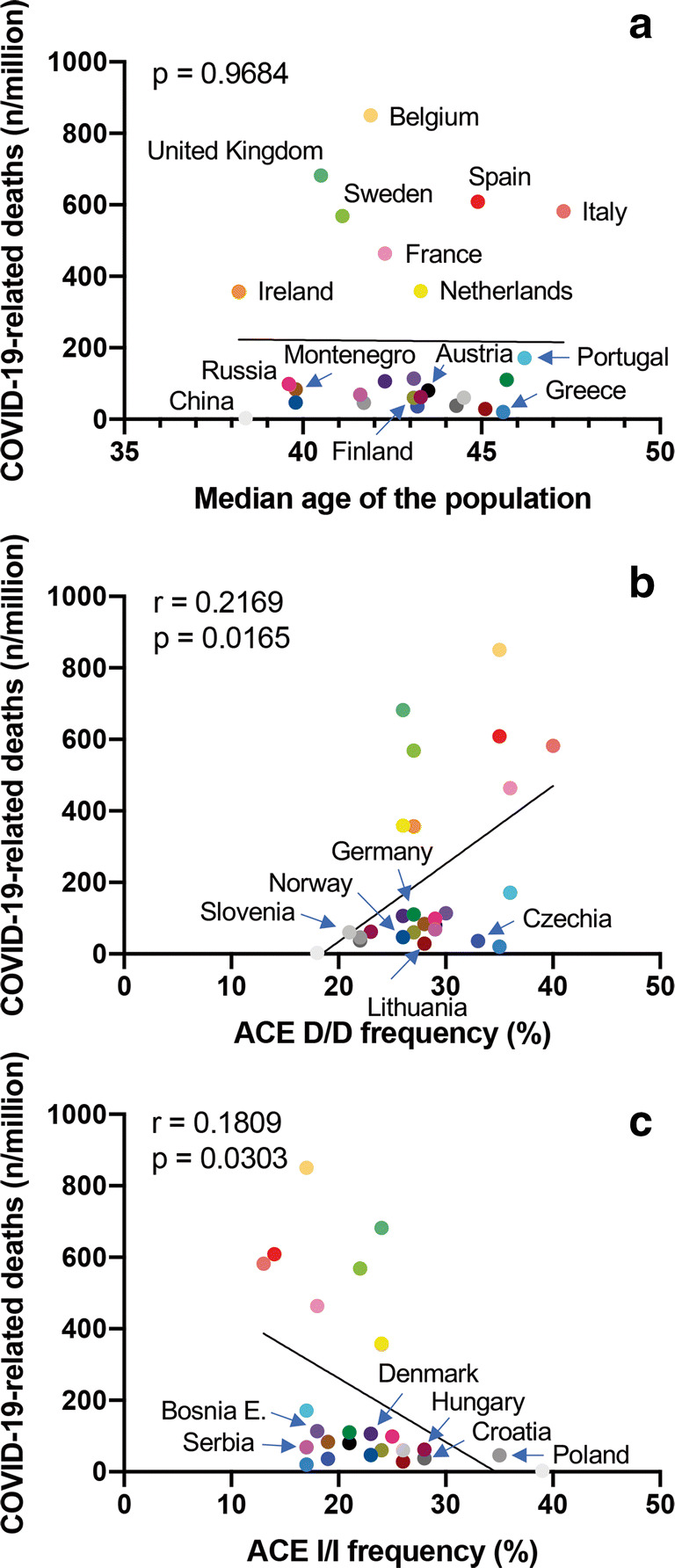
Prevalence of COVID-19-related deaths (number of deaths/106 inhabitants) versus median age of the population of the specified country (a), ACE Del/Del polymorphisms expressed in percentage (b), and ACE Ins/Ins polymorphisms expressed in percentage (c). Numbers in panels refer to R squared (r) and p value (p) for simple linear regression. Nations are reported in color code: Austria, black; Belgium, cantaloupe; Bosnia and Erzegovina, grape; China, mercury; Czechia, blueberry; Croatia, iron; Denmark, eggplant; Finland, asparagus; France, carnation; Germany, moss; Greece, aqua; Hungary, maroon; Ireland, tangerine; Italy, salmon; Lithuania, cayenne; Montenegro, mocha; Netherlands, lemon; Poland, nickel; Portugal, turquoise; Russia, strawberry; Serbia, magenta; Slovenia, magnesium; Spain, maraschino; Sweden, lime; UK, sea foam
The renin-angiotensin-aldosterone system (RAAS) is under scrutiny in the coronavirus COVID-19 pandemic [7] because the angiotensin-converting enzyme 2 (ACE2) is the main receptor for the SARS-CoV-2 on alveolar epithelial cells [8]. ACE2 and the serine protease TMPRSS2, which is necessary for spike protein priming, are also expressed in several other tissues, including blood vessels, olfactory epithelium, brain, heart, kidney, and intestine, thus explaining the multiorgan dysfunction observed in COVID-19 patients [9, 10].
Increased expression of ACE2 in elder males has been put forward to explain the increased SARS-CoV-2 aggressiveness in this subpopulation [9]. However, treatment with ACE inhibitors or angiotensin receptor blockers, which may also cause increased ACE2 expression, does not associate with more severe COVID-19 [11–15]. Additionally, ACE2 counterbalances deleterious vasoconstrictive, proinflammatory, and profibrotic effects of angiotensin (Ang) II by generating downstream peptides such as the hypotensive metabolite Ang1–7 [7]. Indeed, targeted disruption of ACE2 in mice causes severe cardiac contractility defects, increased Ang II levels, and upregulation of hypoxia-induced genes in the heart [16]. ACE2 also exerts a protective effect by limiting leukocyte accrual during acute respiratory distress syndrome in mice, and recombinant ACE2 can protect mice from lung injury [17]. Finally, it has been recently reported that soluble human ACE2 can inhibit SARS-Cov-2 infection in human blood vessel organoids and human kidney organoids [18]. Thus, the role of the RAAS in COVID-19 is far from being elucidated.
ACE2 polymorphisms have been investigated with no evidence of an association with the aggressiveness of severe acute respiratory syndrome [19]. The ACE gene contains an insertion/deletion (Ins/Del) polymorphism (rs4646994; ref. [20]), which associates with higher serum ACE levels [20], obesity [21], hypertension [22], increased cardiovascular risk [23], and thrombophilia [24]; all clinical conditions correlated with more aggressive COVID-19 [1]. Additionally, the ACE Del/Del polymorphism has been associated with mortality in acute respiratory distress syndrome (ARDS) [25]. Our hypothesis is that increased availability of ACE due to ACE Del/Del polymorphism might help explain why SARS-CoV-2 is hitting so hard in Southern Europe.
We found that the distribution of ACE polymorphisms varied among populations, and Del/Del was much more represented in Italy, especially in the eldest, than in China. A gradient in Del/Del polymorphism was also apparent moving from the south to the north of Europe (Table 1). As reported in Fig. 1b, a simple linear regression showed an association between Del/Del polymorphism and COVID-19-related deaths in 25 European countries for which we were able to retrieve comparable information on ACE polymorphisms (e.g., gender distribution, age, samples analyzed). Because data appeared bimodally distributed, they were also analyzed by Spearman’s correlation. The association between ACE Del/Del polymorphism and COVID-19-related deaths remained significant (r 0.4024 (95% 0.005720 to 0.6896), p (two tailed) = 0.0416).
A potential association between ACE polymorphisms and COVID-19 was already investigated by Delanghe and colleagues, who compared the Del-allele frequency of ACE with the mortality of COVID-19 in 25 different European countries [26]. They found a significant correlation between COVID-19-related deaths and the prevalence of the ACE D-allele (Spearman r = − 0.510, p = 0.01). However, focusing on D-allele, the authors aggregated Ins and Del polymorphisms in the analysis, thus diluting the negative effect of Del/Del into the positive effects of Ins/Ins polymorphisms. Indeed, in our analysis, Ins/Ins polymorphism inversely correlated with COVID-19-related deaths (Fig. 1c, Spearman: r = − 0.4898 (95%, − 0.7427 to − 0.1145), p (two tailed) = 0.0111). A correlation between Ins/Del polymorphism and COVID-19-related deaths was not found (Table 1; p = 0.9061). Altogether, these findings suggest a pathogenic role of ACE in COVID-19. This finding might also help explain why in Southern Europe COVID-19-associated mortality has been so high. ACE polymorphisms remain one of probably many genetic and non-genetic factors influencing COVID-19 outcomes.
Abundance of ACE in the blood of Del/Del COVID-19 patients might favor the generation of Ang II. Additionally, ACE2 receptor downregulation caused by SARS-Cov-2 engagement increases Ang II availability, and its deleterious effects downstream of the Ang II type 1 receptor [17]. ACE2 downregulation might also facilitate neutrophil recruitment in the lungs, eventually leading to increased tissue damage. Additionally, Ang II is involved in platelet activation and aggregation [27], which may occur in COVID-19 patients [1]. Dysregulation of RAAS due to excessive ACE might also explain the increased susceptibility of elders to COVID-19 [28], in which the Del/Del polymorphism is particularly high (Table 1). Thus, serum ACE levels and/or Del/Del polymorphism might be predictive of more aggressive disease.
Based on these premises, ACE Del/Del polymorphism and serum ACE levels should be investigated as predictive biomarkers of COVID-19 aggressiveness, as it has been already proposed in ARDS patients [29], and ACE and Ang II might be potential therapeutic targets in COVID-19 patients.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
The work has not been previously presented.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A Review. Jama. 2020;324:782. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 2.Worldmeter COVID-19 Coronavirus pandemic. https://www.worldometersinfo/coronavirus/. Accessed 5 Aug 2020
- 3.Statista number of tests for COVID-19 in most impacted countries worldwide as of Aug. 5, 2020. https://www.statistacom/statistics/1028731/covid19-tests-select-countries-worldwide/. Accessed 5 Aug 2020
- 4.Conticini E, Frediani B, Caro D. Can atmospheric pollution be considered a co-factor in extremely high level of SARS-CoV-2 lethality in Northern Italy? Environ Pollut. 2020;261:114465. doi: 10.1016/j.envpol.2020.114465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fattorini D, Regoli F. Role of the chronic air pollution levels in the Covid-19 outbreak risk in Italy. Environ Pollut. 2020;264:114732. doi: 10.1016/j.envpol.2020.114732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li H, Xu XL, Dai DW, Huang ZY, Ma Z, Guan YJ. Air pollution and temperature are associated with increased COVID-19 incidence: a time series study. Int J Infect Dis. 2020;97:278–282. doi: 10.1016/j.ijid.2020.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280 e278. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muus C, Luecken MD, Eraslan G, Waghray A, Heinberg G, Sikkema L, Kobayashi Y, Vaishnav ED, Subramanian A, Smilie C et al Integrated analyses of single-cell atlases reveal age, gender, and smoking status associations with cell type-specific expression of mediators of SARS-CoV-2 viral entry and highlights inflammatory programs in putative target cells BioRxiv. 10.1101/2020.04.19.049254
- 10.Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, Cao Y, Yousif AS, Bals J, Hauser BM, Feldman J, Muus C, Wadsworth MH II, Kazer SW, Hughes TK, Doran B, Gatter GJ, Vukovic M, Taliaferro F, Mead BE, Guo Z, Wang JP, Gras D, Plaisant M, Ansari M, Angelidis I, Adler H, Sucre JMS, Taylor CJ, Lin B, Waghray A, Mitsialis V, Dwyer DF, Buchheit KM, Boyce JA, Barrett NA, Laidlaw TM, Carroll SL, Colonna L, Tkachev V, Peterson CW, Yu A, Zheng HB, Gideon HP, Winchell CG, Lin PL, Bingle CD, Snapper SB, Kropski JA, Theis FJ, Schiller HB, Zaragosi LE, Barbry P, Leslie A, Kiem HP, Flynn JAL, Fortune SM, Berger B, Finberg RW, Kean LS, Garber M, Schmidt AG, Lingwood D, Shalek AK, Ordovas-Montanes J, Banovich N, Barbry P, Brazma A, Desai T, Duong TE, Eickelberg O, Falk C, Farzan M, Glass I, Haniffa M, Horvath P, Hung D, Kaminski N, Krasnow M, Kropski JA, Kuhnemund M, Lafyatis R, Lee H, Leroy S, Linnarson S, Lundeberg J, Meyer K, Misharin A, Nawijn M, Nikolic MZ, Ordovas-Montanes J, Pe’er D, Powell J, Quake S, Rajagopal J, Tata PR, Rawlins EL, Regev A, Reyfman PA, Rojas M, Rosen O, Saeb-Parsy K, Samakovlis C, Schiller H, Schultze JL, Seibold MA, Shalek AK, Shepherd D, Spence J, Spira A, Sun X, Teichmann S, Theis F, Tsankov A, van den Berge M, von Papen M, Whitsett J, Xavier R, Xu Y, Zaragosi LE, Zhang K (2020) SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 181:1016–1035 e1019 [DOI] [PMC free article] [PubMed]
- 11.Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020;382:2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, Hausvater A, Newman JD, Berger JS, Bangalore S, Katz SD, Fishman GI, Kunichoff D, Chen Y, Ogedegbe G, Hochman JS. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N Engl J Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jarcho JA, Ingelfinger JR, Hamel MB, D’Agostino RB, Sr, Harrington DP. Inhibitors of the renin-angiotensin-aldosterone system and Covid-19. N Engl J Med. 2020;382:2462–2464. doi: 10.1056/NEJMe2012924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel AB, Verma A (2020) COVID-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: what is the evidence? Jama. 10.1001/jama.2020.4812 [DOI] [PubMed]
- 15.Fosbol EL, Butt JH, Ostergaard L, Andersson C, Selmer C, Kragholm K, Schou M, Phelps M, Gislason GH, Gerds TA, et al. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality. Jama. 2020;324:168–177. doi: 10.1001/jama.2020.11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santos AJ, da Costa J, Zhang L, Pei Y, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 17.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA, Fukamizu A, Hui CC, Hein L, Uhlig S, Slutsky AS, Jiang C, Penninger JM. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monteil V, Kwon H, Prado P, Hagelkruys A, Wimmer RA, Stahl M, Leopoldi A, Garreta E, Hurtado Del Pozo C, Prosper F, et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181:905–913 e907. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiu RW, Tang NL, Hui DS, Chung GT, Chim SS, Chan KC, Sung YM, Chan LY, Tong YK, Lee WS, et al. ACE2 gene polymorphisms do not affect outcome of severe acute respiratory syndrome. Clin Chem. 2004;50:1683–1686. doi: 10.1373/clinchem.2004.035436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86:1343–1346. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riera-Fortuny C, Real JT, Chaves FJ, Morales-Suarez-Varela M, Martinez-Triguero ML, Morillas-Arino C, Hernandez-Mijares A. The relation between obesity, abdominal fat deposit and the angiotensin-converting enzyme gene I/D polymorphism and its association with coronary heart disease. Int J Obes. 2005;29:78–84. doi: 10.1038/sj.ijo.0802829. [DOI] [PubMed] [Google Scholar]
- 22.He Q, Fan C, Yu M, Wallar G, Zhang ZF, Wang L, Zhang X, Hu R. Associations of ACE gene insertion/deletion polymorphism, ACE activity, and ACE mRNA expression with hypertension in a Chinese population. PLoS One. 2013;8:e75870. doi: 10.1371/journal.pone.0075870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berge KE, Berg K. Cardiovascular risk factors in people with different genotypes in the insertion/deletion (I/D) polymorphism at the locus for angiotensin I-converting enzyme (ACE) Clin Genet. 1997;52:422–426. doi: 10.1111/j.1399-0004.1997.tb02562.x. [DOI] [PubMed] [Google Scholar]
- 24.Philipp CS, Dilley A, Saidi P, Evatt B, Austin H, Zawadsky J, Harwood D, Ellingsen D, Barnhart E, Phillips DJ, et al. Deletion polymorphism in the angiotensin-converting enzyme gene as a thrombophilic risk factor after hip arthroplasty. Thromb Haemost. 1998;80:869–873. doi: 10.1055/s-0037-1615379. [DOI] [PubMed] [Google Scholar]
- 25.Marshall RP, Webb S, Bellingan GJ, Montgomery HE, Chaudhari B, McAnulty RJ, Humphries SE, Hill MR, Laurent GJ. Angiotensin converting enzyme insertion/deletion polymorphism is associated with susceptibility and outcome in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2002;166:646–650. doi: 10.1164/rccm.2108086. [DOI] [PubMed] [Google Scholar]
- 26.Delanghe JR, Speeckaert MM, De Buyzere ML. The host’s angiotensin-converting enzyme polymorphism may explain epidemiological findings in COVID-19 infections. Clin Chim Acta. 2020;505:192–193. doi: 10.1016/j.cca.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poplawski A. The effect o angiotensin II on the platelet aggregation induced by adenosine diphosphate, epinephrine and thrombin. Experientia. 1970;26:86. doi: 10.1007/BF01900409. [DOI] [PubMed] [Google Scholar]
- 28.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A, for the COVID-19 Lombardy ICU Network Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. Jama. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsantes AE, Kopterides P, Bonovas S, Bagos P, Antonakos G, Nikolopoulos GK, Gialeraki A, Kapsimali V, Kyriakou E, Kokori S, Dima K, Armaganidis A, Tsangaris I. Effect of angiotensin converting enzyme gene I/D polymorphism and its expression on clinical outcome in acute respiratory distress syndrome. Minerva Anestesiol. 2013;79:861–870. [PubMed] [Google Scholar]


