Abstract
Purpose:
Quality measures represent the standards of appropriate treatment agreed upon by experts in the field and often supported by data. The extent to which providers in the community adhere to quality measures in radiation therapy (RT) is unknown.
Methods and materials:
The Comparative Effectiveness Analysis of Surgery and Radiation study enrolled men with clinically localized prostate cancer in 2011 and 2012. Patients completed surveys and medical records were reviewed. Patients were risk-stratified according to D’Amico classification criteria. Patterns of care and compliance with 8 quality measures as endorsed by national consortia as of 2011 were assessed.
Results:
Overall, 926 men underwent definitive RT (69% external beam radiation therapy [EBRT]), 17% brachytherapy (BT), and 14% combined EBRT and BT with considerable variation in radiation techniques across risk groups. Most men who received EBRT had dose-escalated EBRT (>75 Gy; 93%) delivered with conventional fractionation (<2 Gy; 95%), intensity modulated RT (76%), and image guided RT (85%). Most men treated with BT received I125 (77%). Overall, 73% of the men received EBRT that was compliant with the quality measures (dose-escalation, image-guidance, appropriate use of androgen deprivation therapy, and appropriate treatment target) but only 60% of men received BT that was compliant with quality measures (postimplant dosimetry and appropriate dose). African-American men (64%) and other minorities (62%) were less likely than white men (77%) to receive EBRT that was compliant with quality measures.
Conclusions:
Most men who received RT for localized prostate cancer were treated with an appropriately high dose and received image guidance and intensity modulated RT. However, compliance with some nationally recognized quality measures was relatively low and varied by race. There are significant opportunities to improve the delivery of RT and especially for men of a minority race.
Introduction
With the passage of the Patient Protection and Affordable Care Act, there is renewed emphasis on improving the quality of medical care while containing costs.1,2 This is particularly relevant in prostate cancer (PCa) care where considerable variations in the quality of cancer care exist,3‐5 and the costs of care are expected to increase at least 35% over the next decade.6 Quality measures are tools that evaluate health care processes that are associated with high-quality health care.7 Quality measures for PCa radiation therapy (RT) have largely been identified by a combination of dedicated research groups and consensus recommendations.8,9 These groups have set standards with regard to radiation doses and techniques.
Although considerable effort has been made to identify radiation oncology quality measures,10–12 contemporary RT practice patterns and compliance with quality measures have not been well-characterized for PCa. Therefore, we evaluated radiation practice patterns and characterized treatment compliance with radiation quality measures among men who enrolled in the prospective population-based Comparative Effectiveness Analysis of Surgery and Radiation (CEASAR) study.
Methods and materials
Patient population
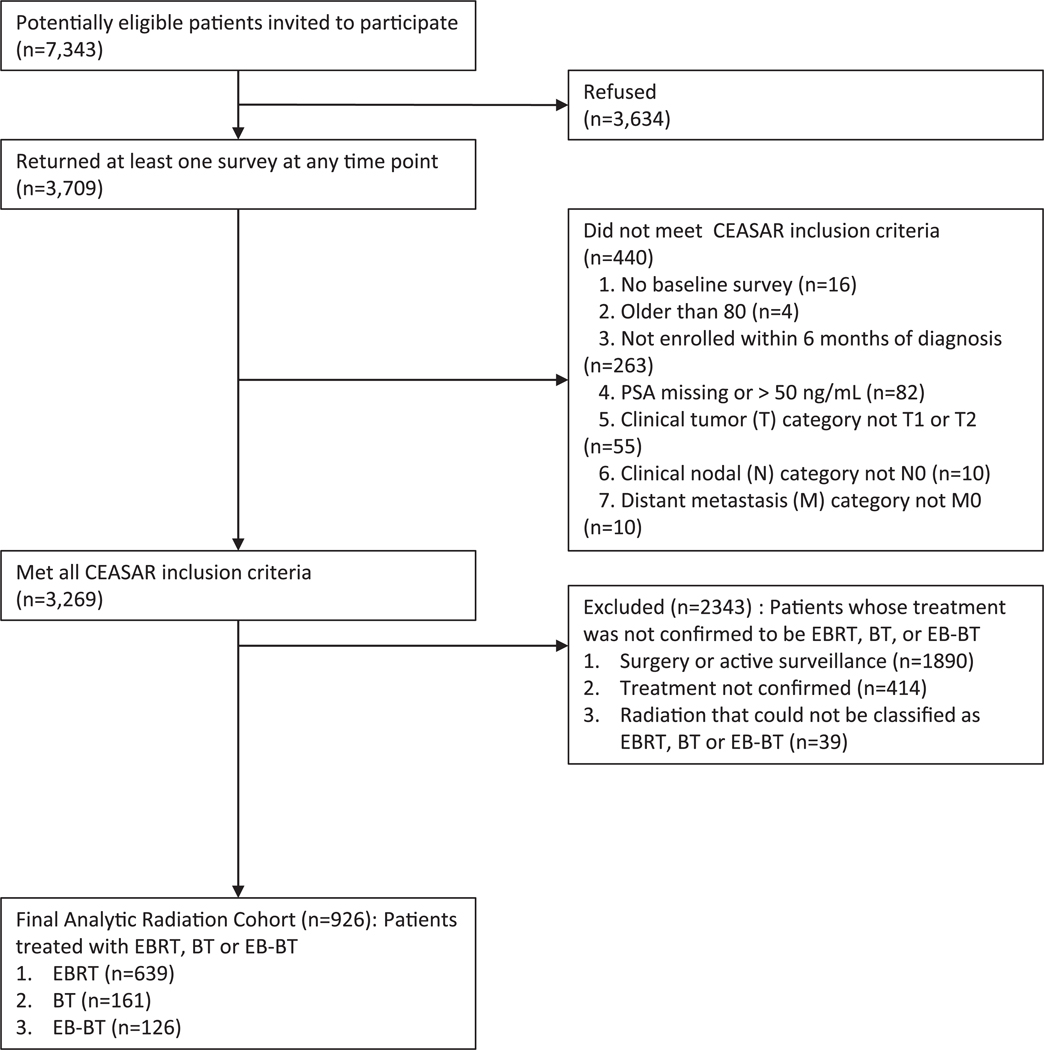
The CEASAR study enrolled men from January 2011 to February 2012 who were <80 years of age with clinically localized PCa and a prostate-specific antigen level <50 ng/mL. Patients were recruited from 5 Surveillance, Epidemiology, and End Results Program (SEER) registries (Atlanta, Los Angeles, Louisiana, New Jersey, and Utah) and a PCa patient registry (Cancer of the Prostate Strategic Urologic Research Endeavor).13 Details of the study design and objectives of the CEASAR study were described previously.14 The 926 men who underwent definitive RT for their PCa were evaluated for this analysis (Fig. 1).
Figure 1.
Diagram of the Assembly of the Comparative Effectiveness Analyses of Surgery and Radiation (CEASAR) Study Radiation Cohort. Abbreviations: EBRT=External beam radiation therapy, BT=Brachytherapy, EB-BT=Combined EBRT and BT.
Data collection
Baseline surveys that were completed by the study subjects captured sociodemographic data and comorbidity as previously described.14 Treatment details were obtained from medical chart abstraction that was performed 1 year after enrollment. The records of a total of 878 of 926 men underwent medical chart abstraction. Comorbidity was scored in accordance with the Total Illness Burden Index for Prostate Cancer.15 Race and ethnicity was categorized into Caucasian, African-American (AA), and other races/ethnicities on the basis of patient reports or, when missing, registry data.
Quality measures
Five quality measures for external beam radiation therapy (EBRT) and 3 for brachytherapy (BT) were selected from the recommendations of the 2011 National Comprehensive Cancer Network Prostate Cancer guidelines,16 American Brachytherapy Society guidelines,17 Quality Research in Radiation Oncology (QRRO),9,18 Physician Quality Reporting Initiative,19 and National Radiation Oncology Registry 20 (Table 1). Radiation treatment guidelines change over time so compliance was measured as adherence to the guidelines that were established at the time of study enrollment as of 2011. However, we evaluated the more inclusive BT doses as recommended by the American Brachytherapy Society that were published during the enrollment period rather than the more stringent BT doses as recommended by the 2011 National Comprehensive Cancer Network guidelines.
Table 1.
Patient characteristics
| EBRT (n = 639) | BT (n = 161) | EB+BT (n = 126) | Combined (n = 926) | P-value | |
|---|---|---|---|---|---|
| Age at time of diagnosis, median (IQR) | 69.0 (64.0, 73.0) | 66.0 (63.0, 71.0) | 67.0 (61.0, 72.0) | 68.0 (63.0, 73.0) | .001 |
| Race | |||||
| White | 446 (70%) | 133 (84%) | 92 (74%) | 671 (73%) | .007 |
| Black | 119 (19%) | 16 (10%) | 25 (20%) | 160 (17%) | |
| Other | 68 (11%) | 9 (6%) | 8 (6%) | 85 (9%) | |
| TIBI score | |||||
| 0–2 | 99 (17%) | 42 (27%) | 25 (21%) | 166 (19%) | .028 |
| 3–6 | 379 (66%) | 86 (55%) | 82 (68%) | 547 (64%) | |
| ≥7 | 99 (17%) | 29 (18%) | 14 (12%) | 142 (17%) | |
| Education | |||||
| High school graduate or less | 209 (36%) | 47 (30%) | 33 (28%) | 289 (34%) | .296 |
| Some college | 130 (23%) | 46 (29%) | 28 (24%) | 204 (24%) | |
| College graduate | 118 (21%) | 33 (21%) | 27 (23%) | 178 (21%) | |
| Graduate/professional school | 117 (20%) | 31 (20%) | 31 (26%) | 179 (21%) | |
| Income | |||||
| <$30,000 | 167 (31%) | 34 (23%) | 19 (17%) | 220 (28%) | .002 |
| $30,001-$50,000 | 118 (22%) | 31 (21%) | 34 (30%) | 183 (23%) | |
| $50,001-$100,000 | 142 (27%) | 59 (39%) | 29 (26%) | 230 (29%) | |
| >$100,000 | 104 (20%) | 27 (18%) | 30 (27%) | 161 (20%) | |
| Insurance | |||||
| Uninsured, VA, or Medicaid | 37 (6%) | 5 (3%) | 6 (5%) | 48 (5%) | .044 |
| Medicare | 425 (67%) | 94 (59%) | 78 (62%) | 597 (65%) | |
| Private/HMO | 169 (27%) | 61 (38%) | 42 (33%) | 272 (30%) | |
| Marital status | |||||
| Not married | 150 (26%) | 32 (21%) | 27 (22%) | 209 (25%) | .302 |
| Married | 422 (74%) | 123 (79%) | 93 (78%) | 638 (75%) | |
| Clinical tumor stage | |||||
| T1 | 465 (73%) | 133 (83%) | 95 (75%) | 693 (75%) | .039 |
| T2 | 173 (27%) | 28 (17%) | 31 (25%) | 232 (25%) | |
| Biopsy Gleason score | |||||
| ≤6 | 220 (35%) | 104 (65%) | 36 (29%) | 360 (39%) | < .001 |
| 3+4 | 216 (34%) | 41 (25%) | 45 (36%) | 302 (33%) | |
| 4+3 | 93 (15%) | 11 (7%) | 14 (11%) | 118 (13%) | |
| ≥8 | 107 (17%) | 5 (3%) | 31 (25%) | 143 (15%) | |
| Prostate-specific antigen level | |||||
| 0–4 | 87 (14%) | 27 (17%) | 18 (14%) | 132 (14%) | < .001 |
| 4–10 | 417 (65%) | 126 (78%) | 94 (75%) | 637 (69%) | |
| 10–20 | 101 (16%) | 8 (5%) | 12 (10%) | 121 (13%) | |
| 20–50 | 34 (5%) | 0 (0%) | 2 (2%) | 36 (4%) | |
| D’Amico risk criteria | |||||
| Low | 185 (29%) | 95 (59%) | 33 (26%) | 313 (34%) | < .001 |
| Intermediate | 294 (46%) | 57 (35%) | 57 (45%) | 408 (44%) | |
| High | 157 (25%) | 9 (6%) | 36 (29%) | 202 (22%) |
BT, brachytherapy; EBRT, external beam radiation therapy; EB+BT combined external beam radiation therapy with brachytherapy; HMO, health maintenance organization; IQR, interquartile range; TIBI, total illness burden index; VA, Veterans Affairs
Men who received EBRT alone (without BT) were evaluated for adherence with: 1) Prescription dose ≥75 Gy if treated with conventional fractionation9,16,18,20; 2) treatment with image guided radiation therapy (IGRT)9,16,18,20; 3) receipt of androgen deprivation therapy (ADT) if high-risk disease9,16,18,20; 4) no ADT if low-risk disease9,16,18,20; and 5)no pelvic radiation if low-risk disease.16,20 Men who received low-dose rate (LDR) BT alone (without EBRT) were evaluated for: 1) Documentation of postimplant dosimetry9,16,18,20; 2) prescription dose of 140 Gy to 160 Gy for iodine 125; (I125)16 and 3) prescription dose of 110 Gy to 125 Gy for palladium 103 (Pd103).16 These quality measures were selected in part because they could be reliably extracted from the medical record.
Statistical analysis
Patients’ demographic and clinical characteristics were summarized by RT received (EBRT, BT, and EBRT+BT). Treatment-specific compliances and practice pattern outcomes were summarized by individual factors and overall. Patient characteristics among the treatment groups were compared using the Wilcoxon rank sum (continuous variables) and χ2 tests (categorical variables). Differences in compliances and practice pattern outcomes among the different levels of sociodemographic factors were compared similarly. To further evaluate the effect of sociodemographic factors on compliance to recommended treatment strategies for RT, multivariable logistic regressions were used adjusted for age, race (black vs white, other vs white), education level (high school or less vs some college vs college graduate vs graduate/professional school), insurance status (Medicaid/no insurance/Veterans Affairs vs private insurance/health maintenance organization vs Medicare), and D’Amico risk category21 (low, intermediate, high). Odds ratio (OR) and 95% confidence intervals (CIs) were reported as the measure of the effects of these factors on the outcomes. In multivariable analyses, a multiple imputation approach was used to take into account the missing covariate values. 22 Statistical significance was considered for all 2-sided P-values ≤5%. All analyses were conducted using R software version 3.3.
Results
Clinical and patient characteristics
The clinical and patient characteristics at the time of diagnosis are shown in Table 1. The median age was 68 years (interquartile range [IQR]: 63–73 years). Thirty four percent of the men had low-risk, 44% had intermediate-risk, and 22% had high-risk disease. Seventeen percent were African-American and 9% were Hispanic/Latino, Asian, or other. With regard to education status and income, 34% completed high school or less and approximately 28% earned <$30,000 per year.
Adherence with quality measures
Men who were treated with EBRT had relatively high compliance with the selected quality measures (Table 2). Most men who were treated with conventional fractionation received dose-escalated radiation of N75 Gy (93%) and most received IGRT (85%). The majority of men with low-risk PCa did not receive pelvic radiation (96%) and most did not receive ADT (92%). Eighty-one percent of patients with high-risk PCa received ADT. Overall 73% of men who received EBRT had treatment that complied with all relevant quality measures for men in their risk group: 66% for men with low-risk, 80% for intermediate-risk, and 68% for high-risk disease.
Table 2.
Compliance with quality metrics
| Metric | N | Overall (n = 633) | White (n = 446) | Black (n = 119) | Other (n = 68) | P-value a |
|---|---|---|---|---|---|---|
| External beam radiation therapy alone | ||||||
| 1. IGRT utilization | 552 | .02 b | ||||
| Compliant | 85% (471) | 87% (341) | 88% (86) | 73% (44) | ||
| Noncompliant | 15% (81) | 13% (53) | 12% (12) | 27% (16) | ||
| 2. Dose prescription for conventional fractionation >75 Gy | 546 | .004 c | ||||
| Compliant | 93% (507) | 95% (363) | 87% (87) | 88% (57) | ||
| Noncompliant | 7% (39) | 5% (18) | 13% (13) | 12% (8) | ||
| 3. No pelvic field irradiation for low-risk disease | 170 | < .001 b | ||||
| Compliant | 96% (163) | 99% (120) | 80% (24) | 100% (19) | ||
| Noncompliant | 4% (7) | 1% (1) | 20% (6) | 0% (0) | ||
| ADT utilization | ||||||
| 4. No ADT for low-risk disease | 179 | .144 b | ||||
| Compliant | 92% (164) | 94% (121) | 88% (28) | 83% (15) | ||
| Noncompliant | 8% (15) | 6% (8) | 12% (4) | 17% (3) | ||
| 5. ADT for high-risk disease | 155 | .036 b | ||||
| Compliant | 81% (125) | 78% (78) | 77% (27) | 100% (20) | ||
| Noncompliant | 19% (30) | 22% (22) | 23% (8) | 0% (0) | ||
| Care compliant with all guidelines for EBRT | 568 | .005 c | ||||
| Compliant | 73% (414) | 77% (306) | 64% (68) | 62% (40) | ||
| Noncompliant | 27% (154) | 23% (92) | 36% (38) | 38% (24) | ||
| Low-dose rate brachytherapy alone | ||||||
| Postbrachytherapy implant CT dosimetry | ||||||
| 6. Postimplant CT dosimetry | 72 | |||||
| Compliant | 68% (49) | 66% (39) | 78% (7) | 75% (3) | ||
| Noncompliant | 32% (23) | 34% (20) | 22% (2) | 25% (1) | ||
| Low-dose rate BT dosages | ||||||
| 7. I125 dose 140–160 Gy | 80 | |||||
| Compliant | 90% (72) | 88% (59) | 100% (10) | 100% (3) | ||
| Noncompliant | 10% (8) | 12% (8) | 0% (0) | 0% (0) | ||
| 8. Pd103 dose 110–125 Gy | 26 | |||||
| Compliant | 92% (24) | 95% (18) | 83% (5) | 100% (1) | ||
| Noncompliant | 8% (2) | 5% (1) | 17% (1) | 0% (0) | ||
| Care compliant with all guidelines for BT | 81 | |||||
| Compliant | 60% (49) | 58% (39) | 70% (7) | 75% (3) | ||
| Noncompliant | 40% (32) | 42% (28) | 30% (3) | 25% (1) |
ADT, androgen deprivation therapy; BT, brachytherapy; CT, computed tomography; EBRT, external beam radiation therapy, I125, iodine 125; IGRT: image guidance radiation therapy; Pd103, palladium 103
Statistical tests were not attempted to low-dose rate BT-alone metrics due to limited sample size.
Fisher’s exact test
Pearson’s χ2 test
For men undergoing low dose BT alone, 68% had postimplant dosimetry performed. Of the men who received I125 BT seed implants, 90% received 140 Gy to 160 Gy and for those who received Pd103 implants, 92% received 110 Gy to 125 Gy (Appendix 1). Sixty percent of men who received BT in this cohort received BT that was compliant with both quality measures. Radiation records were obtained for all patients undergoing BT at least 90 days after their implant.
Treatment details
Sixty-nine percent of patients received EBRT, 13% received LDR BT, 4% received high-dose rate BT, and 14% received combined EBRT+BT (Table 1). Treatment details for EBRT, BT, and EB+BT are shown in Table 3. Most of the men undergoing EBRT (Table 3) received intensity modulated radiation therapy (IMRT, 76%) that was delivered with IGRT (85%). Nearly all patients (95%) underwent conventional fractionation (<2 Gy per fraction) but only 1% received moderate hypofractionation (>2–3 Gy) and 4% received ultra-hypofractionation (>3 Gy). Only 3 men undergoing EBRT (0.5%) did not complete their radiation treatment: Two patients because they were not able to tolerate the procedure and 1 patient because of patient choice.
Table 3.
Treatment details for radiation treatment by D’Amico risk criteria
| Low risk | Intermediate risk | High risk | Overall | P-value a,b | |
|---|---|---|---|---|---|
| EBRT (n) | 185 | 294 | 157 | 636 | |
| Use of ADT | |||||
| % receiving ADT, (n) | |||||
| Yes | 8% (15) | 44% (128) | 81% (127) | 43% (270) | < .001 |
| No | 92% (166) | 56% (166) | 19% (30) | 57% (362) | |
| Dose prescription for EBRT | |||||
| Conventional fractionation | 91% (157) | 95% (251) | 98% (141) | 95% (549) | .006 |
| Median dose (IQR), Gy | 79.2 (76.0, 79.2) | 79.2 (77.4, 79.2) | 79.0 (77.4, 79.2) | 79.2 (76.0, 79.2) | .516 |
| % receiving >75 Gy, (n) | 139 (89%) | 237 (94%) | 134 (95%) | 510 (93%) | .041 |
| Moderate fractionation | 1% (1) | 2% (6) | 1% (1) | 1% (8) | |
| Ultra-hypofractionation | 8% (14) | 3% (7) | 1% (2) | 4% (23) | |
| Radiation fields for EBRT | |||||
| % receiving pelvic radiation, (n) | |||||
| Yes | 4% (7) | 13% (36) | 40% (59) | 17% (102) | < .001 |
| No | 96% (165) | 87% (232) | 60% (87) | 83% (484) | |
| Use of image guidance | |||||
| % with IGRT, (n) | |||||
| Yes | 85% (132) | 85% (218) | 87% (125) | 85% (475) | .737 |
| No | 15% (24) | 15% (39) | 13% (18) | 15% (81) | |
| BT | |||||
| No. receiving BT | 95 | 57 | 9 | 161 | |
| Low-dose rate BT (n) | 81 | 34 | 7 | 122 | |
| I125 | 84% (67) | 68% (23) | 43% (3) | 77% (93) | .057 |
| Median dose (IQR), Gy | 145.0 (145.0, 145.0) | 144.0 (144.0, 145.0) | 111.2(94.3, 128.1) | 145.0 (144.0, 145.0) | .006 |
| dose 140–160 Gy | |||||
| Yes | 92% (54) | 91% (20) | 50% (1) | 90% (75) | |
| No | 8% (5) | 9% (2) | 50% (1) | 10% (8) | |
| Pd103 | 15% (12) | 29% (10) | 57% (4) | 21% (26) | |
| Median dose (IQR), Gy | 125.0 (125.0, 125.0) | 125.0 (125.0, 125.0) | 125.0 (125.0, 125.0) | 125.0 (125.0, 125.0) | .649 |
| Dose 110–125 Gy | |||||
| Yes | 83% (10) | 100% (10) | 100% (4) | 92% (24) | |
| No | 17% (2) | 0% (0) | 0% (0) | 8% (2) | |
| Cs131 | 1% (1) | 3% (1) | 0% (0) | 2% (2) | |
| High-dose rate BT (N) | 14 | 23 | 2 | 39 | |
| Median dose (IQR), Gy | 39.0 (39.0, 39.0) | 39.0 (39.0, 39.0) | — | 39.0 (39.0, 39.0) | .376 |
| Fractionation, Median | 6.5 (6.5, 7.3) | 6.5 (6.5, 6.5) | — | 6.5 (6.5, 6.5) | .565 |
| dose (IQR), Gy | |||||
| EB+BT | |||||
| No. receiving combined EB+BT | 33 | 57 | 36 | 126 | |
| IMRT | |||||
| Yes | 73% (24) | 93% (53) | 89% (32) | 87% (109) | .022 |
| No | 27% (9) | 7% (4) | 11% (4) | 13% (17) | |
| Median dose (IQR), Gy | 45.0 (45.0, 52.5) | 45.0 (45.0, 52.5) | 45.0 (45.0, 52.5) | 45.0 (45.0, 52.5) | .461 |
| No. with IGRT, (%) | |||||
| Yes | 86% (25) | 81% (44) | 71% (25) | 80% (94) | .31 |
| No | 14% (4) | 19% (10) | 29% (10) | 20% (24) | |
| EB+BT (low-dose rate) | 32 | 48 | 27 | 107 | |
| 1125 | 97% (29/30) | 81% (38/47) | 78% (21/27) | 85% (88/104) | .089 |
| Median dose (SD), Gy | 80.0 (80.0, 106.5) | 97.0 (80.0, 110.0) | 90.0 (80.0, 110.0) | 90.0 (80.0, 110.0) | .376 |
| EB-BT (high-dose rate) | 1 | 9 | 9 | 19 | |
| Median dose (IQR), Gy | 19.0 (19.0, 19.0) | 21.0 (19.0, 22.0) | 22.0 (22.0, 22.0) | 22.0 (19.9, 22.0) | .009 |
| Fractionation, Mean dose (SD), Gy | — | 7.0 (5.5, 9.5) | 5.5 (5.5, 5.5) | 5.5 (5.5, 7.0) | .036 |
ADT, androgen deprivation therapy; BT, brachytherapy; Cs131, Cesium 131; EBRT, external beam radiation therapy; EB+BT, combined external beam radiation therapy with brachytherapy; I125, iodine 125; IGRT, image guidance radiation therapy; IMRT, intensity modulated radiation therapy; IQR, interquartile range; Pd103, palladium 103; PSA, prostate-specific antigen; SD, standard deviation; VMAT, volumetric arc therapy
Pearson’s χ2 test for categorical variables and Wilcoxon rank sum test for continuous variables unless otherwise noted
Fisher’s exact test
Factors associated with compliance
African-American and other men of a minority race were less likely to receive compliant care with all guidelines for EBRT compared with white men (Table 2). Seventy-seven percent of Caucasian men received EBRT that met all quality measures compared with 64% of AA men and 62% of other men of a minority race (P < .01). There was some variation in the association of race/ethnicity with compliance characteristics. AA men (80%) were less likely to avoid pelvic irradiation for low-risk disease than Caucasian men (99%) or men from other minority groups (100%; P < .01). Also, both AA men (87%) and men from other minority groups (88%) were less likely to receive dose-escalated EBRT compared with Caucasian men (95%; P = .004). Hispanic men and men from other race groups (73%) were less likely to receive IGRT than Caucasian men (87%) or AA men (88%; P = .02).
Men with more education (at least some college or more education) were more likely to avoid unnecessary pelvic radiation for low-risk disease compared with men with a high-school education (100% vs 88%; P < .01). Men with at least some college education more commonly received EBRT that was compliant with all quality measures but the difference was not statistically significant (76% vs 69%; P = .07). Men with a high school education or less were more likely to receive BT that meets all quality measures (81%) than men with at least a college education or more (51%; P = .008). There were no significant associations between compliance with quality measures for either EBRT or BT and insurance status.
In multivariable analyses on compliance with the quality measures among patients who underwent EBRT, age, education, and insurance status were not significantly associated with the outcomes (See Table 4). However, compared with white men, AA and other men of a minority race had 46% (OR: 0.54; 95% CI, 0.32–0.89; P < .001) and 51% (OR: 0.49; 95% CI, 0.27–0.91; P = .007) decreased odds, respectively, of receiving EBRT that met all quality measures. Compared with the low-risk criteria, men with intermediate-risk disease had a 108% increase (OR: 2.08; 95% CI, 1.3–3.33; P = .002) in odds of receiving EBRT that met all quality measures.
Table 4.
Factors associated with compliance among patients who received EBRT (estimated from multivariable models)
| Compliant with all guidelines | Compliant with IGRT utilization | Compliant with adequate dose | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Factors | OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value |
| Age (Q3 vs Q1) | 1.05 | (0.78–1.41) | .765 | 0.97 | (0.66–1.41) | .857 | 0.74 | (0.45–1.22) | .234 |
| Race | |||||||||
| White | 1 (ref) | 1 (ref) | 1 (ref) | ||||||
| Black | 0.54 | (0.32–0.9) | .018 | 1.15 | (0.56–2.35) | .704 | 0.22 | (0.1–0.51) | < .001 |
| Other | 0.49 | (0.27–0.9) | .022 | 0.45 | (0.22–0.91) | .025 | 0.26 | (0.1–0.7) | .007 |
| D’Amico risk criteria | |||||||||
| Low | 1 (ref) | 1 (ref) | 1 (ref) | ||||||
| Intermediate | 2.08 | (1.3–3.33) | .002 | 1.08 | (0.61–1.9) | .797 | 2.63 | (1.21–5.69) | .014 |
| High | 1.38 | (0.82–2.33) | .222 | 1.41 | (0.71–2.81) | .329 | 3.1 | (1.19–8.05) | .02 |
| Education | |||||||||
| High school graduate or less | 1 (ref) | 1 (ref) | 1 (ref) | ||||||
| Some college | 1.43 | (0.8–2.58) | .228 | 0.99 | (0.48–2.01) | .967 | 0.7 | (0.26–1.9) | .482 |
| College graduate | 1.32 | (0.73–2.39) | .358 | 1.44 | (0.68–3.06) | .338 | 0.79 | (0.28–2.21) | .647 |
| Graduate/professional school | 0.86 | (0.49–1.52) | .609 | 0.89 | (0.44–1.83) | .761 | 0.49 | (0.18–1.31) | .155 |
| Insurance | |||||||||
| Uninsured, VA, or Medicaid | 1 (ref) | 1 (ref) | 1 (ref) | ||||||
| Medicare | 1.5 | (0.62–3.66) | .372 | 1.98 | (0.71–5.5) | .189 | 0.91 | (0.18–4.64) | .905 |
| Private/HMO | 1.31 | (0.53–3.24) | .564 | 1.71 | (0.61–4.78) | .31 | 0.84 | (0.16–4.46) | .838 |
CI, confidence interval; HMO, health maintenance organization; IGRT, image guided radiation therapy; OR, odds ratio; ref, reference; Q, quarter; VA, Veterans Affairs
Discussion
The majority of men treated with RT for localized PCa in this population-based cohort study underwent EBRT, primarily dose-escalated IMRT delivered with IGRT, and conventional fractionation. Although there was an 80% to 90% compliance rate with most of the individual RT quality measures, 19% of men with high-risk disease did not receive ADT. Additionally, 27% of EBRT and 40% of BT did not adhere to all evaluated quality measures. There were racial disparities as nonwhite men were much less likely to receive guideline-concordant RT.
Although EBRT was the most common technique across all risk groups, there were variatios in the RT techniques by risk categorization. BT as a monotherapy was predominately used in low-risk PCa while combined EBRT+BT was more commonly administered for intermediate- and high-risk disease. Evolving EBRT techniques during this time period including proton radiation, ultra-hypofractionation, and CyberKnife were utilized for low-risk disease. However, the proportion of patients who received these techniques was small and each represented 6% to 8% of treatments for low-risk patients.
We found less frequent use of moderate hypofractionation than reported in a National Cancer Data Base (NCDB) study of men who were treated during the same time period. 23 This difference may be explained in part because the NCDB is a hospital-based registry 24 while our study patients were recruited from population-based registries. Additionally, our cohort is smaller than from the NCDB and both cohorts are drawn from different geographic areas. The rate of medical claims for SBRT in a population-based SEER-Medicare study during this time period25 was similar to the frequency of CyberKnife and ultra-hypofractionation that was identified in our study that obtained RT details from medical chart reviews.
There was high compliance with the majority of individual quality measures for EBRT. Most men treated with conventional fractionation received dose-escalated radiation (>75 Gy), which improves PCa control.26–29 The majority of men also received IGRT, which can improve the accuracy of targeting of the prostate while limiting toxicity to adjacent organs. Additionally, most men with low-risk disease appropriately did not receive unnecessary ADT or pelvic radiation, which can cause toxicity but does not improve outcomes.30–34 Our study demonstrates the increased adoption of dose-escalated EBRT and IGRT compared with the QRRO Survey of men with PCa who were treated in 2007.9
A significant portion of men with high-risk PCa did not receive ADT with radiation. The addition of ADT to EBRT improves PCa survival for men with high-risk disease 35–37 but there is concern that ADT may increase cardiovascular morbidity and mortality. 38–40 We could not determine why ADT was not administered to some men with high-risk disease in our study. The possibility exists that there was under-ascertainment of ADT administration from the medical chart abstraction. However, population-based studies that analyzed medical claims data also have found a significant portion of men with high-risk PCa who do not receive ADT. 41,42
Most men who were treated with BT received I125 LDR implants and approximately two-thirds had documentation of postimplant dosimetry. Postimplant dosimetry provides an assessment of implant quality and allows for feedback on continual technical improvement.17 The QRRO survey9 found similar utilization rates of I125 and Pd103; however, they reported significantly higher rates of postimplant dosimetry. These differences could reflect the fact that radiation centers agreed to participate in the QRRO study and report their radiation details whereas this population-based study pursued radiation records for all enrolled men. Our medical record abstraction may have underestimated the utilization of postimplant dosimetry if they did not have access to postimplant dosimetry documentation.
There was racial variation in the receipt of RT that complied with the evaluated quality measures. Men of a minority race were less likely to receive dose-escalated EBRT and Hispanic men were less likely to receive IGRT. Lack of compliance with quality measures that improve PCa control and reduce treatment toxicity may play a role in the disparity of PCa outcome as seen in men of a minority race.43 Previous population-based studies of men undergoing radical prostatectomy and RT for PCa found no evidence of racial disparity.44,45 However, these studies were based on available claims data and not able to capture specific details on the radiation techniques where we identified disparities.
The strengths of this study are that it is a population-based study that reflects how radiation is delivered in the community and that comprehensive medical chart reviews captured granular radiation technical details. Some items are worth noting. First, medical chart abstraction may underestimate the level of compliance with quality measures if treatment details are not accurately documented by providers. However, documentation of procedural and process measures is an essential component of quality medical care provisions and can itself be a proxy for quality care because it allows for accurate measurement, comparison, and improvement of outcomes. Second, we could not determine why care did not adhere to quality measures.
Third, the CEASAR study was designed to evaluate process and outcome measures and did not capture many structural measures. Although structural measures such as the resources of hospitals and providers can influence outcomes, the main measures were selected because they were endorsed by several consortia. We were unable to impact hospital type or facility volume. Fourth, we did not evaluate whether adherence to these quality measures impacted cancer control or treatment toxicity but this will be investigated in future studies.
Fifth, our cohort was enrolled from 5 SEER registries and the Cancer of the Prostate Strategic Urologic Research Endeavor registry and does not reflect geographic and regional treatment differences outside of these catchment areas. Finally, medical evidence and guidelines evolve over time. Randomized trials that demonstrate similar efficacy and toxicity for moderate hypofractionation compared with conventional fractionation for PCa46,47 and a randomized trial that demonstrates a biochemical progression-free survival benefit for combined EBRT+BT over EBRT for men with intermediate- and high-risk PCa48,49 were published after the study period. The utilization of moderate hypofractionation and combined EBRT+BT may increase in more recent years as a consequence of these publications.
The ability to measure patterns and quality of care, evaluate and compare performance, and identify opportunities for improvement in care delivery is increasingly important with the current movement toward shared accountability and value-based payment models. Large administrative data registries and prospective trials often lack granular patient-level details and heterogeneous patient populations to assess contemporary practice patterns or quality of care provided.50 This study leverages its diverse patient population, wide array of providers from academic and community centers, and fine patient details from medical chart abstraction to show important practice patterns and significant gaps in care in the management of PCa that can be improved. In addition, we were able to identify potential areas for quality improvement and particularly for men of a minority race, which can have an impact on improving the disparities in health outcomes.
Conclusions
In this population-based cohort study, most men treated with RT for localized PCa received dose-escalated IMRT that was delivered with IGRT and conventional fractionation. Although most treatment complied with individual RT quality measures, compliance varied by race. There are opportunities to improve the quality of RT for localized PCa and especially for men of a minority race.
Supplementary Material
Acknowledgments
Sources of Support: Funding for the study was provided by grants 1R01HS019356 and 1R01HS022640 from the Agency for Healthcare Research and Quality as well as IJL1TR000011 from the National Center for Advancing Translational Sciences to the Vanderbilt Institute of Clinical and Translational Research. The research reported in this article was partially funded through Patient-centered Outcomes Research Institute award CE12-11-4667.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.prro.2018.04.009.
References
- 1.Choudhry NK, Rosenthal MB, Milstein A. Assessing the evidence for value-based insurance design. Health Aff (Millwood). 2010;29: 1988–1994. [DOI] [PubMed] [Google Scholar]
- 2.Fisher ES, Shortell SM. Accountable care organizations: accountable for what, to whom, and how. JAMA. 2010;304:1715–1716. [DOI] [PubMed] [Google Scholar]
- 3.McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348: 2635–2645. [DOI] [PubMed] [Google Scholar]
- 4.Schmid M, Meyer CP, Reznor G, et al. Racial differences in the surgical care of Medicare beneficiaries with localized prostate cancer. JAMA Oncol. 2016;2:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samuel CA, Landrum MB, McNeil BJ, Bozeman SR, Williams CD, Keating NL. Racial disparities in cancer care in the Veterans Affairs health care system and the role of site of care. Am J Public Health. 2014;104:S562–S571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103:117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Medicare and Medicaid Services. Quality Measures. Available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/QualityMeasures/index.html?redirect=/QualityMeasures/03_ElectronicSpecifications.asp. Accessed September 1, 2017.
- 8.Albert JM, Das P. Quality indicators in radiation oncology. Int J Radiat Oncol Biol Phys. 2013;85:904–911. [DOI] [PubMed] [Google Scholar]
- 9.Zelefsky MJ, Lee WR, Zietman A, et al. Evaluation of adherence to quality measures for prostate cancer radiotherapy in the United States: Results from the Quality Research in Radiation Oncology (QRRO) survey. Pract Radiat Oncol. 2013;3:2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehman M, Hayden AJ, Martin JM, et al. FROGG high-risk prostate cancer workshop: Patterns of practice and literature review. Part I: Intact prostate. J Med Imaging Radiat Oncol. 2014;58:257–265. [DOI] [PubMed] [Google Scholar]
- 11.Webber C, Brundage MD, Siemens DR, Groome PA. Quality of care indicators and their related outcomes: A population-based study in prostate cancer patients treated with radiotherapy. Radiother Oncol. 2013;107:358–365. [DOI] [PubMed] [Google Scholar]
- 12.Brundage M, Danielson B, Pearcey R, et al. A criterion-based audit of the technical quality of external beam radiotherapy for prostate cancer. Radiother Oncol. 2013;107:339–345. [DOI] [PubMed] [Google Scholar]
- 13.Lubeck DP, Litwin MS, Henning JM, et al. The CaPSURE database: A methodology for clinical practice and research in prostate cancer. CaPSURE Research Panel. Cancer of the Prostate Strategic Urologic Research Endeavor. Urology. 1996;48:773–777. [DOI] [PubMed] [Google Scholar]
- 14.Barocas DA, Chen V, Cooperberg M, et al. Using a population-based observational cohort study to address difficult comparative effectiveness research questions: The CEASAR study. J Comp Eff Res. 2013;2:445–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Litwin MS, Greenfield S, Elkin EP, Lubeck DP, Broering JM, Kaplan SH. Assessment of prognosis with the total illness burden index for prostate cancer: Aiding clinicians in treatment choice. Cancer. 2007;109:1777–1783. [DOI] [PubMed] [Google Scholar]
- 16.National Comprehensive Cancer Network. The NCCN Clinical Practice Guidelines in Oncology Prostate Cancer (Version 4.2011). Available at: http://www.nccn.org. Accessed September 12, 2016.
- 17.Davis BJ, Horwitz EM, Lee WR, et al. American Brachytherapy Society consensus guidelines for transrectal ultrasound-guided permanent prostate brachytherapy. Brachytherapy. 2012;11:6–19. [DOI] [PubMed] [Google Scholar]
- 18.American Brachytherapy Society. Quality research in radiation oncology guidelines. Available at: http://www.qrro.org/Prostate_CPM.pdf. Accessed September 12, 2016.
- 19.Penson DF. Assessing the quality of prostate cancer care. Curr Opin Urol. 2008;18:297–302. [DOI] [PubMed] [Google Scholar]
- 20.Efstathiou JA, Nassif DS, McNutt TR, et al. Practice-based evidence to evidence-based practice: Building the National Radiation Oncology Registry. J Oncol Pract. 2013;9:e90–e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. [DOI] [PubMed] [Google Scholar]
- 22.Harrell F Regression Modeling Strategies with Applications to Linear Models, Logistic and Ordinal Regression and Survival Analysis. 2nd ed New York, NY: Springer-Verlag; 2015. [Google Scholar]
- 23.Stokes WA, Kavanagh BD, Raben D, Pugh TJ. Implementation of hypofractionated prostate radiation therapy in the United States: A National Cancer Database analysis. Pract Radiat Oncol. 2017;7:270–278. [DOI] [PubMed] [Google Scholar]
- 24.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: A powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halpern JA, Sedrakyan A, Hsu WC, et al. Use, complications, and costs of stereotactic body radiotherapy for localized prostate cancer. Cancer. 2016;122:2496–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peeters ST, Heemsbergen WD, Koper PC, et al. Dose-response in radiotherapy for localized prostate cancer: Results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol. 2006;24:1990–1996. [DOI] [PubMed] [Google Scholar]
- 27.Dearnaley DP, Sydes MR, Graham JD, et al. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: First results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2007;8:475–487. [DOI] [PubMed] [Google Scholar]
- 28.Kuban DA, Tucker SL, Dong L, et al. Long-term results of the MD Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:67–74. [DOI] [PubMed] [Google Scholar]
- 29.Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: A randomized controlled trial. JAMA. 2005;294:1233–1239. [DOI] [PubMed] [Google Scholar]
- 30.Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med. 2011;365:107–118. [DOI] [PubMed] [Google Scholar]
- 31.Wirth MP, See WA, McLeod DG, et al. Bicalutamide 150 mg in addition to standard care in patients with localized or locally advanced prostate cancer: results from the second analysis of the early prostate cancer program at median follow up of 5.4 years. J Urol. 2004;172:1865–1870. [DOI] [PubMed] [Google Scholar]
- 32.Pommier P, Chabaud S, Lagrange JL, et al. Is there a role for pelvic irradiation in localized prostate adenocarcinoma? Preliminary results of GETUG-01. J Clin Oncol. 2007;25:5366–5373. [DOI] [PubMed] [Google Scholar]
- 33.Lawton CA, DeSilvio M, Roach M, et al. An update of the phase III trial comparing whole pelvic to prostate only radiotherapy and neoadjuvant to adjuvant total androgen suppression: Updated analysis of RTOG 94–13, with emphasis on unexpected hormone/radiation interactions. Int J Radiat Oncol Biol Phys. 2007;69:646–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asbell SO, Krall JM, Pilepich MV, et al. Elective pelvic irradiation in stage A2, B carcinoma of the prostate: analysis of RTOG 77–06. Int J Radiat Oncol Biol Phys. 1988;15:1307–1316. [DOI] [PubMed] [Google Scholar]
- 35.Pilepich MV, Winter K, Lawton CA, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma–long-term results of phase III RTOG 85–31. Int J Radiat Oncol Biol Phys. 2005;61:1285–1290. [DOI] [PubMed] [Google Scholar]
- 36.Hanks GE, Pajak TF, Porter A, et al. Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytor-eduction and radiotherapy in locally advanced carcinoma of the prostate: The Radiation Therapy Oncology Group Protocol 92–02. J Clin Oncol. 2003;21:3972–3978. [DOI] [PubMed] [Google Scholar]
- 37.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): A phase III randomised trial. Lancet. 2002;360:103–106. [DOI] [PubMed] [Google Scholar]
- 38.Levine GN, D’Amico AV, Berger P, et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: A science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. Circulation. 2010;121:833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nanda A, Chen MH, Braccioforte MH, Moran BJ, D’Amico AV. Hormonal therapy use for prostate cancer and mortality in men with coronary artery disease-induced congestive heart failure or myocardial infarction. JAMA. 2009;302:866–873. [DOI] [PubMed] [Google Scholar]
- 40.Tsai HK, D’Amico AV, Sadetsky N, Chen MH, Carroll PR. Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst. 2007;99:1516–1524. [DOI] [PubMed] [Google Scholar]
- 41.Muralidhar V, Catalano PJ, Reznor G, et al. Variation in national use of long-term ADT by disease aggressiveness among men with unfavorable-risk prostate cancer. J Natl Compr Cancer Netw. 2016;14:421–428. [DOI] [PubMed] [Google Scholar]
- 42.Dell’Oglio P, Abou-Haidar H, Leyh-Bannurah SR, et al. Assessment of the rate of adherence to international guidelines for androgen deprivation therapy with external-beam radiation therapy: A population-based study. Eur Urol. 2016;70:429–435. [DOI] [PubMed] [Google Scholar]
- 43.Gross CP, Smith BD, Wolf E, Andersen M. Racial disparities in cancer therapy: Did the gap narrow between 1992 and 2002? Cancer. 2008;112:900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spencer BA, Miller DC, Litwin MS, et al. Variations in quality of care for men with early-stage prostate cancer. J Clin Oncol. 2008;26: 3735–3742. [DOI] [PubMed] [Google Scholar]
- 45.Ellis SD, Blackard B, Carpenter WR, et al. Receipt of National Comprehensive Cancer Network guideline-concordant prostate cancer care among African American and Caucasian American men in North Carolina. Cancer. 2013;119:2282–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17:1047–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Incrocci L, Wortel RC, Alemayehu WG, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): Final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17:1061–1069. [DOI] [PubMed] [Google Scholar]
- 48.Sathya JR, Davis IR, Julian JA, et al. Randomized trial comparing iridium implant plus external-beam radiation therapy with external-beam radiation therapy alone in node-negative locally advanced cancer of the prostate. J Clin Oncol. 2005;23:1192–1199. [DOI] [PubMed] [Google Scholar]
- 49.Hoskin PJ, Rojas AM, Bownes PJ, Lowe GJ, Ostler PJ, Bryant L. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother Oncol. 2012;103:217–222. [DOI] [PubMed] [Google Scholar]
- 50.MacLean CH, Louie R, Shekelle PG, et al. Comparison of administrative data and medical records to measure the quality of medical care provided to vulnerable older patients. Med Care. 2006;44:141–148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.