Graphical abstract
Keywords: COVID-19, Nanoceria, Cytokine storm, Lung fibrosis, SARS-CoV-2
Abstract
The COVID-19 pandemic has emerged as an unprecedented global healthcare emergency and has devastated the global economy. The SARS-CoV-2 virus replicates in the host cells and is seemingly much more virulent compared to other flu viruses, as well as the SARS-CoV-1. The respiratory complications of the disease include acute respiratory distress syndrome (ARDS), cytokine storm, systemic inflammation, and pulmonary fibrosis. Nanoceria (NC) is a versatile rare earth nanoparticle with remarkable catalase and superoxide dismutase mimetic redox regenerative properties. Interestingly, NC possesses promising anti-inflammatory, antioxidant and anti-fibrotic properties, making it an attractive tool to fight against the SARS-CoV-2 as well as the associated systemic complications. Until now, there is no clinically approved vaccine or drug for the treatment of COVID-19, and the conquest to find a novel therapy for this global havoc is being undertaken at a warlike pace. Herein, based on preclinical evidence, we hypothesize that NC owing to its unique pharmacological properties, might be an attractive preclinical candidate to win the battle over COVID-19. Further, it may be used as a prevention or treatment strategy in combination with other drugs.
Introduction
The COronaVIrusDiease-2019 (COVID-19) has emerged as an unprecedented global health crisis [1]. The SARS-CoV-2 virus enters the host cell through the angiotensin-converting enzyme 2 (ACE-2) receptor. Till date, more than nineteen million people have been infected with the SARS-CoV-2, and more than 0.7 million patients have succumbed to death. As of August 09, 2020, there is no approved vaccine to provide a robust clinical solution to this rapidly spreading viral disease [2]. Further, sadly it may still take up to 2 years for the pharmaceutical companies to come up with a clinically approved vaccine.
For the management of COVID-19, it is important to halt further viral replication as well as provide symptomatic relief from the systemic complications, particularly the respiratory complications. Clinically, a large number of antiviral drugs as well as anti-parasitic drugs have been tried in the past few months. However, the clinical benefit of drugs like remdesivir, hydroxychloroquine, and chloroquine have been questionable [[3], [4], [5]]. Further, the patients are given various medications such as steroids, nonsteroidal anti-inflammatory drugs (NSAIDs), and others for the management of systemic complications [6].
The acute respiratory distress syndrome (ARDS) is a critical complication of COVID-19. It is mediated by a complex interplay of multiple signaling pathways like activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) pathway, enhanced inflammation, cytokine storm, mitogen-activated protein kinases (MAPKs) and others [7,8]. Moreover, patients with prior chronic lung inflammation like emphysema may suffer from pulmonary fibrosis which is driven by induction of epithelial-to-mesenchymal transition (EMT) by fibrogenic growth factors like transforming growth factor-beta (TGF-β) [[9], [10], [11]]. Nanoceria (NC) has emerged as a remarkably safe, redox regenerative, rare earth-based nanomedicine against acute and chronic inflammatory diseases owing to its unique ability to interchange between +3 and +4 oxidation states [[12], [13], [14], [15], [16], [17], [18], [19], [20]]. NC is postulated to be a robust antioxidant, possessing catalase and superoxide dismutase mimetic activity [21,22]. Further, NC possesses promising anti-inflammatory properties by inhibition of NFκB and MAPK pathways [[23], [24], [25]]. On the other hand, NC has been shown to reduce fibrogenic signaling by inhibition of the TGF-β signaling pathway [26]. NC can be administered in various dosage forms, including aerosol or transdermal patches based on the condition and compliance of the patient.
Hypothesis
We hypothesize that NC could be a novel therapeutic for the management of COVID-19 by halting the progression of systemic inflammatory complications due to the ability of NC to inhibit NFκB, MAPKs and TGF-β signaling pathways.
Justification of hypothesis
Nanoceria can inhibit acute and chronic inflammatory response and fibrosis during COVID-19
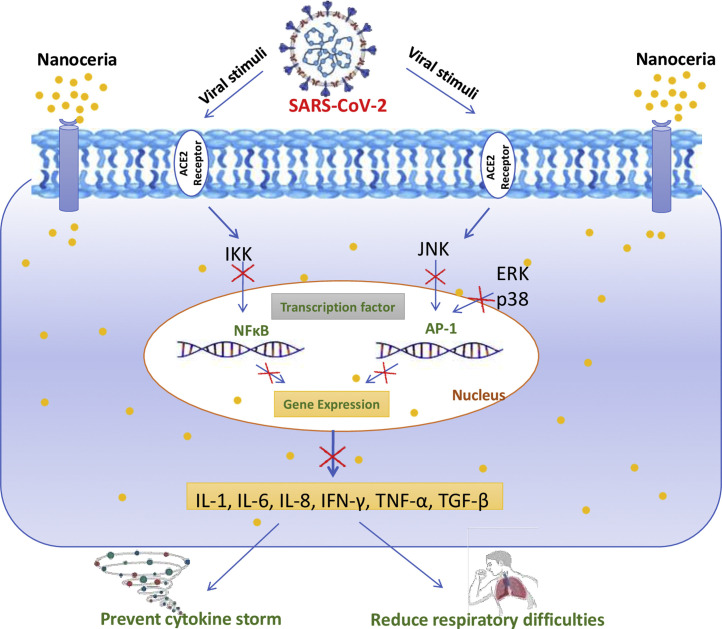
Cytokine storm and severe inflammatory response to COVID-19 pose significant risks to the health of the patients. Pharmacotherapeutics, which can suppress the cytokine storm and reduce overall inflammation, can be of benefit [27]. NC is an excellent nanomedicine for combating acute inflammatory insult. It was shown to reduce severe sepsis-related mortality by inhibition of NFκB signaling and suppressed lipopolysaccharide-induced MAPK signaling [23,28]. Manne et al., showed that NC is effective against peritonitis, proving its effective anti-inflammatory activity [29]. Inflammatory cells, like neutrophils and macrophages, are the major players during the pathological response. It is well known that cytokines such as interleukin (IL)-1β, IL-6, IL-8, tumor necrosis factor (TNF)-α and TGF-β play a profound role in the pathogenesis of COVID-19. Pathogenic viral stimuli trigger the synthesis and release of cytokines by inducing IκB kinase (IKK) and c-Jun N-terminal kinase (JNK) protein complex, which further stimulate the production of excessive cytokines by amplifying the transcriptional activity of NFκB as well as activator protein 1 (AP1) [30]. NC has been shown to attenuate cytokine signaling by affecting multiple cytokine sources including the autocrine and paracrine pathways. NC can directly decrease cytokine synthesis or suppress activity by blocking the receptor interaction of cytokines. Nanoceria is reported to show cytokine inhibition by modulation of p65-NFκB, MAP kinase/NFκB and Nrf2/NFκB pathways (Fig. 1 ) [24,25,31]. Based on this, intervention with NC could significantly reverse elevated levels of cytokines during COVID-19 and could halt the disease progression.
Fig. 1.
Putative mechanistic involvement of nanoceria in the proposed inhibition of cytokines production stimulated by SARS-CoV-2: SARS-CoV-2 enters into the host cell by ACE-2 cell surface receptors, and modulates the IKK and JNK activity which potentiates NFκB as well as AP1 protein transcription, respectively. These events stimulate the expression of more than 100 genes, including the gene coding for cytokine production, which in turn results in a high level of cytokine release, technically known as "cytokine storm" in COVID-19. Nanoceria may inhibit these changes and could be implied as an adjuvant treatment for COVID-19 owing to its strong anti-inflammatory effects. The red “ ” indicates the inhibition of cytokine synthesis by nanoceria. ACE2- Angiotensin-converting enzyme 2, AP1- Activation protein 1, IKK- IκB kinase enzyme complex, JNK- c-Jun N-terminal kinases. Part of the figure was adapted and reproduced from reference [57] under the Creative Commons Attribution License (CCBY).
” indicates the inhibition of cytokine synthesis by nanoceria. ACE2- Angiotensin-converting enzyme 2, AP1- Activation protein 1, IKK- IκB kinase enzyme complex, JNK- c-Jun N-terminal kinases. Part of the figure was adapted and reproduced from reference [57] under the Creative Commons Attribution License (CCBY).
Recently, reports have emerged indicating neurological damage caused by SARS-CoV-2. To improve the outcomes in neurodegenerative diseases where oxidative stress and chronic inflammation play pathological role in conjunction, classical antioxidants have achieved limited success. In this context, NC exhibits promising effects on chronic inflammation and may be effective against COVID-19 associated neuroinflammation. The seminal work of Rzigalinski et al., in 2003 and subsequent studies showed that NC improved longevity of brain cells and provided protection from free radicals and mechanical trauma [[32], [33], [34], [35]]. Owing to its regenerative potential and efficacy at low dose, NC has been pharmacologically effective against neurodegenerative diseases [36,37]. In a stroke model, NC reduced ischemic cell death in brain slices by more than 50 %, reduced the levels of nitric oxide and superoxide. The outcomes in models of ischemia and traumatic brain injury associated neuroinflammation entails that NC is a plausible candidate against chronic inflammation [[38], [39], [40]].
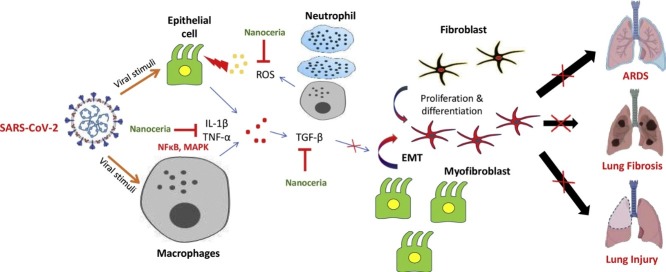
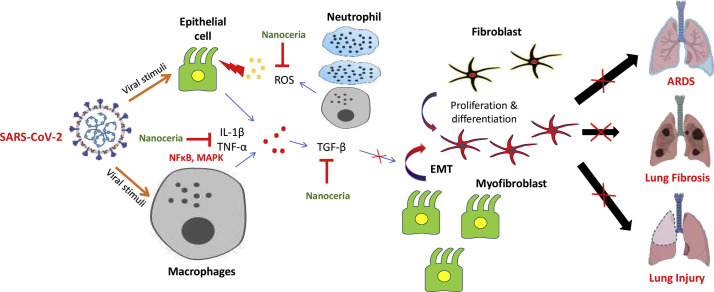
During ARDS, severe inflammation encompasses the lungs rapidly and increases mortality risk. The cyanosis of skin and short/rapid breathing are some of the important clinical features of ARDS for which patients require ventilator support. Lung images of affected patients have revealed characteristic white patches with jelly like fluid accumulation (ground glass) within the lungs, which resembles the pathological lungs after drowning [41]. Further, lung fibrosis is one of the observed complications of COVID-19 patients with prior respiratory diseases like emphysema [42,43]. Without any respiratory support, lung fibrosis can potentially make the patients incapable of breathing [44,45]. Physiologically, TGF-β is an important growth factor required for normal growth and development. Its excessive inhibition may have side effects like reduced apoptosis of Th17 cells, increased TNF-α expression, developmental disorders, cardiotoxicity and hyperkeratosis [[46], [47], [48]]. Pathologically, TGF-β induces the EMT which promotes the transformation of fibroblasts to highly mobile and secretory myofibroblasts [49]. These myofibroblasts secrete excessive extracellular matrix (ECM), resulting in scarring and fibrosis-related injuries [50,51]. However, a large pool of scientific evidences indicate that pharmacological inhibition of excessive TGF-β can be a promising strategy for the management of fibrotic disorders. In pre-clinical studies, it has been revealed that NC can potentially attenuate fibrosis progression by inhibiting TGF-β signaling. NC was shown to effectively reduce liver fibrosis, with marked reduction in collagen deposition and EMT activation [52]. Inhibition of TGF-β by NC makes it a promising adjuvant for COVID-19 treatment therapy. Moreover, Arya et al., reported protective role of NC against hypobaric hypoxia-induced oxidative damage and inflammation via modulation of oxidative stress [53]. These findings indicate that NC could aid in lung protection in COVID-19. Fig. 2 provides the rationale for the pharmacological benefits of NC for the alleviation of respiratory complications.
Fig. 2.
Schematic mechanism of lung injury in COVID-19: The SARS-CoV-2 sensitizes the lung epithelial cells and macrophages, which in response generate inflammatory signals, producing oxidative stress and release a massive amount of cytokines including TGF-β. TGF-β in turn, promotes the conversion of fibroblast to myofibroblast and provokes epithelial-to-mesenchymal transition (EMT). All these alterations advance the fibrosis mechanism, which causes lung injuries. The red “ ” indicates the inhibition of regulatory step in disease progression. EMT- Epithelial-to-mesenchymal transition, MAPK- Mitogen-activated protein kinase, NFκB- Nuclear factor kappa-light-chain-enhancer of activated B cells, TGF-β- Transforming growth factor-beta. Part of the figure was adapted and reproduced from reference [57] under the Creative Commons Attribution License (CCBY).
” indicates the inhibition of regulatory step in disease progression. EMT- Epithelial-to-mesenchymal transition, MAPK- Mitogen-activated protein kinase, NFκB- Nuclear factor kappa-light-chain-enhancer of activated B cells, TGF-β- Transforming growth factor-beta. Part of the figure was adapted and reproduced from reference [57] under the Creative Commons Attribution License (CCBY).
Nanoceria scavenges reactive oxygen species (ROS)
Due to redox imbalance, the homeostasis between pro-oxidants and antioxidants is disturbed which results in cellular damage promoting COVID-19 related systemic complications. The mechanisms which lie behind the ROS generation are macromolecular damage and impeded thiol redox circuit that result in impaired redox control and aberrant cellular signaling. Altogether, these make the disease more intense. NC being a potent reactive oxygen species (ROS) scavenger, reduces pathological damage in various diseases [54]. NC can reduce lipid peroxidation, improve physiological glutathione levels, as well as elicits SOD and catalase mimetic activity which make it a potential candidate for the management of systemic complications of COVID-19 [22,25].
Possible formulations of nanoceria for COVID-19 treatment therapy
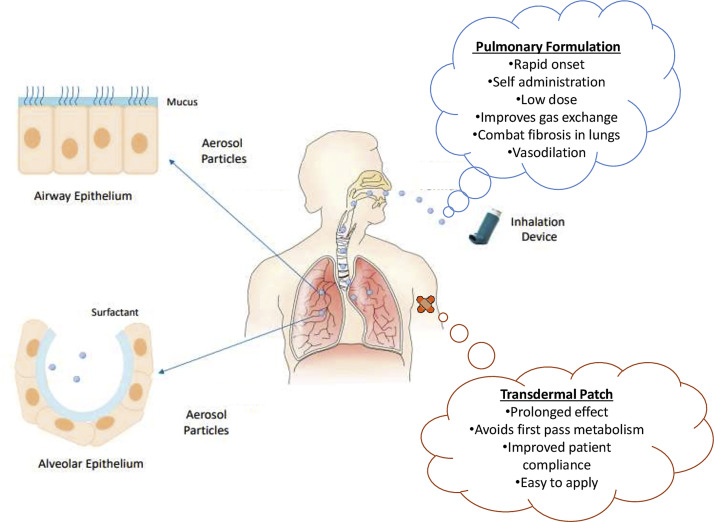
The route of administration and dosage form of a drug play key role in effective disease management. So, it is important to design a suitable dosage form to achieve desired therapeutic outcome. In this connection, nanotechnology provides versatile options to researchers and the pharmaceutical industry. Since, lungs are the primary target in COVID-19 infection, local targeted drug delivery to the lungs might be an excellent therapeutic approach for effective treatment. To serve this purpose, aerosol based inhalational spray is a promising approach that is not only patient compliant but also imparts quick relief for a defined time period [55]. Aerosols can deliver NC directly to the lungs and may reduce the disease severity caused by the novel coronavirus, with probable efficacy in the amelioration of ARDS related complications. While many drugs and macromolecules suffer from temperature and pressure generated through the nebulization process, this will not affect inorganic nanocrystals of NC which have a dense and robust crystal structure. Additionally, its catalytic behavior makes it effective at lower doses for a long period of time, thus it can be nebulized at very low concentration, hence preventing any change to aggregation during transportation into the alveoli. In cases, where patients are unable to administer the drug on their own, NC can be impregnated into a thin film and could be administered as transdermal patches which might provide effect for sustained and prolonged duration without any significant first-pass metabolism. Further, addition of other NSAIDs in combination with NC would serve a synergistic approach for effective COVID-19 management [56]. Fig. 3 shows the mentioned routes of administration with their specific advantages.
Fig. 3.
Diagrammatic representation of nanoceria based formulation for administration through various routes with specific advantages to combat COVID-19. Part of the figure were adapted and reproduced from reference [58] under the Creative Commons Attribution License (CCBY).
Therapeutic implications of the hypothesis
Our hypothesis provides pre-clinical rationale for the management of COVID-19 with NC. It demonstrates the basis on which NC may attenuate the cytokine storm progression, reduce the incidence of ARDS, and modulate the EMT signaling. Theoretically, NC can significantly improve the respiratory functions during COVID-19 infection. The preclinical rationale sheds light on the futuristic approaches based on this novel rare earth based nanomedicine for the management of COVID-19. Thus, we envisage that this hypothesis may serve as a stimulus for further research in this direction.
Author contributions
Prince Allawadhi and Amit Khurana conceptualized and wrote the manuscript. Sachin Allwadhi and Kamaldeep Joshi prepared the artworks and wrote the manuscript. Gopinath Packirisamy provided valuable inputs on nanoceria, wrote the manuscript and approved the final version. Kala Kumar Bharani wrote the manuscript and approved the final version. Part of Fig. 1, Fig. 2 were prepared using BioRender.com.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgments
The authors would like to thank the Dean, Faculty of Veterinary Science, PVNR Telangana Veterinary University, Hyderabad.
Biographies

Mr. Prince Allawadhi has completed his M.Pharmacy (Pharmacology) from Jamia Hamdard (Hamdard University), New Delhi, India. He did his graduation in pharmaceutical sciences (B.Pharmacy) from Vaish Institute of Pharmaceutical Education and Research (VIPER), Rohtak, Haryana, India. He has multiple publications in international peer reviewed journals and has 6 book chapters to his credit in Thieme and Springer Nature publishing house. He is recipient of India's prestigious Rajnibhai V Patel Trust PharmInnova Best Master Thesis Award in the Pharmacology category (2019). His research interests are in the field of nanomedicine, chronic and acute pancreatitis, inflammation, immunology and organoids based 3D culture.

Dr. Amit Khurana is jointly working as a Research Scientist at the Centre for Biomedical Engineering (CBME), IIT-Delhi and the Department of Veterinary Pharmacology and Toxicology, College of Veterinary Science, Hyderabad, India. He did his Ph.D in Pharmacology and Toxicology from NIPER-Hyderabad, India. His research interests include novel therapeutics for chronic inflammatory disorders and artificial skin substitutes for burn injury. He has authored more than thirty publications in reputed international journals. He is recipient of numerous awards like DST-DAAD Doctoral Fellowship, DBT-CNPq Indo-Brazil Fellowship, PC Dandiya Award of the Indian Pharmacological Society and Merit Award from Delhi Pharmacy Council.

Mr. Sachin Allwadhi has completed his B.Tech. in Computer Science and Engineering from Kurukshetra University, Kurukshetra, Haryana, India and M.Tech. in Computer Science and Engineering from Maharshi Dayanand University (M.D.U.), Rohtak, Haryana, India. Currently, he is pursuing Ph.D in Computer Science and Engineering from M.D.U., Rohtak, Haryana, India. His research interests include Image Processing, Image Steganography, Artificial Intelligence, Bioinformatics and Machine Learning. He has been a scholar throughout and has also authored multiple publications in internationally reputed journals.

Dr. Kamaldeep Joshi did his B.Tech., M.Tech. and PhD in Computer Science and Engineering from Maharshi Dayanand University, Rohtak, Haryana, India. Currently, he is working as an Assistant Professor in Department of Computer Science and Engineering, University Institute of Engineering and Technology, Maharshi Dayanand University, Rohtak, Haryana, India. His work and research interests include Image Processing, Neural Network, and Information Security. Dr. Joshi has authored over 20 articles in international peer reviewed journals. He has attended various national and international conferences and contributed research papers in their proceedings. He is also a member of many international societies.

Dr. Gopinath Packirisamy is an Associate Professor in the Department of Biotechnology at Indian Institute of Technology (IIT), Roorkee, India. He earned his Ph.D. in Biotechnology from Indian Institute of Technology, Guwahati, India. He did his postdoctoral research at University of Rochester Medical Center, New York, USA. Currently his research group at IIT Roorkee is working on the development of polymer based nanocarriers for delivery of various therapeutic agents. He has authored more than 100 research publications, ten patents and has done one technology transfer. He has received numerous awards for his scientific contributions in the area of nanobiotechnology.

Dr. Kala Kumar Bharani is currently working as Professor and Head, Department of Veterinary Pharmacology and Toxicology, College of Veterinary Science, Rajendranagar, Hyderabad, India. His area of expertise include, novel formulation based approaches for veterinary diseases, production pharmacology, mastitis, and toxicology of insecticides & pesticides. He has successfully completed eight industrially sponsored projects, three government funded (ICAR, ICMR and DBT) extramural projects. He is recipient of the prestigious Dr. S B Pandey Oration Award and Dr. Lalitha Kameswaran Oration Award.
References
- 1.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lurie N., Saville M., Hatchett R., Halton J. Developing Covid-19 vaccines at pandemic speed. N. Engl. J. Med. 2020 doi: 10.1056/NEJMp2005630. [DOI] [PubMed] [Google Scholar]
- 3.Cortegiani A., Ingoglia G., Ippolito M., Giarratano A., Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J. Crit. Care. 2020 doi: 10.1016/j.jcrc.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erickson T., Chai P., Boyer E. Chloroquine, hydroxychloroquine and COVID-19. Toxicol. Commun. 2020;4(1):40–42. doi: 10.1080/24734306.2020.1757967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.-X. Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell B., Moss C., Rigg A., Van Hemelrijck M. COVID-19 and treatment with NSAIDs and corticosteroids: should we be limiting their use in the clinical setting? Ecancermedicalscience. 2020;14 doi: 10.3332/ecancer.2020.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., Ji R., Wang H., Wang Y., Zhou Y. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int. J. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu Y., Sun J., Dai Z., Deng H., Li X., Huang Q., Wu Y., Sun L., Xu Y. Prevalence and severity of corona virus disease 2019 (COVID-19): a systematic review and meta-analysis. J. Clin. Virol. 2020 doi: 10.1016/j.jcv.2020.104371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H., Zhou P., Wei Y., Yue H., Wang Y., Hu M., Zhang S., Cao T., Yang C., Li M. Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient with COVID-19. Ann. Intern. Med. 2020;172(9):629–632. doi: 10.7326/M20-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J., Wang B., Yang J., Wang M., Chen C., Luo G., He W. Advances in the research of mechanism of pulmonary fibrosis induced by Corona Virus Disease 2019 and the corresponding therapeutic measures. Zhonghua Shao Shang za zhi= Zhonghua Shaoshang Zazhi= Chin. J. Burns. 2020;36 doi: 10.3760/cma.j.cn501120-20200307-00132. E006-E006. [DOI] [PubMed] [Google Scholar]
- 11.Chanda D., Otoupalova E., Smith S.R., Volckaert T., De Langhe S.P., Thannickal V.J. Developmental pathways in the pathogenesis of lung fibrosis. Mol. Aspects Med. 2019;65:56–69. doi: 10.1016/j.mam.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casals E., Zeng M., Parra‐Robert M., Fernández‐Varo G., Morales‐Ruiz M., Jiménez W., Puntes V., Casals G. Cerium oxide nanoparticles: advances in Biodistribution, toxicity, and preclinical exploration. Small. 2020 doi: 10.1002/smll.201907322. [DOI] [PubMed] [Google Scholar]
- 13.Deshpande S., Patil S., Kuchibhatla S.V., Seal S. Size dependency variation in lattice parameter and valency states in nanocrystalline cerium oxide. Appl. Phys. Lett. 2005;87(13) [Google Scholar]
- 14.Kuiry S.C., Patil S.D., Deshpande S., Seal S. Spontaneous self-assembly of cerium oxide nanoparticles to nanorods through supraaggregate formation. J. Phys. Chem. B. 2005;109(15):6936–6939. doi: 10.1021/jp050675u. [DOI] [PubMed] [Google Scholar]
- 15.Singh R., Karakoti A.S., Self W., Seal S., Singh S. Redox-sensitive cerium oxide nanoparticles protect human keratinocytes from oxidative stress induced by glutathione depletion. Langmuir. 2016;32(46):12202–12211. doi: 10.1021/acs.langmuir.6b03022. [DOI] [PubMed] [Google Scholar]
- 16.Barkam S., Das S., Saraf S., McCormack R., Richardson D., Atencio L., Moosavifazel V., Seal S. The change in antioxidant properties of dextran‐coated redox active nanoparticles due to synergetic photoreduction–oxidation. Chem. Eur. J. 2015;21(36):12646–12656. doi: 10.1002/chem.201500868. [DOI] [PubMed] [Google Scholar]
- 17.von Montfort C., Alili L., Teuber-Hanselmann S., Brenneisen P. Redox-active cerium oxide nanoparticles protect human dermal fibroblasts from PQ-induced damage. Redox Biol. 2015;4:1–5. doi: 10.1016/j.redox.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dowding J., Song W., Bossy K., Karakoti A., Kumar A., Kim A., Bossy B., Seal S., Ellisman M., Perkins G. Cerium oxide nanoparticles protect against A β-induced mitochondrial fragmentation and neuronal cell death. Cell Death Differ. 2014;21(10):1622–1632. doi: 10.1038/cdd.2014.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grulke E., Reed K., Beck M., Huang X., Cormack A., Seal S. Nanoceria: factors affecting its pro-and anti-oxidant properties. Environ. Sci. Nano. 2014;1(5):429–444. [Google Scholar]
- 20.Kumar A., Das S., Munusamy P., Self W., Baer D.R., Sayle D.C., Seal S. Behavior of nanoceria in biologically-relevant environments. Environ. Sci. Nano. 2014;1(6):516–532. [Google Scholar]
- 21.Heckert E.G., Karakoti A.S., Seal S., Self W.T. The role of cerium redox state in the SOD mimetic activity of nanoceria. Biomaterials. 2008;29(18):2705–2709. doi: 10.1016/j.biomaterials.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pirmohamed T., Dowding J.M., Singh S., Wasserman B., Heckert E., Karakoti A.S., King J.E., Seal S., Self W.T. Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem. Commun. 2010;46(16):2736–2738. doi: 10.1039/b922024k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selvaraj V., Nepal N., Rogers S., Manne N.D., Arvapalli R., Rice K.M., Asano S., Fankhanel E., Ma J.J., Shokuhfar T. Inhibition of MAP kinase/NF-kB mediated signaling and attenuation of lipopolysaccharide induced severe sepsis by cerium oxide nanoparticles. Biomaterials. 2015;59:160–171. doi: 10.1016/j.biomaterials.2015.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khurana A., Tekula S., Godugu C. Nanoceria suppresses multiple low doses of streptozotocin-induced Type 1 diabetes by inhibition of Nrf2/NF-κB pathway and reduction of apoptosis. Nanomedicine. 2018;13(15):1905–1922. doi: 10.2217/nnm-2018-0085. [DOI] [PubMed] [Google Scholar]
- 25.Khurana A., Anchi P., Allawadhi P., Kumar V., Sayed N., Packirisamy G., Godugu C. Superoxide dismutase mimetic nanoceria restrains cerulein induced acute pancreatitis. Nanomedicine. 2019;14(14):1805–1825. doi: 10.2217/nnm-2018-0318. [DOI] [PubMed] [Google Scholar]
- 26.Kumari P., Saifi M.A., Khurana A., Godugu C. Cardioprotective effects of nanoceria in a murine model of cardiac remodeling. J. Trace Elem. Med. Biol. 2018;50:198–208. doi: 10.1016/j.jtemb.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 27.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selvaraj V., Manne N.D., Arvapalli R., Rice K.M., Nandyala G., Fankenhanel E., Blough E.R. Effect of cerium oxide nanoparticles on sepsis induced mortality and NF-κB signaling in cultured macrophages. Nanomedicine. 2015;10(8):1275–1288. doi: 10.2217/nnm.14.205. [DOI] [PubMed] [Google Scholar]
- 29.Manne N.D., Arvapalli R., Nepal N., Thulluri S., Selvaraj V., Shokuhfar T., He K., Rice K.M., Asano S., Maheshwari M. Therapeutic potential of cerium oxide nanoparticles for the treatment of peritonitis induced by polymicrobial insult in Sprague-Dawley rats. Crit. Care Med. 2015;43(11):e477–e489. doi: 10.1097/CCM.0000000000001258. [DOI] [PubMed] [Google Scholar]
- 30.Taghizadeh-Hesary F., Akbari H. The powerful immune system against powerful COVID-19: a hypothesis. Med. Hypotheses. 2020 doi: 10.1016/j.mehy.2020.109762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selvaraj V., Nepal N., Rogers S., Manne N.D.P.K., Arvapalli R., Rice K.M., Asano S., Fankhanel E., Ma J.J., Shokuhfar T., Maheshwari M., Blough E.R. Inhibition of MAP kinase/NF-kB mediated signaling and attenuation of lipopolysaccharide induced severe sepsis by cerium oxide nanoparticles. Biomaterials. 2015;59:160–171. doi: 10.1016/j.biomaterials.2015.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rzigalinski B.A., Bailey D., Chow L., Kuiry S., Patil S., Merchant S., Seal S. Cerium oxide nanoparticles increase the lifespan of cultured brain cells and protect against free radical and mechanical trauma. FASEB J. 2003:A606. Federation Amer Soc Exp Biol 9650 Rockville Pike, Bethesda, MD 20814-3998 USA. [Google Scholar]
- 33.B.A. Rzigalinski, S. Seal, D. Bailey, S. Patil, Cerium oxide nanoparticles and use in enhancing cell survivability, Google Patents, 2009.
- 34.Singh N., Cohen C.A., Rzigalinski B.A. Treatment of neurodegenerative disorders with radical nanomedicine. Ann. N. Y. Acad. Sci. 2007;1122(1):219–230. doi: 10.1196/annals.1403.015. [DOI] [PubMed] [Google Scholar]
- 35.Halliwell B. Oxidative stress and neurodegeneration: where are we now? J. Neurochem. 2006;97(6):1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 36.Rzigalinski B.A., Carfagna C.S., Ehrich M. Cerium oxide nanoparticles in neuroprotection and considerations for efficacy and safety, Wiley interdisciplinary reviews. Nanomed. Nanobiotechnol. 2017;9(4) doi: 10.1002/wnan.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou D., Fang T., Lu L.-q., Yi L. Neuroprotective potential of cerium oxide nanoparticles for focal cerebral ischemic stroke. J. Huazhong Univ. Sci. Technol. 2016;36(4):480–486. doi: 10.1007/s11596-016-1612-9. [DOI] [PubMed] [Google Scholar]
- 38.Estevez A.Y., Pritchard S., Harper K., Aston J.W., Lynch A., Lucky J.J., Ludington J.S., Chatani P., Mosenthal W.P., Leiter J.C., Andreescu S., Erlichman J.S. Neuroprotective mechanisms of cerium oxide nanoparticles in a mouse hippocampal brain slice model of ischemia. Free Radic. Biol. Med. 2011;51(6):1155–1163. doi: 10.1016/j.freeradbiomed.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Schubert D., Dargusch R., Raitano J., Chan S.W. Cerium and yttrium oxide nanoparticles are neuroprotective. Biochem. Biophys. Res. Commun. 2006;342(1):86–91. doi: 10.1016/j.bbrc.2006.01.129. [DOI] [PubMed] [Google Scholar]
- 40.Bailey Z.S., Nilson E., Bates J.A., Oyalowo A., Hockey K.S., Sajja V.S.S.S., Thorpe C., Rogers H., Dunn B., Frey A.S. Cerium oxide nanoparticles improve outcome after in vitro and in vivo mild traumatic brain injury. J. Neurotrauma. 2020;37(12):1452–1462. doi: 10.1089/neu.2016.4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X., Bucci E., Piacentini M., Ippolito G., Melino G. Nature Publishing Group; 2020. COVID-19 Infection: The Perspectives on Immune Responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun R., Liu H., Wang X. Mediastinal emphysema, giant bulla, and pneumothorax developed during the course of COVID-19 pneumonia. Korean J. Radiol. 2020;21(5):541. doi: 10.3348/kjr.2020.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spagnolo P., Balestro E., Aliberti S., Cocconcelli E., Biondini D., Della Casa G., Sverzellati N., Maher T.M. Pulmonary fibrosis secondary to COVID-19: a call to arms? Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marini J.J., Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020 doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 45.Ñamendys-Silva S.A. Respiratory support for patients with COVID-19 infection. Lancet Respir. Med. 2020;8(4):e18. doi: 10.1016/S2213-2600(20)30110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dobolyi A., Vincze C., Pál G., Lovas G. The neuroprotective functions of transforming growth factor beta proteins. Int. J. Mol. Sci. 2012;13(7):8219–8258. doi: 10.3390/ijms13078219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morris J.C., Tan A.R., Olencki T.E., Shapiro G.I., Dezube B.J., Reiss M., Hsu F.J., Berzofsky J.A., Lawrence D.P. Phase I study of GC1008 (fresolimumab): a human anti-transforming growth factor-beta (TGFβ) monoclonal antibody in patients with advanced malignant melanoma or renal cell carcinoma. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0090353. e90353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanford L.P., Ormsby I., Gittenberger-de Groot A.C., Sariola H., Friedman R., Boivin G.P., Cardell E.L., Doetschman T. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development. 1997;124(13):2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pohlers D., Brenmoehl J., Löffler I., Müller C.K., Leipner C., Schultze-Mosgau S., Stallmach A., Kinne R.W., Wolf G. TGF-β and fibrosis in different organs—molecular pathway imprints. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2009;1792(8):746–756. doi: 10.1016/j.bbadis.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 50.Ihn H. Pathogenesis of fibrosis: role of TGF-β and CTGF. Curr. Opin. Rheumatol. 2002;14(6):681–685. doi: 10.1097/00002281-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 51.Kendall R.T., Feghali-Bostwick C.A. Fibroblasts in fibrosis: novel roles and mediators. Front. Pharmacol. 2014;5:123. doi: 10.3389/fphar.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oró D., Yudina T., Fernández-Varo G., Casals E., Reichenbach V., Casals G., de la Presa B.G., Sandalinas S., Carvajal S., Puntes V. Cerium oxide nanoparticles reduce steatosis, portal hypertension and display anti-inflammatory properties in rats with liver fibrosis. J. Hepatol. 2016;64(3):691–698. doi: 10.1016/j.jhep.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 53.Arya A., Sethy N.K., Singh S.K., Das M., Bhargava K. Cerium oxide nanoparticles protect rodent lungs from hypobaric hypoxia-induced oxidative stress and inflammation. Int. J. Nanomed. 2013;8:4507. doi: 10.2147/IJN.S53032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhushan B., Nandhagopal S., Kannan R.R., Gopinath P. Therapeutic nanozyme: antioxidative and cytoprotective effects of nanoceria against hydrogen peroxide induced oxidative stress in fibroblast cells and in zebrafish. ChemistrySelect. 2016;1(11):2849–2856. [Google Scholar]
- 55.Wong J.P., Christopher M.E., Viswanathan S., Schnell G., Dai X., Van Loon D., Stephen E.R. Aerosol and nasal delivery of vaccines and antiviral drugs against seasonal and pandemic influenza. Expert Rev. Respir. Med. 2010;4(2):171–177. doi: 10.1586/ers.10.15. [DOI] [PubMed] [Google Scholar]
- 56.Mehta N., Mazer-Amirshahi M., Alkindi N., Pourmand A. Pharmacotherapy in COVID-19; A narrative review for emergency providers. Am. J. Emerg. Med. 2020 doi: 10.1016/j.ajem.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Šmelcerović A., Kocić G., Gajić M.Z., Tomović K., Djordjević V., Stanković-Djordjević D., Marko A. DPP-4 inhibitors in the prevention/treatment of pulmonary fibrosis, heart and kidney injury caused by COVID-19-a therapeutic approach of choice in type 2 diabetic patients? Front. Pharmacol. 2020;11:1185. doi: 10.3389/fphar.2020.01185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Orienti I., Gentilomi G.A., Farruggia G. Pulmonary delivery of fenretinide: a possible adjuvant treatment in COVID-19. Int. J. Mol. Sci. 2020;21(11):3812. doi: 10.3390/ijms21113812. [DOI] [PMC free article] [PubMed] [Google Scholar]