Abstract
Background
As the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has emerged as a viral pandemic, data on the clinical characteristics and outcomes of patients with SARS-CoV-2 infection undergoing solid organ transplant are emerging. The objective of this systematic review was to assess currently published literature relating to the management, clinical course, and outcome of SARS-CoV-2 infection in liver, kidney, and heart solid organ transplant recipients.
Methods
We conducted a systematic review to assess currently published literature relating to the management, clinical course, and outcome of SARS-CoV-2 infection in liver, kidney, and heart solid organ transplant recipients. Articles published through June 2020 were searched in the MEDLINE, ClinicalTrials.gov, and PubMed databases. We identified 49 eligible studies comprising a total of 403 solid organ transplant recipients.
Results
Older age, male sex, and preexisting comorbidities, including hypertension and/or diabetes, were the most common prevailing characteristics among the solid organ transplant recipients. Clinical presentation ranged from mild to severe disease, including multiorgan failure and death. We found an overall mortality rate of 21%.
Conclusion
Our analysis suggests no increase in overall mortality or worse outcome in solid organ transplant recipients receiving immunosuppressive therapy compared with mortality in the general surgical population with SARS-CoV-2. Our findings suggest that transplant surgery and its immunosuppressive effects should not be a deterrent to proper surgical care for patients in the SARS-CoV-2 era.
The World Health Organization (WHO) declared the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), that causes coronavirus disease 2019 (COVID-19) a pandemic disease on March 11, 2020 [1], and as of September 5, 2020, the WHO reported 26,468,031 cases and 871,166 deaths related to SARS-CoV-2 infection globally [2]. Despite the extraordinary burden and stress the healthcare system is experiencing because of the disease, the vast majority of surgical care cannot be delayed or indefinitely withheld. Although current data on the clinical characteristics and outcomes of patients with SARS-CoV-2 infection undergoing surgery are sparse [3], it has been postulated that major surgery combined with SARS-CoV-2 infection may induce significant inflammatory stress, imparting an increased risk of postoperative complications and mortality [4,5].
Although many institutions are delaying elective surgeries, transplant surgeries are designated as tier 3b (“do not postpone”) by the Centers for Medicare and Medicaid Services [6]. Despite this designation, these solid organ transplant (SOT) recipients represent an extremely vulnerable surgical cohort: in frequent contact with healthcare personnel, chronically immunosuppressed, and having other concomitant medical conditions [7,8]. The surgical management and outcomes of SARS-CoV-2 in SOT recipients remain unclear [9], because published reports on SARS-CoV-2 positive SOT recipients and their outcomes are limited and largely unknown [[9], [10], [11]]. Case reports from Asia, Europe, and the United States suggest a wide range in severity of clinical symptoms from mild and nonspecific to severe respiratory distress and pneumonia [[11], [12], [13]]. Furthermore, reports of atypical presentations with an absence of respiratory symptoms may confound the diagnosis [[12], [13], [14]].
Although the American Society of Transplant Surgeons has recommended best practice guidelines for transplantation in the SARS-CoV-2 era, regional and institutional variation in transplant practice persists [15,16]. In addition, limitation and regional variance in testing pose a significant difficulty in the early identification of suspected SARS-CoV-2 cases in SOT recipients. A recent survey of 111 transplant centers in the United States found a marked reduction in transplant activity despite the tier 3b designation, a wide variation in SARS-CoV-2 testing practices, and substantial differences in the use of off-label and investigational therapies for treatment [17].
There is an urgent need to better understand the effects of SARS-CoV-2 on SOT recipients. We reviewed published literature in this rapidly evolving field to examine the current management practice; the clinical course of the disease; and the outcomes of SARS-CoV-2 infection in liver, kidney, and heart SOT recipients.
Materials and Methods
We conducted a review of SARS-CoV-2 infection in SOT recipients according to the recommended Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines.
Study Search
Articles published through June 6, 2020, were searched in the MEDLINE, ClinicalTrials.gov, and PubMed databases. A combination of the following Medical Subject Heading terms was used to identify articles discussing SARS-CoV-2 infection in solid organ transplant recipients: “coronavirus,” “SarsCov,” “SarsCov2,” “SARS-Cov-2,” “Severe Acute Respiratory Syndrome,” “COVID,” “COVID-19,” “kidney,” “heart,” “liver,” “solid organ transplant,” “transplant,” “transplantation,” “outcome,” and “immunosuppressant.”
Inclusion and Exclusion Criteria
Only case reports, case series, and prospective and retrospective cohort studies published between 2019 and 2020 were included for final analysis and discussion. No restriction was placed on the publication status of the article. All non-English, investigational, animal, in vitro, and cadaveric studies were excluded. In addition, book chapters, conference abstracts, review articles, management guidelines, and any article that did not include discussion of clinical course, treatment, or outcomes of SARS-CoV-2 infection in SOT recipients were also excluded.
Data Collection and Analysis
Articles were screened independently by the authors. Any disagreements were reconciled through discussion between reviewers. Data extracted from each article included study type, year and month of publication, study country, number of patient cases, SOT type (heart, kidney, liver, or multiple), patient demographics, presence of comorbidities, immunosuppressant medications, time from transplant to initial presentation, initial presenting symptoms, treatment, clinical course, and outcomes (Table 1 ). Reporting of all of the above variables was not a requirement for article inclusion, and any unavailable variables were documented as “not reported.” Data were reported using the median and interquartile range (IQR) for non-normally distributed continuous variables and absolute counts and percentages for categorical variables.
Table 1.
Summary of Clinical Outcomes of Severe Acute Respiratory Syndrome Coronavirus 2–positive Solid Organ Transplant Recipients, by Study
| SOT | Author [reference] | Location | No. of Cases (n) | Age and Sex | Comorbidities | Immunosuppressive Regimen |
Time From Transplant | Initial Presentation (Symptoms) | Treatment | Clinical Course | Outcomes | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Multiple SOT types | Tschopp et al [18] | Switzerland | 21 Kidney (48%) Liver (24%) >1 organ (14%) Pancreas (5%) Lung (5%) Heart (5%) |
Median 56 years 71% male |
HTN (67%) DM (43%) Obesity (24%) |
Tac (86%) Prednisone (43%) MMF (17%) CSA (10%) Aza (10%) mTOR (5%) |
Median 47 months |
Fever (76%), dry cough (57%), nausea (33%) and diarrhea (33%). |
Immunosuppressant modified in 14 pts (67%); HCQ, azithromycin lopinavir/ritonavir |
20 pts (95%) admitted 5 pts (25%) to ICU |
16 pts (80%) discharged 3 pts (15%) remain hospitalized 2 pts (10%) died |
|
| Fernández-Ruiz et al [11] | Spain | 18 Kidney (44%) Liver (33%) Heart (22%) |
Median 71 years 77% male |
HTN (72%) DM (50%) Cirrhosis (28%) Obesity (11%) |
Prednisone (67%) MMF/MPA (61%) Tac (56%) EVE (22%) CSA (17%) Aza (6%) mTOR (6%) |
Median 9.3 years |
Fever (83%), gastrointestinal symptoms (28%), respiratory failure (28%) | Lopinavir/ritonavir ± HCQ (50%) HCQ monotherapy (28%) Interferon-β (17%) |
2 pts (11%) required ICU and invasive mechanical ventilation 4 pts (22%) developed progressive respiratory failure 1 (6%) pt had improvement in condition |
5 pts died (28%) 5 pts (28%) remain hospitalized 8 pts (44%) discharged |
||
| Pereira et al [8] | United States | 90 Kidney (51%) Lung (19%) Liver (14%) Heart (10%) Heart-kidney (3%) Liver-kidney (1%) Kidney-pancreas (1%) |
Median 57 years 59% male |
HTN (64%) DM (46%) CKD (63%) Chronic lung disease (19%) Dialysis (6%) Obesity (6%) Cancer (3%) HIV (1%) |
CNI (86%) MMF (72%) Steroid (59%) Aza (4%) Belatacept (6%) IVIG ± pheresis (3%) mTOR (7%) |
Median 6.64 years | Fever (70%), cough (59%), dyspnea (43%), fatigue (28%), myalgias (24%), diarrhea (31%) | Immunosuppressant held or reduced in majority of hospitalized pts HCQ (91%) Azithromycin (66%) Remdesivir (3%) Tocilizumab (21%) Bolus steroid (24%) |
22 (24%) required outpatient care 68 pts (76%) admitted; of these, 27 (30%) had severe disease requiring intubation or admission to ICU |
16 pts (18%) died 37 pts (54%) discharged |
||
| Travi et al [19] | Italy | 13 Liver (54%) Kidney (31%) Heart/kidney (15%) |
Median 59 years 69% male |
HTN (54%) DM (31%) |
Tac (54%) CSA (38%) MMF (38%) Steroid (46%) Belatacept (8%) |
Median 5.3 years | Respiratory symptoms | 62% had reduction or change to immunosuppressant medication HCQ (62%) HCQ + lopinavir/ritonavir (23%) Remdesivir (8%) High-dose steroids (23%) Tocilizumab (15%) |
69% developed respiratory failure | 1 pt died | ||
| Fung et al [20] | United States | 10 Kidney (70%) 7 Lung (10%) 1 Heart (10%) 1 Liver (10%) 1 |
Median 56.5 years 60% male 6 |
HTN, DM, cardiovascular disease | Triple immunosuppression (70%) 7 |
Median 6.1 years |
Fever (80%), cough (80%), dyspnea (80%), myalgia (60%), fatigue (50%) | Immunosuppressive medications decreased in 8 (80%) 2 (20%) enrolled in RCT 3 (30%) with either HCQ, azithromycin, lopinavir/ritonavir, 7 (70%) abx |
70% hospitalized 30% required ICU admission; all developed ARDS and shock |
5 pts (50%) discharged 2 pts (20%) remain hospitalized |
||
| Hoek et al [21] | Netherlands | 23 Kidney (65%) 15 Heart (13%) 3 Lung (13%) 3 Liver (4%) 1 Kidney-heart (4%) 1 |
Mean 59 years 78% male 18 |
HTN (83%) 19, DM (43%) 10, obese (22%) 5 | CNI + MMF (61%) 14 CNI, MMF + steroid (26%) 6 Steroid (4%) 1 EVE (4%) 1 |
<1 year (4%) >1 year (96%) |
Fever (81%) 19, cough (71%) 16, dyspnea (59%) 14 |
57% remained on immunosuppressive medications 13 All hospitalized pts received abx HCQ (13%) 3 |
83% required hospitalization 19 13% monitored at home without additional treatment 3 2 pts (9%) admitted to ICU requiring ventilation |
5 (22%) died 14 (61%) recovered and discharged 4 (17%) with clinical improvement |
||
| Hsu et al [22] | Los Angeles, CA | 1 heart/kidney | 39 years, male | DM, HTN, obesity, chronic foot ulcer | Tac, MMF, prednisone | 3 years | Fever, headache, sore throat, dry cough, dyspnea, fatigue, myalgias | HCQ Enrolled in clinical trial |
Tac, prednisone, continued for entirety of illness course, MMF held starting SD 4 Presented to ED on SD 2; home quarantine SD 3; worsening symptoms and hospitalization SD 4, discharge SD 5; readmission SD 8; worsening hypoxia and transfer to ICU ID 9; transferred out of ICU; discharged SD 15 |
Alive, discharged | ||
| Yi et al [23] | Houston, TX | 21 Kidney (57%) 12 Liver (14%) 3 Lung (10%) 2 Heart-lung (5%) 1 Liver-kidney (5%) 1 Heart-kidney (5%) 1 Kidney-pancreas (5%) 1 |
Mean 54.8 years 62% male 13 |
90% with either HTN, DM, obesity, chronic lung disease, CVD | Triple immunosuppression (81%) 17 |
Median of 5.58 years | 95% with fever, cough SOB 20 43% with diarrhea, vomiting, abdominal pain 9 |
Immunosuppressive medications adjusted daily based on organ type Azith ± HCQ, tolicuzimab remdesivir, ribavirin |
33% treated as outpatients 7 67% hospitalized 14 50% hospitalized pts admitted to the ICU, 36% of hospitalized requiring ventilatory support 7 ICU, 5 vent |
1 pt (5%) died (heart-kidney) 4 (19%) remain in ICU 6 (29%) discharged |
||
| Heart SOT | Holzhauser et al [24] | United States | 2 | Pt 1: 59 years/female Pt 2: 75 years/male |
Pt 1: HTN, DM, CKD Pt 2: HTN, DM, CKD, and CAV |
Pt 1: Tac, MPA Pt 2: CSA, MMF |
Pt 1: 8 years Pt 2: 20 years |
Pt 1: Fever, myalgia, fatigue, diarrhea, productive cough Pt 2: Fever, cough, diarrhea, fatigue, anorexia |
Pt 1: Cefepime, vancomycin, oseltamivir, HCQ, tocilizumab, doxycycline, IVIG, lopinavir/ritonavir, micafungin, SMZ-TMP, tobramycin, linezolid Immunosuppressants held Pt 2: HCQ, tocilizumab, methylprednisolone MMF held |
Pt 1: Respiratory failure, renal failure, and ARDS requiring intubation Pt 2: Required noninvasive respiratory support; clinical improvement over course of hospitalization |
Pt 1: Died Pt 2: Alive, discharged |
|
| Li et al [25] | China | 2 | Pt 1: 51 years/male Pt 2: 43 years/male |
Pt 1: HTN Pt 2: Hyperlipidemia, IGT |
Pt 1: Tac, MMF Pt 2: Tac, MMF |
Pt 1: 17 years Pt 2: 3 years |
Pt 1: Fever, chills, fatigue, anorexia, diarrhea Pt 2: Fever |
Pt 1: Levofloxacin ribavirin, moxifloxacin, ganciclovir, IVIG, methylprednisolone, Umifenovir Pt 2: Ceftriaxone, ganciclovir, moxifloxacin, Umifenovir |
Pt 1: Hospital admission MMF and Tac held 5 days Pt 2: Home quarantine followed by hospitalization for 5 days |
Pt 1: Alive, discharged Pt 2: Alive, discharged |
||
| Russell et al [26] | United States | 1 | 3 years/female | EBV | Tac | 25 months | Productive cough, rhinorrhea, nasal congestion | IVIG | Hospital admission; remained clinically stable with mild clinical course | Alive, discharged | ||
| Latif et al [27] | United States | 28 | Median 64 years 79% male |
HTN (71%) DM (61%), CAV (57%) Obesity (25%) |
CNI (96%), MMF (68%) Steroid (68%) Sirolimus/EVE (18%) |
Median 8.6 years | Fever (83%), dyspnea/cough (91%), gastrointestinal symptoms (48%) | 22 pts (79%) had change in immunosuppressant medications on hospitalization HCQ (78%), High-dose steroid (47%) IL-6-ra (26%) |
6 pts (21%) managed outpatient 22 pts (79%) hospitalized 7 pts (25%) required mechanical ventilation |
7 admitted pts (25%) died 11 admitted pts (50%) discharged 4 admitted pts (18%) remain hospitalized |
||
| Kidney SOT | Alberici et al [28] | Italy | 20 | Not reported | Not reported | Not reported | Not reported | Not reported | HCQ (95%) Dexamethasone (55%) Tocilizumab (30%) |
4 pts (20%) admitted to ICU | 5 pts (25%) died 3 pts (15%) discharged |
|
| Banerjee et al [29] | England | 7 | Median age 54 years (range, 45-69) Pt 1: 48/male Pt 2: 67/female Pt 3: 54/female Pt 4: 65/male Pt 5: 69/female Pt 6: 54/male Pt 7: 45/male |
Pt 1: HTN Pt 2: DM, HTN Pt 3: Post-transplant diabetes mellitus, CMV Pt 4: HTN, wheelchair bound Pt 5: DM, HTN Pt 6: HTN, hemolytic anemia Pt 7: HTN |
Pt 1: Aza, prednisolone Pt 2: Tac, MMF, prednisolone Pt 3: Tac, MMF, prednisolone Pt 4: Tac, MMF, prednisolone Pt 5: Tac, MMF, prednisolone Pt 6: Tac, MMF Pt 7: Tac, Aza, prednisolone |
Pt 1: 31 years Pt 2: 1 year Pt 3: 3 months Pt 4: 2 years Pt 5: 2 months Pt 6: 7 years Pt 7: 3 years (second transplant) |
Respiratory symptoms (cough, shortness of breath) and fever Pt 5 presented with respiratory symptoms, fever plus vomiting and diarrhea |
Pt 1: Aza, prednisolone continued Pt 2: MMF stopped; Tx with broad-spectrum abx in ICU; Tac d/c 1 day before death Pt 3: Tac and MMF stopped; Tx with broad-spectrum abx, oseltamivir; Empiric tx for pneumocystis with high-dose cotrimoxazole Pt 4: MMF stopped Pt 5: MMF stopped; Tx with doxycycline, piperacillin-tazobactam, paracetamol, furosemide, and blood transfusion Pt 6: MMF stopped Pt 7: Aza stopped, Tac dose reduced, prednisolone dose increased |
Pt 1: Remained at home Pt 2: Hypoxic, transferred to ICU, required ventilation; developed AKI, severe metabolic acidosis Pt 3: Hypoxic on presentation, started on CPAP; rapid deterioration of respiratory status requiring ventilation Pt 4: Admitted to ICU; stepped down to medical ward Pt 5: Brief ICU stay for respiratory support, not intubated; stepped down to ward Pt 6: Developed AKI, continued to remain symptomatic, and MMF stopped Pt 7: Admitted, managed in the ward; developed severe AKI, required one hemodialysis session |
Pt 1: Full recovery Pt 2: Died 12 days after hospitalization Pt 3: Alive, remains on ventilation Pt 4: Alive, requires 4 to 6 L oxygen to maintain saturation Pt 5: Alive, in inpatient ward Pt 6: Stayed at home; alive with continued cough and some flulike symptoms Pt 7: Alive, in inpatient ward |
||
| Arpali et al [30] | Turkey | 1 | 28 years/female | Not reported | Tac and prednisone | 6 months | Fever, malaise, sore throat, rhinorrhea | Continued on Tac and prednisone; oseltamivir given at second ED visit | Initially presented to ED, treated with amoxicillin, no SARS-CoV-2 testing done; presented following day to ED with high fever, swabbed for SARS-CoV-2, sent home; 6 days later, testing result positive and returned to hospital to be monitored; discharged after 24 hours | Alive, at home, reports no symptoms | ||
| Guillen et al [31] | Spain | 1 | 50 years/male | HTN | Tac, EVE, prednisone | 4 years (third deceased donor transplant) | Fever, vomiting | Ceftriaxone, azithromycin, ceftaroline, meropenem, lopinavir/ritonavir, HCQ, interferon-β, Tac and EVE held due to potential DDI | Presented to ED and discharged with presumptive viral gastroenteritis; presented to ED 5 days later with persistent fever and productive cough, dx with CAP; tested positive for SARS-CoV-2, was placed in isolation; respiratory status worsened, requiring intubation | Remains in ICU with respiratory support | ||
| Zhu et al [32] | China | 1 | 52 years/male | Not reported | Tac, MMF, prednisone | 12 years | Fatigue, dyspnea, tightness and chest pain, nausea, loss of appetite, intermittent abdominal pain, occasional dry coughs, fever, headache | Tac, MMF, prednisone discontinued; restarted at full dose 3 days prior to discharge Umifenovir, moxifloxacin, methylprednisolone, IVIG, interferon alpha, carbapenem, pantoprazole |
Presented to fever clinic, laboratory findings and chest CT suggestive of SARS-CoV-2 Symptoms worsened at home and admitted to hospital on SD 8; required oxygen via NC; symptoms improved over course of hospitalization; discharged on SD 21 |
Alive, discharged to home | ||
| Marx et al [33] | France | 1 | 58 years/male | Not reported | Belatacept, MMF, prednisone | 3 years | Fever, mild dyspnea, cough | MMF and belatacept discontinued on admission to hospital; CSA started but plan to d/c this and restart MMF and belatacept at next date of infusion | Pt admitted to hospital; treated for possible bacterial superinfection but reported to have mild hospital course | Alive, resolution of fever and respiratory symptoms 5 days after discharge | ||
| Gandolfini et al [34] | Italy | 2 | Pt 1: 75 years/male Pt 2: 52 years/female |
Pt 1: COPD, heart disease, HTN, obesity Pt 2: HTN |
Pt 1: Tac, MMF, steroid Pt 2: Tac, MMF, steroid |
Pt 1: 120 months Pt 2: 8 months |
Cough, myalgia, fever, dyspnea | MMF and Tac were discontinued on the day of admission; both patients received hydroxychloroquine and lopinavir/ritonavir or darunavir/cobicistat Pt 2: Colchicine |
Both patients required noninvasive ventilation Pt 1: Abrupt worsening of respiratory conditions and died 5 days after admission Pt 2: Respiratory symptoms worsened and received colchicine; respiratory symptoms improved after drug initiation |
Pt 1: Died Pt 2: Alive, remained on noninvasive ventilation |
||
| Akalin et al [35] | United States | 36 | Median of 60 years 72% males |
HTN (94%), DM (70%) History of smoking tobacco or current smokers (36%) CVD (17%) |
Tac (97%) Prednisone (94%) MMF (86%) |
Not reported | Fever (58%), diarrhea (22%) | Of hospitalized pts: Antimetabolite held in 86% Tac held in 21% HCQ (86%) 21% received leronlimab on a compassionate-use basis 7% received tocilizumab |
8 pts (22%) in stable condition were monitored at home 28 pts (78%) were admitted to the hospital; 11 pts (39%) received mechanical ventilation, 6 pts (21%) received renal replacement therapy |
10 (28%) pts died, including 2 pts who had been monitored as outpatients 12 pts (43%) remained hospitalized 10 pts (36%) hospitalized discharged to home |
||
| Chen et al [36] | China | 1 | 49 years/male | HTN | Tac, MMF, prednisone | 7 years | Loss of appetite, fever | MMF, Tac, and prednisone held Umifenovir, methylprednisolone, moxifloxacin, IVIG, ribavirin |
Progressive worsening of cough, shortness of breath, hypoxic, fever; required inhaled oxygen and transferred to respiratory intensive care; symptoms gradually improved over course of hospitalization | Alive, discharged to home | ||
| Fontana et al [37] | Italy | 1 | 61 years/male | CKD, malignancy, coagulopathy, Parkinson disease | CSA, steroid | 15 years | Fever/chills | CSA held, steroid increased HCQ, tocilizumab, azithromycin, meropenem |
Remained hemodynamically stable throughout hospitalization | Alive, discharged to home | ||
| Zhang et al [38] | China | 5 | Mean 45 years 80% male 4 |
HTN (40%), 2 DM (40%), 2 Malignancy (20%), 1 |
MMF, CNI, and steroid (80%) 4 | Range of 2 months to 4 years | Fever (100%), cough (100%), myalgia/fatigue (60%), 3 Sputum (60%) 3 |
Oseltamivir or arbidol (100%) Abx (20%) 1 IVIG (20%) 1 |
Immunosuppressant modified after symptom onset All pts hospitalized; resolution of symptoms in 4 (80%) None required intubation or ICU admission |
2 (40%) discharged 3 (60%) remain hospitalized |
||
| Abrishami et al [39] | Iran | 12 | Mean 47.66 years 75% male |
HTN (17%) | All on triple therapy (steroid, CNI/sirolimus, MMF/Aza) | Not reported | Fever (75%), cough (75%), dyspnea (42%) | HCQ, lopinavir/ritonavir, abx (100%) IVIG given if pt hypoxic |
Immunosuppressant modified for all 100% pts hospitalized; 10 (83%) admitted to ICU; 90% in ICU were intubated |
8 (67%) died 4 (33%) discharged |
||
| Columbia University Kidney Transplant Program [40] | United States | 15 | Median 51 years 65% male 10 |
Not reported | Tac (93%) 14 MMF/MPA (80%) 12 Prednisone (67%) 10 Belatacept (13%) 2 Leflunomide (7%) 1 Aza (7%) 1 |
Median 49 months | Fever (87%), 13 Cough (60%), 9 Diarrhea (20%), 3 Myalgias (13%) 2 |
93% had immunosuppressant regimen changed 14 HCQ ± azithromycin (87%) 13 Tocilizumab (7%) 1 |
4 (27%) required intubation 6 (40%) developed AKI |
2 (13%) died 8 (53%) discharged 6 (40%) remain hospitalized |
||
| Nair et al [41] | United States | 10 | Median 57 years 60% male 6 |
HTN (100%), majority also with DM | Tac + MMF/MPA (90%) 9 Steroid (70%) 7 7 |
Median 7.7 years | Fever, cough, myalgia, fatigue, diarrhea | Hospitalized patients had antimetabolite agent stopped HCQ + azithromycin (100%) Antibiotic (60%) |
90% hospitalized 9 5 (50%) admitted to ICU 5 (50%) developed acute kidney injury. |
3 (30%) died 7 (70%) discharged |
||
| Zhu et al [32] | China | 10 | Age between 24 and 65 years 80% male |
HTN, CAD, COPD, atrial fibrillation, HF (60%) | Tac (90%) MMF (90%) Steroid (70%) CSA (10%) Mizoribine (10%) |
6 mo to 12 years | Fever (90%), cough (90%), shortness of breath (90%), fatigue (90%), diarrhea (30%) | Immunosuppressant medication modified in 90% Methylprednisolone (80%) IVIG (70%) Antiviral (100%) |
Mild symptoms in 20% Severe symptoms in 50% Critical symptoms in 30% 100% received NC 30% required noninvasive mechanical ventilation None underwent intubation |
80% recovered 1 (10%) remained hospitalized 1 (10%) died |
||
| Machado et al [42] | Brazil | 1 | 69 years/male | HCV, DM, HTN | Tac, MMF, prednisone | 6 years | Fever, fatigue, confusion, diarrhea, decreased urine output | MMF held, Tac decreased, prednisone increased on hospitalization HCQ, nitazoxanide, ceftriaxone, azithromycin |
Developed mild AKI and severe metabolic acidosis; did not require supplemental oxygen; improved over course of hospitalization | Alive, discharged | ||
| Kim et al [43] | Korea | 2 | Pt 1: 37 years/male Pt 2: 56 years/male |
Not reported | Pt 1: Tac, MMF, prednisolone Pt 2: Tac, MMF, prednisolone |
Pt 1: 4 years Pt 2: 8 years |
Pt 1: Fever, cough, rhinorrhea, diarrhea, and decreased urine output Pt 2: Asymptomatic |
Pt 1: MMF, tac held; Lopinavir/ritonavir and HCQ Pt 2: MMF held; HCQ with azithromycin |
Pt 1: Improvement in clinical course and kidney function; did not require supplemental oxygen Pt 2: Remained hemodynamically stable with mild symptoms (cough); did not require supplemental oxygen |
Pt 1: Recovered Pt 2: Recovered |
||
| Seminari et al [44] | Italy | 1 | 50 years/male | HTN, DM | Tac, MMF | 4 years | Fever, cough | Ceftriaxone | Improvement in clinical course | Alive, discharged | ||
| Wang et al [45] | China | 1 | 49 years/male | HTN, DM | CSA, MMF, prednisone | 2 years | Fever, respiratory symptoms | Immunosuppressant medications continued Lopinavir/ritonavir, ribavirin, interferon-α2b, methylprednisolone |
Required supplemental oxygen; respiratory status improved over course of admission | Recovered | ||
| Billah et al [46] | United States | 1 | 44years/M | Not reported | Tac, MMF, prednisone | 7 years | Dyspnea | Immunosuppressant medications continued Methylprednisolone |
Developed AKI requiring dialysis; Intubated for respiratory failure | Remains both dialysis and ventilator dependent | ||
| Cheng et al [47] | China | 2 | Pt 1: 48 years/male Pt 2: 65 years/female |
Pt 1: Not reported Pt 2: Not reported |
Pt 1: Tac, MMF, prednisone Pt 2: Tac, MMF, prednisone |
Pt 1: 11 years Pt 2: 9 years |
Pt 1: Fever, chest tightness Pt 2: Fever, cough, chest tightness, myalgia |
Pt 1: Immunosuppressant medications held; methylprednisolone Pt 2: Immunosuppressant medications held; moxifloxacin, Umifenovir, IVIG, methylprednisolone |
Pt 1: Symptomatic supportive treatment with improvement in clinical course Pt 2: Respiratory symptoms initially deteriorated; required supplementary oxygen; gradual improvement in clinical course |
Pt 1: Alive, discharged Pt 2: Alive, discharged |
||
| Crespo et al [48] | Spain | 16 | Median 73.6 years 75% male 12 |
HTN (88%) 14, DM (50%) 8, heart disease (50%) 8, obesity (44%) 7, malignancy (31%) 5, lung disease (19%) 3 |
CNI (88%) 14 prednisone (81%) 13, MMF (50%) 8, mTOR (31%) 5, TCDA (19%) 3 | Not reported | Fever (100%), dyspnea (75%) 12 myalgia (50%) 8, diarrhea (25%) 4 |
Tac held in 70%, MMF and mTOR held in all 16 Abx (88%), 14 HCQ (81%) 13, steroid (38%) 6 ritonavir-lopinavir/darunavir (31%), 5 tocilizumab (25%) 4 |
15 pts (94%) hospitalized 6 pts (40%) required ICU admission |
8 pts (53%) died | ||
| Ning et al [49] | China | 1 | 29 years/male | HTN | MMF, CSA, methylprednisolone | 2 years | Fever/chills, fatigue | Immunosuppressant medications continued SMZ-TMP, moxifloxacin, lopinavir/ritonavir |
Developed oliguria and hyponatremia; clinical course improved over course of admission | Resolution and discharge | ||
| Bush et al [50] | United States | 1 | 13 years/male | Chronic severe constipation, rectal prolapse, cecostomy, colostomy with colonic resection | Sirolimus, MMF | 6 years | Rhinorrhea, cough, fever | MMF and sirolimus reduced Antibiotics |
Required NC; remained hemodynamically stable | Alive, discharged to home | ||
| Kumar et al [51] | United States | 1 | 50 years/male | HIV, HTN, asthma, steatohepatitis | Tac, MMF | 14 months | Fever/chills, nasal congestion, cough | Not reported | Not admitted, enrolled in COVID home monitoring program | Health improved to baseline | ||
| Liver SOT | Maggi et al [52] | Italy | 2 | Pt 1: 61 years/male Pt 2: 69 years/M |
Pt 1: Not reported Pt 2: HIV |
Basiliximab, prednisolone, and Tac | Pts developed SARS-CoV-2 infection during hospitalization for transplant | Pt 1: Fever POD 9 Pt 2: Not reported |
Not reported | Pt 1: Presented with fever POD 9 but with normal chest x-ray findings Pts 2: Tested positive for SARS-CoV-2 on POD 22 |
Pt 1: Alive Pt 2: Died on POD 30 |
|
| Bhoori et al [53] | Italy | 3 | >65 years/male | HTN, hyperlipidemia, DM (100%) | CSA (67%) Tac (33%) |
>10 years | Respiratory symptoms similar to CAP | Not reported | 100% required supplementary oxygen at admission but rapidly developed severe respiratory distress syndrome that required mechanical ventilation | 100% died between 3 and 12 days after the onset of pneumonia Authors report 3 recently (within last 2 years) transplanted patients with positive test result for SARS-CoV-2 (on full imuunosuppression); all experienced uneventful course of disease (no further details about this cohort provided) |
||
| D’Antiga et al [54] | Italy | 3 | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | None developed clinical pulmonary disease | Not reported | ||
| Qin et al [55] | China | 1 | 37 years/male | Not reported | Tac, glucocorticoid | Pt developed SARS-CoV-2 infection during hospitalization for transplant | Fever following chemoembolization on day 3 of hospitalization; persistent fever noted 2 days after transplant (transplant occurred on day 7 of hospitalization) | Osteltamivir, rh-GCSF, IVIG started after confirmation of infection Tac and glucocorticoids titrated to lower dose and then increased on day 40 of hospitalization given concerns for acute cellular rejection |
Presented with fever following hepatic arterial chemoembolization; continued to have persistent fever 2 days following embolization; RT-PCR confirmed infection; fever subsided on day 33 of hospitalization | Alive, discharged to home | ||
| Lagana et al [56] | United States | 1 | 6 months/female | Not reported | Not reported | Pt developed SARS-CoV-2 infection during hospitalization for transplant | Respiratory distress, fever, diarrhea Notably, donor tested positive on POD 2 (symptoms not reported) |
HCQ | Fever with increased work of breathing on POD 4; admitted to ICU | Pt remained in hospital with mild respiratory symptoms | ||
| Huang et al [57] | China | 1 | 59 years/male | Hepatitis B | Tac, MMF | 3 years | Fever, cough, chills, fatigue, diarrhea, jaundice, ascites, splenomegaly | Nebulized α-interferon, umifenovir, lopinavir/ritonavir, methylprednisolone, albumin, blood, plasma, IVIG; multiple antimicrobials, including caspofungin, voriconazole, piperacillin tazobactam, cefoperazone -sulbactam, meropenem Tac and MMF dosages halved due to DDI with lopinavir/ritonavir |
Respiratory failure on day 4 of hospitalization, placed on NC; hypoxemia worsened requiring intubation; on day 12, blood cx positive for Candida, pleural fluid positive for Pseudomonas; ECMO on day 15 due to worsened respiratory status; condition deteriorated to multiorgan failure | Pt died on day 45 of admission | ||
| Bin et al [58] | China | 1 | 50 years/male | Not reported | Tac | 3 years | Fever | Umifenovir, lopinavir/ritonavir, methylprednisolone, IVIG, alpha interferon, antibiotics Tac held on admission to hospital; increased to full dose on discharge |
Pt became progressively dyspneic requiring NC on day 5 of hospitalization; symptoms resolved on day 21; discharged after 4 weeks of hospitalization | Alive, at home | ||
| Lee et al [59] | United States | 38 | Median 60 years | For hospitalized pts (n = 24): CKD (71%) 17 HTN (71%), 17 DM (50%), 12 cardiovascular disease (42%), 10 obesity (42%),10 |
For hospitalized pts (n = 24): Tac (96%) 23 CSA (4%) 1 MPA (54%) 13 Steroid (50%) 12 |
Not reported | Gastrointestinal symptoms (42%) 10 | Immunosuppression was decreased in 79% of hospitalized patients 19 18 (75%) received HCQ + azithromycin 5 (21%) received glucocorticoid 8 (33%) received anticoagulant |
63% hospitalized 18 (75%) required supplemental oxygen 8 (33%) required mechanical ventilation |
7 (29%) died 3 (13%) remain hospitalized 14 (58%) discharged |
||
| Patrono et al [60] | Italy | 10 | Pt 1: 69 years/male Pt 2: 59 years/male Pt 3: 56 years/male Pt 4: 58 years/male Pt 5: 64 years/female Pt 6: 64 years/male Pt 7: 64 years/male Pt 8: 62 years/male Pt 9: 75 years/male Pt 10: 85 years/female |
Pt 1: None Pt 2: Obesity Pt 3 through Pt 10: Not reported |
Pt 1: MMF, Tac, prednisone Pt 2: Tac, EVE Pt 3: Tac, EVE Pt 4: MMF, Tac, prednisone Pt 5: Tac, prednisone Pt 6: MMF, Tac Pt 7: MMF, Tac Pt 8: MMF, Tac Pt 9: MPA, Tac Pt 10: Tac |
Pt 1: 5 days Pt 2: 8 months Pt 3: 3 years Pt 4: 2 months Pt 5: 4 years Pt 6: 8 years Pt 7: 9 years Pt 8: 11 years Pt 9: 11 years Pt 10: 22 years |
Pt 1: Cough Pt 2: Fever, diarrhea, dyspnea Pt 3: Fever, odonyphagia, cough Pt 4: Asymptomatic Pt 5: Fever, anorexia, diarrhea Pt 6: Fever Pt 7: Fever Pt 8: Fever Pt 9: Fever, diarrhea, myalgia, cough Pt 10: Asymptomatic |
6 patients were administered HCQ, 3 high-dose steroids, and 2 antivirals (lopinavir/ritonavir and darunavir/ritonavir) 6 patients were administered HCQ, 3 high-dose steroids and 2 antivirals (lopinavir/ritonavir and darunavir/ritonavir) 6 (60%) HCQ, 3 (30%) high dose steroids, 2 (20%) antivirals |
Pt 1: Asymptomatic Pt 2: Required supplemental oxygen; gradual symptom improvement Pt 3: Mild symptoms followed by dyspnea requiring supplemental oxygen; clinical course improved Pt 4: Tested positive 2 months after discharge for transplant Pt 8: Contracted infection during hospitalization for head trauma Pt 10: Incidentally found to be positive Pt 5-7, 9: Not reported |
Pt 1: Alive Pt 2: Alive Pt 3: Alive Pt 4: Alive Pt 5: Alive Pt 6: Alive Pt 7: Alive Pt 8: Died (unrelated to SARS-CoV-2) Pt 9: Died Pt 10: Alive |
||
| Hammami et al [61] | United States | 1 | 63 years/male | ESRD, DM, HTN, HF, PVD | Tac | 10 years | Fever, dry cough, fatigue, headache | HCQ, ceftriaxone, azithromycin, cefepime, vancomycin, tocilizumab | Waxing and waning fever; day 10 of hospitalization developed pleuritic chest pain and severe periumbilical pain, with improvement after tocilizumab; remained afebrile thereafter | Alive | ||
| Modi et al [62] | United States | 1 | 32 years/male | HIV | Tac, MMF, prednisone | 7 years | Fatigue, fever, headache, dry cough | MMF held, Tac reduce, prednisone continued HCQ |
Admitted with mild symptoms which gradually improved over course of hospitalization | Discharge home | ||
| Morand et al [63] | France | 1 | 4 years/female | EBV | Tac | 5 months | Rhinitis, fever, cough | Tac dose reduced Antipyretic |
Improvement in clinical symptoms during hospitalization | Recovered | ||
Abbreviations: Abx, antibiotics; AKI, acute kidney injury; ARDS, acute respiratory distress syndrome; Aza, azathioprine; CAD, coronary artery disease; CAP, community-acquired pneumonia; CAV, cardiac allograft vasculopathy; CNI, calcineurin inhibitor; CMV, cytomegalovirus; CPAP, continuous positive airway pressure; CSA, cyclosporine; Cx, culture; CMV, cytomegalovirus; CVD, cardiovascular disease; Dx, diagnosis; d/c, discontinued; DDI, drug–drug interaction; DM, diabetes mellitus; EBV, Epstein-Barr virus; ED, emergency department; ESRD, end-stage renal disease; EVE, everolimus; HCQ, hydroxychloroquine; HCV, hepatitis C virus; HF, heart failure; HIV, human immunodeficiency virus; HTN, hypertension; ICU; intensive care unit; IGT, impaired glucose tolerance; IL-6-ra, interleukin 6 receptor antagonist; IVIG, intravenous immunoglobulin; MMF, mycophenolate mofetil; MPA; mycophenolate acid; mTOR, mammalian target of rapamycin; NC, nasal cannula; Pt(s), patient(s); POD, postoperative day; PVD, peripheral vascular disease; RCT, randomized controlled trial; rh-GCSF, recombinant human granulocyte colony-stimulating factor; SD, symptom day; SMZ-TMP; sulfamethoxazole-trimethoprim; Tac, tacrolimus; TCDA, T-cell–depleting agents; Tx, treatment.
Results
Study Selection
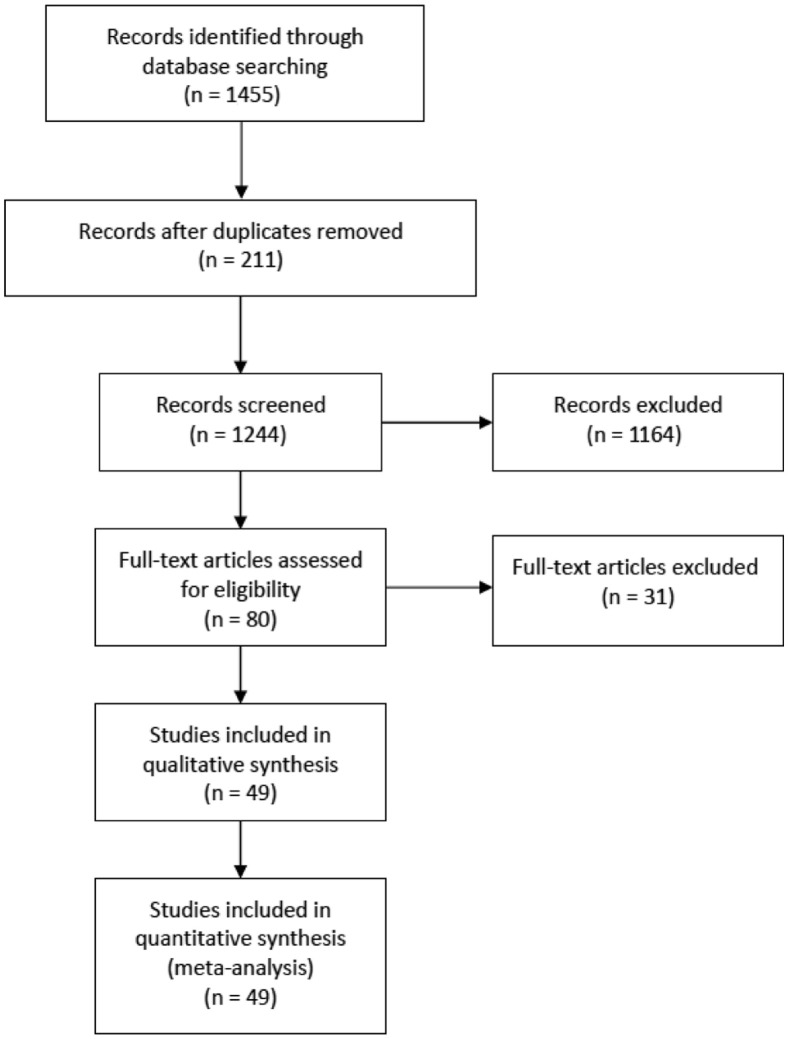
A total of 1455 citations were identified in the initial search. After removing 211 duplicates, a total of 1244 studies were screened by title and abstract (Fig 1 ). Studies were excluded if they did not mention SOT, SARS-CoV-2 infection, or associated clinical course and outcomes or did not fulfill the inclusion criteria. After excluding 1164 studies, we completed a full-text assessment of the remaining 80 studies. Forty-nine studies were included in our final analysis after the exclusion of 31 studies after a full-text screen. Exclusion of these 31 studies at the full-text review included the following reasons: discussed management and recommendations (n = 10), review articles (n = 6), discussed impact of pandemic on transplant program volumes (n = 3), descriptive studies (n = 3), non-English (n = 1), discussed non-SOT transplant (n = 1), did not discuss SOT (n = 2), discussed SARS-CoV-2 infection in transplant surgeon or donor (n = 2), discussed investigational therapy (n = 1), discussed immunologic response (n = 1), or discussed non–transplant-related guideline (n = 1).
Fig 1.
PRISMA flowchart.
Study Characteristics
Of the 49 studies included, 22 were case reports, 8 were case series, and 19 were cohort studies. Four studies discussed heart SOT, 25 discussed kidney SOT, 12 discussed liver SOT, and 8 included multiple SOTs. A total of 433 SOTs were reported among all studies (Table 2 ). The most common SOT was the kidney with 252 (58.2%), followed by liver with 89 (20.6%), heart with 51 (11.8%), lung with 24 (5.5%), and pancreas with 1 (0.2%). Seventeen individuals (3.9%) received more than one SOT. A majority were men (n = 264; 61%). The median age was 54 years (IQR, 45-64), and the median time from transplant was 48 months (IQR, 12-108). Overall mortality was reported as 21% (Table 3 ).
Table 2.
Characteristics of Total Solid Organ Transplant Recipients With Severe Acute Respiratory Syndrome Coronavirus 2 Infection
| No. | % | |
|---|---|---|
| Location | ||
| United States | 249 | 57.51% |
| Italy | 55 | 12.7% |
| China | 26 | 6% |
| Organ transplanted | ||
| Kidney | 252 | 58.2% |
| Liver | 89 | 20.6% |
| Heart | 51 | 11.8% |
| Other organ∗ | 42 | 9.6% |
| Sex | ||
| Male | 264 | 61.0% |
| Comorbidity | ||
| HTN | 249 | 57.5% |
| DM | 159 | 36.7% |
| Obesity | 44 | 10.2% |
| CKD | 77 | 17.8% |
| Immunosuppressive | ||
| Tac | 160 | 37.0% |
| CNI | 122 | 28.2% |
| Prednisone or other steroid | 217 | 50.1% |
| MMF/MPA | 214 | 49.4% |
| Other immunosuppressive† | 125 | 28.8% |
Abbreviations: CKD, chronic kidney disease; CNI, calcineurin inhibitor; DM, diabetes mellitus; HTN, hypertension; MMF, mycophenolate mofetil; MPA, mycophenolic acid; Tac, tacrolimus.
Includes lung, pancreas, and multiple solid organ transplant.
Includes mammalian target of rapamycin, belatacept, leflunomide, mizoribine, cyclosporine, azathioprine, intravenous immunoglobulin/pheresis, basiliximab, T-cell–depleting agents, CNI + MMF, and triple therapy.
Table 3.
Presentation, Clinical Course, and Outcome of Total Solid Organ Transplant Recipients
| No. | % | |
|---|---|---|
| Initial presentation | ||
| Fever | 291 | 67.2% |
| Cough | 220 | 50.8% |
| Gastrointestinal symptoms | 120 | 27.7% |
| Dyspnea | 169 | 39.0% |
| Asymptomatic | 3 | 0.7% |
| Treatment | ||
| Immunosuppressant modified | 235 | 54.3% |
| Antibiotics | 178 | 41.1% |
| HCQ | 242 | 55.9% |
| Methylprednisolone or other steroid | 78 | 18.0% |
| Clinical course | ||
| Hospitalized | 283 | 65.4% |
| Outpatient | 50 | 11.5% |
| Respiratory failure | 18 | 4.2% |
| Transfer to ICU | 78 | 18.0% |
| Outcome∗ | ||
| Death (all studies) | 91 | 21.0% |
| Kidney | 39 | 26.0% |
| Heart | 8 | 24.2% |
| Liver | 14 | 26.4% |
Abbreviations: HCQ, hydroxychloroquine; ICU, intensive care unit.
Death for all studies includes studies for multiple solid organ transplant (SOT) type, including those reporting lung, pancreas, and multiple SOT, whereas death for kidney, heart, and liver SOT recipients was determined solely from studies discussing each individual organ separately.
Characteristics, Clinical Course, and Outcomes by SOT Type
Kidney
Among the 25 studies reporting solely kidney SOT, 150 recipients with SARS-CoV-2 infection were identified. Ninety-five (63.3%) were male. The most common comorbidities were hypertension (55.3%) and diabetes mellitus (26.7%). Tacrolimus (52%), mycophenolate mofetil (MMF) (56%), and prednisone/steroid (64.7%) were the most commonly used maintenance immunosuppressants. Additional immunosuppressant regimens included unspecified calcineurin inhibitors (CNIs) (12%), mTOR inhibitors (4.6%), and belatacept (2%). Fever was the most common presenting symptom (71.3%), followed by cough (39.3%) and dyspnea (26%). Ninety-three individuals (62%) were hospitalized, and 10.7% developed acute kidney injury. Mechanical ventilation, supplemental oxygen, and transfer to an intensive care unit (ICU) for a higher level of care were required in 20%, 11.3%, and 19.3% of the individuals, respectively. Nearly half (46.7%) of those reported had their maintenance immunosuppressant reduced when the infection was suspected or confirmed. The most commonly used treatments were hydroxychloroquine (HCQ) (65.3%), antibiotics (43.3%), steroids (20.7%), and lopinavir/ritonavir (15.3%). Thirty-three patients were reported as alive (22%), discharged to home (n = 45; 30%), or remaining hospitalized (non-ICU, n = 27 [18%]; ICU, n = 3 [2%]), and 26% of individuals died (n = 39).
Liver
Fifty-three liver SOT recipients were identified from 12 studies reporting liver SOT, and males comprised 28.3% of the population (n = 15). Hypertension, chronic kidney disease, and diabetes were the most common comorbidities (39.6%, 32.1%, and 30.2%, respectively). Tacrolimus (79.2%), MMF/mycophenolic acid (MPA) (39.6%), and steroids (35.8%) were the most commonly used maintenance immunotherapies. Fever and gastrointestinal symptoms were the 2 most common initial presenting symptoms, followed by cough (28.3%, 28.3%, and 18.9%, respectively). Thirty-four individuals (64.2%) were hospitalized, and 45.3% subsequently had their maintenance immunosuppressant medication reduced. HCQ and antibiotics were used in 39.6% and 39.6%, respectively, for treatment of SARS-CoV-2 infection. In addition, 47.2% of individuals required supplemental oxygen during hospitalization, and 14 (26.4%) individuals died after the onset of illness.
Heart
Thirty-three individuals who underwent heart SOT were reported in 4 studies; 25 (75.8%) were male. The most common comorbidities were hypertension (69.7%), diabetes (57.6%), and cardiac allograft vasculopathy (48.5%). The most commonly used maintenance immunotherapies were CNI (81.8%) and MMF/MPA (69.7%). Fever (81.8%), cough (94.8%), dyspnea (75.8%), and gastrointestinal symptoms (48.5%) were the most common initial presenting symptoms. Twenty-seven (81.8%) patients were hospitalized, and intubation/mechanical ventilation was required in 24.2% of those individuals. Twenty-four (72.7%) patients received HCQ, and high-dose steroids were administered to 15 patients (45.5%). Maintenance immunotherapy was modified in 75.8% of the cases. Fifteen (45.5%) were reported as discharged, and 24.2% of the individuals died during their illness.
Discussion
As the number of SARS-CoV-2 infections continues to grow worldwide, clinical data in SOT recipients are emerging, and our study showed overall mortality of 21% with no substantial variations among the different types of SOT (Table 3). The mortality rate is in concordance with published data in terms of outcomes reported in patients undergoing acute care surgery and cancer surgery: Lei et al, Liang et al, and the COVIDSurg Collaborative group reported mortality in the general surgical population of 20.5%, 39%, and 23.8%, respectively [4,64,65].
Older age, male sex, and preexisting conditions such as hypertension and diabetes were the most common characteristics among the SOT recipients. As predicted, we saw a broad spectrum of clinical courses ranging from having only a few mild symptoms to multiorgan failure leading to death. Despite the concerns of atypical disease presentation in immunocompromised patients, the most common presenting symptoms were similar to general population symptoms [7,66,67]; however, there were some variations in the incidence of the initial presenting symptoms among the different SOT types (Table 1).
Modification of immunosuppressant therapy at confirmation or suspicion of SARS-CoV-2 infection was reported in 54.3% of the patients, reflecting individualized adjustment based on the severity of the disease, type of transplanted organ, interval time since transplant, and risk of rejection [8]. On a similar note, the American Association for the Study of Liver Diseases recently published management guidelines for liver transplant recipients in the COVID era [68]: continuing the routine immunosuppressive regimen in nonsymptomatic recipients and reducing the immunosuppression regimen, including prednisone, azathioprine, or MMF and CNI in symptomatic patients with COVID-19. Our study suggests that the current practice of reducing immunosuppression upon the diagnosis of SARS-CoV-2 infection appears to be an appropriate measure without causing significant short-term adverse effects on graft function while maintaining patient survival comparable to that of the general population.
The median time from transplant to infection was 48 months in our study; the majority of the studies focused on patients who had received SOTs many years ago. Although it is a small number, we identified 4 cases in which the SOT recipient contracted SARS-CoV-2 infection during the transplant perioperative period, and we found no significant difference in their initial presentation, clinical course, and outcome when compared with a cohort of patients who received a transplant more than 1 year ago.
Although our study provides a general overview of SOT recipients’ clinical course and outcomes with SARS-CoV-2 infection, we recognize several limitations of the study. First, the inclusion of early case reports may be biased toward those with increased severity of disease and worse outcome, leading to publication bias with overinterpretation. Second, the inclusion of a mixed transplant population and a wide heterogeneity in study inclusion criteria may not be a true representation of the study samples and therefore precluded the ability to derive causality. Furthermore, data were based on absolute counts and therefore can be used only for descriptive purposes. Last, a certain degree of reporting bias inevitably played a role because SOT recipients are trained to be more vigilant with their health conditions and have a low threshold for seeking medical attention. This reporting bias could have led to more disease diagnosis in our study group than in the general population.
In conclusion, SARS-CoV-2 infection in SOT recipients in general appears to have similar presentation, clinical course, and outcome as in the general non-SOT surgical population. We found that the patient demographics, preexisting risk factors, and outcomes were similar within each SOT type, and we saw no substantial differences in mortality rate among the different SOT types. Although our data show that the overall short-term survival is about the same, long-term patient survival and graft function data are needed to fully understand the impact of COVID in SOT patients.
References
- 1.WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020. 2020. https://web.archive.org/web/20200418151429/https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 [accessed 29.06.20]
- 2.WHO coronavirus disease (COVID-19) dashboard. https://covid19.who.int/?gclid=EAIaIQobChMIyN6ygr3k6QIVy8DACh0R6A_HEAAYASAAEgL2TvD_BwE [accessed 04.09.20]
- 3.Aziz H., Filkins A., Kwon Y.K. Review of COVID-19 outcomes in surgical patients. Am Surg. 2020;86(7):741–745. doi: 10.1177/0003134820934395. [DOI] [PubMed] [Google Scholar]
- 4.Lei S., Jiang F., Su W. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. 2020;21:100331. doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Besnier E., Tuech J.J., Schwarz L. We asked the experts: Covid-19 outbreak: is there still a place for scheduled surgery? “Reflection from pathophysiological data. World J Surg. 2020;44(6):1695–1698. doi: 10.1007/s00268-020-05501-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aziz H, James T, Remulla D, et al. Effect of COVID-19 on surgical training across the United States: a national survey of general surgery residents [e-pub ahead of print]. J Surg Educ https://doi.org/10.1016/j.jsurg.2020.07.037, accessed May 6, 2020. [DOI] [PMC free article] [PubMed]
- 7.Fishman J.A. Infection in organ transplantation. Am J Transplant. 2017;17(4):856–879. doi: 10.1111/ajt.14208. [DOI] [PubMed] [Google Scholar]
- 8.Pereira M.R., Mohan S., Cohen D.J. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20(7):1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang J., Lin H., Wu Y. COVID-19 in posttransplant patients-report of 2 cases. Am J Transplant. 2020;20(7):1879–1881. doi: 10.1111/ajt.15896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michaels M.G., La Hoz R.M., Danziger-Isakov L. Coronavirus disease 2019: implications of emerging infections for transplantation. Am J Transplant. 2020;20(7):1768–1772. doi: 10.1111/ajt.15832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernández-Ruiz M., Andrés A., Loinaz C. COVID-19 in solid organ transplant recipients: a single-center case series from Spain. Am J Transplant. 2020;20(7):1849–1858. doi: 10.1111/ajt.15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kates O.S., Fisher C.E., Stankiewicz-Karita H.C. Earliest cases of coronavirus disease 2019 (COVID-19) identified in solid organ transplant recipients in the United States. Am J Transplant. 2020;20(7):1885–1890. doi: 10.1111/ajt.15944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong Z., Zhang Q., Xia H. Clinical characteristics and immunosuppressants management of coronavirus disease 2019 in solid organ transplant recipients. Am J Transplant. 2020;20(7):1916–1921. doi: 10.1111/ajt.15928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fishman J.A., Grossi P.A. Novel coronavirus-19 (COVID-19) in the immunocompromised transplant recipient: #Flatteningthecurve. Am J Transplant. 2020;20(7):1765–1767. doi: 10.1111/ajt.15890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romanelli A., Mascolo S. Crucial aspects of the management of solid organ transplant patient with COVID-19: a narrative review. J Biomed Res Rev. 2020;3(1):32–36. [Google Scholar]
- 16.Moris D., Shaw B.I., Dimitrokallis N., Barbas A.S. Organ donation during the coronavirus pandemic: an evolving saga in uncharted waters. Transpl Int. 2020;33(7):826–827. doi: 10.1111/tri.13614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyarsky B.J., Chiang T.P.Y., Werbel W.A. Early impact of COVID-19 on transplant center practices and policies in the United States. Am J Transplant. 2020;20(7):1809–1818. doi: 10.1111/ajt.15915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tschopp J, L’Huillier AG, Mombelli M, et al. First experience of SARS-CoV-2 infections in solid organ transplant recipients in the Swiss Transplant Cohort Study [e-pub ahead of print]. Am J Transplant https://doi.org/10.1111/ajt.16062, accessed May 6, 2020. [DOI] [PMC free article] [PubMed]
- 19.Travi G., Rossotti R., Merli M. Clinical outcome in solid organ transplant recipients with COVID-19: a single-center experience. Am J Transplant. 2020;20(9):2628–2629. doi: 10.1111/ajt.16069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fung M, Chiu CY, DeVoe C, et al. Clinical outcomes and serologic response in solid organ transplant recipients with COVID-19: a case series from the United States [e-pub ahead of print]. Am J Transplant https://doi.org/10.1111/ajt.16079, accessed May 6, 2020. [DOI] [PMC free article] [PubMed]
- 21.Hoek RAS, Manintveld OC, Betjes MGH, et al. COVID-19 in solid organ transplant recipients: a single-center experience [e-pub ahead of print]. Transpl Int https://doi.org/10.1111/tri.13662, accessed May 6, 2020. [DOI] [PMC free article] [PubMed]
- 22.Hsu J.J., Gaynor P., Kamath M. COVID-19 in a high-risk dual heart and kidney transplant recipient. Am J Transplant. 2020;20(7):1911–1915. doi: 10.1111/ajt.15936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yi SG, Rogers AW, Saharia A, et al. Early experience with COVID-19 and solid organ transplantation at a US high-volume transplant center [e-pub ahead of print]. Transplantation https://doi.org/10.1097/TP.0000000000003339, accessed May 6, 2020. [DOI] [PMC free article] [PubMed]
- 24.Holzhauser L, Lourenco L, Sarswat N, Kim G, Chung B, Nguyen AB. Early experience of COVID-19 in 2 heart transplant recipients: case reports and review of treatment options [e-pub ahead of print]. Am J Transplant https://doi.org/10.1111/ajt.15982, accessed May 6, 2020. [DOI] [PMC free article] [PubMed]
- 25.Li F., Cai J., Dong N. First cases of COVID-19 in heart transplantation from China. J Heart Lung Transplant. 2020;39(5):496–497. doi: 10.1016/j.healun.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russell M.R., Halnon N.J., Alejos J.C., Salem M.M., Reardon L.C. COVID-19 in a pediatric heart transplant recipient: emergence of donor-specific antibodies. J Heart Lung Transplant. 2020;39(7):732–733. doi: 10.1016/j.healun.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Latif F, Farr MA, Clerkin KJ, et al. Characteristics and outcomes of recipients of heart transplant with coronavirus disease 2019 [e-pub ahead of print]. JAMA Cardiol https://doi.org/10.1001/jamacardio.2020.2159, accessed May 6, 2020. [DOI] [PMC free article] [PubMed]
- 28.Alberici F., Delbarba E., Manenti C. Management of patients on dialysis and with kidney transplant during SARS-COV-2 (COVID-19) pandemic in Brescia. Italy. Kidney Int Rep. 2020;5(5):580–585. doi: 10.1016/j.ekir.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banerjee D., Popoola J., Shah S., Ster I.C., Quan V., Phanish M. COVID-19 infection in kidney transplant recipients. Kidney Int. 2020;97(6):1076–1082. doi: 10.1016/j.kint.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arpali E., Akyollu B., Yelken B., Tekin S., Turkmen A., Kocak B. Case report: a kidney transplant patient with mild COVID-19. Transpl Infect Dis. 2020;22(4) doi: 10.1111/tid.13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guillen E., Pineiro G.J., Revuelta I. Case report of COVID-19 in a kidney transplant recipient: does immunosuppression alter the clinical presentation? Am J Transplant. 2020;20(7):1875–1878. doi: 10.1111/ajt.15874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu L., Xu X., Ma K. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression. Am J Transplant. 2020;20(7):1859–1863. doi: 10.1111/ajt.15869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marx D., Moulin B., Fafi-Kremer S. First case of COVID-19 in a kidney transplant recipient treated with belatacept. Am J Transplant. 2020;20(7):1944–1946. doi: 10.1111/ajt.15919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gandolfini I., Delsante M., Fiaccadori E. COVID-19 in kidney transplant recipients. Am J Transplant. 2020;20(7):1941–1943. doi: 10.1111/ajt.15891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akalin E., Azzi Y., Bartash R. Covid-19 and kidney transplantation. N Engl J Med. 2020;382(25):2475–2477. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen S., Yin Q., Shi H. A familial cluster, including a kidney transplant recipient, of coronavirus disease 2019 (COVID-19) in Wuhan, China. Am J Transplant. 2020;20(7):1869–1874. doi: 10.1111/ajt.15903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fontana F., Alfano G., Mori G. COVID-19 pneumonia in a kidney transplant recipient successfully treated with tocilizumab and hydroxychloroquine. Am J Transplant. 2020;20(7):1902–1906. doi: 10.1111/ajt.15935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H., Chen Y., Yuan Q. Identification of kidney transplant recipients with coronavirus disease 2019. Eur Urol. 2020;77(6):742–747. doi: 10.1016/j.eururo.2020.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abrishami A., Samavat S., Behnam B., Arab-Ahmadi M., Nafar M., Sanei Taheri M. Clinical course, imaging features, and outcomes of COVID-19 in kidney transplant recipients. Eur Urol. 2020;78(2):281–286. doi: 10.1016/j.eururo.2020.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Columbia University Kidney Transplant Program Early description of coronavirus 2019 disease in kidney transplant recipients in New York. J Am Soc Nephrol. 2020;31(6):1150–1156. doi: 10.1681/ASN.2020030375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nair V., Jandovitz N., Hirsch J.S. COVID-19 in kidney transplant recipients. Am J Transplant. 2020;20(7):1819–1825. doi: 10.1111/ajt.15967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Machado DJB, Ianhez LE. COVID-19 pneumonia in kidney transplant recipients – where we are? [e-pub ahead of print]. Transpl Infect Dis https://doi.org/10.1111/tid.13306, accessed May 6, 2020. [DOI] [PMC free article] [PubMed]
- 43.Kim Y., Kwon O., Paek J.H. Two distinct cases with COVID-19 in kidney transplant recipients. Am J Transplant. 2020;20(8):2269–2275. doi: 10.1111/ajt.15947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seminari E., Colaneri M., Sambo M. SARS CoV-2 infection in a renal-transplanted patient: a case report. Am J Transplant. 2020;20(7):1882–1884. doi: 10.1111/ajt.15902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J., Li X., Cao G., Wu X., Wang Z., Yan T. COVID-19 in a kidney transplant patient. Eur Urol. 2020;77(6):769–770. doi: 10.1016/j.eururo.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Billah M, Santeusanio A, Delaney V, Cravedi P, Farouk SS. A catabolic state in a kidney transplant recipient with COVID-19 [e-pub ahead of print]. Transpl Int https://doi.org/10.1111/tri.13635, accessed May 6, 2020. [DOI] [PMC free article] [PubMed]
- 47.Cheng DR, Wen JQ, Liu ZZ, Lv TF, Chen JS. Coronavirus disease 2019 in renal transplant recipients: report of two cases [e-pub ahead of print]. Transpl Infect Dis https://doi.org/10.1111/tid.13329, accessed May 6, 2020. [DOI] [PMC free article] [PubMed]
- 48.Crespo M, José Pérez-Sáez M, Redondo-Pachón D, et al. COVID-19 in elderly kidney transplant recipients [e-pub ahead of print]. Am J Transplant https://doi.org/10.1111/ajt.16096, accessed May 6, 2020. [DOI] [PMC free article] [PubMed]
- 49.Ning L., Liu L., Li W. Novel coronavirus (SARS-CoV-2) infection in a renal transplant recipient: case report. Am J Transplant. 2020;20(7):1864–1868. doi: 10.1111/ajt.15897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bush R, Johns F, Acharya R, Upadhyay K. Mild COVID-19 in a pediatric renal transplant recipient [e-pub ahead of print]. Am J Transplant https://doi.org/10.1111/ajt.16003, accessed May 6, 2020. [DOI] [PMC free article] [PubMed]
- 51.Kumar RN, Tanna SD, Shetty AA, Stosor V. COVID-19 in an HIV-positive kidney transplant recipient [e-pub ahead of print]. Transpl Infect Dis https://doi.org/10.1111/tid.13338, accessed May 6, 2020. [DOI] [PMC free article] [PubMed]
- 52.Maggi U., De Carlis L., Yiu D. The impact of the COVID-19 outbreak on liver transplantation programmes in Northern Italy. Am J Transplant. 2020;20(7):1840–1848. doi: 10.1111/ajt.15948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhoori S., Rossi R.E., Citterio D., Mazzaferro V. COVID-19 in long-term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol. 2020;5(6):532–533. doi: 10.1016/S2468-1253(20)30116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D’Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transpl. 2020;26(6):832–834. doi: 10.1002/lt.25756. [DOI] [PubMed] [Google Scholar]
- 55.Qin J, Wang H, Qin X, et al. Perioperative presentation of COVID-19 disease in a liver transplant recipient [e-pub ahead of print]. Hepatology https://doi.org/10.1002/hep.31257, accessed May 6, 2020. [DOI] [PubMed]
- 56.Lagana SM, De Michele S, Lee MJ, et al. COVID-19 associated hepatitis complicating recent living donor liver transplantation [e-pub ahead of print]. Arch Pathol Lab Med https://doi.org/10.5858/arpa.2020-0186-SA, accessed May 6, 2020. [DOI] [PubMed]
- 57.Huang J.-F., Zheng K.I., George J. Fatal outcome in a liver transplant recipient with COVID-19. Am J Transplant. 2020;20(7):1907–1910. doi: 10.1111/ajt.15909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bin L., Yangzhong W., Yuanyuan Z., Huibo S., Fanjun Z., Zhishui C. Successful treatment of severe COVID-19 pneumonia in a liver transplant recipient. Am J Transplant. 2020;20(7):1891–1895. doi: 10.1111/ajt.15901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee B.T., Perumalswami P.V., Im G.Y., Florman S., Schiano T.D. COVID-19 in liver transplant recipients: an initial experience from the U.S. epicenter. Gastroenterology. 2020;159(3) doi: 10.1053/j.gastro.2020.05.050. 1176-8.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Patrono D, Lupo F, Canta F, et al. Outcome of COVID-19 in liver transplant recipients: a preliminary report from northwestern Italy [e-pub ahead of print]. Transpl Infect Dis https://doi.org/10.1111/tid.13353, accessed May 6, 2020. [DOI] [PMC free article] [PubMed]
- 61.Hammami M.B., Garibaldi B., Shah P. Clinical course of COVID-19 in a liver transplant recipient on hemodialysis and response to tocilizumab therapy: a case report. Am J Transplant. 2020;20(8):2254–2259. doi: 10.1111/ajt.15985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Modi AR, Koval CE, Taege AJ, et al. Coronavirus disease 2019 in an orthotopic liver transplant recipient living with human immunodeficiency virus [e-pub ahead of print]. Transpl Infect Dis https://doi.org/10.1111/tid.13351, accessed May 6, 2020. [DOI] [PMC free article] [PubMed]
- 63.Morand A., Roquelaure B., Colson P. Child with liver transplant recovers from COVID-19 infection: a case report. Arch Pediatr. 2020;27(5):275–276. doi: 10.1016/j.arcped.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liang W., Guan W., Chen R. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.COVIDSurg Collaborative Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study [published correction appears in Lancet. 2020 Jun 9] Lancet. 2020;396(10243):27–38. doi: 10.1016/S0140-6736(20)31182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Romero F.A., Razonable R.R. Infections in liver transplant recipients. World J Hepatol. 2011;3(4):83–92. doi: 10.4254/wjh.v3.i4.83. [DOI] [PMC free article] [PubMed] [Google Scholar]