Abstract
Objective
To determine predictors of in-hospital mortality related to COVID-19 in older patients.
Design
Retrospective cohort study.
Setting and Participants
Patients aged 65 years and older hospitalized for a diagnosis of COVID-19.
Methods
Data from hospital admission were collected from the electronic medical records. Logistic regression and Cox proportional hazard models were used to predict mortality, our primary outcome. Variables at hospital admission were categorized according to the following domains: demographics, clinical history, comorbidities, previous treatment, clinical status, vital signs, clinical scales and scores, routine laboratory analysis, and imaging results.
Results
Of a total of 235 Caucasian patients, 43% were male, with a mean age of 86 ± 6.5 years. Seventy-six patients (32%) died. Nonsurvivors had a shorter number of days from initial symptoms to hospitalization (P = .007) and the length of stay in acute wards than survivors (P < .001). Similarly, they had a higher prevalence of heart failure (P = .044), peripheral artery disease (P = .009), crackles at clinical status (P < .001), respiratory rate (P = .005), oxygen support needs (P < .001), C-reactive protein (P < .001), bilateral and peripheral infiltrates on chest radiographs (P = .001), and a lower prevalence of headache (P = .009). Furthermore, nonsurvivors were more often frail (P < .001), with worse functional status (P < .001), higher comorbidity burden (P < .001), and delirium at admission (P = .007). A multivariable Cox model showed that male sex (HR 4.00, 95% CI 2.08-7.71, P < .001), increased fraction of inspired oxygen (HR 1.06, 95% CI 1.03-1.09, P < .001), and crackles (HR 2.42, 95% CI 1.15-6.06, P = .019) were the best predictors of mortality, while better functional status was protective (HR 0.98, 95% CI 0.97-0.99, P = .001).
Conclusions and implications
In older patients hospitalized for COVID-19, male sex, crackles, a higher fraction of inspired oxygen, and functionality were independent risk factors of mortality. These routine parameters, and not differences in age, should be used to evaluate prognosis in older patients.
Keywords: Mortality, COVID-19, older patients
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) pneumonia is a new form of viral pneumonia first described at the end of 2019.1 During the first 3 months of 2020, many descriptive articles about this new viral infection have led to a better understanding of the disease.2, 3, 4, 5 Older patients in the general population are at higher risk of mortality.6 In a study reporting the clinical characteristics of patients infected with SARS-CoV-2, the fatality rate was 18.8% for patients older than 80 years7 whereas the overall fatality rate is estimated at up to 5%.2 , 8 Observational Chinese cohort studies reported that the presence of comorbidities, old age, and male sex were associated with a higher rate of severe disease course and mortality. The 3 most commonly found comorbidities were hypertension, cardiovascular disease, and diabetes.9, 10, 11 Although most of the studies were not stratified by age groups, the authors found that patients older than 65 years had a higher prevalence of comorbidities, more severe symptoms, more laboratory abnormalities, and were more likely to develop multiorgan failure and die.12 A recent Chinese study including 244 adults with a median age of 67 years, and no patient older than 72 years, showed that age and lower lymphocyte count were associated with in-hospital death.13 Moreover, the median number of days from the occurrence of the first symptom to death tended to be shorter among people older than 70 years as compared to younger people.14 This study aims to determine the risk factors for in-hospital mortality related to COVID-19 in the older patients. We hypothesized that functionality, comorbidities, and frailty at hospital admission are better predictors of mortality in older patients than age per se.
Methods
Design, Setting, and Study Population
This retrospective monocentric cohort study included all patients aged ≥65 years with a confirmed diagnosis of COVID-19 hospitalized between March 13 and April 14, 2020. This hospital system served as the referral center for all acutely ill patients with COVID-19 requiring medical inpatient care during the SARS-CoV-2 pandemic (criteria for hospitalization as listed, eg, in Supplementary Method 1). The University Hospitals are composed of 8 hospitals in the region, with 1800 beds. The Geriatric Hospital is one of those hospitals, composed of 296 beds, 196 beds for acute geriatric care and 100 for geriatric rehabilitation. During the period of the study, 176 beds from these acute care units were used for COVID-19 acute hospitalizations. The hospital covers a region with approximately 500,000 inhabitants. Older patients ineligible for intensive care according to goals of care determinations were oriented to acute geriatric wards. Patients were ineligible for intensive care unit admission because they did not wish or were deemed ineligible for potential admission to intensive care, according to a global assessment taking into consideration the severity of the disease, underlying comorbidities, and the patient's and his or her proxy's wishes. These wards are managed by medical staff trained in internal medicine and geriatrics and can provide general acute medical care, such as intravenous treatments and noninvasive oxygen support (nasal cannula and face mask, allowing an increase of the fraction of inspired oxygen from 24% to 65%). Criteria for quitting the unit was an improvement of the acute illness with subsequent transfer to rehabilitation wards or discharge at home directly. For patients presenting a worsening of symptoms and evolution, end-of-life care was implemented in the wards together with palliative mobile teams.
The diagnosis of COVID-19 was defined by a positive reverse transcriptase–polymerase chain reaction (RT-PCR) for the SARS-CoV-2 on nasopharyngeal swabs. Patients with negative virus detection in the RT-PCR but with a high clinical suspicion were also diagnosed with COVID-19. High clinical suspicion was defined as the presence of suggestive clinical symptoms and radiologic findings consistent with COVID-19.15 Furthermore, COVID-19 pneumonia was defined as the presence of cough and at least 1 associated respiratory sign and/or symptom, with fever for more than 4 days, chest imaging consistent with COVID-19.
The data were collected and analyzed once all included cases either died or were discharged alive from acute geriatric care.
Data Collection
The data of this retrospective study was retrieved from the electronic patient record system. For each included patient, collected data were categorized according to the following domains: demographics, clinical history, previous treatment, clinical status, vital signs, comorbidities, clinical scales and scores, routine laboratory analysis, chest imaging, and antiviral treatments according to local therapeutic guidelines. In addition, we documented COVID-19–associated complications such as acute respiratory distress syndrome (ARDS; presence of respiratory failure symptoms, with compatible chest radiograph findings and moderate to severe hypoxemia),16 acute kidney injury (KDIGO recommendations),17 and acute cardiac injury (presence of acute electrocardiography or echocardiography modifications, followed or not by an increase of high-sensitivity cardiac troponin I levels).
The following scores and scales were computed and documented in medical records: delirium screening by Confusion Assessment Method (CAM),18 comorbidity burden by Cumulative Illness Rating Scale–Geriatric (CIRS-G),19 functional state by Functional Independence Measure (FIM),20 frailty by Clinical Frailty Scale (CFS),21 and severity of pneumonia by Pneumonia Severity Index22 and CURB-65.23 With the exception of the CFS, all scales mentioned above are performed within the first 24h after hospitalization according to our local guidelines. All scales were performed by clinicians in charge of the patient. They are integrated in the geriatric assessment performed by the interdisciplinary team as part of our routine protocols. All teams receive the same training to do this assessment. The CFS was performed retrospectively by clinicians in charge and documented in medical records before data collection for this study, as a 9-point scale based on clinical judgment varying from 1 = very fit to 9 = terminally ill. The CIRS-G measures the chronic medical illness (“morbidity”) burden in 14 individual body systems and grades each from 0 (no disease) to 4 (very severe). The total score ranges from 0 to 56 points. The CAM is the standard screening tool to detect delirium in medical and surgical settings. It integrates information from clinical assessment and diagnostic criteria to determine whether delirium is present. The FIM takes into account physical, psychological, and social functions, such as activities of self-care, sphincter control, locomotion, mobility or transfer, and social cognition. For each evaluated activity, the score ranges from 1 = totally dependent to 7 = totally independent. The scoring system ranges from 18 points (extreme disability) to 126 points (complete independence). Finally, the Pneumonia Severity Index is a prognostic score for community-acquired pneumonia, with 5 categories based on clinical, laboratory, and imaging factors that predict mortality and CURB-65 is a 4-item score to estimate the mortality related to community-acquired pneumonia and to help determine the need for inpatient vs outpatient treatment.
Vital signs were automatically extracted from the electronic patient records, including measures performed during the first 24 hours from admission. Oxygen support was categorized as (1) nasal cannula and (2) face mask, allowing an increase of the fraction of inspired oxygen (FiO2) from 24% to 65%. The highest FiO2 was documented during the first 24 hours of hospitalization. Imaging features from chest radiograph performed on admission or the date closest to admission were collected for the analysis.
Statistical Analysis
The Shapiro-Francia test was applied to assess whether the distribution of continuous variables was Gaussian. Lymphocyte count was the only variable that needed to be normalized using a natural log transformation. Baseline continuous variables were presented as means ± SD, and categorical and binary variables as absolute numbers and proportions. They were compared between survivors and nonsurvivors by Fisher exact, Mann-Whitney U, or 2-tailed t tests, according to the variable type. The uncorrected P values were presented in the tables along with an asterisk (∗), when they were below the significance threshold determined by the Benjamini-Hochberg method,24 a correction for multiple analyses, which was applied by domains. Significant variables were used as independent variables in univariate and sex-adjusted Cox regressions to predict survival time. The remaining significant predictors were then pooled by domains, and stepwise forward Cox survival models were built with a significance level for addition to the model set at P < .10 and a probability to be removed, set at .20. To finalize the variable reduction process, we performed a last stepwise forward Cox model with a P to enter set at <.05 and a P to remove set at <.10. Results are presented as hazard ratios (HRs) along with their 95% confidence intervals (CIs).
The proportional-hazards assumption was tested with Schoenfeld residuals (eg, Supplementary Method 2). Kaplan-Meier curves were drawn for the best predictors and compared using log-rank tests. For this analysis, we considered functional status according to FIM in 3 categories: 0-49, 50-99, and 100-126.25 Stepwise forward competing-risks regression were also performed using Stata's stcrreg command, the 2 competing risks being death and discharged alive.
Multiple logistic regression models were used to predict mortality with the same data reduction process. Results are presented as odds ratio along with their 95% CIs. Then, we studied the association with the best predictors by calculating the area under the receiver operating characteristic (AUC) curves, using Stata's roccomp command. We calculated sensitivity, specificity, positive predictive value, and negative predictive value according to the optimum criterion value determined by the Youden index (sensitivity + specificity – 1). Data analyses were performed with Stata, version 16.1 (StataCorp, College Station, Texas, 2019).
Power analyses are defined in Supplementary Method 3.
Patient and Public Involvement
Because of the urgent need to develop knowledge regarding COVID-19, patients and members of the public were not directly involved in the study conception.
Results
Characteristics of the Study Population
The COVIDAge study included 235 Caucasian patients, 102 (43%) male, and mean age 86 ± 6.5 years (Table 1 ). The youngest patient was 65 years old and the oldest 101 years old. In total, 76 patients died (28 women, 48 men), resulting in a high mortality rate of 32% (women 21% and men 47%). The mean length of stay was 12.8 ± 7.6 days. The RT-PCR was positive in 92.8% of cases, and a diagnosis of SARS-CoV-2 pneumonia was documented in 65.2% of cases.
Table 1.
Characteristics of the Study Population and Comparison Between COVID-19 Survivors and Nonsurvivors
| n | Total (N = 235) | Survivors (n = 159) | Nonsurvivors (n = 76) | P value | BH | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, y | 235 | 86.3 ± 6.5 | 86.0 ± 6.5 | 86.9 ± 6.4 | .30 | |
| Length of stay, d | 235 | 12.8 ± 7.6 | 14.8 ± 7.6 | 8.7 ± 5.7 | <.001 | ∗ |
| Female sex | 235 | 133 (56.6) | 105 (66.0) | 28 (36.8) | <.001 | ∗ |
| Hospitalization in the past 6 mo | 235 | 103 (44.2) | 71 (45.2) | 32 (42.1) | .68 | |
| Exposure history with direct contact to known COVID-19 patients | 235 | 50 (21.6) | 39 (25.0) | 11 (14.7) | .26 | |
| Number of medications | 235 | 7.5 ± 4.1 | 7.1 ± 4.1 | 8.2 ± 3.8 | .04 | ∗ |
| Clinical history | 235 | |||||
| Positive RT-PCR for SARS-CoV-2 | 235 | 218 (92.8) | 146 (91.8) | 72 (94.7) | .59 | |
| Heart failure | 235 | 66 (28.1) | 38 (23.9) | 28 (36.8) | .044 | ∗ |
| COPD | 235 | 25 (10.6) | 17 (10.7) | 8 (10.5) | >.99 | |
| Smoking | 235 | .85 | ||||
| No smoking | 235 | 153 (65.7) | 103 (65.2) | 50 (66.7) | ||
| Past | 235 | 68 (29.2) | 47 (29.7) | 21 (28.0) | ||
| Present | 235 | 12 (5.2) | 8 (5.1) | 4 (5.3) | ||
| Chronic kidney disease | 235 | 62 (26.5) | 41 (25.8) | 21 (28.0) | .75 | |
| Chronic liver disease | 235 | 23 (9.8) | 13 (8.2) | 10 (13.2) | .25 | |
| Diabetes under treatment | 235 | 54 (23.0) | 31 (19.5) | 23 (30.3) | .07 | |
| Stroke | 235 | 46 (19.8) | 31 (19.6) | 15 (20.3) | >.99 | |
| Parkinson's disease | 235 | 8 (3.5) | 6 (3.8) | 2 (2.8) | >.99 | |
| Cognitive disorders | 235 | 119 (50.6) | 81 (50.9) | 38 (50.0) | >.99 | |
| Known swallowing disorders | 235 | 23 (9.8) | 14 (8.9) | 9 (11.8) | .49 | |
| Active neoplasia | 235 | 22 (9.4) | 13 (8.2) | 9 (11.8) | .47 | |
| Immunosuppression | 235 | 12 (5.1) | 8 (5.0) | 4 (5.3) | >.99 | |
| History of coronary syndrome | 235 | 34 (14.5) | 19 (11.9) | 15 (19.7) | .12 | |
| Peripheral artery disease | 235 | 27 (11.5) | 12 (7.5) | 15 (19.7) | .009 | ∗ |
| Atrial fibrillation | 235 | 58 (24.7) | 37 (23.3) | 21 (27.6) | .52 | |
| VTE/DVT | 235 | 23 (9.8) | 15 (9.4) | 8 (10.5) | .82 | |
| Hypertension | 235 | 168 (71.5) | 112 (70.4) | 56 (73.7) | .65 | |
| Dyslipidemia | 235 | 84 (35.7) | 56 (35.2) | 28 (36.8) | .88 | |
| Scales and scores | ||||||
| CIRS-G19 | 235 | 19.1 ± 6.2 | 18.1 ± 6.3 | 21.2 ± 5.3 | <.001 | ∗ |
| FIM20 | 183 | 72.0 ± 29.4 | 76.8 ± 27.9 | 56.8 ± 29.1 | <.001 | ∗ |
| CAM18 | 220 | 27 (12.3) | 12 (8.0) | 15 (21.4) | .007 | ∗ |
| Frailty | 235 | 5.8 ± 1.6 | 5.5 ± 1.5 | 6.5 ± 1.4 | <.001 | ∗ |
| Frailty ≥ 5 | 235 | 185 (80.4) | 114 (73.5) | 71 (94.7) | <.001 | ∗ |
| PSI | 235 | 126.9 ± 55.8 | 122.7 ± 65.2 | 135.8 ± 25.7 | .029 | ∗ |
| Clinical status | 235 | |||||
| Time from first symptoms to admission, days | 235 | 4.4 ± 5.1 | 5.0 ± 5.6 | 3.3 ± 3.6 | .007 | ∗ |
| Asymptomatic | 235 | 18 (7.8) | 15 (9.6) | 3 (4.0) | .19 | |
| Cough | 235 | 144 (61.3) | 95 (59.7) | 49 (64.5) | .57 | |
| Sputum production | 235 | 47 (20.0) | 28 (17.6) | 19 (25.0) | .22 | |
| Myalgia | 235 | 26 (11.2) | 19 (12.2) | 7 (9.2) | .66 | |
| Tiredness | 235 | 104 (44.3) | 72 (45.3) | 32 (42.1) | .68 | |
| Headache | 235 | 19 (8.1) | 18 (11.3) | 1 (1.3) | .009 | ∗ |
| Anorexia | 235 | 48 (20.6) | 39 (24.7) | 9 (12.0) | .025 | ∗ |
| Rhinorrhea | 235 | 20 (8.5) | 15 (9.4) | 5 (6.6) | .62 | |
| Diarrhea | 235 | 28 (11.9) | 23 (14.5) | 5 (6.6) | .09 | |
| Nausea and vomiting | 235 | 23 (9.8) | 15 (9.5) | 8 (10.5) | .82 | |
| Asthenia | 235 | 117 (49.8) | 71 (44.7) | 46 (60.5) | .026 | |
| Dyspnea | 235 | 82 (34.9) | 48 (30.2) | 34 (44.7) | .040 | ∗ |
| Crackles | 235 | 157 (66.8) | 93 (58.5) | 64 (84.2) | <.001 | ∗ |
| Heart failure signs | 235 | 42 (18.0) | 21 (13.4) | 21 (27.6) | .011 | ∗ |
| Vital signs | 235 | |||||
| Body temperature, °C | 235 | 38.1 ± 0.8 | 38.1 ± 0.8 | 38.2 ± 0.9 | .23 | |
| Respiratory rate, breath/minute | 235 | 26.2 ± 8.9 | 25.0 ± 8.7 | 28.6 ± 8.9 | .005 | ∗ |
| O2 mode | 235 | <.001 | ∗ | |||
| No O2 support | 235 | 118 (50.2) | 94 (59.1) | 24 (31.6) | ||
| O2 nasal cannula | 235 | 99 (42.1) | 60 (37.7) | 39 (51.3) | ||
| O2 mask | 235 | 18 (7.7) | 5 (3.1) | 13 (17.1) | ||
| FiO2, % | 235 | 26.3 ± 9.5 | 24.2 ± 5.6 | 30.9 ± 13.5 | <.001 | ∗ |
| HR, bit/minute | 235 | 93.1 ± 17.1 | 90.9 ± 15.0 | 97.7 ± 20.4 | .011 | ∗ |
| BP mean_min, mmHg | 235 | 75.0 ± 14.4 | 75.1 ± 14.8 | 74.8 ± 13.7 | .87 | |
| BP mean_max, mmHg | 235 | 103.2 ± 19.8 | 102.6 ± 18.8 | 104.6 ± 21.8 | .48 | |
| Laboratory | ||||||
| Logn(Lymphocytes), log 109/L | 225 | 0.09 ± 0.04 | 0.01 ± 0.04 | 0.28 ± 0.09 | .008 | |
| Lymphocytes <1.0 × 109/L | 95 | 95 (42.2) | 76 (49.4) | 19 (26.8) | .001 | ∗ |
| Lymphocytes ≥1.0 × 109/L | 130 | 130 (57.8) | 78 (50.6) | 52 (73.2) | ||
| Thrombocytes, 109/L | 234 | 215.1 ± 99.3 | 222.7 ± 98.2 | 199.0 ± 100.2 | .09 | |
| C-reactive protein, mg/L | 229 | 66.3 ± 69.9 | 52.4 ± 51.3 | 96.0 ± 92.3 | <.001 | ∗ |
| Urea, mmol/L | 229 | 10.1 ± 9.3 | 9.3 ± 9.4 | 11.8 ± 8.7 | .05 | |
| Creatinine, μmol/L | 231 | 110.3 ± 81.4 | 99.4 ± 59.6 | 133.4 ± 111.7 | .016 | ∗ |
| eGFR, mL/min per 1.73 m2 | 231 | 55.6 ± 21.6 | 58.2 ± 20.4 | 50.2 ± 23.0 | .013 | ∗ |
| Imaging | ||||||
| Infiltrate | 225 | 131 (58.2) | 79 (51.6) | 52 (72.2) | .004 | ∗ |
| Unilateral | 225 | 55 (24.4) | 39 (25.5) | 16 (22.2) | .62 | |
| Bilateral | 225 | 76 (33.8) | 40 (26.1) | 36 (50.0) | .001 | ∗ |
| Peripheral | 225 | 107 (47.6) | 61 (39.9) | 46 (63.9) | .001 | ∗ |
| Central | 225 | 53 (23.6) | 31 (20.3) | 22 (30.6) | .10 |
BH, Benjamini-Hochberg; BP, blood pressure; COPD, chronic obstructive pulmonary disease; PSI, Pneumonia Severity Index21; VTE/DVT, venous thromboembolism/deep vein thrombosis.
Data are expressed as n (%) or mean ± standard deviation. P values were calculated by Fisher exact test, t test or Mann-Whitney U test according to variable type. BH correction for multiple analysis was applied, ∗ meaning that P values remained statistically significant.
Up to half of our patients benefited from formal help in activities of daily living, and 55.1% lived alone before hospitalization. Although 21.1% lived in nursing homes, 4.7% of patients lived in enriched housing, where formal help in activities of daily living is available during the day but not systematically introduced to all residents (Supplementary Table 1). A hospitalization in the past 6 months was documented in 44.2% of cases. The overall comorbidity burden was high (19 points at CIRS-G), associated with higher functional decline (72 points from total of 126 at FIM) and frailty (80.4% of patients were considered frail with a CFS score ≥5) at admission.
Hypertension was present in 71.5% of patients; cognitive impairment in 50%; dyslipidemia in 35.7%; and heart failure, chronic kidney disease, atrial fibrillation, and diabetes were present in 28.1%, 26.5%, 24.7%, and 23% of the patients, respectively. Patients took an average of 7.5 ± 4.1 different medications per day before admission. Presenting complaints included cough (61.3%), tiredness (41.3%), dyspnea (34.9%), and anorexia (20.6%). Most of the patients were nonsmokers (65.7%). The majority had crackles at admission (66.8%). Arthralgia, anosmia, ageusia, sore throat, conjunctivitis, and abdominal or chest pain occurred in less than 5% of the cases and were not different in survivors and nonsurvivors. Initial laboratory values showed a mean C-reactive protein level of 66.3 ± 69.9 mg/L (0-10), albumin of 3.66 ± 1.17 g/dL (3.5-5.0), and creatinine of 110.3 ± 81.4 μmol/L (50-110). Lymphocytes were under <1.0 × 109/L in 42.2% of the patients. The most common radiologic features at admission were the presence of an interstitial infiltrate in 58.2% of cases, bilateral in 33.8%, and peripheral in 47.6% (Table 1). Of the total of 159 patients alive by the end of this study, 32 were transferred to rehabilitation wards, 30 to nursing homes, 62 were discharged home directly from acute wards, and 30 were still hospitalized in acute wards.
Comparison Between Survivors and Nonsurvivors
Most of the deceased patients were male (63.2% vs 36.8%, P < .001). There was no significant difference in age between survivors and nonsurvivors (P = .30). Living conditions were similar between survivors and nonsurvivors, as measured by the prevalence of formal help in activities of daily living and instrumental activities of daily living (49.7% and 46.1%, P = .68) and the prevalence of patients living alone (55.5% and 54.4%, P > .99) and in nursing homes (19.2% and 25.0%, P = .07). Patients who died had a higher comorbidity burden (CIRS-G: 21.2 ± 5.3 vs 18.1 ± 6.3, P < .001), with an increased prevalence of heart failure (P = .044) and peripheral artery disease (P = .009). They also had a worse functional status (FIM: 56.8 ± 29.1 vs 76.8 ± 27.9, P < .001), were more frail (CFS score ≥5: 94.7% vs 73.5%, P < .001), and had increased prevalence of delirium at admission (CAM scale positive for 21.4% vs 8%, P = .007). Time between appearance of the first symptoms and admission to hospital was shorter in the group of nonsurvivors (P = .007). Asthenia (P = .026), dyspnea (P = .04), a higher heart rate (P = .011), a higher respiratory rate (P = .005), need for a higher FiO2 (P < .001), and the presence of signs of heart failure (P = .011) and crackles (P < .001) were associated with increased risk of in-hospital mortality. Other comorbidities and clinical findings were not significantly different between the 2 groups (Supplementary Table 1).
Patients who died during their hospital stay took more medications prior to admission than patients who were discharged alive (P = .038). However, there were no differences between the 2 groups regarding the intake of nonsteroidal anti-inflammatory drugs, corticosteroids, immunosuppressants, angiotensin-converting enzyme 2 (ACE2) inhibitors, or angiotensin receptor-II antagonists and anticoagulants (Supplementary Table 1).
The group of nonsurvivors had higher CRP and creatinine values with lower eGFR and serum albumin. The natural logarithm of the number of lymphocytes as well as lymphopenia, defined as number of lymphocytes less than 1.0 × 109/L, were significantly associated with death. On chest radiographs, bilateral and peripheral infiltrates rather than unilateral and central infiltrates, were more frequent in the group of patients who died (P < .001).
COVID-19–Related Complications
The diagnosis of ARDS was present in 10% of patients at admission. A diagnosis of concomitant bacterial pneumonia was documented in 15.2% of cases, whereas acute kidney injury and acute cardiac injury were detected in 20.7% and 12.9% of patients, respectively. There was no significant difference between survivors and nonsurvivors regarding the prevalence of these complications.
Specific Antiviral Treatment
Lopinavir or ritonavir alone had been administered to 8% of the patients and hydroxychloroquine alone to 7%, and 3% of the patients received both drugs. Additionally, 69.36% of patients received antibiotics at admission. There was no significant difference between the 2 groups of patients.
Risk Factors of Mortality
With the first step of the data selection process, the number of variables was reduced from 107 to 30, as described in the Methods section. These variables were then analyzed in univariate Cox models (Model 1, Table 2 ) and after adjustment for sex (Model 2, Table 2). In the univariate models, the risk of death increased by 6% for every point added in the CIRS-G, whereas being frail increased the risk by 5 times. The presence of either delirium, peripheral arterial disease, lymphocytes <1.0 × 109/L, or pulmonary infiltrate doubled the risk of dying. Multivariable Cox regression model showed that male sex (HR 4.00, 95% CI 2.08-7.71, P < .001), the need for an increased FiO2 (HR 1.06, 95% CI 1.03-1.09, P < .001), and the presence of crackles at admission (HR 2.42, 95% CI 1.15-6.06, P = .019) were predictors of mortality, whereas the absence of functional decline was protective (HR 0.98, 95% CI 0.97-0.99, P = .001).
Table 2.
Univariate and Sex-Adjusted Cox Regressions to Predict Survival Time
| n | Model 1: Univariate |
Model 2: Adjusted for Sex |
|||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | R2 % | HR (95% CI) | P value | R2 % | ||
| Male sex | 235 | 2.65 (1.66-4.24) | <.001 | 12.8 | — | — | — |
| No. of medications | 230 | 1.45 (1.01-2.09) | .045 | 3.6 | 1.32 (0.92-1.88) | .13 | 3.6 |
| Peripheral artery disease | 235 | 2.08 (1.18-3.66) | .011 | 3.6 | 1.72 (0.97-3.05) | .06 | 3.6 |
| Heart failure | 235 | 1.54 (0.96-2.45) | .07 | 1.7 | 1.51 (0.95-2.40) | .09 | 1.7 |
| Cough | 235 | 1.18 (0.74-1.88) | .50 | 0.4 | 1.13 (0.71-1.81) | .61 | 0.4 |
| Headache | 234 | 0.13 (0.02-0.95) | .044 | 6.0 | 0.15 (0.02-1.09) | .06 | 6.0 |
| Anorexia | 233 | 0.49 (0.24-0.98) | .044 | 3.0 | 0.47 (0.23-0.94) | .034 | 3.0 |
| Dyspnea | 235 | 1.51 (0.96-2.37) | .08 | 1.6 | 1.63 (1.03-2.56) | .036 | 1.6 |
| Crackles | 235 | 2.93 (1.58-5.42) | .001 | 5.3 | 2.54 (1.36-4.73) | .003 | 10.6 |
| Cardiac failure signs | 233 | 1.78 (1.07-2.95) | .027 | 2.7 | 1.77 (1.07-2.94) | .03 | 2.8 |
| Asthenia | 235 | 1.43 (0.90-2.27) | .13 | 1.1 | 1.40 (0.88-2.22) | .15 | 1.0 |
| CURB-65 | 235 | 1.24 (0.96-1.62) | .11 | 1.2 | 1.18 (0.90-1.55) | .22 | 1.2 |
| CAM18 | 220 | 2.09 (1.18-3.70) | .011 | 3.9 | 2.11 (1.19-3.74) | .011 | 3.9 |
| Frailty | 235 | 1.44 (1.23-1.69) | <.001 | 16.5 | 1.46 (1.24-1.70) | <.001 | 16.5 |
| Frailty ≥5 | 235 | 4.65 (1.70-12.72) | .003 | 1.0 | 4.39 (1.60-12.02) | .004 | 10.5 |
| FIM20 | 183 | 0.98 (0.97-0.99) | <.001 | 18.5 | 0.98 (0.97-0.99) | .001 | 18.5 |
| CIRS-G19 | 235 | 1.06 (1.03-1.10) | .001 | 8.0 | 1.05 (1.01-1.09) | .007 | 8.0 |
| FiO2 | 235 | 1.05 (1.03-1.07) | <.001 | 18.8 | 1.05 (1.04-1.07) | <.001 | 18.8 |
| O2 nasal cannula | 235 | 1.79 (1.07-3.00) | .026 | 12.6 | 1.82 (1.09-3.04) | .020 | 12.6 |
| O2 mask | 235 | 4.72 (2.39-9.32) | <.001 | — | 4.48 (2.27-8.85) | <.001 | — |
| Heart rate | 235 | 1.02 (1.00-1.03) | .009 | 4.6 | 1.02 (1.00-1.03) | .005 | 4.6 |
| Respiratory rate | 235 | 1.03 (1.01-1.04) | .008 | 4.7 | 1.03 (1.01-1.05) | <.001 | 4.7 |
| Lymphocytes <1.0 × 109/L | 225 | 2.08 (1.23-3.52) | .006 | 6.1 | 1.79 (1.05-3.05) | .031 | 18.4 |
| C-reactive protein, mg/L | 229 | 1.01 (1.00-1.01) | <.001 | 14.3 | 1.01 (1.00-1.01) | <.001 | 14.3 |
| Creatinine, μmol/L | 231 | 1.0026 (1.0008-1.0045) | .005 | 3.8 | 1.0015 (0.9994-1.0036) | .16 | 3.8 |
| eGFR, mL/min/1.73m2 | 231 | 0.9883 (0.9778-0.9990) | .036 | 2.8 | 0.9914 (0.9811-1.0017) | .10 | 2.8 |
| Albumin, g/dL | 156 | 0.93 (0.89-0.97) | .002 | 11.9 | 0.94 (0.90-0.98) | .009 | 11.9 |
| Pulmonary infiltrate | 225 | 2.12 (1.26-3.54) | .004 | 6.6 | 1.86 (1.11-3.13) | .020 | 6.5 |
| Bilateral | 225 | 2.50 (1.57-3.97) | <.001 | 11.1 | 2.26 (1.42-3.60) | .001 | 11.2 |
| Peripheral | 225 | 2.26 (1.39-3.65) | .001 | 8.7 | 2.04 (1.26-3.32) | .004 | 8.7 |
R2, coefficient of determination.
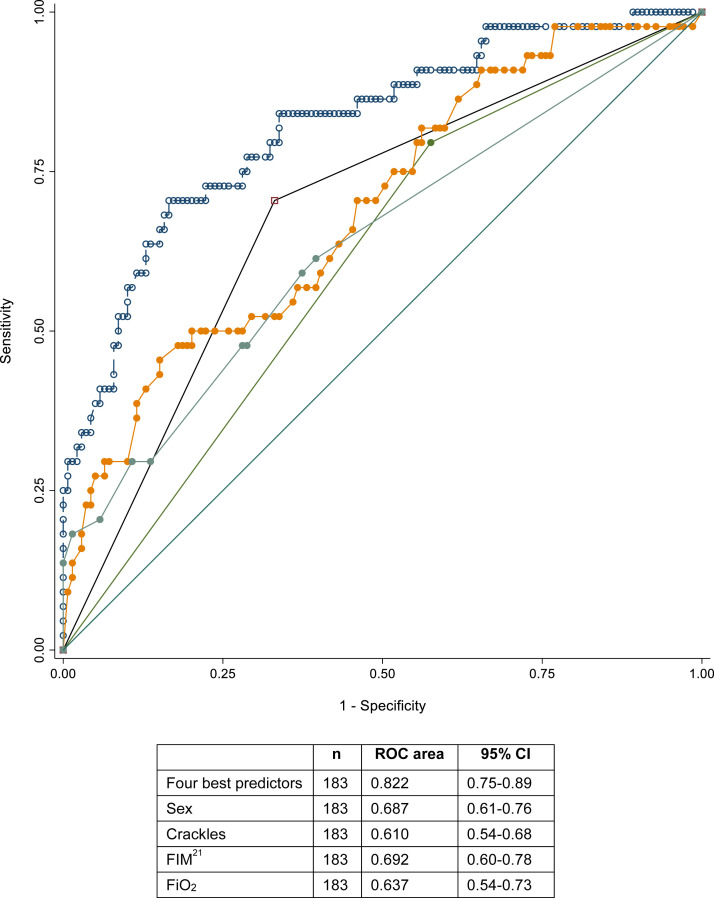
We performed univariate and multivariable logistic regression models to evaluate whether mortality could be predicted on an individual basis. We obtained the same best predictors as when using the Cox model, the pseudo-R 2 was 27.4%, the area under the receiver operating characteristic curve 0.822, sensitivity 0.409, specificity 0.935, positive predictive value 0.667, and negative predictive value 0.833 (Figure 1 ). This was not sufficient to predict mortality on a per-patient level. Stepwise forward competing-risks regression selected the same best predictors and resulted in a similar model (Supplementary Table 2).
Fig. 1.
Receiver operating characteristic (ROC) curve from the logistic regression models to predict mortality, including 4 predictors:  Male sex;
Male sex;  Crackles;
Crackles;  FiO2 %;
FiO2 %;  FIM; and
FIM; and  the 4 best predictors together.
the 4 best predictors together.
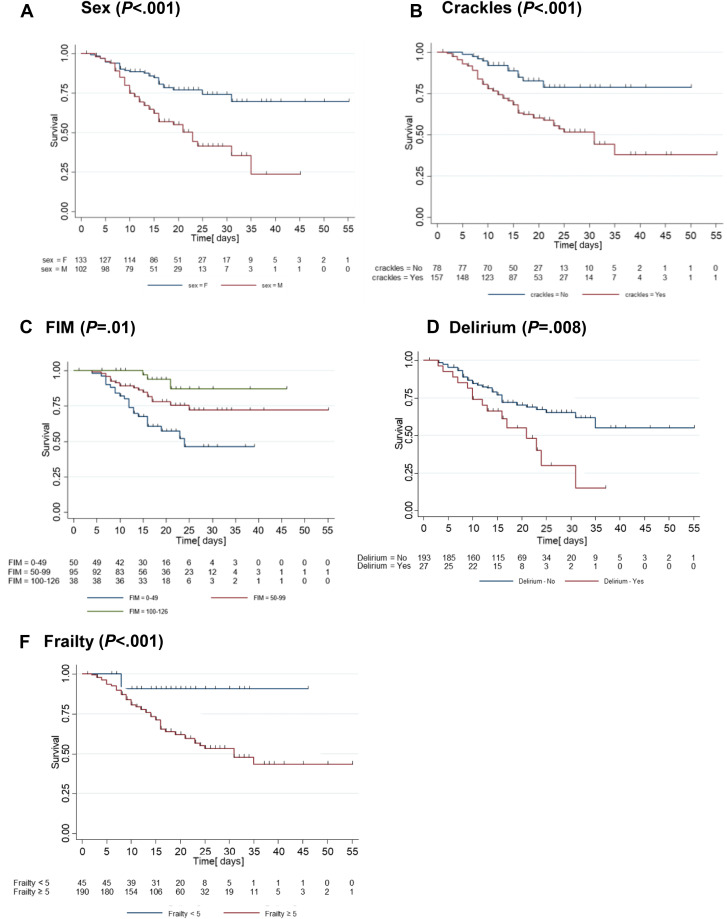
Survival rates analyses by Kaplan-Meier curves showed that female sex and absence of crackles were associated with a 70% or higher chance of surviving until discharge (Figure 2 ). Frailty score lower than 5 and FIM greater than 100 were associated with a chance of survival higher than 85%, whereas the presence of delirium was associated with a survival rate of only 10% after 30 days. The Kaplan-Meier survival estimates for the other binary or ordinal variables are available in the Supplemental Material (Supplementary Figure 1).
Fig. 2.
Kaplan-Meier survival curves.
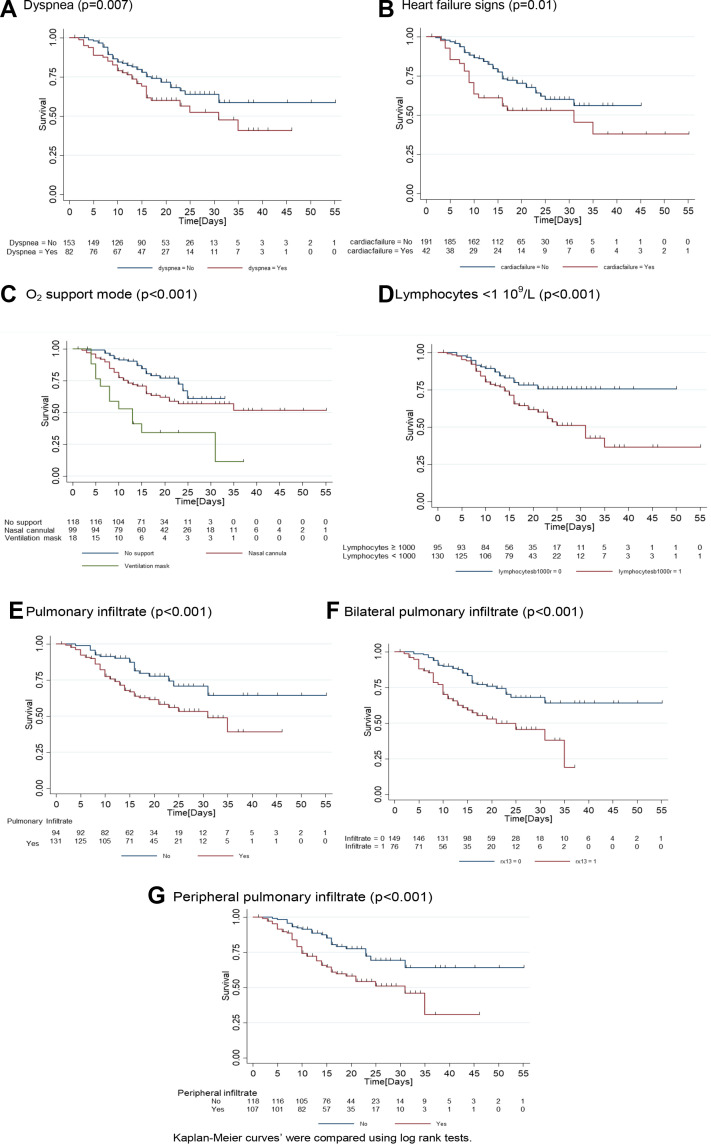
Supplementary Fig. 1.
Kaplan-Meier curves of survival. Tick marks represents patient discharged which were censored. (A) Dyspnea (P = .007); (B) heart failure signs (P = .01); (C) O2 support mode (P < .001); (D) lymphocytes < 1 × 109/L (P < .001); (E) pulmonary infiltrate (P < .001); (F) bilateral pulmonary infiltrate (P < .001); and (G) peripheral pulmonary infiltrate (P < .001). Kaplan-Meier curves were compared using log rank tests.
Discussion
We report an elevated mortality rate of 32%, which is consistent with many previous studies of patients with COVID-19 that underline the marked impact of age on mortality and fatality ratios across the life span, resulting in much higher death rates in the very old.12 , 13 , 26 However, within our population of old hospitalized patients, chronological age alone was no longer a predictor of fatal outcome. This was an important finding that confirms prior studies in other very old populations where comorbidities, functional status, and frailty play a crucial role compared with chronological age alone.27, 28, 29, 30, 31, 32 Studies on risk factors of mortality in community-acquired pneumonia underlined the significant interplay with comorbidities.28 , 30 , 31 Functional status is often associated with multiple chronic diseases that together will impact health outcomes,32 thus representing the key factors for determining prognosis in very old patients.29 In COVIDAge, each additional point on the FIM (score range 18-126) decreased the risk of dying by 2%. The importance of comorbidities and functionality as predictors of mortality suggests that clinical decisions regarding triage workflows and treatment options should incorporate a comprehensive assessment of these factors.
Functional metrics in acute care are often the result of many factors beyond functional status alone. In this study, we used the FIM, which reflects both physical function and activities of daily living, being the result of the severity of the acute illness (mobility restrictions due to dyspnea, presence of delirium, etc), but also chronic conditions exacerbated or not by the acute disease. As for many variables in geriatric assessment, we may observe multicollinearity between measures, as FIM may be a proxy for the severity of acute illness, but also the overall comorbidity burden, the severity of dementia in a population with a high prevalence of this condition, as well as frailty, delirium, etc. Although these variables are mutually associated, metrics documented inside the hospital are reliable indicators in well-trained teams such as ours. However, the fact that worse functionality according to FIM is associated with mortality does not mean that functional decline is the main pathophysiological mechanism underlying the cause of death. Frailty, delirium, and severity of respiratory symptoms are all associated with this measure. We believe that there are 2 questions to be answered: (1) Which mechanism is associated with the increased mortality observed in older patients with COVID-19? and (2) Which measures from clinical routine assessment in acute geriatric settings may have prognostic value in older patients with COVID-19? Our results point out concrete directions to answer the second question and provide hypothesis to be analyzed by future longitudinal studies for the first one.
The best predictive model of in-hospital mortality in the COVIDAge study included 4 clinical variables that can be measured easily on admission: male sex, presence of crackles, need for increased oxygen support, and functional decline. This model explained 59% of the variability related to risk of death; 18.5% of the variability was explained by the FIM (slightly more than the predictive power of FiO2). It was consistent with the view that marked variability in health status of older patients, and consequently mortality was better explained by functionality.27 The strong effect of sex was an expected finding given the results of other studies of COVID-19–related mortality in populations of all ages.3 , 19 Male patients were up to 3 times more likely to die than females. A higher rate of SARS-CoV-2 IgG in female patients has been highlighted,33 but the exact mechanism contributing to this difference needs further investigation. Recent publications addressed the hypothesis of sex and age differences regarding ACE2 expression that is higher in females.34 , 35 Hormonal mediators play a role in immune responses and could lead to a greater protection in women.36
The 2 other predictive items in the multivariable Cox model were related to the presence and severity of pneumonia, the most common pathology leading to complications and death in SARS-CoV-2 infection. Every 2% increase in FiO2 added 7% of risk of dying. This was in accordance with a previous study showing that symptoms related to hypoxemia were more common in patients who died.9
Frail patients are at increased risk of worse outcomes in the acute setting such as disability, institutionalization, and death.37 Recently, frailty assessment according to the CFS was proposed by several institutions as a triage instrument in the COVID-19 pandemic.38 , 39 In the COVIDAge study, patients with low scores on the CFS had very high survival rates (90%). However, physicians caring for older patients should proceed with caution before applying this score for medical decisions such as triage to intermediate or intensive care. Despite the high mortality of the COVID-19 population, it is important to note that 45% of those identified as frail (CFS score ≥ 5) survived the acute phase of COVID-19. No scale should be used as a sole indicator in older patients' triage. Although some of those metrics seem to have a strong association with mortality and other prognostic factors, we believe the global geriatric assessment, combined with preferences of patients and their proxies in the process of shared decision making, is crucial to determine goals of care. However, it is important to acknowledge the need for easy, reliable, and reproducible instruments that can be performed by first-line professionals such as in the emergency division, who are not necessarily trained in geriatric care. Specifically, during the COVID-19 crisis when we observed the fast increase in hospital admissions, combining patients' needs with the resources available became a priority question for almost all health systems' organizations. Accurate triage is challenging in geriatrics because of the atypical presentations, higher prevalence of cognitive impairment, and polypharmacy, making it more difficult to quickly establish prognosis in urgent scenarios. The availability of consultants in geriatrics is also a way to help decision making in complex cases through different settings.
A striking result was that only 10% of patients with delirium on admission survived to discharge from acute care. This underlines the importance of screening older hospitalized patients for delirium with simple instruments such as the CAM and is in agreement with other studies that recognized delirium as an independent marker of disease severity.40 , 41
As in previous studies in older patients,42 lymphopenia was frequent in patients with COVID-19 and associated with poor outcome3 , 13 independently of sex. Lymphocyte counts at admission whether treated as a transformed continuous variable or a binary variable were significantly associated with in-hospital mortality but, in contrast to a recent study by Sun,13 was not retained in our best predictive model. This is perhaps related to the high frequency of lymphopenia in Sun's study.
Limitations
The main limitation of COVIDAge is its retrospective monocentric design, with the utilization of information extracted from medical records. Consequently, missing data could not be retrieved. The primary endpoint was in hospital mortality without a fixed follow-up period. However, we were careful to collect data once all inpatients had either died or been discharged from acute geriatric care. We can equally not exclude some variability in clinical scale ratings taking into consideration subjective judgment even though our clinical teams are well trained for this assessment. Importantly, the instruments used to evaluate functional status, comorbidity burden, and frailty have all been proven reliable and have all been validated for use in older populations.
Our results were obtained in Caucasian hospitalized older patients, who did not wish or were deemed ineligible for potential admission to intensive care and they are not generalizable to other populations of advanced age. Risk factors of mortality may differ according to goals of care determined at admission and their consequent limitations of medical treatment. Our results should be interpreted with caution because data from specific populations and clinical scenarios are not suitable to support decisions in triage toward intensive care hospitalizations for example. Further studies comparing older patients' trajectories including those admitted to intensive care will build up the knowledge on worse outcomes' predictors in older patients through different settings.
Conclusion and Implications
Male sex, crackles on lung examination, a need for a higher fraction of inspired oxygen, and functional decline were independent risk factors and the strongest predictors of mortality in Caucasian older patients with COVID-19. Interestingly chronological age alone was not a significant prognostic indicator within this old age group. Our results indicate that practice recommendations for the clinical management of COVID-19 in older patients should not focus on age alone.
Acknowledgments
We acknowledge all health care workers involved in the diagnosis and treatment of patients in Trois-Chêne Hospital site of University Hospitals of Geneva. We thank Drs Lucia Filtri, Maureen Goethals, Viktor Kreyenbuhl-Baptista and Philippe Wollenstein and Mrs Véronique Lachat, Marie-Pierre Meynet and Serenella Ferro-Rojas, for their assistance in data collection. The COVIDAge study did not benefit from any specific funding source.
Footnotes
The authors declare no conflicts of interest.
A.M. and C.S. are co–first authors.
Appendices
Supplementary Table 1.
Characteristics of the Study Population
| n | Total (N = 235) | Survivors (n = 159) | Nonsurvivors (n = 76) | P value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Lives alone | 235 | 102 (55.1) | 71 (55.5) | 31 (54.4) | >.99 |
| Formal help in ADL/IADL | 235 | 114 (48.5) | 79 (49.7) | 35 (46.1) | .68 |
| Uses public transport | 235 | 5 (2.2) | 3 (1.9) | 2 (2.7) | .90 |
| Place of living | 235 | .07 | |||
| Home | 235 | 172 (74.1) | 121 (77.6) | 51 (67.1) | |
| Nursing home | 235 | 49 (21.1) | 30 (19.2) | 19 (25.0) | |
| Enriched housing | 235 | 11 (4.7) | 5 (3.2) | 6 (7.9) | |
| Previous treatment | |||||
| NSAID | 235 | 19 (5.7) | 10 (4.4) | 9 (8.7) | .79 |
| Corticosteroids | 235 | 29 (8.7) | 20 (8.8) | 9 (8.7) | .13 |
| Immunosuppressants | 235 | 4 (1.2) | 3 (1.3) | 1 (1.0) | >.99 |
| ACEi/ARAII | 235 | 83 (25.0) | 58 (25.4) | 25 (24.0) | >.99 |
| Anticoagulant | 235 | 77 (23.2) | 54 (23.7) | 23 (22.1) | .89 |
| Symptoms | |||||
| Arthralgia | 235 | 13 (5.6) | 9 (5.7) | 4 (5.3) | >.99 |
| Agueusia | 235 | 3 (1.3) | 3 (1.9) | 0 (0.0) | .55 |
| Anosmia | 235 | 4 (1.7) | 2 (1.3) | 2 (2.6) | .60 |
| Sore throat | 235 | 7 (3.0) | 6 (3.8) | 1 (1.3) | .44 |
| Conjunctivitis | 235 | 2 (0.9) | 2 (1.3) | 0 (0.0) | >.99 |
| Abdominal pain | 235 | 17 (7.3) | 11 (6.9) | 6 (8.0) | .79 |
| Chest pain | 235 | 10 (4.3) | 5 (3.2) | 5 (6.7) | .30 |
| Score | |||||
| CURB 65 | 235 | .036 | |||
| 1 | 48 (20.4) | 37 (23.3) | 11 (14.5) | ||
| 2 | 104 (44.3) | 72 (45.3) | 32 (42.1) | ||
| 3 | 73 (31.1) | 45 (28.3) | 28 (36.8) | ||
| 4 | 8 (3.4) | 3 (1.9) | 5 (6.6) | ||
| 5 | 2 (0.9) | 2 (1.3) | 0 (0.0) | ||
| Clinical Status | |||||
| BMI | 235 | .31 | |||
| <20 | 235 | 48 (22.3) | 35 (23.8) | 13 (19.1) | |
| 20-24.9 | 235 | 79 (36.7) | 56 (38.1) | 23 (33.8) | |
| 25-29.9 | 235 | 53 (24.7) | 32 (21.8) | 21 (30.9) | |
| 30+ | 235 | 35 (16.3) | 24 (16.3) | 11 (16.2) | |
| Vital signs | |||||
| SO2, % | 235 | 93.0 ± 4.1 | 93.4 ± 3.7 | 92.3 ± 4.9 | .08 |
| BP systolic_min, mmHg | 235 | 111.3 ± 21.9 | 112.6 ± 21.9 | 108.5 ± 21.9 | .18 |
| BP diastolic_min, mmHg | 235 | 55.5 ± 13.5 | 54.9 ± 14.2 | 56.7 ± 11.8 | .31 |
| BP systolic_max, mmHg | 235 | 148.0 ± 23.5 | 148.4 ± 22.2 | 147.1 ± 26.2 | .72 |
| Laboratory | |||||
| Arterial pH | 150 | 7.4 ± 0.1 | 7.4 ± 0.1 | 7.4 ± 0.1 | .84 |
| Paco2, kPa | 150 | 4.7 ± 1.0 | 4.8 ± 1.0 | 4.6 ± 1.1 | .45 |
| Pao2, kPa | 150 | 9.6 ± 5.9 | 9.3 ± 3.6 | 10.2 ± 8.3 | .43 |
| Lactates, IU/L | 134 | 1.5 ± 1.0 | 1.3 ± 0.8 | 1.7 ± 1.2 | .05 |
| Hemoglobin, g/dL | 232 | 119.4 ± 21.8 | 121.2 ± 21.4 | 115.8 ± 22.3 | .09 |
| Hematocrit, % | 232 | 35.5 ± 6.0 | 35.9 ± 5.7 | 34.8 ± 6.4 | .20 |
| Leukocytes, 109/L | 232 | 7.1 ± 4.1 | 6.8 ± 3.7 | 7.8 ± 4.8 | .14 |
| Thrombocytes <150 109/L | 53 | 53 (22.6) | 22 (29.3) | 31 (19.5) | .10 |
| Thrombocytes ≥150 109/L | 181 | 181 (77.4) | 128 (80.5) | 53 (80.5) | |
| 25-hydroxy vitamin D, IU/L | 102 | 57.8 ± 25.7 | 57.7 ± 25.6 | 58.2 ± 26.7 | .93 |
| AST, U/L | 208 | 43.0 ± 54.4 | 36.1 ± 23.2 | 58.0 ± 89.6 | .06 |
| ALT, U/L | 209 | 28.5 ± 56.2 | 24.2 ± 15.5 | 37.8 ± 97.3 | .26 |
| Thromboplastin time | 142 | 33.6 ± 14.4 | 32.4 ± 14.7 | 36.4 ± 13.6 | .12 |
| Chest radiograph | |||||
| Pleural effusion | 225 | 53 (23.8) | 31 (20.5) | 22 (30.6) | .13 |
| Consolidation | 225 | 17 (7.6) | 8 (5.2) | 9 (12.5) | .06 |
ADL/IADL, activities of daily living and instrumental activities of daily living; ARB, angiotensin II receptor blockers; ACEi, angiotensin-converting enzyme inhibitors; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BMI, body mass index; BP, blood pressure; NSAIDs, nonsteroidal anti-inflammatory drugs.
Data are expressed as n (%) or mean ± standard deviation. P values were calculated by Fisher exact test, t test, or Mann-Whitney U test according to variable type.
Supplementary Table 2.
Competing-risks Regression Models to Predict Mortality
| M1: Univariate Model |
M2: Multiple Variable Model |
|||
|---|---|---|---|---|
| SHR (95% CI) | P value | SHR (95% CI) | P value | |
| Male sex | 3.82 (1.96-7.45) | <.0001 | 3.84 (1.96-7.54) | <.0001 |
| Crackles | 2.89 (1.29-6.46) | .010 | 3.02 (1.31-6.95) | .009 |
| FiO2 % | 1.08 (1.06-1.10) | <.0001 | 1.07 (1.05-1.09) | <.0001 |
| FIM | 0.98 (0.97-0.99) | <.0001 | 0.98 (0.97-0.99) | <.0001 |
FIM, Functional Independence Measure; FiO2, fraction of inspired oxygen; SHRs, subhazard ratios.
Supplementary Method 1. Criteria for Hospitalization
The criteria for hospitalization in acute geriatric care for SARS-CoV-2 infection were as follows: (1) age ≥65 years; (2) goals of care established or updated on admission indicating ineligibility or unwillingness to be transferred to intensive care if clinical deterioration occurred; (3) any 1 of the following: (a) pneumonia with a severity assessed by CURB-65 score ≥2, (b) new dependence on oxygen or increase of oxygen needs, (c) a respiratory rate ≥20, (d) decompensated chronic diseases, (e) severely altered general state of health, and (f) deteriorating clinical course.
Supplementary Method 2. Statistical Analysis—Quality of Prediction
The quality of Cox models' prediction was evaluated with the coefficient of determination (pseudo-R 2), which was computed according to the method described by Nagelkerke (1991), modified by O'Quigley, Xu, and Stare (2005), and revised by Royston.7
Supplementary Method 3. Power Analysis
For regression models predicting binary outcomes (logistic, Cox), a sample size of 235 with an expected event rate of 30%, allows the inclusion of up to 7 variables in the models with unbiased results.8
References
- 1.Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of a novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W.J., Ni Z.Y., Hu Y. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu X.W., Wu X.X., Jiang X.G. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: Retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team The epidemiological characteristics of an outbreak of 2019 novel coronavirus disease (COVID-19)-China 2020. China CDC Wkly. 2020;2:113–122. [PMC free article] [PubMed] [Google Scholar]
- 7.Niu S., Tian S., Lou J. Clinical characteristics of older patients infected with COVID-19: A descriptive study. Arch Gerontol Geriatr. 2020;89:104058. doi: 10.1016/j.archger.2020.104058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahase E. Coronavirus covid-19 has killed more people than SARS and MERS combined, despite lower case fatality rate. BMJ. 2020;368:m641. doi: 10.1136/bmj.m641. [DOI] [PubMed] [Google Scholar]
- 9.Chen T., Wu D., Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan W.J., Liang W.H., Zhao Y. Comorbidity and its impact on 1590 patients with Covid-19 in China: A nationwide analysis. Eur Respir J. 2020;55:2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen T., Dai Z., Mo P. Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID-19) in Wuhan, China (2019): A single-centered, retrospective study. J Gerontol A Biol Sci Med Sci. 2020;75:1788–1795. doi: 10.1093/gerona/glaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun H., Ning R., Tao Y. Risk factors for mortality in 244 older adults with COVID-19 in Wuhan, China: A retrospective study. J Am Geriatr Soc. 2020;68(6):E19–E23. doi: 10.1111/jgs.16533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W., Tang J., Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J Med Virol. 2020;92:441–447. doi: 10.1002/jmv.25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fatima S., Ratnani I., Husain M., Surani S. Radiological findings in patients with COVID-19. Cureus. 2020;12:e7651. doi: 10.7759/cureus.7651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferguson N.D., Fan E., Camporota L. The Berlin definition of ARDS: An expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38:1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 17.Kellum J.A., Lameire N. Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1) Crit Care. 2013;17:204. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inouye S.K., van Dyck C.H., Alessi C.A. Clarifying confusion: The confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 19.Salvi F., Miller M.D., Grilli A. A manual of guidelines to score the modified cumulative illness rating scale and its validation in acute hospitalized elderly patients. J Am Geriatr Soc. 2008;56:1926–1931. doi: 10.1111/j.1532-5415.2008.01935.x. [DOI] [PubMed] [Google Scholar]
- 20.Linacre J.M., Heinemann A.W., Wright B.D. The structure and stability of the Functional Independence Measure. Arch Phys Med Rehabil. 1994;75:127–132. [PubMed] [Google Scholar]
- 21.Rockwood K., Song X., MacKnight C. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fine M.J., Auble T.E., Yealy D.M. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 23.Lim W.S., van der Eerden M.M., Laing R. Defining community acquired pneumonia severity on presentation to hospital: An international derivation and validation study. Thorax. 2003;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benjamini Y., Drai D., Elmer G. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 25.Chumney D., Nollinger K., Shesko K. Ability of Functional Independence Measure to accurately predict functional outcome of stroke-specific population: Systematic review. J Rehabil Res Dev. 2010;47:17–29. doi: 10.1682/jrrd.2009.08.0140. [DOI] [PubMed] [Google Scholar]
- 26.Verity R., Okell L.C., Dorigatti I. Estimates of the severity of coronavirus disease 2019: A model-based analysis. Lancet Infect Dis. 2020;20:669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zekry D., Loures Valle B.H., Lardi C. Geriatrics index of comorbidity was the most accurate predictor of death in geriatric hospital among six comorbidity scores. J Clin Epidemiol. 2010;63:1036–1044. doi: 10.1016/j.jclinepi.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 28.Cillóniz C., Dominedò C., Ielpo A. Risk and prognostic factors in very old patients with sepsis secondary to community-acquired pneumonia. J Clin Med. 2019;8:961. doi: 10.3390/jcm8070961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zekry D., Frangos E., Graf C. Diabetes, comorbidities and increased long-term mortality in older patients admitted for geriatric inpatient care. Diabetes Metab. 2012;38:149–155. doi: 10.1016/j.diabet.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Hespanhol V., Barbara C. Pneumonia mortality, comorbidities matter? Pulmonology. 2020;26:123–129. doi: 10.1016/j.pulmoe.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Cillóniz C., Polverino E., Ewig S. Impact of age and comorbidity on cause and outcome in community-acquired pneumonia. Chest. 2013;144:999–1007. doi: 10.1378/chest.13-0062. [DOI] [PubMed] [Google Scholar]
- 32.Wei M.Y., Kabeto M.U., Galecki A.T., Langa K.M. Physical functioning decline and mortality in older adults with multimorbidity: Joint modeling of longitudinal and survival data. J Gerontol A Biol Sci Med Sci. 2019;74:226–232. doi: 10.1093/gerona/gly038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng F., Dai C., Cai P. A comparison study of SARS-CoV-2 IgG antibody between male and female COVID-19 patients: A possible reason underlying different outcome between sex. J Med Virol. 2020 May 8 doi: 10.1002/jmv.25989. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng H., Wang Y., Wang G.Q. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J Med Virol. 2020;92:726–730. doi: 10.1002/jmv.25785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sward P., Edsfeldt A., Reepalu A. Age and sex differences in soluble ACE2 may give insights for COVID-19. Crit Care. 2020;24:221. doi: 10.1186/s13054-020-02942-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaillon S., Berthenet K., Garlanda C. Sexual dimorphism in innate immunity. Clin Rev Allergy Immunol. 2019;56:308–321. doi: 10.1007/s12016-017-8648-x. [DOI] [PubMed] [Google Scholar]
- 37.Clegg A., Young J., Iliffe S. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hubbard R.E., Maier A.B., Hilmer S.N. Frailty in the face of COVID-19. Age Ageing. 2020;49:499–500. doi: 10.1093/ageing/afaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chong E., Chan M., Tan H.N., Lim W.S. COVID-19: Use of the Clinical Frailty Scale for critical care decisions. J Am Geriatr Soc. 2020;68:E30–E32. doi: 10.1111/jgs.16528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witlox J., Eurelings L.S., de Jonghe J.F. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: A meta-analysis. JAMA. 2010;304:443–451. doi: 10.1001/jama.2010.1013. [DOI] [PubMed] [Google Scholar]
- 41.Ely E.W., Shintani A., Truman B. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 42.Liu K., Chen Y., Lin R., Han K. Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients. J Infect. 2020;80:e14–e18. doi: 10.1016/j.jinf.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- 1.Salvi F., Miller M.D., Grilli A. A manual of guidelines to score the modified cumulative illness rating scale and its validation in acute hospitalized elderly patients. J Am Geriatr Soc. 2008;56:1926–1931. doi: 10.1111/j.1532-5415.2008.01935.x. [DOI] [PubMed] [Google Scholar]
- 2.Inouye S.K., van Dyck C.H., Alessi C.A. Clarifying confusion: The confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 3.Rockwood K. Frailty and its definition: A worthy challenge. J Am Geriatr Soc. 2005;53:1069–1070. doi: 10.1111/j.1532-5415.2005.53312.x. [DOI] [PubMed] [Google Scholar]
- 4.Linacre J.M., Heinemann A.W., Wright B.D. The structure and stability of the Functional Independence Measure. Arch Phys Med Rehabil. 1994;75:127–132. [PubMed] [Google Scholar]
- 5.Fine M.J., Auble T.E., Yealy D.M. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 6.Lim W.S., van der Eerden M.M., Laing R. Defining community acquired pneumonia severity on presentation to hospital: An international derivation and validation study. Thorax. 2003;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Royston P. Explained variation for survival models. Stata J. 2006;6:83–96. [Google Scholar]
- 8.Peduzzi P., Concato J., Kemper E. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]