Abstract
Cardiac side effects associated with immune checkpoint inhibitors (ICIs) are an uncommon but serious complication with a relatively high mortality. We experienced a case of cardiomyopathy induced by nivolumab. Echocardiography showed diffuse hypo-kinesis of the left ventricular cardiac wall and a significant decrease in the ejection fraction, like dilated cardiomyopathy. The myocardial biopsy showed non-inflammatory change; cardiac function gradually improved after treatment of acute heart failure without a corticosteroid. Although non-inflammatory left ventricular dysfunction induced by ICIs is rare, it is a reported cardiovascular toxicity. Physicians should consider this complication when treating patients with ICIs for malignant diseases.
Keywords: lung cancer, dilated cardiomyopathy, immune checkpoint inhibitor, nivolumab, immune-related adverse events, heart failure
Introduction
After the programmed cell death-1 (PD-1) gene was cloned (1), an anti-PD-1 antibody (2) was rapidly developed as an immune checkpoint inhibitor (ICI). ICIs have become an important treatment option for malignant tumors (2, 3). As a rheostat for immune responses, PD-1 is a powerful target for immunological therapy, with highly effective clinical applications for cancer treatment (4). Recently, ICI monotherapy was established for patients with non-small-cell-lung cancer (NSCLC) (5-9), and combination treatment using an ICI and cytotoxic agents has rapidly developed for NSCLC and small cell lung cancer (10-13).
However, as ICI therapy has been frequently used, various immune-related adverse events (irAEs) have been reported (14). Although cardiac effects associated with ICIs are rare among irAEs, they are often serious complications with high mortality. We experienced a case of non-inflammatory diffuse left ventricular dysfunction like dilated cardiomyopathy induced by nivolumab monotherapy and describe it herein.
Case Report
A 79-year-old man was diagnosed as having squamous NSCLC stage IVA, cT3N2M1a in October 2017 in our hospital. He was started on docetaxel therapy (50 mg/m2) in November 2017. After 6 cycles, right pleural effusion increased, and docetaxel was discontinued based on the diagnosis of disease progression. Therefore, he was started on nivolumab therapy bi-weekly (1 to 10 cycles: 3 mg/kg and from 11 cycles: 240 mg/body) as second-line treatment in March 2018. After 3 cycles of nivolumab, palliative radiotherapy (30 Gy) was administered for bone metastasis that was located in the fifth vertebra as a new lesion. Because of the patient's wishes, nivolumab was continued until 17 cycles without discontinuation of treatment. Right pleural effusion disappeared at about 7 cycles and the patient's general condition improved.
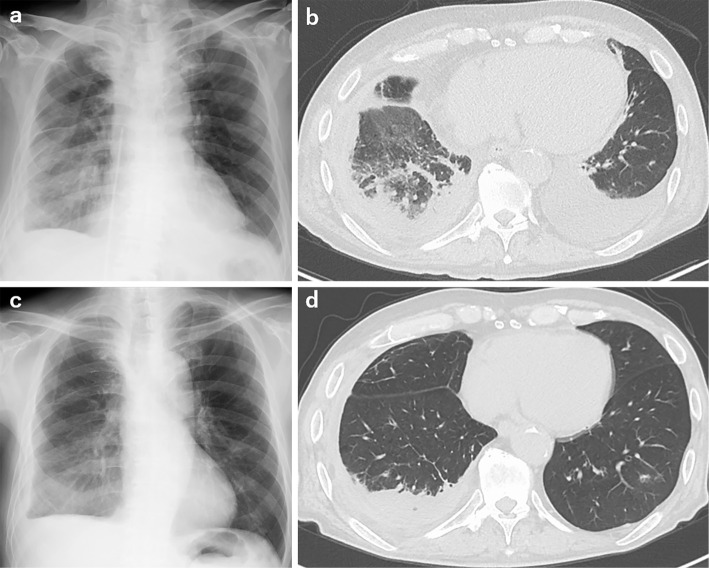
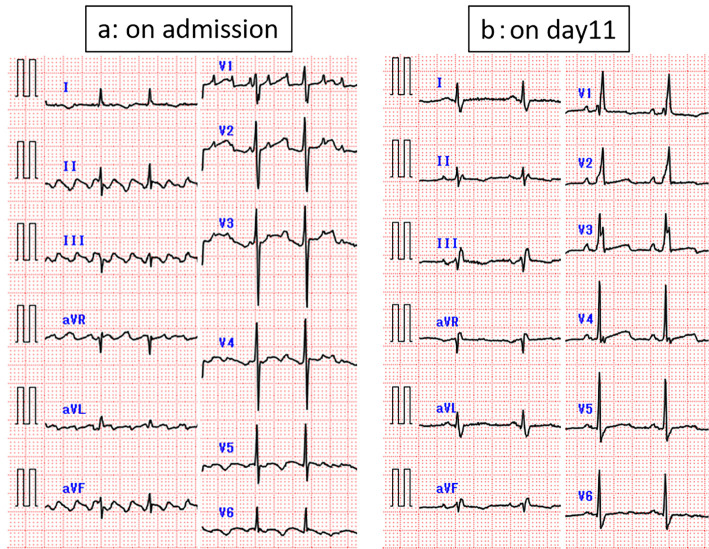
At 20 days after 17 cycles of nivolumab (day 1), he developed dyspnea and was urgently admitted to our hospital. Vital signs on admission were as follows: consciousness, clear; body temperature, 36.5℃; blood pressure, 94/58 mmHg; pulse rate, 86 beats/min and regular; oxygen saturation on room air, 93% at rest and 85% on light exertion; respiratory rate, 28 breaths/min. The physical examination on admission revealed the following: New York Heart Association functional class, 4; cardiac sounds, no murmur; chest auscultation, coarse crackles in both lungs; and body surface findings, swelling of both jugular veins and pitting edema of both lower extremities. The chest X-ray and computed tomography on admission showed edema predominantly in the right lung, bilateral slight pulmonary effusion, and cardiac dilatation (Fig. 1a, b). The electrocardiogram revealed atrial flutter (Fig. 2a). Findings of the laboratory analysis were as follows: white blood cell count, 8,400/μL (with normal differential); red blood cell count, 463×104/μL; hemoglobin level, 15.0 g/dL; platelet count, 29.9×104/μL; C-reactive protein level, 0.4 mg/dL; creatinine kinase (CK), 76 IU/L (normal range: 62-287 IU/L); CK-myocardial band (MB), 15 IU/L (normal range: 0-23); troponin I level, 92.7 pg/mL (normal range: 0-26 pg/mL); and brain natriuretic peptide (BNP) level, 1,061.5 pg/mL (normal range: <18.4 pg/mL). Echocardiography showed diffuse hypo-kinesis of the left ventricular cardiac wall with left ventricular ejection fraction (LVEF) of 20% (Fig. 3a, b), which significantly decreased in comparison to that of 73% in June 2017. Additionally, thicknesses of the interventricular septum and left ventricular posterior wall were 9.5 mm and 9.6 mm, respectively, and they were within normal range. On the basis of these findings, we diagnosed the patient with acute heart failure.
Figure 1.
a and b: Chest X-ray and computed tomography scan showing cardiomegaly and bilateral pleural effusion. c and d: Cardiomegaly and pleural effusion are improved after treatment of heart failure.
Figure 2.
a: Electrocardiogram at the time of admission showing atrial flutter. b: The finding has normalized on day11.
Figure 3.
a and b: Echocardiogram (a: systolic phase and b: diastolic phase) at the time of admission showing a low left ventricular ejection fraction (LVEF). c and d: Echocardiogram (c: systolic phase and d: diastolic phase) at 4 months after discharge showing normal LVEF.
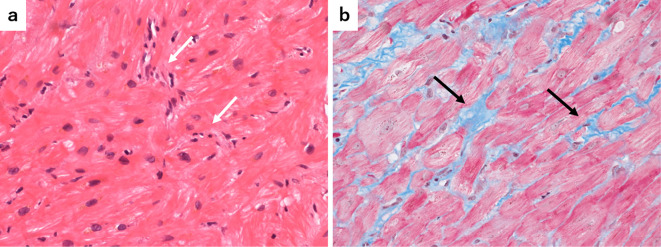
Coronary angiography revealed no significant stenosis of the coronary arteries, and cardiac magnetic resonance imaging did not show inflammatory changes or cardiac fibrosis. Further, a myocardial biopsy was performed from the endocardial side of the right ventricle. Pathological findings of the biopsy showed, slight infiltration of inflammatory cells, and interstitial fibrosis between the myocardial fibers (Fig. 4a, b), which was not compatible with myocarditis. Finally, the patient's heart failure was diagnosed as cardiomyopathy due to nivolumab.
Figure 4.
Histological findings of the myocardial biopsy specimen (a: Hematoxylin and Eosin staining, ×100 magnification, b: Masson trichrome staining, ×100 magnification) showing fibrosis of the myocardial tissue, a little infiltration of inflammatory cells (white arrows), and interstitial fibrosis between the myocardial fibers (black arrows).
The heart failure was started treatment with continuous infusion of furosemide (40 mL/day), infusion of dopamine 4 μg/kg/min and oral tolvaptan 7.5 mg once daily. On day 4, his oxygen saturation was recovered 93% to 97% on room air, body weight was reduced 58 kg to 53 kg and edema was disappeared, therefore infusion of dopamine and oral tolvaptan were stopped and furosemide infusion was transferred to oral furosemide 20 mg once daily. Furthermore, the bisoprolol 1.25 mg, spironolactone 25 mg and enalapril 1.25 mg once daily were prescribed as well. At the same time, atrial flutter on admission was reverted to normal sinus rhythm without cardioversion (Fig. 2b). On day10, furosemide 20 mg was reduced to 10 mg once daily and bisoprolol was changed to carvedilol 2.5 mg twice daily, because brood pressure dropped to 80/44 mmHg and bisoprolol was not tolerated. His heart failure gradually improved (Fig. 1c, d), and he was discharged on day 44 with medication of carvedilol 2.5 mg twice daily, furosemide 10 mg once daily and spironolactone 25 mg once daily.
About 2 months after the onset of heart failure, LVEF was slightly recovered to 35% from 20% on admission. Carvedilol could not be increased and enalapril was quit, because he was sometimes aware of orthostatic hypotension. About 3 months after the onset of heart failure, because his heart failure was well controlled and orthostatic hypotension was disappeared, carvedilol was increased to 5 mg twice daily. About 4 months after the onset of heart failure, LVEF was recovered to 61% on the echocardiogram, and Troponin I and BNP levels also returned to normal (Fig. 3c, d, Table). Furosemide and spironolactone were gradually eliminated.
Table.
Laboratory and Echocardiographic Data.
| Before Chemotherapy |
Day1 | Day11 | Day52 | Day80 | Day108 | Day136 | Day192 | Day248 | |
|---|---|---|---|---|---|---|---|---|---|
| Laboratory data | |||||||||
| WBC (103/µL) | 6.4 | 8.4 | 5.4 | - | 6.7 | 7.7 | 5.7 | 4.1 | 4.9 |
| CRP (mg/dL) | 0.10 | 0.40 | 1.96 | - | - | 6.79 | 2.38 | 0.47 | 0.76 |
| BNP (pg/mL) | - | 1,061.5 | - | - | 62.3 | 48.4 | 52.7 | 57.0 | 68.9 |
| Troponin I (pg/mL) | - | 92.7 | 48.1 | - | 10.4 | 3.2 | 1.7 | 3.2 | 2.4 |
| CK (U/L) | - | 76 | 25 | - | 27 | 22 | 31 | 31 | 38 |
| CK-MB (U/L) | - | 15 | 6 | - | - | - | - | - | - |
| Echocardiographic data | |||||||||
| LVDd (mm) | 43 | 48 | 52 | 45 | - | 50 | 44 | 43 | 42 |
| LVDs (mm) | 25 | 44 | 47 | 38 | - | 41 | 30 | 31 | 27 |
| IVSTd (mm) | 10.7 | 9.5 | 9.0 | 8.0 | - | 7.0 | 8.8 | 7.0 | 8.7 |
| PWTd (mm) | 9.6 | 9.6 | 9.0 | 8.3 | - | 8.5 | 10.2 | 9.0 | 8.7 |
| LAD (mm) | 33 | 36 | 33 | 24 | - | 24 | 26 | 24 | 28 |
| LVEF (%) | 73 | 20 | 22 | 35 | - | 37 | 61 | 56 | 68 |
WBC: white blood cells, CRP: C-reactive protein, BNP: brain natriuretic peptide, CK-MB: creatinine kinase-myocardial band, LVDd: left ventricular end-diastolic diameter, LVDs: left ventricular end-systolic diameter, IVSTd: interventricular septal end-diastolic thickness, PWTd: posterior wall end-diastolic thickness, LVEF: left ventricular ejection fraction, LAD: left atrial diameter
Discussion
ICIs can have significant cardiac adverse effects, such as myocarditis, cardiomyopathy, heart failure, arrhythmia, and cardiac arrest (15). According to the safety databases of Bristol-Myers Squibb Corporate, about the occurrence of events in patients treated with nivolumab, ipilimumab or both, cardiac toxicity induced by ICIs treatment has been estimated to occur in less than 1% (16). Combination therapy with both drugs was associated with more severe and frequent myocarditis than nivolumab alone (0.27% versus 0.06%) (16). Thus, cardiotoxicities in patients treated with nivolumab alone would be very rare. Herein, we described a patient who developed nivolumab-induced cardiomyopathy at 10 months after initial administration of the drug. Emerging data suggest that cardiotoxic effects usually occur early after exposure to an ICI. Escudier and colleagues (17) reported 30 cases of ICI-mediated cardiotoxicity, among which the median time from the start of treatment to the presentation of cardiotoxic effects was 65 days, but it ranged widely from 2 to 454 days. As our case indicates, delayed cardiotoxic effects can also occur.
We diagnosed our patient as having dilated cardiomyopathy based on following findings: echocardiography showed diffuse hypo-kinesis of the left ventricular cardiac wall with a significant decrease in the LVEF of 20% in comparison with the previous finding; absence of significant stenosis of the coronary arteries; result of the cardiac muscle biopsy specimen showed myocardial interstitial fibrosis without inflammatory changes; and cardiac function gradually improved after treatment of acute heart failure without administration of a corticosteroid. We do not know the onset of cardiomyopathy because we did not assess the patient's cardiac function frequently until the development of heart failure. Lyon et al. (18) reviewed ICIs and cardiovascular toxicity. In their article, non-inflammatory left ventricular dysfunction was reported as one type of cardiovascular toxicity. Our patient did not have apical ballooning syndrome, which was reported in several case reports (19-22). Dilated cardiomyopathy has been rarely reported as a type of cardiovascular toxicity due to an ICI until now.
The mechanism of cardiomyopathy related to ICIs is not clear. Nishimura et al. (23) reported that a PD-1 knockout (KO) mouse developed spontaneous severe dilated cardiomyopathy with sudden death. Furthermore, they found that the PD-1 KO mice had deposition of immunogloblin G on the surface of cardiomyocytes, which was later recognized to be an anti-cardiac troponin I antibody by Okazaki et al. (24). Cytotoxic T-lymphocyte antigen-4 (CTLA-4) KO mice also develop autoimmune myocarditis with infiltration of CD4+ and CD8+ T cells in the myocardium (25). Baban et al. (26) reported that cardiomyocyte programmed cell death ligand-1 (PD-L1) expression is upregulated in cardiac stress and disease, including ischemia-reperfusion injury and left ventricular hypertrophy in preclinical models. PD-L1 signaling might have cardioprotective action that suppresses excessive myocardial inflammation. We speculate that the inhibition of CTLA-4, PD-1 or PD-L1 can result in autoimmune T-cell mediated cardiotoxicity, and direct inhibition of PD-L1 might also accelerate pre-existing heart disease, and potentially cause non-inflammatory cardiomyocyte dysfunction.
Although early detection of immunotherapy-mediated cardiotoxic effects before severe, life-threatening complications develop is important, there is no evidence-based algorithm for surveillance of these effects. Mahmood et al. reported that 94% cases of ICI-associated myocarditis showed elevation of the troponin level, and assessing such levels at baseline and at each cycle may be valuable (27). However, another study reported that troponin was not always elevated in patients who had nivolumab-induced cardiotoxicity (17). Conversely, Mahmood et al. reported that the concentration of BNP or N-terminal BNP was increased in only 66% of patients (27). Although left ventricular systolic dysfunction was common, severe systolic dysfunction with an LVEF <35% was present in only 46% of cases (17, 27). Thus, there is no useful methods for specific and early detection of ICI-related cardiotoxicity. In our case, troponin and BNP levels were elevated, but CK and CK-MB levels were normal on admission. We think these findings indicated that cardiomyopathy progressed gradually after the initiation of nivolumab. Elevation of the troponin or BNP level indicates damage to the myocardium, even if symptoms are not present. If cardiotoxicity is suspected, physicians should consider discontinuing ICI treatment in the early stage, depending on the severity of the patient's condition.
This case report has two limitations. First, we did not prove the presence of the anti-cardiac troponin I antibody in plasma, deposition of the antibody on the surface of cardiomyocytes, or expression of PD-L1 in cardiomyocytes. Second, it is difficult to evaluate the effect of other treatments, i.e., docetaxel or palliative radiotherapy, on cardiac function.
Conclusion
Although there are some case reports about ICI-mediated myocarditis, a case of dilated cardiomyopathy without inflammatory change induced by nivolumab is rare. When physicians encounter acute heart failure in patients treated with nivolumab, they need to have a high index of clinical suspicion of this disease and make an early diagnosis.
In the present case, we made the final diagnosis based on the myocardial biopsy findings, but we needed to carefully consider the indication for the biopsy because of its invasive nature. Cardiac function gradually improved after discontinuation of nivolumab and treatment of acute heart failure. In similar cases, we think that it is important to make an early assessment of cardiac function and to consider discontinuing the administration of nivolumab.
Written informed consent is available.
The authors state that they have no Conflict of Interest (COI).
Atsuhiko Iuchi and Yumiko Samejima contributed equally to this work.
Acknowledgement
The authors would like to thank this patient.
References
- 1.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 11: 3887-3895, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 28: 3167-3175, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366: 2443-2454, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol 14: 1212-1218, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373: 123-135, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373: 1627-1639, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387: 1540-1550, 2016. [DOI] [PubMed] [Google Scholar]
- 8.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389: 255-265, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375: 1823-1833, 2016. [DOI] [PubMed] [Google Scholar]
- 10.Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378: 2078-2092, 2018. [DOI] [PubMed] [Google Scholar]
- 11.Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379: 2040-2051, 2018. [DOI] [PubMed] [Google Scholar]
- 12.Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378: 2288-2301, 2018. [DOI] [PubMed] [Google Scholar]
- 13.Horn L, Mansfield AS, Szczesna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 379: 2220-2229, 2018. [DOI] [PubMed] [Google Scholar]
- 14.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 36: 1714-1768, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varricchi G, Galdiero MR, Marone G, et al. Cardiotoxicity of immune checkpoint inhibitors. ESMO Open 2: e000247, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 375: 1749-1755, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Escudier M, Cautela J, Malissen N, et al. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation 136: 2085-2087, 2017. [DOI] [PubMed] [Google Scholar]
- 18.Lyon AR, Yousaf N, Battisti NML, Moslehi J, Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol 19: e447-e458, 2018. [DOI] [PubMed] [Google Scholar]
- 19.Geisler BP, Raad RA, Esaian D, Sharon E, Schwartz DR. Apical ballooning and cardiomyopathy in a melanoma patient treated with ipilimumab: a case of takotsubo-like syndrome. J Immunother Cancer 3: 4, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinzerling L, Ott PA, Hodi FS, et al. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J Immunother Cancer 4: 50, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson RD, Brooks M. Apical takotsubo syndrome in a patient with metastatic breast carcinoma on novel immunotherapy. Int J Cardiol 222: 760-761, 2016. [DOI] [PubMed] [Google Scholar]
- 22.Ederhy S, Cautela J, Ancedy Y, Escudier M, Thuny F, Cohen A. Takotsubo-like syndrome in cancer patients treated with immune checkpoint inhibitors. JACC Cardiovasc Imaging 11: 1187-1190, 2018. [DOI] [PubMed] [Google Scholar]
- 23.Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 291: 319-322, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Okazaki T, Tanaka Y, Nishio R, et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med 9: 1477-1483, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Love VA, Grabie N, Duramad P, Stavrakis G, Sharpe A, Lichtman A. CTLA-4 ablation and interleukin-12 driven differentiation synergistically augment cardiac pathogenicity of cytotoxic T lymphocytes. Circ Res 101: 248-257, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Baban B, Liu JY, Qin X, Weintraub NL, Mozaffari MS. Upregulation of programmed death-1 and its ligand in cardiac injury models: interaction with GADD153. PLoS One 10: e0124059, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 71: 1755-1764, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]