Abstract
COVID-19 has raised worldwide concern as spiraling into a pandemic. Reports about comprehensive investigation of COVID-19 viremia are extremely scanty. Herein, we present four COVID-19 patients with positive SARS-CoV-2 nucleic acid test in blood, accounting for 12.12% of 33 detected cases. Rapid deterioration of these cases with septic shock, accompanying with lung CT images enlarged rapidly, decrease of blood oxygen, heart rate drop (with asynchrony of hypoxemia) accompanied with SARS-CoV-2 viremia. It indicates that massive replication and releasing into blood of SARS-CoV-2 and secondary inflammation storm may lead to injury of multiple organs and poor prognosis. So, positive COVID-19 nucleic acid test in blood may be a good forecasting marker of rapid deterioration of COVID-19 pneumonia. In addition, clearance of viremia may indicate tendency for recovery.
Keywords: SARS-CoV-2, Viremia, Predict, Deterioration
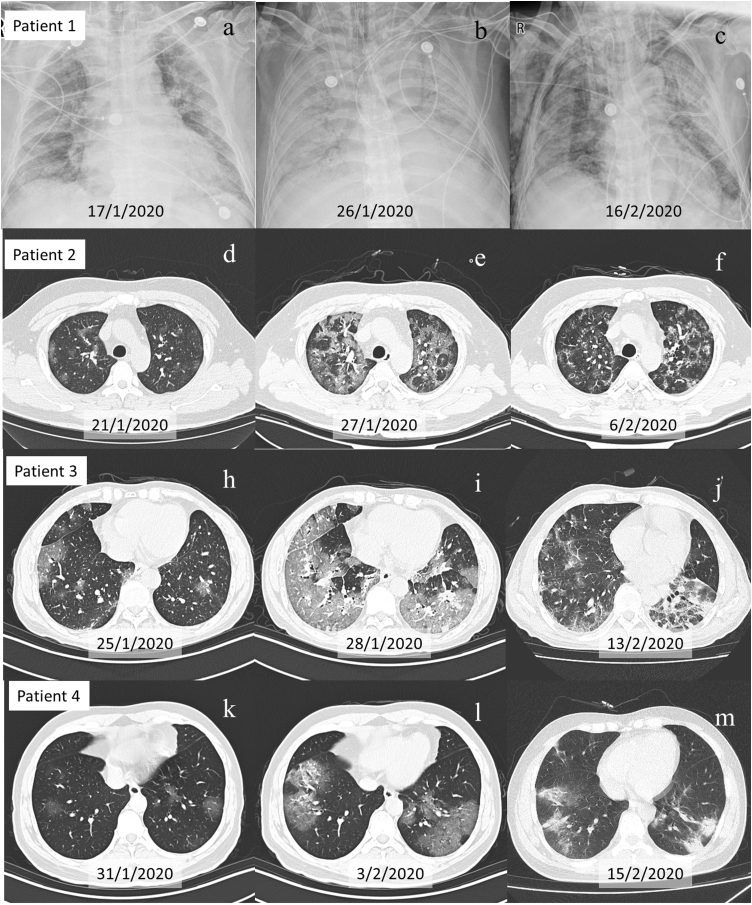
Since December 2019, a novel coronavirus disease (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread rapidly from Asia to other continents and spiraled into pandemic.1 As of June 2nd 2020, there were 83,002 confirmed patients and 4634 deaths in 31 provinces in China,1 with a mortality rate of 5.58%. Globally,2 there were 6,140,934 confirmed cases and 373,548 confirmed deaths in 216 countries, areas or territories in June 2nd 2020, with a mortality rate of 6.08%. Detection of SARS-CoV-2 by real time polymerase chain reaction (RT-PCR) is an essential diagnostic tool. However, a large proportion of the COVID-19 reports focuses mainly SARS-CoV-2 detection on respiratory or gastrointestinal tracts, ignoring the possibility of testing other sources, particularly the blood. Reports about comprehensive investigation of COVID-19 viremia are extremely scanty. Herein, we present four COVID-19 patients with positive SARS-CoV-2 nucleic acid test in blood, accounting for 12.12% of 33 detected cases. The four patients were all male, coming from Wuhan, aged 78, 60, 44, and 36 years old, respectively. Initially, positive SARS-CoV-2 nucleic acid test were found only in nasopharynx. However, after rapid deterioration of these cases with septic shock, lung CT images enlarged rapidly (Fig. 1), decrease of blood oxygen, and heart rate drop (with asynchrony of hypoxemia), SARS-CoV-2 nucleic acid test was detected from blood changed (Table1). SARS-CoV-2 viremia was the only pathogen detected in blood samples. With improvement, SARS-CoV-2 nucleic acid of patients 2, 3 and 4 was no longer detected in blood within three days. Nonetheless, in patient 1 SARS-CoV-2 nucleic acid continued to be detected in blood for 10 days, and then was detected in stool, urine and pleural effusion. It is suggested that the rapid deterioration of COVID-19 may be related to massive replication of SARS-CoV-2, releasing into blood, and then leading to injury of multiple organs. Although we did not isolate live virus from blood sample timely, live virus strains of SARS-CoV-2 had been isolated and cultured from feces of patient 1 in later stage, indicating the possibility of migration from lung to blood circulation to digestive tract. SARS-CoV-2 viremia of patient 1 have persisted for 10 days and finally resulted in multiple organs dysfunction.
Fig. 1.
Evolution of chest X-ray and CT scans of four patients with viremia. Figure (a–c), Figure (d–f), Figure (h–j), and Figure (l–n) show chest X-ray and CT scans of patient 1, 2, 3 and 4, respectively. Figure (a), Figure (d), Figure (h), and Figure (k) show mild lesions on admission. Figure (b), Figure (e), Figure (i) and Figure (l) show excessive new ground-glass exudate on CT scans the same or next day positive SARS-CoV-2 nucleic acid test in blood was first detected. Figure (c), Figure (f), Figure (j) and Figure (m) demonstrated recovery of pneumonia after SARS-CoV-2 nucleic acid becoming negative in blood. For patient 1, even after pneumonia was some what improved in Figure (c), other organs dysfunction persistently was observed due to extended viremia.
Table 1.
Clinical data during disease course.
| Patient 1 |
Patient 2 |
Patient 3 |
Patient 4 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Admission | Exacerbation | Progression | Admission | Exacerbation | Recovery | Admission | Exacerbation | Recovery | Admission | Exacerbation | Recovery | |
| Date | 17-Jan | 26-Jan | 16-Feb | 21-Jan | 26-Jan | 6-Feb | 25-Jan | 27-Jan | 13-Feb | 30-Jan | 3-Feb | 15-Feb |
| Vital sign | ||||||||||||
| Heart rate | 67 | 42 | 88 | 95 | 68 | 72 | 82 | 66 | 84 | 110 | 58 | 72 |
| Respiratory rate | 30 | 12 (muscle relaxation) | 20 (sedation) | 16 | 29 | 18 | 17 | 35 | 22 | 18 | 32 | 19 |
| Blood pressure, mmHg | 105/65 | 92/52 | 110/60 | 123/85 | 84/57 | 125/65 | 126/76 | 90/60 | 100/60 | 126/90 | 105/60 | 106/62 |
| Temperature, oC | 37.5 | 36.4 | 36.5 | 38 | 36.5 | 36.7 | 37.8 | 38.6 | 36.5 | 37.9 | 38.5 | 36.5 |
| SpO2, % | 92 | 94 | 99 | 95 | 92 | 97 | 96 | 88 | 96 | 97 | 86 | 98 |
| Oxygen support | HFNC (50 L/min, FiO2 40%) | Mechanical ventilation + VV-ECMO | Mechanical ventilation + VV-ECMO | Room air | HFNC (45 L/min, FiO2 45%) | Nasal cannula | Room air | Mechanical ventilation | HFNC (25 L/min, FiO2 45%) | Room air | HFNC (40 L/min, FiO2 50%) | Nasal cannula |
| PaO2/FiO2 | 220.5 | 64.2 | 187.5 | 467.6 | 184.4 | 329.4 | 455.7 | 61.27 | 302 | 452.9 | 208 | 371.4 |
| PaCO2, mmHg | 29.4 | 44.3 | 45 | 42.3 | 40.9 | 41.4 | 36.4 | 27.8 | 41.9 | 36.9 | 35.2 | 35.9 |
| pH | 7.402 | 7.412 | 7.316 | 7.372 | 7.379 | 7.421 | 7.42 | 7.494 | 7.442 | 7.406 | 7.378 | 7.415 |
| Lactic acid, mmol/L | 1.9 | 1.5 | 2.2 | 1.8 | 3.2 | 1.1 | 2 | 2.4 | 1.4 | 2.4 | 1.7 | 2 |
| White blood cell count, ×109/L | 9.9 | 9.24 | 19.52 | 6.2 | 8.25 | 4.59 | 4.7 | 6.34 | 6.26 | 4.77 | 4.9 | 4.05 |
| Neutrophil cell count, ×109/L | 9.49 | 7.94 | 14.42 | 4.31 | 7.38 | 2.16 | 2.94 | 4.1 | 3.87 | 3.47 | 3 | 2.42 |
| Lymphocyte count, ×109/L | 0.3 | 0.59 | 1.98 | 1.48 | 0.45 | 1.77 | 1.08 | 1.73 | 1.33 | 0.88 | 1.7 | 1.04 |
| Procalcitonin, ng/mL | <0.1 | 0.73 | 1.12 | <0.1 | <0.1 | <0.1 | <0.1 | 0.17 | 0.1 | 0.19 | <0.1 | 0.15 |
| C-reactive protein, mg/L | 154.3 | 206.2 | 102.96 | 58.17 | 11.64 | 2.18 | 17.07 | 59.28 | 5.68 | 46.15 | 105.34 | 9.17 |
| Total bilirubin, μmol/L | 6.3 | 47.56 | 21.77 | 9.4 | 16.12 | 11.1 | 8.64 | 33.81 | 7.46 | 11.2 | 25.08 | 7.46 |
| Albumin, g/L | 32.9 | 38.8 | 51.3 | 41.1 | 35.4 | 42.8 | 39.5 | 35.2 | 41.9 | 40.8 | 38.5 | 43.5 |
| Lactate dehydrogenase, U/L | 351 | 286 | 745 | 271 | 260 | 166 | 177 | 283 | 229 | 203 | 399 | 196 |
| Creatine kinase, U/L | 32 | 28 | 71 | 47 | 41 | 24 | 123 | 167 | 24 | 108 | 463 | 50 |
| Troponin I, ng/mL | <0.01 | 0.024 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| B-type natriuretic Peptide, pg/mL | 1210 | 4657 | 3100 | 263 | 1660 | 122 | 34 | 2820 | 66 | 171 | 2780 | 411 |
| Creatinine, μmol/L | 76 | 94.4 | 135.4(CRRT) | 52 | 48.4 | 61.4 | 71.5 | 70.8 | 53.5 | 80.8 | 74 | 64.8 |
| Prothrombin time, s | 14.3 | 15.7 | 14.1 | 16.5 | 11.4 | 11.1 | 12.6 | 13.3 | 13.1 | 13.1 | 12.5 | 11.7 |
| Activated partial thromboplastin time, s | 30.4 | 29.7 | 40.7 | 29.6 | 25.5 | 26.9 | 34.9 | 38.1 | 29.6 | 32.7 | 32.2 | 28.3 |
| D-dimer, mmol/L | 460 | 17,308 | 1575 | 93 | 253 | 130 | 415 | 392 | 2954 | 89 | 111 | 472 |
| Potassium, mmol/L | 3.7 | 4.02 | 4.95 | 3.7 | 3.9 | 4.16 | 3.44 | 3.25 | 3.74 | 3.47 | 3.38 | 4.0 |
| Sodium, mmol/L | 136 | 141 | 138 | 138 | 142 | 143 | 135 | 133 | 135 | 137 | 141 | 137 |
| SARS-CoV-2 RT-PCR | ||||||||||||
| Nasal or throat swab | + | + | + | + | + | – | + | + | – | + | + | – |
| Blood | – | + | – | – | + | – | – | + | – | – | + | – |
| Stool | – | + | + | – | + | – | – | + | + | – | + | + |
| Urine | / | + | + | – | – | – | / | – | – | – | – | – |
| Pleural effusion | / | + | + | / | / | / | / | / | / | / | / | / |
| Corticosteroid therapy | 80 mg Methylprednisolone on dayb 6 | 500 mg Methylprednisolone on day 8 | 500 mg Methylprednisolone on day 6 | 250 mg Methylprednisolone on day 7 | ||||||||
| Duration of positive SARS-CoV-2 tested in blood | 10 days | 2 days | 3 days | 3 days | ||||||||
| Duration of Excerbationa | Persistant | 11 days | 15 days | 12 days | ||||||||
| Prognosis | Progress | Recovery | Recovery | Recovery | ||||||||
Duration of exacerbation: time to recover from hemodynamic instability and PaO2/FiO2 less than 300.
Days were counted from admission.
It has been reported that viremia may cause damage of multiple organs in several ways and the patient's condition deteriorated even after no virus was detected in blood.3, 4 A retrospective study of 41 COVID-19 patients found that pro-inflammatory factors were significantly higher in ICU admitted cases, including IL-2, IL-7, IL-10, GSCF, IP10, MCP1, MIP1A, and TNF-α, which suggested that cytokine storm probably contributes to deterioration of patients with this disease. One COVID-19 patient also showed substantially reduced but hyper activated peripheral CD4+ and CD8+ T cells and increased proportion of CCR4+ CCR6 + Th17+ cells in CD4+ T cells, indicating a highly pro-inflammatory effect.5 The long persistence of SARS-CoV-2 viremia in patient 1 may have inevitably triggered the severe immunity disorder and resulted in multiple organs failure. Glucocorticoid is one of the most useful anti-inflammatory medications, but dosage and course in COVID-19 is still controversial. Single high dose of methylprednisolone had been used on patients 2, 3 and 4 after SARS-CoV-2 nucleic acid test became negative in blood, and effective treatment for shock and pulmonary inflammatory exudation was implemented (Table 1, Fig. 1). As for patient 1, due to persistence of SARS-CoV-2 viremia, the dosage of methylprednisolone was reduced.
Notably, all patients showed bradycardia as the disease progressed (Table1). At first, we empirically attributed to hypoxemia but bradycardia remained with adequate oxygen supplementation. It should be pointed out that hypoxemia may not be the only factor responsible for it. Interestingly, after clearance of viremia, the patients returned to normal rhythm. It may be evident that SARS-CoV-2 viremia has an inhibitory effect on cardiac sinus node or on cardiac conduction system. Further investigations are needed.
In summary, positive SARS-CoV-2 nucleic acid test in blood may predict rapid deterioration of COVID-19 patients. More attention should be paid to SARS-CoV-2 viremia.
Availability of data and materials
All data are presented in the manuscript.
Authors’ contributions
SB Li and MZ Chen managed the patient’s care throughout the course. J Liu made treatment decisions. CY Tan and YJ Liang were contributed to literature search and data collection. CY Tan was involved in drafting this manuscript. All authors read, edited, and approved the final manuscript.
Ethics approval and consent to participate
The study was reviewed and approved by Fifth Affiliated Hospital of Sun Yat-sen University. Informed consent was obtained from the patient for publication of this case report and any accompanying images.
Consent for publication
Informed consent was obtained in writing from the patient to publish personal data.
Conflicts of interest
The authors declare no conflicts of interest.
Funding
No.
Acknowledgements
No.
References
- 1.National Health Commission of the People’s Republic of China The latest news on Health Emergency Office. 2020. http://www.nhc.gov.cn/xcs/yqfkdt/202006/3a406ae5bc0c448bbafcc762df8b8969.shtml
- 2.Novel coronavirus (2019-nCoV). Situation report-56. WHO. https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- 3.Peiris J.S., Chu C.M., Cheng V.C., Chan K.S., Hung I.F., Poon L.L., et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beijing Group of National Research Project for SARS Dynamic changes in blood cytokine levels as clinical indicators in severe acute respiratory syndrome. Chin Med J. 2003;116:1283–1287. [PubMed] [Google Scholar]
- 5.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. Epub 2020 Feb 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are presented in the manuscript.