Abstract
Background:
Patient selection for liver transplantation for metastatic neuroendocrine tumors remains a topic of debate. There is no established MELD exception, making it difficult to obtain donor organs.
Methods:
A multicenter database was created assessing outcomes for liver and multivisceral transplantation for metastatic neuroendocrine tumors and identifying prognostic factors for survival. Demographic, transplant, primary tumor site and management, pathology, recurrent disease and survival data were collected and analyzed. Survival probabilities were calculated using the Kaplan–Meier method.
Results:
Analysis included 85 patients who underwent liver transplantation November 1988–January 2012 at 28 centers. One, three, and five-year patient survival rates were 83%, 60%, and 52%, respectively; 40 of 85 patients died, with 20 of 40 deaths due to recurrent disease. In univariate analyses, the following were predictors of poor prognosis: large vessel invasion (P<0.001), extent of extrahepatic resection at liver transplant (P=0.007), and tumor differentiation (P=0.003). In multivariable analysis, predictors of poor overall survival included large vessel invasion (P=0.001), and extent of extrahepatic resection at liver transplant (P=0.015).
Conclusion:
In the absence of poor prognostic factors, metastatic neuroendocrine tumor is an acceptable indication for liver transplantation. Identification of favorable prognostic factors should allow assignment of a MELD exception similar to hepatocellular carcinoma.
Keywords: liver transplantation, neuroendocrine tumor, prognostic indicators
INTRODUCTION
Gastroenteropancreatic neuroendocrine tumors (NET) arise from neuroendocrine cells present throughout the body. They are generally classified as islet cell or carcinoid and can occur in various anatomic sites resulting in a variety of presentations, with 32–47% of patients found to have metastatic disease at the time of diagnosis [1]. The incidence, recently reported at 5.25 per 100,000, has been rising in recent years, and the prevalence is 35 per 100,000 [1,2].
Slow-growing NETs have often metastasized to the liver by the time of diagnosis and negatively impact survival [3–5]. Various medical and surgical therapeutic options exist for metastatic NET to the liver, and the appropriate therapy remains a topic of discussion [6–11]. Liver resection has been shown to improve survival in selected cases [12–16]. Ki-67 index, tumor differentiation, and tumor stage have been identified as prognostic factors for patients who are candidates for resection [4,17,18]. International groups have incorporated these factors into proposed guidelines for the selection of patients for surgical resection [5,8,19]. In cases in which resection is not possible, total hepatectomy and liver transplantation (LT) have been performed for alleviation of tumor burden, symptom control, potential for cure, and for complications from prior therapy. Early reports were limited to single centers with few cases making it difficult to develop specific guidelines for transplant candidacy. Recently, there have been efforts to combine large numbers of cases from multiple centers as well as for single centers to develop and evaluate strategies to better define the utility of LT for metastatic NET.
Increasing interest has focused upon how to apply collective experience of LT for metastatic NET to establish evidence-based selection criteria. Two prospective studies have each achieved high survival outcomes of 78.3% and 87% through the use of strict selection criteria [20,21]. There have been several single center retrospective studies [20–25] as well as several meta-analyses and transplant registry-based studies [26–29] addressing this topic. Combining multicenter data has resulted in the ability to analyze outcomes and prognostic factors in larger populations. The largest and most recent multicentric report involved an analysis of the risk factors and outcomes for 213 patients from 35 European centers who underwent transplant from November 1982 through December 2009 and revealed several negative prognostic factors including hepatomegaly, poor differentiation, and major resection at the time of transplant [30]. When the United Network for Organ Sharing (UNOS) data was analyzed the only factor to achieve statistical significance was waiting time: 5-year overall survival with a waiting time longer than the median (67 days) was 63% versus 36% for patients who waited less than 67 days [26]. However, these results were based upon the limited information in the UNOS system. To date, no large multicenter North American experience including the preoperative and pathologic variables has been published to corroborate and expand upon the European experience.
Our aim was to develop a database of patients who had undergone LT for metastatic NET to identify demographic, histologic, site, and management-specific prognostic factors for overall survival. Secondary endpoints included graft survival as well as disease recurrence and causes of death.
MATERIALS AND METHODS
The patient population was initially identified by querying UNOS regarding centers and dates in which LT was performed for metastatic NET since 1988. After initial contact, we were notified by several centers that there were additional cases, and a request for participation in data collection was extended to both U.S. and Canadian centers. In addition, an attempt to collect global data led to one European center contributing data. Collected data included: demographics, type of transplant (multivisceral versus liver alone), type of donor (live donor versus deceased donor), patient and graft outcomes, disease status at last follow up, and cause of death and/or graft loss. Disease-specific data included location, histology, and management of primary site and medical and surgical therapies for primary site and metastatic disease. All transplant pathology reports were collected and reviewed by an independent pathologist to collect histologic data including cell type, differentiation, lymphatic, and vascular invasion, number of nodules, and tumor burden. Operative and pathology reports were used to corroborate center-supplied data, confirm the diagnosis, and obtain additional information for the database. Disease recurrence at last visit was recorded, however specific data regarding the timing and site of recurrence was rarely provided due to the retrospective nature of the study. Cause of death and cause of graft loss was reported by each center.
In order to assess the effect of timing of resection of primary site as well as extrahepatic surgeries at the time of transplant, the type of surgery was categorized as follows: Type 1=multivisceral transplantation; Type 2=pancreaticoduodenectomy at the time of LT; Type 3=distal pancreatectomy with splenectomy and bowel resection at LT; Type 4=distal pancreatectomy with or without splenectomy at LT; Type 5=bowel resection at LT; Type 6=no additional extrahepatic surgery at LT. The University of Southern California Institutional Review Board approved this study.
Statistical Methods
The primary endpoint considered in the analysis was patient survival. Survival was defined as the duration in time between date of transplant and date of death with patients who were alive censored at the last follow-up date. Besides patient survival, graft survival, which was defined as the duration in time between date of transplant and date of graft loss at retransplant or death, was also analyzed. Death was considered an event in the analysis of graft survival, and patients who were alive without experiencing a retransplant were censored at the last follow-up date. Date of retransplant was unknown for one patient; in the calculation of graft survival, the shortest graft survival duration (which was less than one day) was assigned to that patient.
Survival probabilities were calculated using Kaplan–Meier method with Greenwood standard errors. Univariable and multivariable Cox regression analyses were performed to evaluate the association between patient/disease characteristics and patient survival and to estimate hazard ratios. All P-values reported were two-sided, and a P-value of ≤ 0.05 was considered statistically significant. All analyses were performed in STATA (version 11.0; StataCorp LP College Station, TX).
RESULTS
29 centers (27U.S, one Canadian and one European) contributed 94 cases. All pathology reports were reviewed and only those cases in which NET was confirmed were included in the analysis, resulting in a total of 28 centers contributing 85 patients who underwent LT between November 23, 1988, and January 1, 2012. These included 34 females and 51 males, with median age at transplant of 48 years (range 16–75 years) and median follow up of 2.7 years (range 0.05–21.4 years). Patient and tumor characteristics, location and management of primary site, transplant type, and decade of LT are described in Table I. LT alone was performed in 80% of patients with the remaining 20% undergoing multivisceral transplant. There were 42 pancreaticoduodenal primaries (including ampulla and pylorus), and 24 primaries in the digestive tract. One patient had a primary lung tumor, one had both pancreatic and bowel tumors, one had a choledochal cyst, and the primary site was never identified in 16 patients. Table II shows the timing of resection according to anatomic site.
TABLE I.
Patient and Disease Characteristics
| Variables | Total (N=85) | % |
|---|---|---|
| Age at transplant | ||
| < 50 years | 46 | 54.1 |
| ≥ 50 years | 39 | 45.9 |
| Gender | ||
| Female | 34 | 40.0 |
| Male | 51 | 60.0 |
| Transplant Type | ||
| Liver Alone | 68 | 80.0 |
| Multivisceral | 17 | 20.0 |
| Primary Site of the Tumor | ||
| Duodenum/Pancreas | 42 | 49.4 |
| Digestive Tract | 24 | 28.2 |
| Other/Unknowna | 19 | 22.4 |
| Cell Type | ||
| Carcinoid | 35 | 41.2 |
| Islet Cell | 34 | 40.0 |
| Unknown | 16 | 18.8 |
| Timing of primary tumor resection | ||
| Pre- transplant | 31 | 36.5 |
| At time of transplantb | 38 | 44.7 |
| Otherc | 16 | 18.8 |
| Lymph node invasion | ||
| No | 18 | 21.2 |
| Yes | 34 | 40.0 |
| Unknown | 33 | 38.8 |
| Large vessel invasion | ||
| No | 29 | 34.1 |
| Yes | 18 | 21.2 |
| Unknown | 38 | 44.7 |
| Decade of transplant | ||
| Before 2000 | 29 | 34.1 |
| In or After 2000 | 56 | 65.9 |
| Extent of extrahepatic resection at transplantd | ||
| Type 1 | 17 | 20.0 |
| Type 2 | 9 | 10.6 |
| Type 3 | 3 | 3.5 |
| Type 4 | 5 | 5.9 |
| Type 5 | 7 | 8.2 |
| Type 6 | 44 | 51.8 |
| Differentiation | ||
| Well | 21 | 24.7 |
| Moderate | 6 | 7.1 |
| Poor | 5 | 5.9 |
| Unknown | 53 | 62.4 |
“Other/Unknown” included lung (n=1), choledochal cyst (n=1), pancreas and small bowel (n=1), and unknown (n=16).
This category included six patients whose primary tumor resection was performed pre-transplant and additional extrahepatic tumor resection was performed at transplant.
“Other” included patients who never had resection (n=15) or whose resection was done after transplant (n=1).
“Type 1= multivisceral transplant, Type 2=pancreaticoduodenectomy, Type 3=distal pancreatectomy, splenectomy and bowel resection, Type 4=distal pancreatectomy±splenectomy, Type 5=bowel resection, Type 6=no additional surgery at transplant.
TABLE II.
Timing of Resection According to Anatomic Site
| Site | Resection Pre-transplant | Resection at transplant | Resection Pre- and at transplant | Resection Post-Transplant | No resection |
|---|---|---|---|---|---|
| Pancreas (n = 38) | 12 | 22a | 3 | 1 | 0 |
| Small bowel (n = 16) | 8 | 5a | 3 | 0 | 0 |
| Duodenum (n = 2) | 1 | 1 | 0 | 0 | 0 |
| Ampulla (n = 1) | 1 | 0 | 0 | 0 | 0 |
| Choledochal cyst (n = 1) | 0 | 1 | 0 | 0 | 0 |
| Pylorus (n = 1) | 0 | 1 | 0 | 0 | 0 |
| Gastric (n = 4) | 3 | 1a | 0 | 0 | 0 |
| Large bowel (n = 1) | 1 | 0 | 0 | 0 | 0 |
| Appendix (n = 1) | 1 | 0 | 0 | 0 | 0 |
| Rectum (n = 2) | 2 | 0 | 0 | 0 | 0 |
| Lung (n = 1) | 1 | 0 | 0 | 0 | 0 |
| Pancreas and small bowel (n= 1) | 1 | 0 | 0 | 0 | 0 |
| Unknown (n=16) | 0 | 1a | 0 | 0 | 15 |
12 of 22 pancreatic primaries, 3 small bowel, 1 gastric primary and 1 unknown primary underwent multivisceral transplant.
As of the last follow-up date, 40 patients (47.1%) have died. Recurrent disease was the cause of death in 20 patients, whereas 18 patients died of causes unrelated to NET and two patients died of unknown causes. Disease status was reported in 81 patients, and a total of 46 of these 81 patients were found to have recurrent disease during the follow-up period.
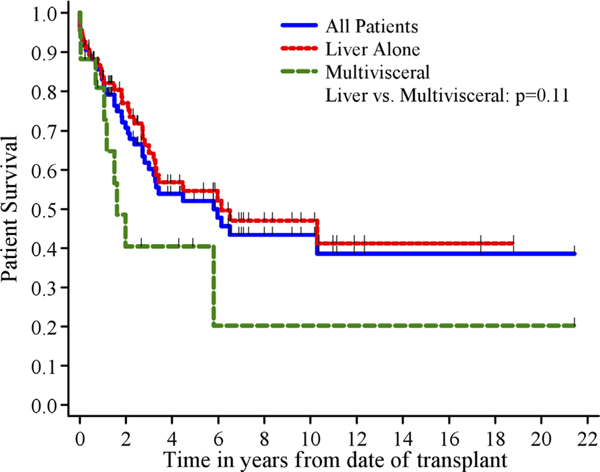
One, three, and five-year patient survival was 83%, 60%, and 52% (Fig. 1). One, three, and five-year graft survival was 79%, 56%, and 43%.
Fig. 1.
Patient survival by transplant type.
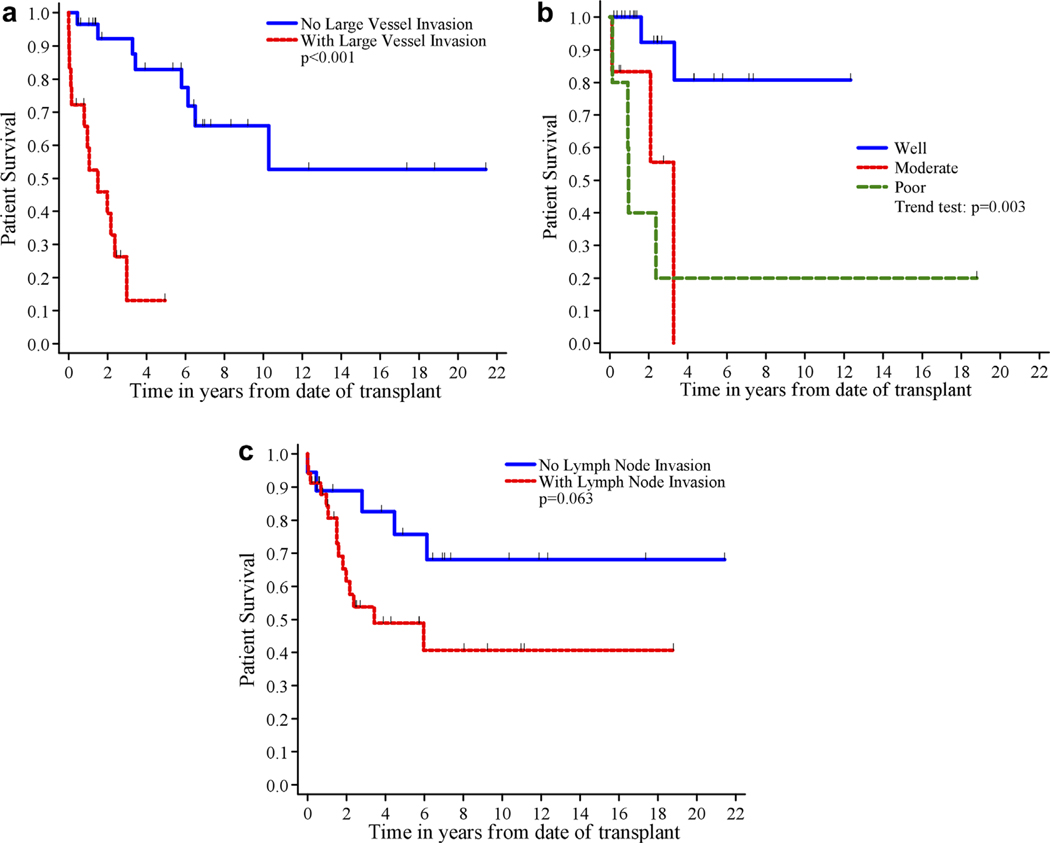
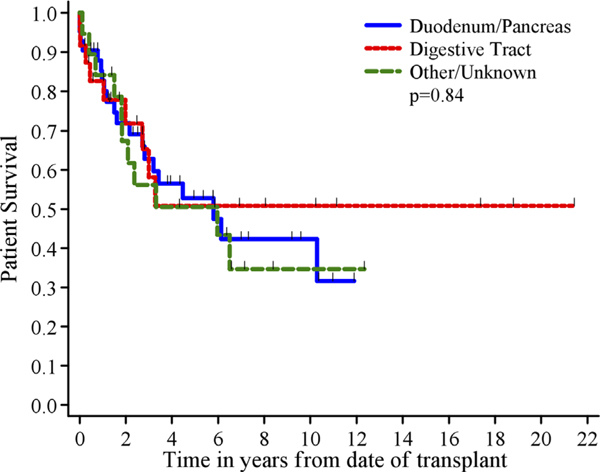
Univariate analysis results are shown in Table III. Large vessel invasion (P < 0.001, Fig. 2a) and differentiation (P=0.003, Fig. 2b) correlated with patient survival. Although it did not achieve statistical significance, five-year patient survival was 76% for patients without lymph node invasion compared to 49% for those with lymph node invasion (P=0.063, Fig. 2c). Transplant decade after year 2000 (P=0.074) trended toward but did not reach statistical significance. When primary site of the tumor was considered, five-year patient survival was 53% for duodenum/pancreas compared to 51% for digestive tract (P=0.84, Fig. 3). Age greater than or equal to 50 approached statistical significance in the subset of patients undergoing liver transplant alone after the year 2000 (P=0.057).
TABLE III.
Patient Survival (PS) and Hazard Ratios (HR) for Patient Subsets (N=85)
| Variables | n | # Events | 1 year PS±SE (%) | 3 year PS±SE (%) | 5 year PS±SE (%) | HR (95%CI) | P |
|---|---|---|---|---|---|---|---|
| Age at transplant | |||||||
| <50 years | 46 | 19 | 78±6% | 65±7% | 59±8% | 1.0 | 0.32 |
| >50 years | 39 | 21 | 90 ±5% | 54±9% | 44±9% | 1.4 (0.73, 2.5) | |
| Gender | |||||||
| Female | 34 | 17 | 82 ±7% | 54±9% | 51 ±9% | 1.0 | 0.63 |
| Male | 51 | 23 | 84 ±5% | 64±7% | 53±8% | 0.86 (0.46, 1.6) | |
| Transplant type | |||||||
| Liver alone | 68 | 31 | 84 ± 5% | 64 ± 6% | 55 ± 7% | 1.0 | 0.11 |
| Multivisceral | 17 | 9 | 81 ± 10% | 40 ± 14% | 40 ± 14% | 1.9 (0.90, 4.0) | |
| Primary site of the tumor | |||||||
| Duodenum/pancreas | 42 | 20 | 83±6% | 63±8% | 53 ± 9% | 1.0 | 0.84 |
| Digestive track | 24 | 9 | 83±8% | 58 ±12% | 51 ±12% | 0.85 (0.38, 1.9) | |
| Other/unknowna | 19 | 11 | 84 ±8% | 56 ±12% | 51 ±12% | 1.1 (0.53, 2.3) | |
| Timing of primary tumor res. | |||||||
| Pre-transplant | 31 | 12 | 90 ±5% | 75±8% | 58 ±10% | 1.0 | 0.19 |
| At time of transplantb | 38 | 19 | 78±7% | 46±9% | 46±9% | 1.9 (0.94, 4.0) | |
| Otherc | 16 | 9 | 81±10% | 61±13% | 54 ±13% | 1.5 (0.63, 3.6) | |
| Lymph node invasion | |||||||
| No | 18 | 5 | 89±7% | 83±9% | 76 ±11% | 1.0 | 0.063 |
| Yes | 34 | 15 | 84 ±6% | 54 ±10% | 49±10% | 2.5 (0.90, 7.0) | |
| Unknown | 33 | 20 | 79±7% | 55±9% | 43±9% | ||
| Large vessel invasion | |||||||
| No | 29 | 8 | 97±3% | 92 ±5% | 83±8% | 1.0 | <0.001 |
| Yes | 18 | 13 | 59±12% | 13 ±11% | 13±11%d | 12 (3.7, 42) | |
| Unknown | 38 | 19 | 84 ±6% | 57±9% | 46±9% | ||
| Decade of transplant | |||||||
| Before 2000 | 29 | 21 | 76 ±8% | 55±9% | 41±9% | 1.0 | 0.074 |
| In or after 2000 | 56 | 19 | 87±5% | 63±7% | 60±8% | 0.56 (0.30, 1.1) | |
| Extent of resection | |||||||
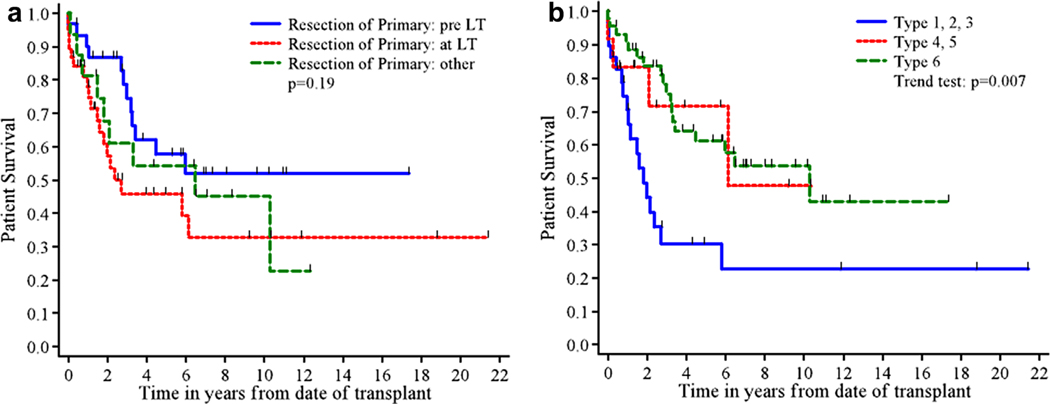
| Type 1 | 17 | 9 | 81±10% | 40±14% | 40±14% | 0.81 (0.70, 0.94)f | 0.007 |
| Type 2 | 9 | 7 | 67±16% | 13 ±12% | 13 ±12% | ||
| Type 3 | 3 | 2 | 33±27% | 33±27% | 33±27% | ||
| Type 4 | 5 | 1 | 100% | 100% | 100% | ||
| Type 5 | 7 | 3 | 71±17% | 48±23% | 48 ±23% | ||
| Type 6 | 44 | 18 | 91 ±4% | 75±7% | 61±8% | ||
| Differentiation | |||||||
| Well | 21 | 2 | 100% | 92±7% | 81 ±13% | 3.1 (1.5, 6.6)g | 0.003 |
| Moderate | 6 | 3 | 83±15% | 56±25% | 56±25%e | ||
| Poor | 5 | 4 | 40 ±22% | 20 ±18% | 20 ±18% | ||
| Unknown | 53 | 31 | 81 ±5% | 56±7% | 50±7% |
SE: Standard Error; HR: Hazard Ratio; CI: Confidence Interval
“Other/Unknown” included lung (n=1), choledochal cyst (n=1), pancreas and small bowel (n=1), and unknown (n=16).
This category included 6 patients whose primary tumor resection was performed pre-transplant and additional extrahepatic tumor resection was performed at transplant.
“Other” included patients who never had resection (n=15) or whose resection was done after transplant (n=1).
No follow-up was available at 5 years. PS at 4 years was reported.
No follow-up was available at 5 years. PS at 3 years was reported.
Extent of resection was evaluated as a continuous variable in the Cox regression models. The HR represents each unit change in grading of extent of resection.
Differentiation was evaluated as a continuous variable in the Cox regression models. The HR represents each unit change in grading of extent of resection. “Unknown” was excluded from the Cox models.
Fig. 2.
a: Patient survival by large vessel invasion. b: Patient survival by differentiation c: Patient survival by lymph node invasion.
Fig. 3.
Patient survival by primary site of tumor.
While one-year patient survival rates were similar for LT alone and for multivisceral transplant, five-year survival was lower for multivisceral transplant (40%) than for LT alone (55%; P=0.11). Patient survival according to timing of resection did not achieve statistical significance although five-year survival was higher in patients who had the primary tumor removed before transplant (58%, P=0.19, Fig. 4a). The extent of resection required for the primary tumor at the time of transplant correlated significantly with five-year survival, with patients undergoing multivisceral transplant or requiring combined pancreatic and bowel resection (Types 1–3) demonstrating significantly lower survival than those who either had no concomitant surgery or underwent an isolated pancreatic or bowel resection (Types 4–6) (P=0.007, Fig. 4b).
Fig. 4.
a: Patient survival by timing of resection. b: Patient survival by extent of resection.
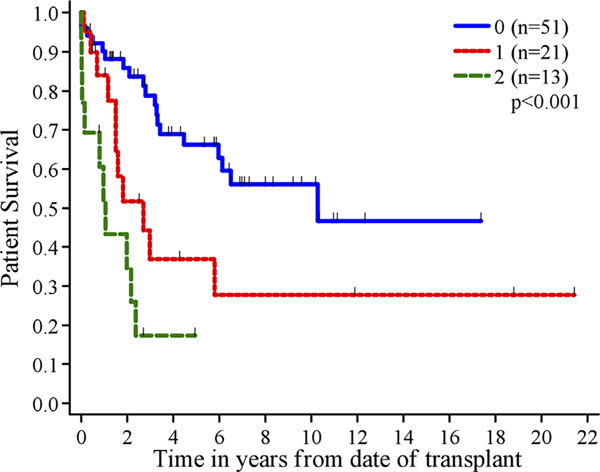
We included lymph node invasion, large vessel invasion, and extent of resection in a multivariable Cox regression model to evaluate their joint association with patient survival. For lymph node invasion and large vessel invasion, patients were grouped into three groups (no, yes, unknown); for extent of resection, we utilized the grading system defined above. In this multivariable analysis, large vessel invasion (yes vs. no; P=0.001) and extent of resection (P=0.015) were both found to be significantly associated with patient survival. The difference between the presence or absence of lymph node invasion was not significant (P=0.24). Differentiation was not included due to the small sample size for which these data are available. To examine whether large vessel invasion and extent of resection could be combined into a scoring system to identify patients more likely to survive post LT, we counted the number of adverse features each patient had. Therefore a score of 0 corresponded to no large vessel invasion and an extent of surgery type 4, 5, or 6. In addition, large vessel invasion was unknown for 38 patients, and those patients were scored 0 for large vessel invasion. A score of 1 point corresponded to large vessel invasion or extent of surgery type 1, 2, or 3 (but not both). A score of 2 points corresponded to larger vessel invasion and extent of surgery type 1, 2, or 3. One, three and five-year patient survivals were 90%, 79%, and 66% for score 0, 84%, 37%, and 37% for score 1, and 52%, 17%, and no follow up for score 2 (Fig. 5). This was statistically significant with a P-value of <0.001. When this analysis was redone excluding the 38 patients with unknown large vessel invasion, the conclusions did not change.
Fig. 5.
Patient survival by scoring system.
DISCUSSION
The overall survival in our patient population is similar to that reported in other large studies. Despite high recurrence rates, the five-year survival is 52%. While this does not compare favorably with survival in the modern era, it is necessary to view this survival rate in the context of the study time period, which began in 1988. In an analysis of outcomes using UNOS data, Gedaly reports 49% five-year survival for LT for metastatic NET compared to 62% for LT for hepatocellular carcinoma [26]. LeTreut reports the European experience including a five-year survival of 52%, which is identical to our own outcomes [30]. Based on our data and the data of other studies, LT remains indicated for metastatic NET in selected cases.
Central to the discussion of the merit of this procedure is the ability to identify consistent prognostic factors. In this study, univariate analysis identified differentiation, large vessel invasion, and extent of concomitant surgery as significant risk factors for survival. Multivariable analysis confirmed large vessel invasion and extent of surgery as significant factors that held up in a scoring system. We further showed that in this clinical setting, there is the potential for developing an effective scoring system for identifying patients who are unlikely to benefit from LT. Decade of transplant, type of transplant, lymph node invasion and age greater than 50 in the subset of patients who underwent LT alone after the year 2000 trended towards significance.
A number of literature reviews, registry-based analyses, single-center analyses, and more recently larger multicenter analyses have established that survival outcomes are good enough to merit LT in the setting of NET which has metastasized to the liver. Discussion now centers upon the appropriate determination and use of predictors of prognosis to guide in the selection of patients.
Reviews and Registry Data
Lehnert reviewed the literature encompassing 103 cases. He reported 5-year survival of 47% and multivariable analysis identified adverse prognostic factors as age greater than 50 years (P<0.03) and transplantation combined with upper abdominal exenteration or pancreaticoduodenectomy (P<0.001) [27].
Gedaly’s analysis of LT performed for metastatic NET from the UNOS database from 1988–2008 reported a 5-year survival of 49%, and the only variable to achieve statistical significant improved 5-year survival was waiting time over 67 days [26]. Nguyen reported data from UNOS for the time period 1988–2011 [29]. The 5-year survivals in the post-MELD era for HCC, metastatic NET and non-malignant diseases were 65.4%, 57.8%, and 73.7%. There was no statistical difference for the two malignancies but the survival for non-malignant diseases was significantly better (P=0.002). These studies, while providing useful information, reflect the limited data in the UNOS database and do not allow for an analysis of the clinical, pathologic, anatomic, and surgical prognostic factors.
Mathe analyzed 89 cases of NET with a pancreaticoduodenal primary site from 20 studies in the literature and reported five-year survival of 44% [28]. Negative prognostic factors included age greater than or equal to 55 years (P=0.0242) and simultaneous pancreatic resection at the time of LT (P=0.0132). With 0, 1, or 2 of these prognostic factors, 5-year survival was 61%, 40%, and 0%, respectively (P=0.0023). Our data confirm the negative prognosis accompanying pancreatic resection at the time of transplant (we showed that distal pancreatectomy did not worsen prognosis unless combined with bowel resection) but again failed to definitively confirm the age factor.
Single Center Studies
Frilling reported 67.2% five-year survival in 15 patients and identified the following positive prognostic factors: Ki67 index <10%, no extrahepatic disease, and the presence of somatostatin receptors on tumor surface [20]. Van Vilsteren reported 87% one-year survival in 17 patients who underwent transplantation after instituting a protocol using the following criteria: complete excision of primary site >6 months prior to LT, exclusion of poorly differentiated tumors and rectal carcinoid primaries [21]. Additional single center studies with small sample sizes reported 5-year survival ranging from 33–90% and/or cited prognostic factors including simultaneous resections and tumor differentiation [22–24,31–43].
Multicenter Studies
LeTreut reported 47% 5-year patient survival in 85 patients from 17 centers in France [44]. On univariate analysis, poor prognostic variables included hepatomegaly, gastrin secretion, upper abdominal exenteration, pancreaticoduodenal primary, positive resection margins, and poor differentiation. Resection of the primary tumor and serotonin secretion were good prognostic variables. On multivariable analysis, primary tumor site in the duodenum or pancreas (P=0.0018), upper abdominal exenteration at surgery (P=0.0034) and hepatomegaly (P=0.0157) were poor prognostic factors. LeTreut also reported an analysis of the risk factors and outcomes for 213 patients from 35 European centers who underwent transplant from November 1982 through December 2009 [30]. Five-year patient survival was 52% [30]. Multivariable analysis revealed the following negative risk factors: major resection at time of transplant (P<0.0001), poor differentiation (P<0.0005), and hepatomegaly (P<0.0003). Five-year survival improved to 59% for the subset of 106 patients who underwent LT after 2000. A separate multivariable analysis on these 106 patients revealed the following risk factors: hepatomegaly (P=0.006), age greater than 45 years (P=0.073) and resection in addition to LT (P=0.058). Utilizing a point system in which one point was given for each of these three prognostic factors, 5-year patient survival was 77%, 79%, 39%, and 33% for 0, 1, 2, and 3 points, respectively.
Our study did not confirm age to be a significant risk factor, however, there was a trend for age to have an impact in the modern era as it did in the European experience. Overall simultaneous resection did not reach significance, but extent of simultaneous resection was significant in our study confirming an impact of extrahepatic resection at the time of LT on outcomes. In addition, our study identified large vessel invasion as a significant prognostic factor.
Combining our data with prior reports, it is clear that certain factors repeatedly stand out as impacting patient survival. Tumor differentiation and extent of simultaneous surgery have been adequately validated. Other factors including age greater than 45–55 depending upon the study and the era, hepatomegaly, large vessel invasion, timing of primary site resection, and transplant era have been shown to be significant in various studies and have trended towards significance in others. The role of age, management of primary site and the role of preoperative imaging to evaluate vascular involvement all warrant further consideration. Hepatomegaly would represent tumor burden and a further look at this issue is required as it is usually the issue preventing other successful modalities such as resection.
The limitations of this study included the inability to determine disease-free survival and the small number of pathology reports stating tumor differentiation and Ki67 status. Although the data demonstrated that long-term survival can be expected even in the face of recurrence, the lack of data pertaining to time, location, and management of disease recurrence made an analysis of disease free survival and prognostic factors for disease recurrence impossible.
There was a lack of uniformity inherent in a retrospective study, as well as the time span covered, in the pathology reports. Overall assessment of the cell type, presence, and extent of vascular or lymphatic invasion, tumor burden and differentiation were not uniformly reported. Differentiation was specifically reported in less than half of the cases with very few reports specifically reporting Ki67. Currently proposed WHO (2010) pathological classification of neuroendocrine tumors (NET), considers the Ki-67 proliferation index as the main criterium for the grading of NET. Continued progress in this field would be facilitated by consistent reporting policies that include the reporting of both positive as well as negative findings.
In recent years targeted medical therapies have been approved in the treatment of NET. Sunitinib, a receptor tyrosine kinase inhibitor, has been shown to prolong progression-free survival over placebo in patients with advanced pancreatic neuroendocrine tumors [45,46]. Everolimus, a mammalian target of rapamycin inhibitor, has also shown increase of progression-free survival over placebo [46]. Everolimus is approved for the prophylaxis of rejection in liver transplant recipients and its role in patients undergoing liver transplant for metastatic NET needs to be determined. The potential exists that with the development of new agents and the implementation of multidisciplinary protocols, the outcomes may be improved.
CONCLUSION
The recent expansion of literature in this field has clarified the role of LT in metastatic NET confirming a role for LT in this disease process. The identification of significant risk factors has allowed us to better select our patients and consideration should be given to the development of a scoring system to allow for MELD exceptions to allow improved access to organs for well-selected patients.[47]
ACKNOWLEDGMENTS
The following centers contributed data to the database:
Cedars–Sinai Medical Center, Los Angeles, CA; Children’s Hospital Los Angeles, Los Angeles, CA; The Cleveland Clinic Foundation. Cleveland, OH; Duke University Hospital, Durham, NC; Indiana University Health, Indianapolis, IN; Jackson Memorial Hospital University of Miami Medical Center, Miami, FL; Keck Hospital of USC, Los Angeles, CA; Lahey Clinic Medical Center, Burlington, MA; Mayo Clinic Florida, Jacksonville, FL; Mayo Clinic Hospital, Scottsdale, AZ; Medical College of Virginia Hospitals, Richmond, VA.; Mount Sinai Medical Center, New York, NY; New York University Medical Center New York, NY; Oregon Health and Science University/Portland VA Medical Center, Portland, OR; Rush University Medical Center, Chicago, IL; Saint Louis University Hospital, St. Louis, MO; Stanford University Medical Center, Stanford, CA; University Hospital of Zürich, Zürich, Switzerland; University of Alabama Hospital, Birmingham, AL; University of Cincinnati Medical Center, Cincinnati, OH, The Nebraska Medical Center, Omaha, NE; Hospital of the University of Pennsylvania, Philadelphia, PA; University of Pittsburgh Medical Center, Pittsburgh, PA; Strong Memorial Hospital, University of Rochester Medical Center, Rochester, NY; University of Toronto, Toronto, ON, Canada; University of Virginia Health Sciences Center, Charlottesville, VA; University of Wisconsin Hospital and Clinics, Madison, WI; Washington University in St. Louis, St. Louis, MO.
GRANTS AND FINANCIAL SUPPORT
Database partially supported by Novartis Pharmaceuticals. Database partially supported by American Society of Transplant Surgeons Roche Student-Mentor Presidential Award. This project was supported in part by award number P30CA014089 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. The data collection and study management for this study were developed using CAFÉ (Common Application Framework Extensible) developed at USC’s Norris Comprehensive Cancer Center with support in part by award number P30CA014089 from the National Cancer Institute. The Biostatistics Core Services at USC’s Norris Comprehensive Cancer Center performed data analysis. Based on OPTN data as of 1/1/2006. This work was supported in part by Health Resources and Services Administration contract 234–2005-370011c. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Grant sponsor: Novartis Pharmaceuticals.; Grant sponsor: American Society of Transplant Surgeons Roche Student-Mentor Presidential Award.; Grant sponsor: National Cancer Institute; Grant number: P30CA014089; Grant sponsor: Health Resources and Services Administration; Grant number: 234–2005-370011c.
Abbreviations:
- LT
liver transplantation
- NET
neuroendocrine tumor
- UNOS
United Network for Organ Sharing
Footnotes
Conflict of Interest: None.
REFERENCES
- 1.Modlin IM,Lye KD, Kidd M: A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003;97:934–959. [DOI] [PubMed] [Google Scholar]
- 2.Yao JC, Hassan M, Phan A, et al. : One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063–3072. [DOI] [PubMed] [Google Scholar]
- 3.Pape U-F, Böhmig M, Berndt U, et al. : Survival and clinical outcome of patients with neuroendocrine tumors of the gastroenteropancreatic tract in a German referral center. Ann NY Acad Sci 2004;1014:222–233. [DOI] [PubMed] [Google Scholar]
- 4.Hentic O, Couvelard A, Rebours V, et al. : Ki-67 index, tumor differentiation, and extent of liver involvement are independent prognostic factors in patients with liver metastases of digestive endocrine carcinomas. Endocr Relat Cancer 2011;18:51–59. [DOI] [PubMed] [Google Scholar]
- 5.Pavel M, Baudin E, Couvelard A, et al. : ENETS consensus guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 2012;95:157–176. [DOI] [PubMed] [Google Scholar]
- 6.Mazzaferro V, Pulvirenti A, Coppa J: Neuroendocrine tumors metastatic to the liver: How to select patients for liver transplantation? J Hepatol 2007;47:460–466. [DOI] [PubMed] [Google Scholar]
- 7.Capurso G, Bettini R, Rinzivillo M, et al. : Role of resection of the primary pancreatic neuroendocrine tumour only in patients with unresectable metastatic liver disease: A systematic review. Neuroendocrinology 2011;93:223–229. [DOI] [PubMed] [Google Scholar]
- 8.Ramage JK, Ahmed A, Ardill J, et al. : Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs). Gut 2012;61:6–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutcliffe R, Maguire D;, Ramage MD, et al. : Management of neuroendocrine liver metastases. Am J Surg 2004;187:39–46. [DOI] [PubMed] [Google Scholar]
- 10.Frilling A, Sotiropoulos GC, Li J, et al. : Multimodal management of neuroendocrine liver metastases. HPB (Oxford) 2010;12:361–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frilling A, Modlin IM, Kidd M, et al. : Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol 2014;15:e8–e21. [DOI] [PubMed] [Google Scholar]
- 12.Mayo SC, de Jong MC, Pulitano C, et al. : Surgical management of hepatic neuroendocrine tumor metastasis: Results from an international multi-institutional analysis. Ann Surg Oncol 2010;17:3129–3136. [DOI] [PubMed] [Google Scholar]
- 13.Musunuru S, Chen H, Rajpal S, et al. : Metastatic neuroendocrine hepatic tumors: Resection improves survival. Arch Surg 2006;141:1000–1004. [DOI] [PubMed] [Google Scholar]
- 14.Touzios JG, Kiely JM, Pitt SC, et al. : Neuroendocrine hepatic metastases. Ann Surg 2005;241:776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elias D, Lasser P, Ducreux M, et al. : Liver resection (and associated extrahepatic resections) for metastatic well-differentiated endocrine tumors: A 15-year single center prospective study. Surgery 2003;133:375–382. [DOI] [PubMed] [Google Scholar]
- 16.Chen H, Hardacre JM, Uzar A, et al. : Isolated liver metastases from neuroendocrine tumors: Does resection prolong survival?. J Am Coll Surg 1998;187:88–92. [DOI] [PubMed] [Google Scholar]
- 17.Panzuto F, Nasoni S, Falconi M, et al. : Prognostic factors and survival in endocrine tumor patients: Comparison between gastrointestinal and pancreatic localization. Endocr Relat Cancer 2005;12:1083–1092. [DOI] [PubMed] [Google Scholar]
- 18.Cho CS, Labow DM, Tang L, et al. : Histologic grade is correlated with outcome after resection of hepatic neuroendocrine neoplasms. Cancer 2008;113:126–134. [DOI] [PubMed] [Google Scholar]
- 19.Steinmuller T, Kianmanesh R, Falconi M, et al. : Consensus guidelines for the management of patients with liver metastases from digestive (neuro) endocrine tumors: Foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 2008;87:47–62. [DOI] [PubMed] [Google Scholar]
- 20.Frilling A, Malago M, Weber F, et al. : Liver transplantation for patients with metastatic endocrine tumors: Single-center experience with 15 patients. Liver Transpl 2006;12:1089–1096. [DOI] [PubMed] [Google Scholar]
- 21.van Vilsteren FG, Baskin-Bey ES, Nagorney DM, et al. : Liver transplantation for gastroenteropancreatic neuroendocrine cancers: Defining selection criteria to improve survival. Liver Transpl 2006;12:448–456. [DOI] [PubMed] [Google Scholar]
- 22.Olausson M, Friman S, Herlenius G, et al. : Orthotopic liver or multivisceral transplantation as treatment of metastatic neuroendocrine tumors. Liver Transpl 2007;13:327–333. [DOI] [PubMed] [Google Scholar]
- 23.Rosenau J, Bahr M, von Wasielewski R, et al. : Ki67, E-cadherin, and p53 as prognostic indicators of long-term outcome after liver transplantation for metastatic neuroendocrine tumors. Transplantation 2002;73:386–394. [DOI] [PubMed] [Google Scholar]
- 24.Chan G, Kocha W, Reid R, et al. : Liver transplantation for symptomatic liver metastases of neuroendocrine tumours. Curr Oncol 2012;19:217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marin C, Robles R, Fernández JA, et al. : Role of liver transplantation in the management of unresectable neuroendocrine liver metastases. Transplant Proc 2007;39:2302–2303. [DOI] [PubMed] [Google Scholar]
- 26.Gedaly R, Daily MF, Davenport D, et al. : Liver transplantation for the treatment of liver metastases from neuroendocrine tumors. Arch Surg 2011;146:953–958. [DOI] [PubMed] [Google Scholar]
- 27.Lehnert T Liver transplantation for metastatic neuroendocrine carcinoma: An analysis of 103 patients. Transplantation 1998;66:1307–1312. [DOI] [PubMed] [Google Scholar]
- 28.Mathe Z, Tagkalos E, Paul A, et al. : Liver transplantation for hepatic metastases of neuroendocrine pancreatic tumors: A survival-based analysis. Transplantation 2011;91:575–582. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen NT, Harring TR, Goss JA, et al. : Neuroendocrine liver metastases and orthotopic liver transplantation: The US experience. Int J Hepatol 2011;2011:742890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Treut YP, Gregoire E, Klempnauer J, et al. : Liver transplantation for neuroendocrine tumors in Europe-results and trends in patient selection: A 213-case European liver transplant registry study. Ann Surg 2013;257:807–815. [DOI] [PubMed] [Google Scholar]
- 31.Bonaccorsi-Riani E, Apestegui C, Jouret-Mourin A, et al. : Liver transplantation and neuroendocrine tumors: Lessons from a single centre experience and from the literature review. Transpl Int 2010;23:668–678. [DOI] [PubMed] [Google Scholar]
- 32.Fernández JA, Robles R, Marín C, et al. : Role of liver transplantation in the management of metastatic neuroendocrine tumors. Transplantation Proc 2003;35:1832–1833. [DOI] [PubMed] [Google Scholar]
- 33.Florman S, Toure B, Kim L, et al. : Liver transplantation for neuroendocrine tumors. J Gastrointest Surg 2004;8:208–212. [DOI] [PubMed] [Google Scholar]
- 34.Ahlman H, Friman S, Cahlin C, et al. : Liver transplantation for treatment of metastatic neuroendocrine tumors. Ann NY Acad Sci 2004;1014:265–269. [DOI] [PubMed] [Google Scholar]
- 35.Ringe B, Lorf T, Döpkens K, et al. : Treatment of hepatic metastases from gastroenteropancreatic neuroendocrine tumors: Role of liver transplantation. World J Surg 2014;697–699. [DOI] [PubMed] [Google Scholar]
- 36.Coppa J, Pulvirenti A, Schiavo M, et al. : Resection versus transplantation for liver metastases from neuroendocrine tumors. Transplant Proc 2001;33:1537–1539. [DOI] [PubMed] [Google Scholar]
- 37.Pascher A, Steinmüller T, Radke C, et al. : Primary and secondary hepatic manifestation of neuroendocrine tumors. Langenbeck Arch Surg 2000;385:265–270. [DOI] [PubMed] [Google Scholar]
- 38.Dousset B, Saint-Marc O, Pitre J, et al. : Metastatic endocrine tumors: Medical treatment, surgical resection, or liver transplantation. World J Surg 1996;20:908–915. [DOI] [PubMed] [Google Scholar]
- 39.Anthuber M, Jauch K, Briegel J, et al. : Results of liver transplantation for gastroenteropancreatic tumor metastases. World J Surg 1996;20:73–76. [DOI] [PubMed] [Google Scholar]
- 40.Alessiani M, Tzakis A, Todo S, et al. : Assessment of five-year experience with abdominal organ cluster transplantation. J Am Coll Surg 1995;180:1–9. [PMC free article] [PubMed] [Google Scholar]
- 41.Routley D, Ramage JK, McPeake J, et al. : Orthotopic liver transplantation in the treatment of metastatic neuroendocrine tumors of the liver. Liver Transplant Surg 1995;1:118–121. [DOI] [PubMed] [Google Scholar]
- 42.Arnold JC, O’Grady JG, Bird GL, et al. : Liver transplantation for primary and secondary hepatic apudomas. Br J Surg 1989;76: 248–249. [DOI] [PubMed] [Google Scholar]
- 43.Makowka L, Tzakis AG, Mazzaferro V, et al. : Transplantation of the liver for metastatic endocrine tumors of the intestine and pancreas. Surg Gynecol Obstet 1989;168:107–111. [PMC free article] [PubMed] [Google Scholar]
- 44.Le Treut YP, Gregoire E, Belghiti J, et al. : Predictors of long-term survival after liver transplantation for metastatic endocrine tumors: An 85-case French multicentric report. Am J Transplant 2008;8:1205–1213. [DOI] [PubMed] [Google Scholar]
- 45.Le Treut YP, Delpero JR, Dousset B, et al. : Results of liver transplantation in the treatment of metastatic neuroendocrine tumors. Ann Surg 1997;225:355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raymond E, Dahan L, Bang YJ, et al. : Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. New Engl JMed 2011;364:502–513. [DOI] [PubMed] [Google Scholar]
- 47.Yao JC, Phan AT, Chang DZ, et al. : Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: Results of a phase II study J Clin Oncol 262008;4311–4318 [DOI] [PMC free article] [PubMed] [Google Scholar]