Abstract
Objective
Pembrolizumab has benefited patients with advanced non-small-cell lung cancer (NSCLC) with a programmed death-ligand (PD-L)1 high expression, but little information is available regarding its safety for patients with interstitial lung disease (ILD). The aim of this study was to assess the efficacy and tolerability of pembrolizumab for patients with advanced NSCLC and preexisting ILD.
Methods
We retrospectively reviewed the medical records of five patients with advanced NSCLC and preexisting ILD who received pembrolizumab monotherapy in a first-line setting.
Patients
All patients had mild ILD and pulmonary emphysema with a forced vital capacity within the normal range. Pembrolizumab was administered at a dose of 200 mg/body on day 1 every 3 weeks.
Results
The overall response rate was 60%. Four patients developed pembrolizumab-induced lung injury, which was improved in all cases by corticosteroid therapy. One patient received pembrolizumab for two years, did not experience lung injury and achieved a complete response.
Conclusion
Pembrolizumab has a high risk of inducing lung injury in patients with preexisting ILD, although it may be very effective in NSCLC patients with a high PD-L1 expression, even concurrent with preexisting ILD. Further large-scale studies are needed to determine risk factors of pembrolizumab-induced lung injury in such patients.
Keywords: non-small-cell lung cancer, interstitial lung disease, pembrolizumab, lung injury
Introduction
Immune checkpoint inhibitors (ICIs) have improved the overall survival of patients with advanced non-small-cell lung cancer (NSCLC). Pembrolizumab is a highly selective humanized monoclonal antibody against programmed death 1 (PD-1) that blocks PD-1's interaction with both programmed death ligand 1 (PD-L1) and PD-L2. Pembrolizumab shows a significantly longer progression-free and overall survival than platinum-based chemotherapy as the first-line therapy, especially in patients with advanced NSCLC and PD-L1 expression on at least 50% of tumor cells and with no sensitizing epidermal growth factor receptor (EGFR) mutations or anaplastic lymphoma kinase (ALK) translocations (1,2). However, individuals with interstitial lung disease (ILD) have been excluded from prospective clinical trials because of the high risk of drug-induced lung injury. Therefore, whether or not pembrolizumab is effective and safe for patients with preexisting ILD (pre-ILD) remains unclear.
We conducted a retrospective analysis of patients with NSCLC and pre-ILD who were treated with pembrolizumab as the first-line therapy.
Materials and Methods
Patients and treatment methods
We retrospectively reviewed the cases of 25 consecutive patients with NSCLC and pre-ILD who had been treated with pembrolizumab as the first-line therapy at Funabashi Municipal Medical Center between March 2017 and December 2018. Overall, five of these patients had pre-ILD. The results were analyzed retrospectively using the medical and radiological records.
Written informed consent was obtained from all of the patients with pre-ILD after disclosing any risks, including the risk of fatal acute exacerbation of ILD (AE-ILD), associated with treatment with pembrolizumab.
Pembrolizumab was administered at a dose of 200 mg/body on day 1 every 3 weeks. Pre-ILD was identified by computed tomography (CT) performed within three months before the initiation of pembrolizumab monotherapy. ILD was defined by the presence of bilateral ground-glass opacities, reticular shadows, nonemphysematous cysts or honeycombing. The presence of pre-ILD was independently evaluated by two pulmonologists. Based on the CT findings, the ILD patterns were divided into a usual interstitial pneumonia (UIP) pattern, nonspecific interstitial pneumonia (NSIP) pattern, respiratory bronchiolitis-interstitial lung disease (RB-ILD) pattern, desquamative interstitial pneumonia (DIP) pattern, cryptogenic organizing pneumonia (OP) pattern, acute interstitial pneumonia (AIP) pattern, lymphoid interstitial pneumonia (LIP) pattern or pleuroparenchymal fibroelastosis (PPFE) pattern, in accordance with the International Consensus Statement (3). The extent of pre-ILD was evaluated using pretreatment CT images on slices taken at the tracheal bifurcation and 2 cm above and below the top of the right diagram in bilateral lung fields, with scores assigned using a 4-point scale (0, none; 1, 1-25% involvement; 2, 26-50% involvement, 3, 51-75% involvement; 4, ≥76% involvement) for each image according to previous reports (4,5). The sum of the scores from each image (range, 0-24) defined the area of pre-ILD involvement for each patient (pre-ILD score).
Clinical evaluations and adverse events
The objective tumor response was assessed according to the Response Evaluation Criteria Solid Tumor (RECIST) (6). Severity of lung injury (pulmonary fibrosis) was graded according to the Common Terminology Criteria for Adverse Events, version 4.0. AE-ILD was defined based on CT findings of newly developed ground-glass opacities and/or consolidation superimposed on a background reticular or honeycombing pattern. Patients with congestive heart failure, inferior respiratory inflammation or pneumonia were excluded.
Results
Baseline patient characteristics
The baseline characteristics of the five patients are summarized in Table 1. One patient was a woman, and the median age was 78 (range, 75-81) years old. All patients were current or past smokers. All patients had an Eastern Cooperative Oncology Group performance status of 1. One patient had stage III disease, and the others had stage IV disease. The median % forced vital capacity (% FVC: FVC/predicted FVC) was 94.6% (range 87.2-113.6%), and the median % forced expiratory volume in 1 second (%FEV1: FEV1/predicted FEV1) was 93.5% (range 60.8-105.6%). All patients had pulmonary emphysema, and 4 were diagnosed with chronic obstructive pulmonary disease (COPD) (FEV1/forced VC ratio <0.7). Based on pretreatment CT scans of the chest, the UIP pattern was observed in one patient; the ILD pattern of the other patients was NSIP. The pre-ILD score in all cases was 3, which means that, in this study, pembrolizumab was administered only to patients with mild pre-ILD.
Table 1.
Patient Characteristics (n=5).
| Case | Sex | Age (y) |
Smoke | Histological type | PD-L1 (%) |
Stage | PS | Pre-ILD | %FVC | FEV1% | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Score | Pattern | ||||||||||
| 1 | F | 81 | Former | Squamous cell ca. | 90 | IIIB | 1 | 3 | NSIP | 93.7 | 68.3 |
| 2 | M | 76 | Former | Pleomorphic ca. | 60 | IV | 1 | 3 | NSIP | 87.2 | 87.1 |
| 3 | M | 78 | Former | Adenocarcinoma | 95 | IV | 1 | 3 | NSIP | 94.6 | 67.4 |
| 4 | M | 81 | Current | Adenosquamous ca. | 90 | IV | 1 | 3 | UIP | 113.6 | 69.0 |
| 5 | M | 75 | Current | Adenocarcinoma | 75 | IV | 1 | 3 | NSIP | 104.7 | 55.4 |
y: years, PD-L1: tumor proportion score of programmed death ligand 1, PS: performance status, ILD: interstitial lung disease, %FVC: % forced vital capacity, FEV1%: ratio of forced expiratory volume in one second to forced vital capacity, M: male, F: female, ca.: carcinoma, NSIP: nonspecific interstitial pneumonia, UIP: usual interstitial pneumonia
Clinical course and chest CT images
The efficacy of pembrolizumab and the clinical course of the five patients are summarized in Table 2. The overall response rate was 60%, and the disease control rate was 80%. Four of the five patients with pre-ILD experienced pembrolizumab-induced lung injury and received steroid therapy, while 1 of the 20 patients without pre-ILD developed lung injury. The duration to the onset of pembrolizumab-induced AE-ILD ranged from 48 to 294 days. Lung injury in all four cases was improved, and one of them received second-line therapy. One patient who did not develop lung injury received pembrolizumab for two years with a complete response (Case 1) (Fig. 1). The clinical course and chest CT findings in each patient who developed lung injury are described below (Case 2-5).
Table 2.
Clinical Course.
| Case | Response | Cycles | LI severity (Grade) |
Time to LI onset (days) |
KL-6 (U/mL) | Treatment for LI | Outcome | |
|---|---|---|---|---|---|---|---|---|
| Baseline | LI onset | |||||||
| 1 | CR | 31 | - | - | 499 | - | - | - |
| 2 | SD | 14 | 2 | 294 | 361 | 232 | Oral steroid | Resolved |
| 3 | PR | 2 | 3 | 48 | 350 | 967 | Steroid pulse, CPA | Resolving |
| 4 | PD | 3 | 3 | 60 | 569 | 1115 | Steroid pulse, CPA | Resolving |
| 5 | PR | 8 | 1 | 89 | 668 | 651 | Oral steroid | Resolved |
LI: lung injury, SD: stable disease, PD: progression disease, PR: partial response, CR: complete response, CPA: cyclophosphamide
Figure 1.
High-resolution computed tomography scan of the chest revealed a drastic response to pembrolizumab treatment in an 81-year-old woman who had squamous cell carcinoma in the right lower lobe with mild interstitial lung disease and emphysema (Case 1).
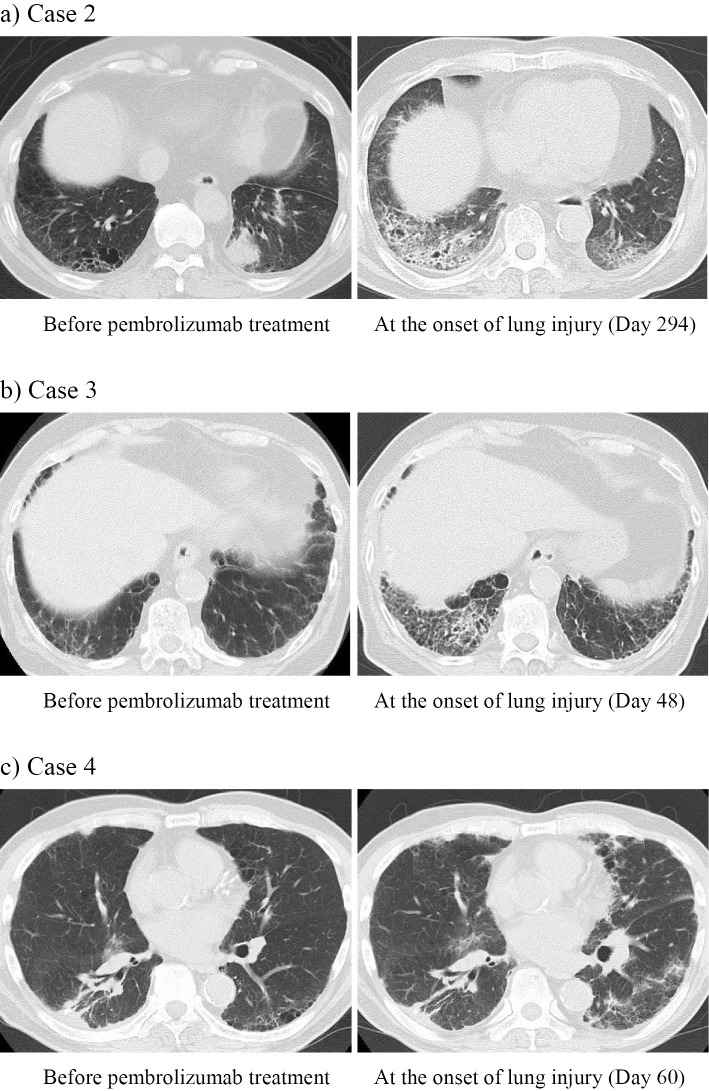
Case 2. The patient with NSCLC (cT4N0 m0) was ultimately diagnosed with pleomorphic carcinoma (pT4N0 m1a) with pleural dissemination after left lower lobectomy. Pembrolizumab monotherapy was initiated 62 days after the surgery. On day 294 of the treatment, the patients complained of cough and anorexia with oxygen desaturation. CT of the chest revealed ground glass opacity (GGO) in the bilateral lung fields (Fig. 2a), suggesting drug-induced lung injury. Oral corticosteroid therapy (prednisolone 30 mg/day) was started, and chest X-ray showed a reduction in the opacity with improved oxygenation.
Figure 2.
Computed tomography scan of the chest before pembrolizumab treatment and at the onset of lung injury in a 76-year-old man with pleomorphic carcinoma (a: Case 2), a 78-year-old man with adenocarcinoma (b: Case 3), and an 81-year-old man with adenosquamous carcinoma (c: Case 4).
Case 3. The patient was diagnosed with stage 4 NSCLC (adenocarcinoma) with pleural dissemination proved by a pleural biopsy with video-assisted thoracoscopic surgery (VATS) 10 days before the start of pembrolizumab monotherapy. On day 48 of the treatment, the patient complained of dyspnea with oxygen desaturation. Chest CT showed GGO predominantly on the right side in both lungs (Fig. 2b). He was admitted and received corticosteroid pulse therapy (methylprednisolone 1,000 mg/day, 3 days) followed by oral corticosteroid therapy (prednisolone 30 mg/day) for suspected drug-related lung injury. However, chest X-ray showed an expansion of the reticular opacity in the both lungs, and he was treated with cyclophosphamide pulse therapy (500 mg/body) on day 53 and a second round of corticosteroid pulse therapy on day 56 followed by oral corticosteroid therapy (prednisolone 60 mg/day). Subsequently, X-ray showed a reduction in the interstitial shadow, and he was discharged with home oxygen therapy and oral corticosteroid therapy on day 78.
Case 4. The patient was diagnosed with stage 4 NSCLC (adenosquamous carcinoma) with pleural dissemination after right lower lobe partial resection and a pleural biopsy. Seventy days after the surgery, pembrolizumab monotherapy was started. On day 60 of pembrolizumab treatment, chest CT showed patchy GGO in the bilateral lung fields (Fig. 2c), with shortness of breath on exertion. The patient had oxygen desaturation and was withdrawn from pembrolizumab with a diagnosis of suspected drug-related lung injury. He was hospitalized and treated with corticosteroid pulse therapy (methylprednisolone 1,000 mg/day, 3 days) followed by cyclophosphamide pulse (500 mg/body) and oral corticosteroid therapy (prednisolone 30 mg/day). X-ray on day 70 showed that the opacity had gradually reduced, and the patient was discharged with home oxygen therapy on day 74.
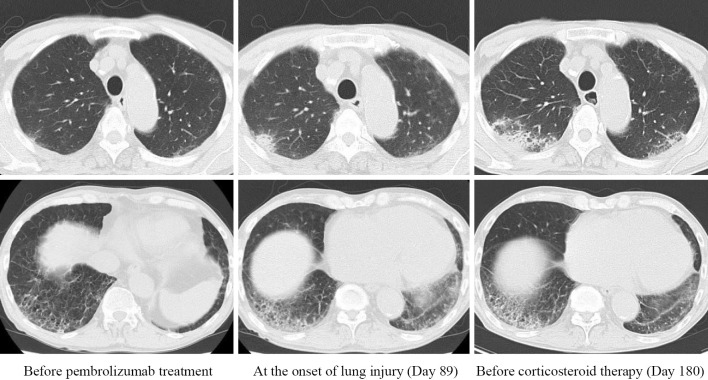
Case 5. The patient was diagnosed with stage 4 NSCLC (adenosquamous carcinoma) with multiple bone metastases. On day 89 of pembrolizumab treatment, chest CT showed a new GGO in the right upper lobe and an expansion of preexisting reticular opacities in the right lower lobe (Fig. 3). Because the patient did not complain of respiratory symptoms at all, he was not withdrawn from pembrolizumab. On day 180, he complained of frequent diarrhea due to immune check point inhibitor (ICI)-related colitis, and oral corticosteroid therapy (prednisolone 30 mg/day) was started. Subsequently, chest CT showed a reduction in the opacities, and the drug-induced lung injury was considered to have resolved.
Figure 3.
Computed tomography scan of the chest before pembrolizumab treatment, at the onset of lung injury and before corticosteroid therapy in a 75-year-old man with adenocarcinoma (Case 5).
Discussion
In the present study, four of five patients with pre-ILD developed pembrolizumab-related AE-ILD, including a severity of grade 3. These findings indicated that treatment with pembrolizumab has a high risk of lung injury in patients with NSCLC and pre-ILD.
Lung injury is one of the most common immune-related adverse events and is the leading adverse event resulting in discontinuation of ICI therapy. In addition, whether ICIs or cytotoxic agents carry a higher risk of inducing AE-ILD has been unclear. In our study, the reasons for administering pembrolizumab to patients with pre-ILD were as follows: 1) Pembrolizumab was expected to be more effective than cytotoxic drugs in NSCLC patients with a high PD-L1 expression, even when concurrent with pre-ILD; 2) Pre-ILD in all patients was mild and inconspicuous on chest X-ray; 3) The patients were over 75 years old and wanted to be treated with pembrolizumab rather than cytotoxic drugs, such as platinum doublet therapy.
ICI-related lung injury is a mostly low-grade event and improves with drug discontinuation and immunosuppression but can also be a potentially life-threatening adverse event (7,8). A recent phase II study evaluated the safety of nivolumab for advanced NSCLC with idiopathic interstitial pneumonia and showed that 2 of 18 previously treated patients developed ICI-related lung injury (9). Compared to that prospective study, the incidence of ICI-related lung injury in the present study was higher. One reason for this might be because the present study had a small sample size. Another reason might be due to thoracic surgery performed prior to the administration of pembrolizumab. In the present study, three of four patients who developed ICI-related lung injury had undergone thoracic surgery before the administration of pembrolizumab. Thoracic surgery including VATS has been known to be a risk factor for AE-ILD (10,11), and immunotherapy following thoracic surgery may carry a high risk of AE-ILD. A third reason might be due to the administration of ICIs as a first-line therapy, which has been reported to carry a high risk of lung injury compared to second- or sequential-line treatment (12). Of note, Khunger et al. reported that no significant differences in the incidence of ICI-related lung injury between treatment-naïve and previously treated patients were observed when the analysis was stratified by PD-1 inhibitors and PD-L1 inhibitors (12). Furthermore, they found that PD-1 inhibitors caused ICI-related lung injury more frequently than PD-L1 inhibitors. Therefore, it may be worth conducting a study to investigate the safety of PD-L1 inhibitors for ILD patients under careful monitoring for early detection of lung injury.
In the present study, all patients had pulmonary emphysema and were diagnosed with combined pulmonary fibrosis and emphysema (CPFE). Patients with CPFE may have a significantly increased risk of lung cancer compared to either patients with pulmonary emphysema or pulmonary fibrosis (13-15), but little is known regarding AE-ILD in chemotherapy for lung cancer associated with CPFE. A low % FVC was reported to be a significant risk factor for chemotherapy-related lung injury in patients with both NSCLC and pre-ILD (16). However, the % FVC of CPFE patients is known to be almost normal (13). In fact, the % FVC of all patients enrolled in the present study was over 80%, and the % FVC was not a risk factor associated with AE-ILD. Recent reports have shown that the size of the pre-ILD area on pretreatment CT was a risk factor for chemotherapy-related lung injury in patients with both NSCLC and pre-ILD (5,17). However, the extent of pre-ILD in all cases in the present study was mild, and whether or not it was risk factor for AE-ILD could not be determined.
In clinical practice, cytotoxic agents are usually selected for treatment of advanced NSCLC with pre-ILD. Carboplatin plus paclitaxel (or nanoparticle albumin-bound-paclitaxel) has been well-evaluated as the most frequently administered first-line regimen for advanced NSCLC with pre-ILD, and the incidence of lung injury was reported to be no more than 10% (18-21). In contrast, the incidence of drug-related lung injury in patients with NSCLC and pre-ILD who received nivolumab was reported to be more than 30% in 2 retrospective studies (22,23). Considering the high frequency of ICI-related lung injury for patients with ILD, cytotoxic drugs seem to be an optimal treatment for such patients. However, most cases of ICI-related lung injury have been manageable (8), while cytotoxic agent-related lung injury often leads to severe and potentially fatal adverse events in patients with pre-ILD (24). In addition, some cases with pre-ILD, including Case 5 in the present study, experienced a good response to ICIs over a long period without ICI-related lung injury (25). Furthermore, when compared to second-line therapy, pembrolizumab monotherapy as a first-line therapy has shown a longer progression-free survival (median of 5.2 months vs. 10.3 months) in patients with advanced NSCLC with a high PD-L1 expression (1,26). Taking these findings into account, we cannot conclude that pembrolizumab monotherapy should be avoided for first-line therapy in patients with NSCLC harboring a high PD-L1 expression concurrent with pre-ILD.
Several limitations associated with the present study warrant mention. First, it was a retrospective analysis performed at a single institution, and the number of patients was very small. Second, all patients were Japanese and 75 years or older. Third, not all pre-ILD was pathologically diagnosed, and the area occupied by pre-ILD was determined semi-quantitatively by experts, not objectively by computer-associated diagnostic techniques.
In conclusion, the administration of pembrolizumab as the first-line therapy was found to carry a high risk of lung injury in patients with pre-ILD. Larger clinical studies are needed to establish the risk factors of pembrolizumab-related AE-ILD in treatment-naïve NSCLC patients with pre-ILD.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The Authors thank Chihiro George Kurokawa of the United States for editing our manuscript.
References
- 1. Reck M, Rodriguez-Abreu D, Robinson AG, et al. . Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375: 1823-1833, 2016. [DOI] [PubMed] [Google Scholar]
- 2. Reck M, Rodriguez-Abreu D, Robinson AG, et al. . Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol 37: 537-546, 2019. [DOI] [PubMed] [Google Scholar]
- 3. Travis WD, Costabel U, Hansell DM, et al. . An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 188: 733-748, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lynch DA, Godwin JD, Safrin S, et al. . High-resolution computed tomography in idiopathic pulmonary fibrosis: diagnosis and prognosis. Am J Respir Crit Care Med 172: 488-493, 2005. [DOI] [PubMed] [Google Scholar]
- 5. Ozawa Y, Akahori D, Koda K, et al. . Distinctive impact of pre-existing interstitial lung disease on the risk of chemotherapy-related lung injury in patients with lung cancer. Cancer Chemother Pharmacol 77: 1031-1038, 2016. [DOI] [PubMed] [Google Scholar]
- 6. Eisenhauer EA, Therasse P, Bogaerts J, et al. . New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45: 228-247, 2009. [DOI] [PubMed] [Google Scholar]
- 7. Suresh K, Voong KR, Shankar B, et al. . Pneumonitis in non-small cell lung cancer patients receiving immune checkpoint immunotherapy: incidence and risk factors. J Thorac Oncol 13: 1930-1939, 2018. [DOI] [PubMed] [Google Scholar]
- 8. Naidoo J, Wang X, Woo KM, et al. . Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol 35: 709-717, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fujimoto D, Yomota M, Sekine A, et al. . Nivolumab for advanced non-small cell lung cancer patients with mild idiopathic interstitial pneumonia: a multicenter, open-label single-arm phase II trial. Lung Cancer 134: 274-278, 2019. [DOI] [PubMed] [Google Scholar]
- 10. Kondoh Y, Taniguchi H, Kitaichi M, et al. . Acute exacerbation of interstitial pneumonia following surgical lung biopsy. Respir Med 100: 1753-1759, 2006. [DOI] [PubMed] [Google Scholar]
- 11. Sato T, Teramukai S, Kondo H, et al. . Impact and predictors of acute exacerbation of interstitial lung diseases after pulmonary resection for lung cancer. J Thorac Cardiovasc Surg 147: 1604-1611 e1603, 2014. [DOI] [PubMed] [Google Scholar]
- 12. Khunger M, Rakshit S, Pasupuleti V, et al. . Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small cell lung cancer: a systematic review and meta-analysis of trials. Chest 152: 271-281, 2017. [DOI] [PubMed] [Google Scholar]
- 13. Jankowich MD, Rounds SIS. Combined pulmonary fibrosis and emphysema syndrome: a review. Chest 141: 222-231, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kitaguchi Y, Fujimoto K, Hanaoka M, Kawakami S, Honda T, Kubo K. Clinical characteristics of combined pulmonary fibrosis and emphysema. Respirology 15: 265-271, 2010. [DOI] [PubMed] [Google Scholar]
- 15. Kurashima K, Takayanagi N, Tsuchiya N, et al. . The effect of emphysema on lung function and survival in patients with idiopathic pulmonary fibrosis. Respirology 15: 843-848, 2010. [DOI] [PubMed] [Google Scholar]
- 16. Enomoto Y, Inui N, Kato T, et al. . Low forced vital capacity predicts cytotoxic chemotherapy-associated acute exacerbation of interstitial lung disease in patients with lung cancer. Lung Cancer 96: 63-67, 2016. [DOI] [PubMed] [Google Scholar]
- 17. Masuda T, Hirano C, Horimasu Y, et al. . The extent of ground-glass attenuation is a risk factor of chemotherapy-related exacerbation of interstitial lung disease in patients with non-small cell lung cancer. Cancer Chemother Pharmacol 81: 131-139, 2017. [DOI] [PubMed] [Google Scholar]
- 18. Minegishi Y, Sudoh J, Kuribayasi H, et al. . The safety and efficacy of weekly paclitaxel in combination with carboplatin for advanced non-small cell lung cancer with idiopathic interstitial pneumonias. Lung Cancer 71: 70-74, 2011. [DOI] [PubMed] [Google Scholar]
- 19. Kenmotsu H, Naito T, Mori K, et al. . Effect of platinum-based chemotherapy for non-small cell lung cancer patients with interstitial lung disease. Cancer Chemother Pharmacol 75: 521-526, 2015. [DOI] [PubMed] [Google Scholar]
- 20. Fujita T, Hiroishi T, Shikano K, et al. . The safety and efficacy of treatment with nab-paclitaxel and carboplatin for patients with advanced squamous non-small cell lung cancer concurrent with idiopathic interstitial pneumonias. Intern Med 57: 1827-1832, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Igawa S, Nishinarita N, Takakura A, et al. . Real-world evaluation of carboplatin plus a weekly dose of nab-paclitaxel for patients with advanced non-small-cell lung cancer with interstitial lung disease. Cancer Manag Res 10: 7013-7019, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yamaguchi T, Shimizu J, Hasegawa T, et al. . Pre-existing pulmonary fibrosis is a risk factor for anti-PD-1-related pneumonitis in patients with non-small cell lung cancer: a retrospective analysis. Lung Cancer 125: 212-217, 2018. [DOI] [PubMed] [Google Scholar]
- 23. Kanai O, Kim YH, Demura Y, et al. . Efficacy and safety of nivolumab in non-small cell lung cancer with preexisting interstitial lung disease. Thorac Cancer 9: 847-855, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kashiwada T, Saito Y, Terasaki Y, et al. . Interstitial lung disease associated with nanoparticle albumin-bound paclitaxel treatment in patients with lung cancer. Jpn J Clin Oncol 49: 165-173, 2019. [DOI] [PubMed] [Google Scholar]
- 25. Ide M, Tanaka K, Sunami S, et al. . Durable response to nivolumab in a lung adenocarcinoma patient with idiopathic pulmonary fibrosis. Thorac Cancer 9: 1519-1521, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Herbst RS, Baas P, Kim DW, et al. . Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387: 1540-1550, 2016. [DOI] [PubMed] [Google Scholar]