Abstract
OBJECTIVE: We investigated the efficacy and tolerability of nicotinamide cream plus an antibacterial adhesive agent and zinc-pyrrolidone carboxylic acid compared to placebo in patients with moderate acne vulgaris (MAV) in Indonesia. METHODS: This was a multicenter, double-blind, randomized, placebo-controlled, parallel-group study conducted in five teaching hospitals in Indonesia from August 2016 to January 2017. Eligible participants included 140 patients with MAV, aged 12 to 50 years, who were enrolled and randomly divided into two groups to receive either adapalene and the study formulation or adapalene and a placebo cream twice daily for six weeks. Clinical response and treatment efficacy were assessed through acne lesion counts, presence of side effects, and patient satisfaction at the second, fourth, and sixth weeks after the first visit. RESULTS: A total of 140 subjects from five different centers (28 subjects in each center) were enrolled. One hundred twenty-seven subjects completed the study, including 63 subjects in the study group and 64 subjects in the placebo group. A significant decrease in the number of noninflammatory lesions in the second week was noted in the study group compared to in the placebo group. There were no significant differences in adverse effects between the two groups in the second and fourth weeks. CONCLUSION: Treatment using nicotinamide plus an antibacterial adhesive agent and zinc-pyrrolidone carboxylic acid was effective in reducing noninflammatory lesions by the second week of therapy. ClinicalTrials.gov registration no. NCT0326298.
Keywords: Moderate acne vulgaris, nicotinamide plus ABA and zinc-PCA, drug efficacy, drug tolerability
Acne vulgaris (AV) is a chronic inflammation of the pilosebaceous follicle characterized by blackheads, whiteheads, papules, pustules, nodes, and cysts, with sequelae of hyperpigmentation and scarring of the predilection sites, such as the face, neck, upper back, shoulders, and upper arms.1 AV is most prevalent in adolescents and young adults aged 11 to 30 years, affecting 80 percent of this population.2
In 2015, Indonesian acne experts held a meeting to establish standardized Indonesian acne grading and treatment guidelines. Based on the meeting, they recommended the classification of acne severity be done based on the Lehmann criteria of mild, moderate, and severe.1 According to the Lehmann criteria, patients with mild AV have less than 20 comedones, less than 15 inflammatory lesions, or less than 30 total lesions; patients with moderate AV have 20 to 100 comedones, 15 to 50 inflammatory lesions, or 30 to 125 total lesions; and severe AV consists of more than five cysts, more than 100 comedones, more than 50 inflammatory lesions, or more than 125 total lesions.3 In the Indonesian treatment guidelines, AV management is divided into first-line and second-line therapy, with dermocosmetic usage indicated as an adjuvant. Topical first-line therapy includes retinoic acids, such as tretinoin and adapalene, with adapalene being milder than tretinoin in terms of side effects.4 Common side effects of topical retinoids include irritation, erythema, desquamation, dry skin, and subjective complaints, such as tightness, itchiness, and a burning sensation, depending on the concentration of the drug.2,5,6
A nonantibiotic combination treatment consisting of the three active ingredients nicotinamide, an antibacterial adhesive agent (ABA), and zinc has demonstrated efficacy in reducing the side effects of retinoic acid.6 Zinc has shown promise in the treatment of AV in a number of clinical trials.2 Although its role in improving AV is not fully understood, current knowledge suggests multiple mechanisms are at play, such as an anti-inflammatory actions, direct inhibition of the proliferation of Propionibacterium acnes, and the limitation of 5α-dihydrotestosterone production, which suppresses sebaceous gland activity.7 Nicotinamide, the physiologically active form of niacin that is the source of the vitamin B3 found in a majority of multivitamin products, has been adopted for its anti-inflammatory properties. Shalita et al8 reported nicotinamide gel, acting through an anti-inflammatory mechanism of action, to be equivalent in terms of efficacy to topical clindamycin for the treatment of AV. ABAs have been demonstrated to have anti-proliferative, antiadhesive, and antibiofilm properties, whose application can help to lower the P. acnes bacterial load while avoiding antibacterial resistance. These agents are believed to exhibit many etiopathogenic properties, such as antibacterial and anti-inflammatory actions to reduce sebum, dryness, irritation, and photosensitivity.7
As such, we performed this multicenter study to investigate the efficacy and tolerability of a combination of nicotinamide, ABA, and zinc-pyrrolidone carboxylic acid (PCA) for the reduction of acne lesions in patients with MAV compared to placebo at five teaching hospitals in Indonesia.
METHODS
Study design and participants. This was a multicenter, double-blind, randomized, placebo-controlled, parallel-group study conducted in five teaching hospitals in Indonesia from August 2016 to January 2017. Eligible participants were male or female patients, aged 12 to 50 years, with MAV (defined according to the Lehmann criteria as 20–100 comedones, 15–50 inflammatory lesions, or 30–125 total lesions).3 The exclusion criteria were: 1) the presence of mild and severe forms of acne; 2) the use of oral retinoids or corticosteroids within 12 weeks prior the study; 3) the use of topical retinoids, anti-inflammatory drugs, corticosteroids, oral antibiotics, or other systemic acne treatments four weeks prior to the study; 4) the use of medicated facial cleansers or other topical medications for acne within one week of the study; 5) the presence of any facial skin conditions that would interfere with clinical evaluations, including acne conglobate or acne fulminans; and 6) pregnancy or lactation during the study.
This study was performed in accordance with the Declaration of Helsinki and was approved by the ethical committee of the Faculty of Medicine at Universitas Indonesia. Subjects were provided with a full explanation of the treatment protocols and study details and all signed an informed consent form before being included.
This study was registered at ClinicalTrials. gov with the registration number NCT0326298 and the title “Efficacy and Tolerability of Nicotinamide Plus Cream for Moderate Acne Vulgaris in Indonesia.”
Randomization and masking. This study recruited 140 patients (28 from each hospital) across five hospitals in Indonesia, including the Adam Malik General Hospital, Medan; Dr. M. Hoesin General Hospital, Palembang; Gatot Subroto Army Hospital, Jakarta; Dr. Sardjito General Hospital, Jogjakarta; and Dr. W. Sudirohusodo General Hospital, Makassar. A sample-size calculation was completed using two proportion formulas. The patients were divided into two treatment groups by block randomization to receive either nicotinamide+ABA+zinc-PCA or placebo. The placebo consisted of adapalene and a basic cream, which was composed of wax, fat, sesame oil, and water. The study group received adapalene and nicotinamide+ABA+zinc-PCA. The study’s statistician performed the randomization. To maintain the integrity of the blinding process, the drug was packaged in two identical tubes using a small sealed bag with a randomization number for each participant. The investigators, trial center staff, clinical monitors, participants, sponsors, and all other trial personnel were unaware of the study medication assignments.
Efficacy and safety assessments. The evaluation of therapy and its side effects was completed every two weeks for six weeks total. The evaluation of therapy effects was conducted subjectively and objectively to assess improvements in the type and number of lesions. The primary outcome was the total reduction of both inflammatory and noninflammatory lesions, while the secondary outcomes included a patient-self assessment and side effects.
Treatment efficacy was determined by two variables: lesion count (i.e., noninflammatory, inflammatory) and patient self-assessment. Objectively, photographs of three positions with the same condition observed per visit were gathered and the calculation of percentage reductions in the number of lesions for both inflammatory and noninflammatory lesions was performed. Subjects reported their self-assessment scores using the following scale: very significantly improved (76%–100%), significantly improved (51%–75%), moderately improved (26%–50%), slightly improved (11%–25%), and no improvement or minimally improved (0%–10%).
The evaluation of side effects was conducted to assess the skin reactions occurring in the subjects using the following four-item assessment rating scheme: 0) none; 1) mild, characterized by itchiness, without any clinical symptoms, and no need for medication; 2) moderate, characterized by itchiness and slight erythema, with no need for medication; and 3) severe, characterized by erythema, irritation, burned sensation, and skin exfoliation requiring medication.
Study protocol. The study subjects in both groups were instructed to apply adapalene to all affected facial areas every night (one hour before bedtime) and to also apply Cream A or B twice daily after washing in the morning and the evening for a period of six weeks. The active nicotinamide+ABA+zinc-PCA treatment and the placebo were provided in the same kind of tube but with different labels of A and B and both physicians and patients were blinded to the type of treatment received. All patients were additionally provided with a gentle skin cleanser and were instructed to use it twice daily.
Patient progress was assessed at the end of the second, fourth, and sixth weeks after the initiation of the treatment period by recording the inflammatory and noninflammatory lesion counts, patient self-assessment scores, presence of side effects, and by taking clinical photographs.
Statistical analysis. At the end of the study, the codes for study allocation were revealed and the collected data were analyzed using the Statistical Package for the Social Sciences version 17 software program (IBM Corp., Armonk, New York). Demographic data were analyzed using the chi-squared test and Mann–Whitney U tests. Treatment efficacy data were assessed using general linear mixed-model tests. Finally, data on side effects were analyzed using the chi-squared test. The significance level was set at 0.05.
RESULTS
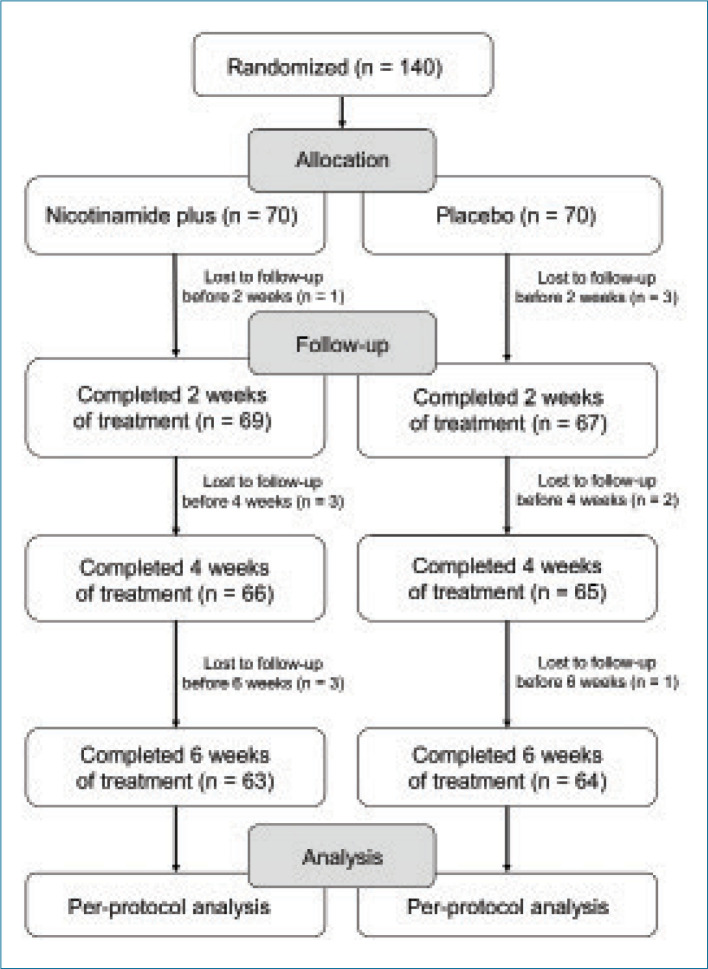
A total of 140 subjects with moderate acne were enrolled in this study and split into two groups, with a total of 70 subjects assigned to apply nicotinamide+ABA+zinc-PCA cream and 70 subjects assigned to apply the placebo cream. Ultimately, 127 subjects, including 47 females participants and 16 male participants, completed the study. Thirteen subjects dropped out, including four subjects who failed to show up to the two-week evaluation visit, five subjects who failed to show up to the four-week evaluation visit, and four subjects who failed to show up to the six-week evaluation visit. Figure 1 includes a flow diagram of the allocation, follow-up, and analysis of study subjects.
FIGURE 1.
Flow diagram of the allocation, follow-up, and analysis of study subjects
Demographics. Demographic characteristics and baseline dermatological scores were comparable between the two treatment groups. Subject differences based on sex, education level, and occupation were tested using the chi-squared test, while those related to age and length of illness was tested using the Mann– Whitney U test. No significant differences were observed (p>0.05).
In this study, subject distribution based on the length of condition median was 12 months in the study group and 24 months in the placebo group. This difference in length between the groups did not impact the results of the intervention. For subjective complaints, 32 subjects (51%) in the placebo group and 31 subjects (48%) in the study group had no complaints and the rates of complaints were not significantly different between the two groups.
The number of subjects who received therapy in the past and those who never received therapy were comparable (i.e., 70 patients in each group). During statistical testing, subjective complaints and treatment history as determined by chi-squared test and the length of illness as determined by the Mann–Whitney U test showed no significant differences between groups (p>0.05).
Therapeutic efficacy. Therapeutic efficacy was subjectively assessed between the two groups in the second, fourth, and sixth weeks and was classified into the categories of minimal improvement, slight improvement, moderate improvement, visible improvement, and highly visible improvement, respectively. The subjective therapeutic efficacy was analyzed statistically using the chi-squared test and revealed a nonsignificant difference between the groups.
Objective clinical improvements as indicated by decreasing noninflammatory lesions in the study group were significantly better only at the second week compared to in the placebo group (p<0.05), with a lower median of initial lesions found in the study group (Mann–Whitney U test). Also, the decrease in noninflammatory lesions in the study group was higher than that in the placebo group. However, no significant differences in inflammatory lesion counts between the groups were observed at baseline and Weeks 2, 4, or 6 (p>0.05).
Side effects. Side effects in the second, fourth, and sixth weeks were classified either as none, mild, moderate, or severe. The side effect profiles were not statistically significantly different between the groups in the second and fourth weeks. In the sixth week, subjects in the study group experienced mild side effects at a statistically significant higher frequency than did those in the placebo group. Most subjects reported no scaling, dryness, stinging, and burning in Weeks 4 and 6 and there were no significant differences in these items between the groups. Nicotinamide+ABA+zinc-PCA appeared to be safe and well-tolerated by the patients.
DISCUSSION
The aim of the present study was to investigate the efficacy and tolerability of nicotinamide cream plus ABA and zinc-PCA when used as an adjuvant treatment in AV. In this study, the combination of adapalene 0.1% and niacinamide, ABA, and zinc-PCA was chosen because, given the various adjuvant therapies available for acne patients to date with different side effect profiles, we hoped to establish the validity of a first-line therapy with minimal side effects that would increase patient adherence to treatment.
Retinoids are the core of topical agents in acne treatment because they are comedolytic, resolve microcomedones (i.e., precursor acne lesions), and are anti-inflammatory.9 Randomized, controlled studies have reported that retinoids reduce comedones and inflammatory lesions by 40 to 80 percent.2,10 Three active agents are available at this time: tretinoin (0.025%–0.1% in a cream, gel, or microsphere gel vehicle), adapalene (0.1% or 0.3% cream or 0.1% lotion), and tazarotene (0.05% or 0.1% cream, gel, or foam).8
The efficacy and safety of adapalene in treating AV have been extensively studied in several clinical trials11,12 either alone or in combination with antimicrobials, consistently demonstrating a more favorable tolerability profile than other topical retinoids, including tretinoin formulations.13,14 In one study, Alirezai et al15 reported that adapalene plus lymecycline therapy led to better results relative to when applying a gel vehicle plus lymecycline for moderate to moderately severe AV once daily for 12 weeks. Adapalene was safe and well-tolerated in their study. Another study from Kawashima et al17 found that adapalene gel 0.1% was more effective and safer than the control gel and well-tolerated for treating acne. Adapalene gel 0.1% produced statistically better reductions in total, inflammatory, and noninflammatory lesions than the control treatment, while also yielding greater subject satisfaction.
Combination therapy is expected to improve adherence to acne therapy.18 In a report of results on a multicentric study in Spain evaluating treatment adherence of patients with mild-to-moderate AV, Kircik et al19 recommended adjuvant therapy be given to improve treatment adherence. In a review conducted by Chee Leok Goh et al,20 combinations of adapalene, nicotinamide, and zinc components examined in some previous in-vivo studies decreased sebum production activity.
Nicotinamide, also known as niacinamide, is an active form of niacin, a source of the vitamin B3 found in many multivitamin products. It has been used because of its anti-inflammatory role. Specifically, the topical use of niacinamide has a stabilizing effect on epidermal barrier function, reduces transepidemal water loss, and improves the moisture content of the horny layer.21,22 Kaymak18 has reported that 4% niacinamide-containing gel achieved good degrees of effectiveness and safety in alleviating the symptoms of mild to moderate acne, with earlier improvement in pustules. Of note, Shalita et al9 found that nicotinamide gel, acting through an anti-inflammatory mechanism of action, is equivalent in efficacy to topical clindamycin for treating AV.7 ABA agents have been known to provide antiproliferative, antiadhesive, and antibiofilm properties and can decrease the amount of P. acnes bacterial load while avoiding antibacterial resistance.20
Dermocosmetics, a term synonymous with “cosmeceuticals,” which are used as adjuvant therapy in the scientific management of a variety of skin disorders, are believed to display several etiopathogenic properties, such as antibacterial and anti-inflammatory, and to reduce sebum, dryness, and irritation.20,21,23 A correct diagnostic approach with appropriate therapeutic options in combination with dermocosmetic agents where appropriate is essential for acne management. Topical nicotinamide plus ABA and zinc-PCA is an oil-in-water emulsion free from perfumes to reduce the possibility of allergies. Previous research suggests the formulation effectively moisturizes the skin with no oily effect and is noncomedogenic.7
In this study, there was a statistically significant decrease in the number of noninflammatory lesions over the study period in the subjects using nicotinamide+ABA+zinc-PCA versus placebo at the second week (p<0.05). The hypothesis of a peak reaction to adapalene occurring during the first two weeks in the skin might play a role to the result. Adapalene can cause initial local irritation, including erythema, burning, burning, stinging, peeling, and xerosis. These symptoms usually peak after two weeks of use, then diminish and resolve once the skin adapts to the use of the product.24
Our results are not different from those of a previous trial from Aulisa et al,7 where nicotinamide plus oil-free was effective after only three weeks of therapy with high success rates of satisfaction among patients with the comedonic and papular types of acne as compared to the papulopustular type. Even after six weeks of treatment, the assessment showed better results among those with the comedonic and papular types than the papulopustular type. In this study, the tolerability of nicotinamide plus was good; there was no statistically significant difference between both groups. The results from this study indicate that the administration of nicotinamide+ABA+zinc-PCA for moderate AV is safe, well-tolerated, and able to significantly reduce noninflammatory lesion counts versus placebo by Week 2. However, the fact that the results at Week 4 and 6 were also reduced is interesting to note despite the statistical nonsignificance. Why this occurred remains unknown. This could be caused by our short period of intervention. The decrease in noninflammatory lesions in the study group was higher than that in the placebo group.
Limitations. There are some limitations in our study. First, we limited our study period to a length of only six weeks. If we aimed to look at the effects of the study agent in inflammatory lesions, a longer intervention period might be needed, and further randomized, placebo-controlled trials are warranted to support our results overall. Second, the study did not analyze cases of mild and severe acne, so our findings might only applicable to patients with moderate AV. Also, we did not follow up with the participants to determine when their acne skin lesions returned to their baseline values after discontinuing treatment. Third, hospital employees were chosen to represent a middle-aged healthy population. Our results might thus be applicable only among middle-aged, otherwise healthy Asian individuals. Finally, a larger sample size might be necessary to produce a better and comprehensive result. This study was conducted during work time to ensure minimal sun exposure and to increase adherence.
Overall, there was a significant decrease in the noninflammatory lesion count in this study between the study group and placebo in the second week. The combination of adapalene, nicotinamide, ABA, and zinc-PCA reduced noninflammatory lesions quickly in the first two weeks of therapy. There was no significant difference in inflammatory lesions found in the fourth and sixth weeks between the two groups.
CONCLUSION
This study demonstrated that, based on the observed decrease in noninflammatory lesions in our participants, nicotinamide+ABA+zinc-PCA cream was more effective compared to placebo, but only in the first two weeks; regarding inflammatory lesions, there was no difference between the groups. Based on the observed side effect profile, there was no significant difference between both groups, so both agents can be said to be well-tolerated.
REFERENCES
- Thiboutout DM, Strauss JS. Diseases of sebaceous glands—acne vulgaris. Fitzpatrick’s Dermatology in General Medicine. In: Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, editors. 6th ed. New York: McGraw-Hill; 2003. pp. 672–687. [Google Scholar]
- Gollnick H, Cunliffe W, Berson D et al. Management of acne: a report from a Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2003;49:S1–S37. doi: 10.1067/mjd.2003.618. 1 Suppl. [DOI] [PubMed] [Google Scholar]
- Lehmann HP, Robinson KA, Andrews JS et al. Acne therapy: a methodologic review. J Am Acad Dermatol. 2002;47(2):231–240. doi: 10.1067/mjd.2002.120912. [DOI] [PubMed] [Google Scholar]
- Wasitaadmadja SM, Arimuko A, Norawati L, Bernadette I, Legiawati L, editors. 2nd edition. Jakarta, Indonesia: Centra Communication; 2016. [Indonesian Cosmetic Dermatology Study Group. Indonesia Acne Treatment Guideline]. Guidelines in Indonesian. [Google Scholar]
- Zanglein AL, Graber EM, Thiboutot DM, Strauss JS. Acne vulgaris and acneiform eruptions. Fitzpatrick’s Dermatology in General Medicine. In: Wolff K, Goldsmith LA, S. IK, Gilchhrest BA, Paller AS, Leffell DJ, editors. 7th ed. New York: McGraw Hill; 2008. pp. 680–703. [Google Scholar]
- Veraldi S, Barbareschi M, Benardon S, Schianchi R. Short contact therapy of acne with tretinoin. J Dermatolog Treat. 2013;24(5):374–376. doi: 10.3109/09546634.2012.751085. [DOI] [PubMed] [Google Scholar]
- Aulisa L, Bizzi B, Caione P et al. Evaluation study on the activity and tolerability of Papulex™ oil-free cream. Nuove Prospettive in Terapia. 2009;1:3–7. S1. [Google Scholar]
- Cervantes J, Eber AE, Perper M et al. The role of zinc in the treatment of acne: a review of the literature. Dermatol Ther. 2018;31(1):1–17. doi: 10.1111/dth.12576. [DOI] [PubMed] [Google Scholar]
- Shalita AR, Smith JG, Parish LC et al. Topical nicotinamide compared with clindamycin gel in the treatment of inflammatory acne vulgaris. Int J Dermatol. 1995;34(6):434–437. doi: 10.1111/j.1365-4362.1995.tb04449.x. [DOI] [PubMed] [Google Scholar]
- Zaenglein AL, Pathy AL, Schlosser BJ et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945–973. doi: 10.1016/j.jaad.2015.12.037. [DOI] [PubMed] [Google Scholar]
- Ravenscroft J. Pharmacy update evidence based update on management of acne. Arch Dis Child Educ Pract Ed. 2005;90:ep98–ep101. [Google Scholar]
- Cunliffe WJ, Poncet M, Loesche C, Verschoore M. A comparison of the efficacy and tolerability of adapalene 0.1% gel versus tretinoin 0.025% gel in patients with acne vulgaris: a meta-analysis of five randomized trials. Br J Dermatol. 1998;139:48–56. doi: 10.1046/j.1365-2133.1998.1390s2048.x. Suppl 52. [DOI] [PubMed] [Google Scholar]
- Waugh J, Noble S, Scott LJ. Adapalene: a review of its use in the treatment of acne vulgaris. Drugs. 2004;64(13):1465–1478. doi: 10.2165/00003495-200464130-00005. [DOI] [PubMed] [Google Scholar]
- Greenspan A, Loesche C, Vendetti N et al. Cumulative irritation comparison of adapalene gel and solution with 2 tazarotene gels and 3 tretinoin formulations. Cutis. 2003;72(1):76–81. [PubMed] [Google Scholar]
- Egan N, Loesche MC, Baker MM. Randomized, controlled, bilateral (split-face) comparison trial of the tolerability and patient preference of adapalene gel 0.1% and tretinoin microsphere gel 0.1% for the treatment of acne vulgaris. Cutis. 2001;68:20–24. 4 Suppl. [PubMed] [Google Scholar]
- Alirezai M, George SA, Coutts I. Daily treatment with adapalene gel 0.1% maintains initial improvement of acne vulgaris previously treated with oral lymecycline. Eur J Dermatol. 2007;17(1):45–51. doi: 10.1684/ejd.2007.0188. [DOI] [PubMed] [Google Scholar]
- Kawashima M, Harada S, Loesche C, Miyachi Y. Adapalene gel 0.1% is effective and safe for Japanese patients with acne vulgaris: a randomized, multicenter, investigator-blinded, controlled study. J Dermatol Sci. 2008;49(3):241–248. doi: 10.1016/j.jdermsci.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Kaymak Y, Onder M. An investigation of efficacy of topical niacinamide for the treatment of mild and moderate acne vulgaris. J Turk Acad Dermatol. 2008;2:jtad82402a. [Google Scholar]
- Kircik LH. Synergy and its clinical relevance in topical acne therapy. J Clin Aesthet Dermatol. 2011;4(11):30–33. [PMC free article] [PubMed] [Google Scholar]
- De Lucas R, Moreno-Arias G, Perez-Lopez M et al. Adherence to drug treatments and adjuvant barrier repair therapies are key factors for clinical improvement in mild to moderate acne: the ACTUO observational prospective multicenter cohort trial in 643 patients. BMC Dermatol. 2015;15:17. doi: 10.1186/s12895-015-0036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh CL, Noppakun N, Micali G et al. Meeting the challenges of acne treatment in Asian patients: a review of the role of dermocosmetics as adjunctive therapy. J Cutan Aesthet Surg. 2016;9(2):85–92. doi: 10.4103/0974-2077.184043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring W. Nicotinic acid/niacinamide and the skin. J Cosmet Dermatol. 2004;3(2):88–93. doi: 10.1111/j.1473-2130.2004.00115.x. [DOI] [PubMed] [Google Scholar]
- Araviiskaia E, Dreno B. The role of topical dermocosmetics in acne vulgaris. Eur Acad Dermatol Venereol. 2016;30(6):926–935. doi: 10.1111/jdv.13579. [DOI] [PubMed] [Google Scholar]
- Kroshinsky D, Shalita AR. Topical retinoids. Acne and Its Therapy. In: Webster Rawlings A., editor. New York, NY: Informa Healthcare; 2007. pp. 130–112. [Google Scholar]