Abstract
AIM
To describe the neuroradiological changes in patients with coronavirus disease 2019 (COVID-19).
MATERIALS AND METHODS
A retrospective review was undertaken of 3,403 patients who were confirmed positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and admitted to Queen Elizabeth Hospital Birmingham, University Hospitals Birmingham NHS Foundation Trust, Birmingham, UK between 1 March 2020 and 31 May 2020, and who underwent neuroimaging. Abnormal brain imaging was evaluated in detail and various imaging patterns on magnetic resonance imaging MRI were identified.
RESULTS
Of the 3,403 patients with COVID-19, 167 (4.9%) had neurological signs or symptoms warranting neuroimaging. The most common indications were delirium (44/167, 26%), focal neurology (37/167, 22%), and altered consciousness (34/167, 20%). Neuroimaging showed abnormalities in 23% of patients, with MRI being abnormal in 20 patients and computed tomography (CT) in 18 patients. The most consistent neuroradiological finding was microhaemorrhage with a predilection for the splenium of the corpus callosum (12/20, 60%) followed by acute or subacute infarct (5/20, 25%), watershed white matter hyperintensities (4/20, 20%), and susceptibility changes on susceptibility-weighted imaging (SWI) in the superficial veins (3/20, 15%), acute haemorrhagic necrotising encephalopathy (2/20, 10%), large parenchymal haemorrhage (2/20, 10%), subarachnoid haemorrhage (1/20, 5%), hypoxic–ischaemic changes (1/20, 5%), and acute disseminated encephalomyelitis (ADEM)-like changes (1/20, 5%).
CONCLUSION
Various imaging patterns on MRI were observed including acute haemorrhagic necrotising encephalopathy, white matter hyperintensities, hypoxic-ischaemic changes, ADEM-like changes, and stroke. Microhaemorrhages were the most common findings. Prolonged hypoxaemia, consumption coagulopathy, and endothelial disruption are the likely pathological drivers and reflect disease severity in this patient cohort.
Introduction
Infection with the novel pathogen, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in a global pandemic, which has claimed the lives of over 630,000 globally (as of 23 July 2020). Severe infection culminating in acute respiratory distress syndrome (ARDS) along with relatively high transmission rates, has led to the greatest global public health crisis in 100 years. There is predominant respiratory involvement; however, multi-organ complications have been reported. Cerebrovascular events and altered mental status have been described as the most common neurological presentations in COVID-19.1 Recent studies have described abnormal brain imaging findings of microhaemorrhages, multifocal white matter hyperintense lesions with variable enhancement, infarcts, haemorrhagic lesions, acute haemorrhagic necrotising encephalopathy, inflammatory CNS syndromes including acute disseminated encephalomyelitis (ADEM), and medial temporal lobe abnormalities.2, 3, 4 As one of the largest UK centres, the present study describes the intracranial imaging findings and likely pathogenesis of patients with COVID-19.
Materials and methods
This retrospective observational study was approved by the institution's review board and the need for ethical approval was waived. Consecutive patients with COVID-19 and neurological manifestations who underwent brain imaging between 1 March 2020 to 31 May 2020 were assessed for inclusion. All patients had a confirmed diagnosis of COVID-19 based on detection of SARS-CoV-2 by reverse transcription polymerase chain reaction (RT-PCR) from sputum or nasopharyngeal swabs. They required hospitalisation with neurological signs or symptoms during inpatient admission, warranting brain imaging. Patients with abnormal brain imaging studies were included (Fig 1 ). Informed consent was obtained from patients or where appropriate, consent was provided by the next of kin.
Figure 1.
Flowchart of patient inclusion and exclusion.
Clinical, laboratory, and imaging data
CT brain imaging was performed using a 64-section multidetector CT system (Siemens, Erlangen, Germany). MRI was performed using a 1.5 T system (Siemens, Erlangen, Germany) with a 32-channel phased-array head coil. The following sequences of the whole-brain were obtained: T1-weighted sagittal, axial T2-weighted, fluid attenuation inversion recovery (FLAIR), susceptibility-weighted imaging (SWI), and diffusion-weighted imaging (DWI), b-value 1,000. Clinical and laboratory data were obtained in those patients with abnormal MRI studies, including demographic information, risk factors, initial presenting symptoms, neurological signs and symptoms, basic laboratory investigations, treatment, and outcome. Where available, chest radiography, electroencephalogram (EEG), and cerebrospinal fluid (CSF) findings were obtained. Clinical indications were recorded for imaging, and MRI was reviewed by two consultant neuroradiologists and consensus was achieved. Patients with age-related, chronic, and incidental findings were excluded with consensus. Continuous variables were presented as mean and standard deviation, while categorical variables were presented as frequencies and proportions.
Results
Between 1 March 2020 and 31 May 2020, 3,403 patients were confirmed positive for SARS-CoV-2 via RT-PCR and hospitalised at Queen Elizabeth Hospital Birmingham, University Hospitals Birmingham NHS Foundation Trust, Birmingham, UK, of which approximately 10% needed intensive care. Neuroimaging studies were performed in 167 patients (CT=172, MRI=36), for various clinical indications (Table 1 ). The most common indications were delirium (n=44), focal neurology (n=37), and altered consciousness (n=34). Brain MRI was abnormal in 20 patients and CT was abnormal in 18 patients. Results of abnormal CT studies are summarised in Table 2 . Findings included subacute infarct, acute infarct, basal ganglia haemorrhage, and subarachnoid haemorrhage, with most patients presenting with focal neurology. The majority of patients presenting with altered consciousness and delirium were investigated by brain MRI. For patients who had an abnormal MRI brain study, the mean age was 59.7 (range 32–91) years, and the male-to-female ratio was approximately 2:1. Sixty percent were of White ethnicity and 25% were from an Asian background. Eighteen of 20 (90%) patients had typical symptoms of COVID-19 and 17/20 (85%) patients had classically described pulmonary changes on chest imaging. D-dimer concentrations were raised in the majority of patients with a mean of 6,795 (range 382–36,381; normal range 0–250) ng/ml, and 76% had vascular risk factors. Most of the neurological complications were noticed later on in the illness, with a mean of 10.1 days from onset of respiratory symptoms to onset of neurological symptoms. Outcome was variable with seven patients discharged home, six undergoing rehabilitation, four patients died, and the remaining three patients continued to remain as inpatients for at least 1 month. Patient demographic and clinical information is summarised in Table 3 .
Table 1.
Indications for brain imaging in hospitalised patients with confirmed COVID-19.
| Indication for brain imaging | No. of patients (%) |
|---|---|
| Delirium/confusion | 44 (26.3) |
| Focal neurological signs | 37 (22.1) |
| Altered consciousness/reduced GCS | 34 (20.3) |
| Trauma | 28 (16.7) |
| Slow return to consciousness | 13 (7.8) |
| Headache | 5 (3.0) |
| Seizure | 3 (1.8) |
| Suspected intracranial infection | 3 (1.8) |
GCS, Glasgow coma score.
Table 2.
Summary of findings for patients with abnormal computed tomography (CT) brain imaging.
| CT head findings | No. of patients (%) |
|---|---|
| Subacute infarct | 8 (44.4) |
| Acute infarct | 7 (38.9) |
| Basal ganglia haemorrhage | 2 (11.1) |
| Subarachnoid haemorrhage | 1 (5.6) |
Table 3.
Summary of demographic and clinical features of the 20 confirmed COVID-19 positive patients with abnormal findings on magnetic resonance imaging (MRI) of the brain.
| Demographic and clinical parameters | Patients with abnormal MRI head study (n=20) |
|---|---|
| Sex, M:F (%) | 13:7, (65:35) |
| Age (years), mean (range) | 59.7 (32–91) |
| Ethnicity | |
| White | 12 (60%) |
| Asian | 5 (25%) |
| Mixed | 1 (5%) |
| Not specified | 2 (10%) |
| Presenting symptoms at admission | |
| Shortness of breath | 16 (80%) |
| Fever | 9 (45%) |
| Cough and shortness of breath | 7 (35%) |
| Focal neurology | 2 (10%) |
| Fall | 1 (5%) |
| Presence of vascular risk factors | 17 (85%) |
| D-dimer level, mean (range) (normal range 0–250) |
6,795 (382–36,381) |
| Lymphocyte count (×109/l), mean (range) (normal range 0.7–4) |
0.74 (0.15–1.40) |
| Onset of neurological signs or symptoms from admission (days), mean (range) | 10.1 (1–39) |
| Outcome | |
| Full recovery/discharged | 7 (35%) |
| Requiring ongoing rehabilitation | 6 (30%) |
| Death | 4 (20%) |
| Slow clinical improvement | 3 (15%) |
MRI brain findings
A number of neuroradiological findings were identified in patients with abnormal MRI brain studies (n=20). MRI patterns and likely pathogenesis are described in detail in Electronic Supplementary Material Table S1. Findings included microhaemorrhages (n=12), watershed white matter hyperintensities (n=4), susceptibility changes on SWI in superficial veins (n=3), acute infarct (n=3), subacute infarct (n=2), acute haemorrhagic necrotising encephalopathy (n=2), large parenchymal haemorrhage (n=2), subarachnoid haemorrhage (n=1), hypoxic–ischaemic changes (n=1), and ADEM-like changes (n=1). Results for MRI findings are summarised in Table 4 .
Table 4.
Summary of findings for patients with abnormal magnetic resonance imaging (MRI) of the brain.
| MRI brain findings | No. of patients (%) |
|---|---|
| Microhaemorrhage | 12 (60) |
| -Parenchymal and callosal | 8 (40) |
| -Callosal only | 4 (20) |
| Watershed white matter hyperintensities | 4 (20) |
| Susceptibility changes on SWI in superficial veins | 3 (15) |
| Acute infarct | 3 (15) |
| Acute haemorrhagic necrotising encephalopathy | 2 (10) |
| Subacute infarct | 2 (10) |
| Large parenchymal haemorrhage | 2 (10) |
| Hypoxic–ischaemic changes | 1 (5) |
| Subarachnoid haemorrhage | 1 (5) |
| ADEM-like changes | 1 (5) |
ADEM, acute disseminated encephalomyelitis; SWI, susceptibility-weighted imaging.
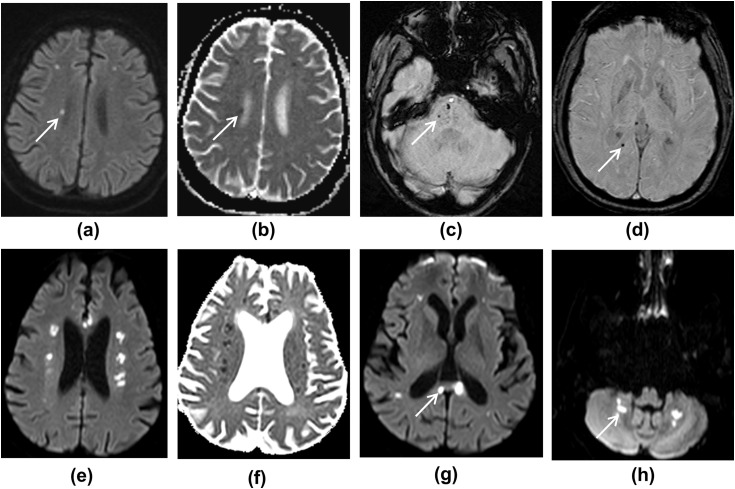
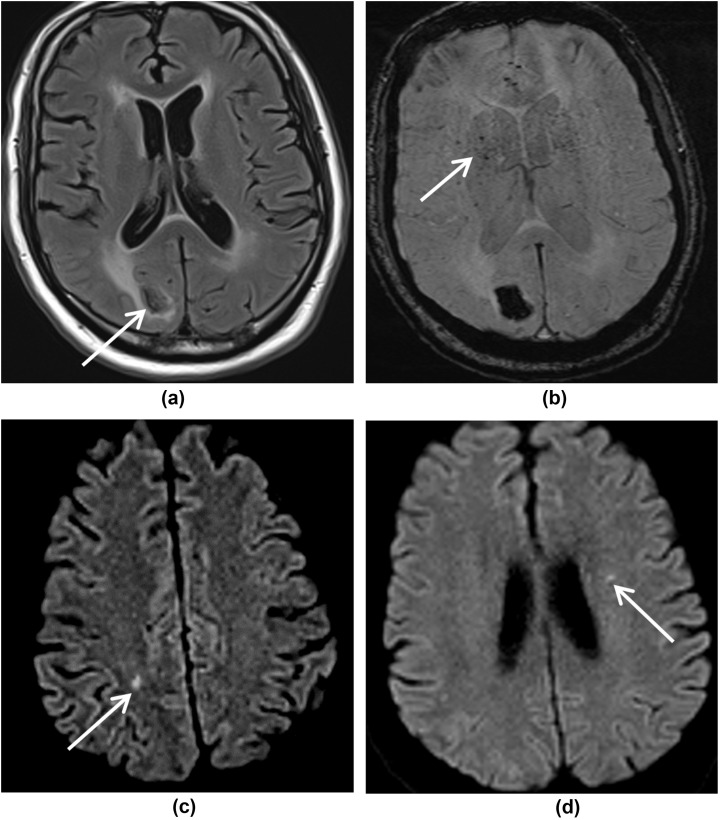
Microhaemorrhage was present in 60% of patients (Figure 2, Figure 3, Figure 4 d ), with all of these patients demonstrating microhaemorrhage in the splenium of corpus callosum. Concurrent parenchymal microhaemorrhage was present in 66% of these patients with callosal microhaemorrhage. One patient had two MRI studies performed within a time interval of 72 h, which showed progression of microhaemorrhages.
Figure 2.
(a–d) MRI images showing deep watershed white matter hyperintensities with microhaemorrhages. (a) DWI shows multiple foci of high diffusion signal in the white matter, and (b) ADC map shows corresponding low ADC. (c,d) SWI images show foci of microhaemorrhage in the pons, right parietal white matter, and right side of the splenium of corpus callosum. (e–h) MRI images from another patient. (e,f) DWI and ADC images showing multiple deep watershed white matter hyperintensities, suggestive of acute infarcts. (g,h) DWI images show white matter hyperintensities in the corpus callosum and dentate nuclei of the cerebellar hemispheres, likely to represent infarcts.
Figure 3.
MRI images showing right occipital lobe haemorrhage. (a) FLAIR image. (b) In addition, SWI image shows microhaemorrhages, and (c,d) DWI images show deep watershed white matter hyperintensities, likely subacute infarcts (ADC was not low, not shown).
Figure 4.
MRI images showing acute haemorrhagic necrotising encephalopathy. (a) FLAIR, (b) DWI, (c) ADC, and (d) SWI images show bilateral symmetrical cortical and subcortical lesions in parieto-occipital lobes, with restricted diffusion and microhaemorrhages, giving a PRES-like appearance. In addition, there is a focus of microhaemorrhage in the splenium of corpus callosum on SWI (bold arrow). (e) FLAIR image showing multiple cerebral infarcts. (f) SWI image shows curvilinear susceptibility artefact, likely to represent microthrombi in the superficial veins. (g,h) FLAIR and SWI images showing focal infarct with microhaemorrhages in the right cerebellar hemisphere.
Watershed white matter hyperintensities on T2/FLAIR were seen in 20% of patients, which demonstrated variable apparent diffusion coefficient (ADC) values. Two patients with vascular risk factors (diabetes, hypertension, and hypercholesterolaemia) showed symmetrical white matter changes in the cerebral deep watershed areas, with restricted diffusion and low ADC values (Fig 2). Similar lesions were also seen in the corpus callosum and cerebellar white matter. In addition, microhaemorrhages were noticed in the splenium of the corpus callosum and brainstem. The other watershed pattern was of scattered DWI high signal lesions in centrum semiovale with micro- and macro-haemorrhages (Fig 3).
Susceptibility changes on SWI were seen in superficial veins in 15% of patients, in conjunction with microhaemorrhages, likely representing microthrombi (Fig 4f). All of these patients had diabetes and two of these three patients also had hypertension as a predisposing risk factor.
Acute and subacute infarcts were seen in 25% of patients, which included middle cerebral artery (MCA), posterior cerebral artery (PCA), brainstem perforator territories, and both unilateral and bilateral in distribution. One patient showed haemorrhagic transformation.
Acute haemorrhagic necrotising encephalopathy was seen in 10% of patients, with bilateral cortical and subcortical lesions in the parieto-occipital lobes showing restricted diffusion and microhaemorrhages (Fig 4). The symmetrical cortical and subcortical involvement in the parieto-occipital lobes appeared similar to a posterior reversible encephalopathy syndrome (PRES)-like pattern. One of the patients had received treatment with extracorporeal membrane oxygenation (ECMO).
Parenchymal haemorrhage was seen in 10% of patients and subarachnoid haemorrhage was seen in 5% of patients. Parenchymal haemorrhage was seen in occipital lobes of two patients (Figure 3, Figure 5 ). One patient demonstrated subarachnoid haemorrhage in bilateral cerebral sulci on SWI and FLAIR sequences and intraventricular haemorrhage in the occipital horns.
Figure 5.
MRI images showing symmetrical white matter signal change. (a) T2W, (b) FLAIR, (c) DWI, and (d) ADC images show white matter hyperintensities in the deep watershed territory bilaterally in the posterior frontal lobes, giving an ADEM-like appearance. (e) SWI image shows small foci of blooming within these lesions indicating microhaemorrhages. In addition, there are subcortical white matter changes in the left occipital lobe with parenchymal haemorrhage on (f) T2W, (g) FLAIR, and (h) SWI images.
Hypoxic–ischaemic changes were seen in one patient who had a cardiac arrest. MRI showed bilateral hypoxic–ischaemic changes with restricted diffusion in the basal ganglia, tail of hippocampi and cerebral peduncles (Fig 6 ). There were also necrotic changes seen in the nigrostriatal tract. Dentate nuclei and thalami were also involved, showing high signal on T2/FLAIR. Microhaemorrhages were seen in the splenium of corpus callosum. This patient had two MRI studies within an interval of 6 days; the first study demonstrated restricted diffusion in the tail of hippocampi and thalami. Second MRI showed more marked changes in the basal ganglia including the nigrostriatal tract. Pseudonormalisation of ADC was noticed in the thalami on this second study, reflecting evolving ischaemic changes.
Figure 6.
MRI images showing bilateral hypoxic–ischaemic changes. (a,b) DWI and ADC images show high diffusion signal with corresponding low ADC in the basal ganglia (thin arrow), tail of hippocampi (bold arrow), and (c) cerebral peduncles. (d) FLAIR image demonstrates high signal in the thalami and (e) dentate nuclei. (f) SWI shows microhaemorrhages in the splenium of the corpus callosum.
ADEM-like changes were seen in one patient who was slow to regain consciousness and had a background of diabetes and hypertension. MRI demonstrated bilateral symmetrical white matter hyperintensities with microhaemorrhages in the posterior frontal lobes (Fig 5). Subcortical white matter changes were also present in the left occipital lobe with parenchymal haemorrhage.
Discussion
There is growing urgency to understand the pathological basis of neurological changes associated with SARS-CoV-2 infection, particularly as these have been associated with disease severity and ongoing neurocognitive symptoms. In the present patient cohort, a number of patterns on brain MRI were seen, which included microhaemorrhages, watershed white matter hyperintensities, susceptibility changes on SWI in superficial veins, hypoxic–ischaemic changes, parenchymal and subarachnoid haemorrhage, acute and subacute infarcts, acute haemorrhagic necrotising encephalopathy, and ADEM-like changes. On CT, patterns consisted of acute and subacute infarcts, basal ganglia haemorrhage, and subarachnoid haemorrhage. There is emerging evidence that the underlying pathogenesis for these presentations is closely linked to a combination of prolonged hypoxaemia, consumption coagulopathy, and endothelial dysfunction.5 , 6
The spike protein of SARS-CoV-2 has a strong affinity for angiotensin-converting enzyme 2 (ACE2) receptor, which allows it to enter host cells. This protein is expressed on alveolar epithelial cells, intestinal enterocytes, and arterial and venous endothelial cells.7 Using electron microscopy, SARS-CoV-2 viral elements have been demonstrated within endothelial cells themselves, associated with profound inflammation and tissue oedema in COVID-19 patients at autopsy.6 A recent neuropathological series of 18 patients has shown that although the virus was detected at low levels in five patients, there was no evidence of encephalitis and the explanation for this could have been due to in situ virions or viral RNA from blood.8 All the patients in this neuropathological series demonstrated acute hypoxic–ischaemic damage. Hypoxic–ischaemic damage has been shown to cause leukoencephalopathy and white matter cytotoxic oedema in critically ill patients. Leukoencephalopathic changes with or without cytotoxic oedema are seen in many other condition including posterior reversible encephalopathy syndrome (PRES), sepsis, ADEM, hypotension, hypoxia, prolonged ventilator support, drug therapy, and toxic metabolic diseases.9 , 10 SARS-CoV-2 infection initiates a pro-inflammatory cytokine storm led by tumour necrosis factor α (TNF-α), interleukin 6 (IL-6), and interleukin 1β (IL-1β).11 This results in a downstream increase in vascular permeability, blood–brain and blood–CSF barrier dysfunction, neuroinflammation and subsequent leukoencephalopathy, thought to be due to oligodendroglial cell death and consequent demyelination predominantly in the deep watershed regions.12 One patient, who had sustained a cardiac arrest, showed restricted diffusion in the basal ganglia bilaterally, in keeping with hypoxic–ischaemic changes. In this patient, the imaging appearances were likely secondary to hypoxia from cardiac arrest, which could have been a complication of COVID-19 infection involving the myocardium. A neuropathological study has also demonstrated similar appearances in the brain after cardiac arrest in one COVID-19 patient, thought to be secondary to hypoxia.13
Microvascular disruption of the endothelium in brain tissue may be responsible for extravasation of red blood cells and extensive microhaemorrhages.6 , 14 Microhaemorrhages have been reported previously in a number of locations including lobar, subcortical, deep, corpus callosum, pontine, and cerebellar.2 , 3 A few studies have reported the splenium of corpus callosum as a predominant location for microhaemorrhages, with or without oedema.4 , 15 Microhaemorrhages in the splenium of the corpus callosum have been reported previously in severe ARDS and high-altitude cerebral oedema, thought to be due to hypoxaemia.16 It is increasingly recognised that respiratory failure may be due to micro-emboli, and these may also affect the cerebral microcirculation resulting in microthrombosis and microvascular ischaemia. Linear structures resembling vessels were observed with susceptibility artefacts on SWI, which are probably microthrombi. This increases the risk of stroke or ischaemia amplifying the cytokine-induced injury to the brain.17
Ischaemic stroke was a common pattern seen on both MRI and CT. Fifteen patients presented with large vessel stroke on CT and five patients on MRI; most were severe, including bilateral MCA infarct, as noted previously.2 Acute stroke seems to be the most common neuroradiological presentation in a large cohort of COVID-19 patients.18 Similar findings with ischaemic changes and microhaemorrhages have also been published in isolated case reports.19 Elevated D-dimer levels were observed in most of the present patients, probably due to a hypercoagulable environment. Four patients presented with intracerebral bleeds on CT and MRI; causes could be due to predisposing vascular risk factors (in 76% of the patient cohort) such as hypertension, anticoagulation treatment as prophylaxis from thromboembolism, and ECMO.
Acute haemorrhagic necrotising encephalopathy was seen in two patients with a similar pattern of bilateral cortical and subcortical lesions in the parieto-occipital lobes showing restricted diffusion and microhaemorrhages, giving a PRES-like appearance. PRES-like appearances in COVID-19 have been noted previously in a few case reports20, 21, 22, 23. The DWI restriction (cytotoxic oedema) observed in these patients is thought to reflect failure of the sodium–potassium pump across cell membranes and vasogenic oedema due to failure of autoregulation in the posterior circulation. Microhaemorrhages were again seen in both of these patients. The pathogenesis of this appearance again could be multifactorial, including endothelial dysfunction, hypoxaemia, cytokine inflammation, and labile blood pressure.
ADEM-like presentation was seen in one patient, with evidence of haemorrhage, also reported in a previous study.2 Although scattered white matter demyelinating lesions and perivenular tracking raises the possibility of a para-infectious ADEM-like process, the authors' impression of the overall phenotype suggested a primarily vascular insult, with secondary white matter injury. A detailed neuropathological examination of one patient has been reported recently,14 which concluded that vascular insult was the primary driver of the neurological sequelae.
At the peak of the pandemic, although delirium and neuro-cognitive symptoms were encountered commonly, it is likely that many patients may not have had neuroimaging performed and this series is likely to be a selected group of patients with acute and sub-acute complications of COVID-19. This was a retrospective study with a bias towards severely ill, hospitalised patients, most of them having predisposing vascular risk factors. In addition, there were logistic limitations in clinical examination, CSF examination, and other laboratory tests as well as organising MRI in these patients. Although CT imaging was performed in a large number of patients, which were apparently normal, small infarcts, micro-haemorrhages, subtle hypoxic changes, or even pre- and para-infectious abnormalities would not have been detected in the absence of MRI. Similar limitations are being observed in other large studies.
The spectrum of MRI findings observed in the present series and other large series reflects a combination of potential mechanisms as described above. The pattern of PRES, ADEM, microhaemorrhages, parenchymal haemorrhages, and large vessel stroke are either atypical or severe, probably due to the combination of a hypercoagulable state, endothelial dysfunction, critical illness, septicaemia, hypoxia, and predisposing risk factors. With the recent limited histological correlates and emerging imaging evidence of a high incidence of reported microhaemorrhages and hypoxic–ischaemic changes, longstanding hypoxaemia, endothelial disruption, and pro-thrombotic conditions appear to be likely contributing factors for most of the imaging appearances in the present study. Understanding evolution of neuropathological processes in SARS-CoV-2 infection is directly relevant in managing these patients both in the early and late phases of the disease course and requires long-term follow-up of these patients.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crad.2020.09.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Varatharaj A., Thomas N., Ellul M.A. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020 Jun 25;S2215–0366(20):30287-X. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paterson R.W., Brown R.L., Benjamin L. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;Jul 8:awaa240. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kremer S., Lersy F., de Sèze J. Brain MRI findings in severe COVID-19: a retrospective observational study. Radiology. 2020 Jun 16:202222. doi: 10.1148/radiol.2020202222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radmanesh A., Derman A., Lui Y.W. COVID-19-associated diffuse leukoencephalopathy and microhemorrhages. Radiology. 2020 May 21:202040. doi: 10.1148/radiol.2020202040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marchandot B., Sattler L., Jesel L. COVID-19 related coagulopathy: a distinct entity? J Clin Med. 2020;9:1651. doi: 10.3390/jcm9061651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varga Z., Flammer A.J., Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamming I., Timens W., Bulthuis M.L.C. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solomon I.H., Normandin E., Bhattacharyya S. Neuropathological features of covid-19. N Engl J Med. 2020 Sep 3;383(10):989–992. doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okumura A., Kidokoro H., Tsuji T. Differences of clinical manifestations according to the patterns of brain lesions in acute encephalopathy with reduced diffusion in the bilateral hemispheres. AJNR Am J Neuroradiol. 2009;30:825–830. doi: 10.3174/ajnr.A1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polito A., Eischwald F., Maho A.L.L. Pattern of brain injury in the acute setting of human septic shock. Crit Care. 2013 Sep 18;17(5):R204. doi: 10.1186/cc12899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta P., McAuley D.F., Brown M. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ginsberg M.D., Hedley-Whyte E.T., Richardson E.P. Hypoxic–ischemic leukoencephalopathy in man. Arch Neurol. 1976;33:5–14. doi: 10.1001/archneur.1976.00500010007002. [DOI] [PubMed] [Google Scholar]
- 13.Jaunmuktane Z., Mahadeva U., Green A. Microvascular injury and hypoxic damage: emerging neuropathological signatures in COVID-19. Acta Neuropathol. 2020 Sep;140(3):397–400. doi: 10.1007/s00401-020-02190-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reichard R.R., Kashani K.B., Boire N.A. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020 Jul;140(1):1–6. doi: 10.1007/s00401-020-02166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitsiori A., Pugin D., Thieffry C. Unusual microbleeds in brain MRI of Covid-19 patients. J Neuroimaging. 2020 doi: 10.1111/jon.12755. 2020 Jul 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riech S., Kallenberg K., Moerer O. The pattern of brain microhemorrhages after severe lung failure resembles the one seen in high-altitude cerebral edema. Crit Care Med. 2015;43(9):e386–e389. doi: 10.1097/CCM.0000000000001150. [DOI] [PubMed] [Google Scholar]
- 17.Jain R., Young M., Dogra S. COVID-19 related neuroimaging findings: a signal of thromboembolic complications and a strong prognostic marker of poor patient outcome. J Neurol Sci. 2020;414:116923. doi: 10.1016/j.jns.2020.116923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jain R. Evolving neuroimaging findings during COVID-19. AJNR Am J Neuroradiol. 2020;41(8):1355–1356. doi: 10.3174/ajnr.A6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poyiadji N., Shahin G., Noujaim D. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: imaging features. Radiology. 2020;296(2):E119–E120. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaya Y., Kara S., Akinci C. Transient cortical blindness in COVID-19 pneumonia; a PRES-like syndrome: case report. J Neurol Sci. 2020;413:116858. doi: 10.1016/j.jns.2020.116858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coolen T., Lolli V., Sadeghi N. Early postmortem brain MRI findings in COVID-19 non-survivors. Neurology. 2020 doi: 10.1212/WNL.0000000000010116. [DOI] [PubMed] [Google Scholar]
- 22.Franceschi A.M., Ahmed O., Giliberto L. Hemorrhagic posterior reversible encephalopathy syndrome as a manifestation of COVID-19 infection. AJNR Am J Neuroradiol. 2020;41(7):1173–1176. doi: 10.3174/ajnr.A6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dixon L., Varley J., Gontsarova A. COVID-19-related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e789. doi: 10.1212/NXI.0000000000000789. Published 2020 May 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.