Graphical abstract
The pleiotropic effects of chloroquine that including anti-malaria, anti-cancer and anti-viral diseases, which is linked with inhibition of autophagy, tumor vascular normalization, lysosomotropic and immunomodulatory property. 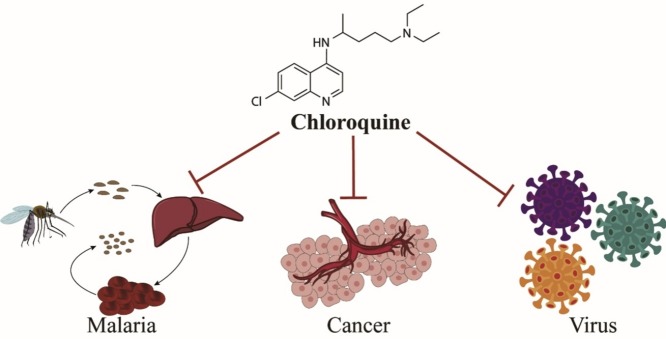
Abstract
Quinoline (QN) derivatives are often used for the prophylaxis and treatment of malaria. Chloroquine (CQ), a protonated, weakly basic drug, exerts its antimalarial effect mainly by increasing pH and accumulating in the food vacuole of the parasites. Repurposing CQ is an emerging strategy for new indications. Given the inhibition of autophagy and its immunomodulatory action, CQ shows positive efficacy against cancer and viral diseases, including Coronavirus 2019 (COVID-19). Here, we review the underlying mechanisms behind the antimalarial, anticancer and antiviral effects of CQ. We also discuss the clinical evidence for the use of CQ and hydroxychloroquine (HCQ) against COVID-19.
Teaser
As a 4-aminoquinoline and a protonated, weakly basic drug, chloroquine shows great potential in the treatment of malaria, cancers and viral diseases, including COVID-19.
Introduction
CQ, a 4-aminoquinoline, has been used as an antimalarial drug for many years, and has also subsequently shown therapeutic effect in systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) [1]. Recently, CQ has also shown potential in the treatment of cancers and viral infections with pleiotropic effects through complex mechanisms 2, 3, 4.
Both CQ and its analog HCQ are characterized by rapid onset, long duration of action, low toxicity, and high tolerance in humans [5]. CQ is partly metabolized into a mono-desethyl metabolite and eliminated mainly through the kidneys. Its long half-life makes it amenable to once-weekly drug delivery for malaria treatment [6]. HCQ is created by replacing an ethyl group in CQ with a hydroxyethyl group; this results in a larger volume of distribution and lower toxicity in humans [7]. They are both easily distributed to different tissues, and can cross the blood–brain barrier (BBB) and the placental barrier with almost no toxicity to pregnant women or their fetuses [8]. However, a long-term dose regimen leads to drug accumulation in lungs, heart, liver, and kidneys at a concentration 10–100 times more than in the plasma, which could be a concern for drug safety [6].
Although taking CQ as a prescribed drug produces few adverse effects, high dosage and long-term administration can lead to severe toxicity, including retinopathy, neuropathy, cardiomyopathy, hypoglycemia, dermatological reactions, and bone marrow suppression. Given the ion activity of CQ and HCQ, they can block potassium channels responsible for ventricular repolarization. QTc prolongation and torsade de pointes (TdP) can occur in patients after both short-term and high-dose administration of CQ and, thus, drug–drug interactions (DDI) with other QTc-prolonging drugs is a cause for concern [9]. Therefore, regular 6-month ophthalmological follow-up examinations, cardiac monitoring, complete blood counts, and blood glucose level tests are advised for patients taking either CQ or HCQ [10].
Here, we review the underlying mechanisms of CQ and HCQ to treat malaria, cancers and viral infections. In addition, we discuss clinical evidence for the use of CQ and HCQ against COVID-19.
Chloroquine as an antimalarial agent
During the first half of the 19th century, QN was successfully extracted from Cinchona bark by French pharmacists, and it became the earliest antimalarial drug. Based on the structure of QN, CQ was first synthesized by Bayer A.G. in Germany in 1934, followed by HCQ in 1944. After drug resistance to CQ was discovered, several other compounds were designed on the basis of the QN parent nucleus to treat malaria [11].
Malaria drug therapy
Malaria is a devastating infectious disease and a public health problem around the world. According to the WHO World Malaria Report 2018, ∼219 million people worldwide were infected with Plasmodium, especially children and pregnant women, and 435 000 individuals died of malaria [12]. Malaria is mainly caused by five common species of protozoan parasites: Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, Plasmodium knowlesi, and Plasmodium malariae. Of these, P. vivax is the most widespread, leading to severe global morbidity and mortality [13].
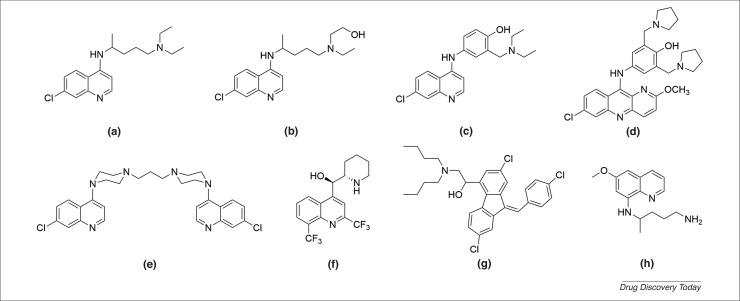
CQ has been a widely used, effective antimalarial therapy for decades. It is often recommended to be co-administered with primaquine to prevent P. vivax recurrence [14]. However, with the appearance of drug-resistant strains of Plasmodium, novel antimalarial agents are urgently needed. A series of common antimalarial quinoline derivatives have been synthesized, all of which have shown some activity against malaria in a single treatment. For example, the 4-aminoquinolines (amodiaquine, pyronaridine, and piperaquine), aminoalcohols (mefloquine and lumefantrine) and 8-aminoquinolines (primaquine) are all promising antimalarial agents [15] (Fig. 1 ).
Figure 1.
The chemical structures of antimalarial quinoline derivatives. (a) Chloroquine (CQ); (b) hydroxychloroquine (HCQ); (c) amodiaquine; (d) pyronaridine; (e) piperaquine; (f) mefloquine; (g) lumefantrine; and (h) primaquine.
After artemisinin was discovered by Tu and colleagues, artemisinin-based combination therapy (ACT) was used as first and second-line treatment for uncomplicated P. falciparum as well as CQ-resistant P. vivax malaria [16]. Amodiaquine, piperaquine, pyronaridine, and lumefantrine are recommended by WHO as partner drugs for artemisinin derivatives. Some common drug combinations include dihydroartemisinin-piperaquine (DHA-PPQ) [17], artesunate-amodiaquine (AS-AQ) [18], pyronaridine-artesunate (PY-AS) [19], and artemether-lumefantrine (AL) [20].
Mechanisms of action of chloroquine against malaria
The quinolines mostly exert their antimalarial effect during the blood stages or liver stages of the life cycle of the parasite [21]. As a protonated, weakly basic drug, CQ increases pH and accumulates in the food vacuole of parasites, where the host erythrocyte hemoglobin degrades, leading to the release of the toxic products. Iron (II) protoporphyrin IX (FeIIPPIX) is automatically oxidized to toxic iron (III) protoporphyrin IX (FeIIIPPIX) or hematin, but the parasites can survive by detoxifying hematin to a dimerized nontoxic hemozoin form. CQ inhibits the polymerization and detoxification of hematin and interferes with the degradation of host erythrocyte hemoglobin, preventing Plasmodium growth [22]. Therefore, CQ causes the accumulation of free hematin that is highly toxic to Plasmodium, resulting in dissolution of the cell membrane and, ultimately, death of the parasites.
Moreover, the peroxidation of parasite lipid membranes, inhibition of lactate dehydrogenase and tyrosine kinase, oxidation of proteins, and damage to DNA also have important roles in the viability of the parasites [23]. CQ can insert into the DNA double helix structure of Plasmodium to form a stable DNA-CQ complex. This complex affects DNA replication and RNA transcription, thus inhibiting Plasmodium growth and reproduction [24]. However, the exact antimalarial mechanisms involved remain controversial.
Drug resistance against antimalarial agents
Although antimalarial agents are successfully and widely used in chemotherapy, drug resistance has hindered their clinical application. Antimalarial drug resistance can involve the evolution and variation of resistant strains of Plasmodium [25]. The drug resistance is closely associated with single nucleotide polymorphisms (SNP) in P. falciparum CQ-resistant transporter (Pfcrt) and P. falciparum multidrug resistance 1 (Pfmdr1) genes [26].
A reduction in drug accumulation is regarded as the primary reason for drug resistance. Several candidate genes related to membrane transport implicate antimalarial drug resistance, and P. falciparum has several genes with sequence similar to ATP-binding cassette (ABC) transporters. The balance between P-gh1 (encoded by Pfmdr1) and PfCRT (encoded by Pfcrt) has a crucial role in regulating drug influx and efflux because of modulation of the anion channel [22]. Thus, drug resistance can arise as a result of the transmembrane protein pump on the cell surface and pH in the food vacuole. The reduced accumulation of drugs can result from an increase in energy-dependent efflux, a decrease in uptake, and the inability of drugs to bind to their target sites [25].
Chloroquine as an anticancer agent
Cancer results in high mortality around the world. Multiple factors regulating tumor invasion and migration could be targets for anticancer drugs [27]. Repurposing drugs is a way to develop new indications for existing compounds. When compared with developing new drugs, repurposing drugs by screening, modifying, or identifying new combinations can reduce development costs and shorten drug development time [28]. Moreover, the mechanisms of action, molecular targets, pharmacological properties, tolerability, and toxicity of approved drugs are generally well understood, allowing them to be used more effectively and safely [29].
At present, repurposing drugs for cancer treatment is considered an alternative strategy and important supplement to drug development. Besides its antimalarial effects, the novel use of CQ as an anticancer agent has drawn great attention. CQ is regarded as an autophagy inhibitor and could be an adjuvant to anticancer chemotherapies. In addition, CQ renders tumor cells more sensitive to a variety of anticancer drugs and enhances their therapeutic activity [7].
Single and combination use of chloroquine
The antitumor effects of CQ alone or in combination with other drugs for different types of cancer have been reported in a series of experimental studies. Research has shown that a single treatment of CQ (25 μM for cells and 50 mg/kg/day for mice) induced prostate apoptosis response-4 (Par-4) protein secretion through the activation of p53, which promoted Rab8b to transport Par-4 from the Golgi to the plasma membrane, playing a crucial role in the inhibition of metastatic tumor growth [30]. However, a randomized and double-blinded trial showed that a single treatment using CQ did not show positive efficacy, which might be attributed to differences in autophagy mechanisms [31]. Blocking autophagy did not necessarily prevent cancer cell growth because glucose starvation or 2-deoxyglucose (2DG) that inhibits hexokinase could prevent CQ-induced lysosomal swelling, damage, and cell death [32]. Compared with monotherapy, combination treatment of CQ with chemotherapy can enhance efficacy, decrease toxicity, and reduce the drug dosage need. A meta-analysis concluded that autophagy inhibitors, such as CQ and HCQ, combined with other anticancer agents, could significantly increase overall response rate (ORR), 1-year overall survival (OS) rate, and 6-month progression-free survival (PFS) rate, improving survival of patients with cancer [33]. In addition, among in vitro and in vivo studies, the combination groups always presented more efficacy against tumors than other groups, which might be because of inhibition of autophagy and induction of apoptosis [7] (Table 1 ).
Table 1.
Summary of in vitro and in vivo studies combining anticancer agents with CQ
| Tumor type | Intervention | Intervention dose | CQ dose | Cell line/animal model | Therapeutic effect | Refs |
|---|---|---|---|---|---|---|
| APL | Arsenic trioxide | 1 μM | 10, 25 uM | NB4 | Suppressing cell growth, inhibiting autophagy, and inducing mitochondrial pathway apoptosis and S phase arrest | [90] |
| BC | Trichostatin A | 0.5 μM | 25 μM | MCF10A, MCF10A-ras | Inducing cell apoptosis by activating FOXO1 and inhibiting mTOR pathway | [91] |
| CSCC | Gefitinib | 5 μM | 50, 100 μM | A431 | Inhibiting protective autophagy and enhancing apoptosis | [92] |
| GC | Tenovin-6 | 0.2, 0.5, 1, 2, 4 μM | 25, 50 μM | AGSEBV, SNU-719, AGS, HGC-27, N87, SNU-1, KATO-III | Inhibiting cell proliferation, inhibiting autophagy flux, and inducing G1 arrest and apoptosis with p53 activation | [93] |
| GC | CMG002 | 100 nM | 10 μM | AGS, NUGC3 | Inducing apoptotic cell death by blocking PI3K/AKT/mTOR pathway | [94] |
| HCC | Doxorubicin | 1 mg/kg 2 times/week | 25 mg/kg/day | Sprague–Dawley rats | Inducing apoptosis by upregulating TRAIL/TRAILR2, caspase-3, and caspase-8 and downregulating Bcl-2 | [95] |
| Hypophysoma | Cabergoline | 100 μM | 20 μM | GH3, MMQ | Increasing cell death, enhancing disruption of autophagy; inducing apoptosis with accumulation of p62/caspase8/LC3-II | [103] |
| 0.5 mg/kg/2 days | 50 mg/kg/day | Athymic nude mice, F344 rats | Suppressing tumor growth | |||
| Melanoma | Temozolomide | 100 μM | 20 μM | Mel MTP, Mel Z and Mel IL | Potentiating TMZ-induced apoptosis, inducing G0/G1 arrest and enhancing cytotoxicity | [96] |
| NEN | Everolimus (RAD001) | 3 mg/kg/day | 60 mg/kg/day | BON1 subcutaneous neoplasm mice | Reducing tumor size and weight, inhibiting autophagy and increasing apoptosis with mTORC1 signaling pathway inhibition | [97] |
| NPC | Cisplatin | 50 μM | 5, 50 μM | C666-1 | Inhibiting cell viability and promoting apoptosis with high-expression of Beclin 1 and LC3B-II | [98] |
| NSCLC | Paclitaxel | 10 nM | 10 μM | A549 | Inhibiting tumor metastasis, reverting paclitaxel resistance and suppressing autophagy via ROS-mediated modulation of AKT activity and downregulation of Wnt/β-catenin pathway | [99] |
| C2-ceramide | 10, 20, 50 μM | 10 μM | H460, H1299 | Inducing cytotoxicity, promoting cell apoptosis, inhibiting autophagy through inhibition of Src and SIRT1 and activation of LAMP2 and LC3-I/II | [104] | |
| 5 μM | 5 μM | Zebrafish xenografts | Inhibiting tumor growth | |||
| Honokiol | 10, 20, 30, 40, 60 μM | 10, 20 μM | A549, H460 | Inhibiting cell proliferation and inducing cell death in caspase-dependent and cathepsin D-involved manner | [105] | |
| 50 mg/kg/day | 100 mg/kg/day | BALB/c nude mice | Reducing tumor growth | |||
| Gefitinib | 100 nM | 10, 20 μM | PC-9/wt, PC-9/gefB4 and PC-9/gefE3 | Inducing apoptosis, inhibiting autophagy and reversing gefitinib resistance | [106] | |
| 50 mg/kg/day | 75 mg/kg/day | BALB/c nude mice | Potentiating gefitinib-induced antitumor activity and reducing tumor growth | |||
| OC | Cisplatin | 5 μM | 5, 10 μM | SKOV3, hey | Inhibiting cell growth, migration, and invasion, inhibiting autophagy | [107] |
| 5 mg/kg/6 days | 60 mg/kg/day | BALB/c nude mice | Reducing tumor volume and tumor weight | |||
| PC | Gambogic acid | 1, 2 μM | 40 μM | PANC-1, BxPC-3 | Inhibiting autophagy by reducing mitochondrial membrane potential and increasing ROS accumulation | [108] |
| 8 mg/kg/3 days | 100 mg/kg first day | BALB/c nude mice | Inhibiting tumor growth | |||
| RCC | ABT-737 | 1 μM | 25 μM | A498, 786-O | Decreasing cell viability, inducing lysosome-dependent cell death by increasing cellular ROS level | [100] |
| Everolimus | 15 μM | 20 μM | A498, RXF393, SN12C, 769P | Inducing cell viability and apoptosis via intrinsic mitochondrial apoptotic pathway activation | [101] | |
| TNBC | Osimertinib | 2.4, 4, 6.5 μM | 10, 30, 75 μM | MDA-MB-231 | Improving effectiveness of osimertinib through autophagy-apoptosis crosstalk pathway (pAKT inhibition and pBad activation) | [102] |
| Isorhamnetin | 10 μM | 20 μM | MDA-MB-231, MCF-7, BT549, MCF-10A | Inhibiting cell proliferation, inducing apoptosis, inducing generation of ROS and inducing mitochondrial fission through phosphorylation of Camk II and Drp1 as well as their mitochondrial translocation | [109] | |
| 20 mg/kg/2 days | 40 mg/kg/2 days | Nude mice | Suppressing tumor growth | |||
Abbreviations: APL, acute promyelocytic leukemia; BC, breast cancer; CSCC, cutaneous squamous cell carcinoma; GC, gastric cancer; HCC, hepatocellular; Int., interventions; NEN, neuroendocrine neoplasms; NPC, nasopharyngeal carcinoma; NSCLC, nonsmall cell lung cancer; OC, ovarian cancer; PC, pancreatic cancer; RCC, renal cell carcinoma; TNBC, triple-negative breast cancer.
Of the 22 clinical trials of CQ in the treatment of cancers on ClinicalTrials.gov (Table 2 ), most of the findings showed that the drug combination was well tolerated and the maximum tolerated dose (MTD) was increased, but there was no significant difference in the treatment groups and control groups, and no significant improvement in OS was observed. Therefore, the efficacy of the combination use of CQ with anticancer agents should be further assessed and explored in larger samples.
Table 2.
Clinical trials investigating the use of CQ and HCQ to treat cancers
| Cancer | NCT number | Phase | Cancer | NCT number | Phase | Cancer | NCT number | Phase |
|---|---|---|---|---|---|---|---|---|
| CQ | ||||||||
| Brain metastasis | NCT01894633 | II | NCT00224978 | III | Myeloma | NCT01438177 | II | |
| NCT01727531 | N/A | NCT03243461 | III | Nonsmall cell lung | NCT02786589 | I/II | ||
| Breast | NCT02333890 | II | Glioma/cholangiocarcinoma/chondrosarcoma | NCT02496741 | I/II | Pancreatic | NCT01777477 | I |
| NCT01446016 | II | Hematological or solid tumor | NCT04333914 | II | Prolactinoma | NCT03400865 | N/A | |
| Ductal carcinoma | NCT01023477 | I/II | Malignant neoplasm | NCT02071537 | I | Small cell lung | NCT01575782 | I |
| Glioblastoma | NCT04397679 | I | NCT02366884 | II | NCT00969306 | I | ||
| NCT02378532 | I | Melanoma | NCT01469455 | I | ||||
| NCT02432417 | II | NCT03979651 | N/A | |||||
| HCQ | ||||||||
| Advanced | NCT01266057 | I | Lymphangioleiomyomatosis | NCT01687179 | I | Adenocarcinoma | NCT01978184 | II |
| Central nervous system tumors | NCT00486603 | I/II | Melanoma | NCT00962845 | I | NCT01128296 | I/II | |
| Breast | NCT04316169 | I | NCT02257424 | I/II | NCT03825289 | I | ||
| NCT02414776 | I | NCT03979651 | N/A | Prolactinoma | NCT03400865 | N/A | ||
| NCT03032406 | II | NCT03754179 | I/II | Prostate | NCT04011410 | II | ||
| NCT03774472 | I/II | NCT01897116 | I | NCT03513211 | I/II | |||
| NCT00765765 | I/II | Myelodysplastic Syndromes | NCT03929211 | I/II | NCT00726596 | II | ||
| NCT03400254 | I/II | Myeloma | NCT01689987 | I | NCT00786682 | II | ||
| NCT01292408 | II | NCT01396200 | I | NCT01828476 | II | |||
| Cholangiocarcinoma | NCT03377179 | II | NCT00568880 | I | NCT02421575 | I | ||
| Colorectal | NCT01006369 | II | NCT04163107 | I | Rectal/colon/adenocarcinoma | NCT01206530 | I/II | |
| NCT02316340 | II | Nephropathy | NCT02765594 | IVIII | Renal cell carcinoma | NCT01144169 | I | |
| NCT03215264 | I/II | Nonsmall cell lung cancer | NCT00809237 | I/II | NCT01550367 | I/II | ||
| Gastrointestinal | NCT04214418 | I/II | NCT01649947 | II | NCT01510119 | I/II | ||
| NCT04145297 | I | NCT01026844 | I | Sarcoma | NCT01842594 | II | ||
| Glioblastoma | NCT04201457 | I/II | NCT02470468 | I/II | Small cell lung | NCT02722369 | II | |
| NCT01602588 | II | NCT00977470 | II | Solid tumor | NCT01417403 | I | ||
| NCT03008148 | II/III | Osteosarcoma | NCT03598595 | I/II | NCT00714181 | I | ||
| Hematological malignancy | NCT04392128 | II | Ovarian cancer | NCT03081702 | I/II | NCT00909831 | I | |
| Hepatocellular | NCT03037437 | II | Pancreatic cancer | NCT04132505 | I | NCT02232243 | I | |
| NCT02013778 | I/II | NCT04386057 | II | NCT01023737 | I | |||
| Leukemia | NCT00771056 | II | NCT01506973 | I/II | NCT01634893 | I | ||
| NCT02631252 | I | NCT01273805 | II | NCT03015324 | I | |||
| NCT01227135 | II | NCT01494155 | II | NCT01480154 | I | |||
| Lung | NCT00728845 | I/II | NCT03344172 | II | NCT00813423 | I | ||
Recent studies found that nanoparticle delivery of CQ combination treatment could further enhance its anticancer effects via accumulation of drugs in the tumor tissue. The most common combination was the co-delivery of doxorubicin (DOX) and CQ in nanoparticles 34, 35.
Inhibition of autophagy in cancer therapy
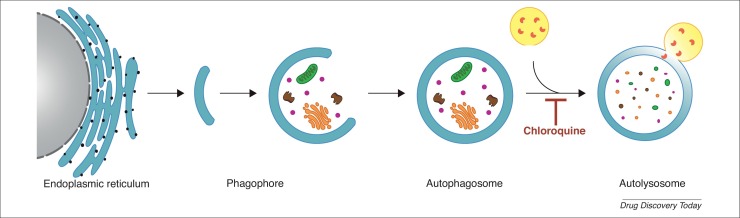
Autophagy, a self-degradative process in eukaryotic cells, removes damaged or dysfunctional cellular organelles and proteins [36]. It is a dynamic process that helps regulate metabolic stress response and maintain cellular homeostasis [37]. Autophagy is typically divided into several stages: initiation, autophagosome formation, fusion, and degradation [38]. More specifically, it starts with the formation of double-membraned vesicles, and then autophagosomes that engulf damaged or dysfunctional cellular organelles and proteins [39]. Autophagosomes and lysosomes fuse to form autolysosomes, and the components are eventually degraded by acidic lysosomal hydrolases [3] (Fig. 2 ).
Figure 2.
The process of autophagy. The phagophore, which originated from the endoplasmic reticulum, is extended to form autophagosomes. The autophagosome can engulf damaged or dysfunctional cellular organelles and proteins. It then fuses with lysosomes to form autolysosomes, and the components are eventually degraded by acidic lysosomal hydrolases, which is inhibited by chloroquine (CQ).
The role of autophagy is interesting but complicated because it can cause unintended consequences (e.g., cancer-promoting or cancer-suppressing effects) [40]. On the one hand, given the genomic instability and degradation of vital components, autophagy inhibits tumor growth and induces apoptosis because of abnormal transformations [41]. On the other hand, the clearance and recycling of dysfunctional organelles and proteins provide energy for tumor cells. It maintains cellular homeostasis and confers resistance of cancer cells against chemotherapy and radiotherapy [42].
Therefore, targeting autophagy could help overcome drug resistance and enhance the clinical efficacy of anticancer therapies for patients [43]. CQ, an autophagy inhibitor, exerts its antitumor effect by inhibiting the fusion of autophagosomes and lysosomes.
Mechanisms of action of chloroquine against cancer
CQ is widely used for sensitizing tumor cells to chemotherapy and radiotherapy. Despite considerable evidence for the efficacy and safety of CQ, the mechanisms behind its antitumor effects remain unclear. CQ not only impairs the anticancer immune response and prevents tumor cell escape [44], but also has advantages in regulating multiple cellular signaling pathways involved in inflammation and cancer [45] compared with other important natural agents, such as curcumin [46], zerumbone [47], thymoquinone [48], and honokiol [49]. It can affect the expression level of inflammatory factors, including nuclear factor-kappa B (NF-κB) and interleukin-1 beta (IL-1β). Here, we focus on autophagy inhibition and tumor vascular normalization.
Tumor cells use autophagy as a crucial compensatory survival mechanism. Autophagy has long been a target of combinational cancer therapeutic strategy. In recent years, it became clear that the underlying mechanisms of CQ-mediated tumor cell death include inhibition of autophagy, disruption of the cell cycle, and induction of cell apoptosis [50]. CQ increases pH in lysosomes and blocks the last step of the autophagy process by impeding the degradation of autophagic proteins, such as light chain 3B-II (LC3B-II). Thus, it prevents the production and recycling of important nutrients and metabolites, including nucleotides, amino acids, and fatty acids, causing tumor cell damage and death 51, 52. When the late stage of autophagy is inhibited, cytotoxic effect is increased by promoting cell apoptosis and cell-cycle arrest [53].
Nonetheless, the antitumor effect of CQ is possibly independent of autophagy inhibition. Using genome editing, deletion of autophagy-related protein 7 (ATG7) did not improve the efficacy of CQ in inhibiting cell proliferation and tumorigenesis in vitro [54]. Thus, the antiproliferation effect might be achieved by damage to mitochondrial membrane permeability and inhibition of ABC family protein and DNA repair [3].
In addition, inhibition of angiogenesis and tumor vascular normalization are attracting attention as emerging strategies for cancer treatment [55]. Tumor blood vessels not only provide nutrients and oxygen for tumor cells, but can also remove metabolic waste, which contributes to the growth and metastasis of cancer. Thus, inhibition of angiogenesis might be an effective anticancer strategy [55]. A study showed that CQ might inhibit angiogenesis via the downregulation of p-AKT, Jagged1, and Ang2, to effectively suppress cancer growth [56]. Some tumor cells are less sensitive to chemotherapy, especially under hypoxic conditions, but tumor vascular normalization reverses this phenomenon. Maes and colleagues demonstrated that reduction of intratumoral hypoxia, invasion, and metastasis was seen after administrating CQ. To some extent, this can be achieved through an autophagy-independent, NOTCH1-reliant mechanism of tumor vascular normalization [57]. Moreover, CQ increases the delivery and response of chemotherapy drugs by reducing blood vessel density and improving cell alignment [58].
Chloroquine as an antiviral agent
The broad-spectrum antiviral action of chloroquine
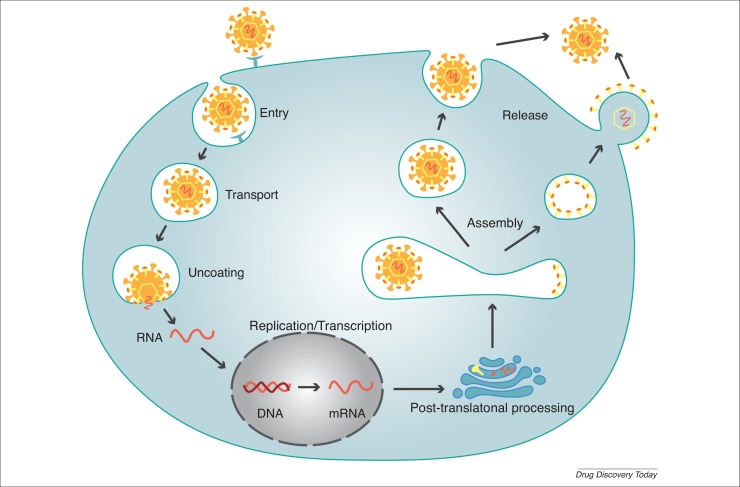
Viral infection is a multistep process involving the fusion of virus with host cell membrane, viral particle transport, nucleic acid replication and transcription, protein glycosylation, viral assembly and viral release [59] (Fig. 3 ). Previous studies showed that CQ increased the pH in organelles, thereby suppressing these steps and abrogating viral replication and further infection [60].
Figure 3.
Viral infection. The process of viral infection has several stages: viral entry, viral particle transport, uncoating, nucleic acid replication and transcription, post-translational processing, virus assembly, and virus release.
CQ has been developed as a nonspecific antiviral drug and exerted direct antiviral effects with inhibiting the replication of several viruses, including flavivirus, retrovirus, and coronavirus families [61]. Previous studies showed the antiviral activity of CQ in Chikungunya virus (CHIKV), dengue virus-2 (DENV-2), hepatitis C virus (HCV), Ebola virus (EBOV), human immunodeficiency virus (HIV), Severe Acute Respiratory Syndrome coronavirus (SARS-CoV), and Middle East Respiratory Syndrome coronavirus (MERS-CoV) [2]. Zika virus (ZIKV) infection causes neonatal microcephaly and neurological disorders, and CQ can prevent maternal to fetal ZIKV transmission. CQ also impedes the release of viral RNA from endosomes and reduces autophagy-dependent virus replication [62].
However, these results are from in vitro or in vivo animal experiments. Clinical trials concerning CQ and HCQ antiviral use are summarized in Table 3 . Unfortunately, the clinical efficacy of both drugs is not yet clear and warrants further study.
Table 3.
Clinical trials of CQ and HCQ for the treatment of viral disease other than COVID-19
| Viral disease | NCT number | Trial phase | Viral disease | NCT number | Trial phase | Viral disease | NCT number | Trial phase |
|---|---|---|---|---|---|---|---|---|
| Intervention: CQ | Intervention: HCQ | |||||||
| AIDS | NCT00972725 | II | HIV | NCT00308620 | II/III | Hepatitis C | NCT01833845 | I/II |
| Autoimmune hepatitis | NCT01980745 | IV | NCT00132535 | N/A | HIV | NCT01232660 | I | |
| NCT02463331 | IV | NCT00819390 | II | NCT01067417 | II | |||
| Chikungunya | NCT00391313 | III | Influenza | NCT01078779 | II | NCT02475915 | I/II | |
| Dengue | NCT00849602 | I/II | Rabies | NCT02564471 | IV | |||
| Hepatitis C virus | NCT02058173 | IV | ||||||
Mechanisms of action of chloroquine against viral infections
The lysosomotropic and immunomodulatory properties of CQ have crucial roles in viral infection and replication. The acidification of endosomes and the activities of host endosomal proteases are indispensable to the survival of viruses. Autophagy inhibitors can potently inhibit the fusion of autophagosomes and lysosomes, resulting in the accumulation of autophagosomes and increased lysosomal pH, which eventually suppress viral infection [63]. CQ can cross the membranes of cells and organelles to accumulate in endosomes, lysosomes, and Golgi vesicles, thereby increasing the pH of cytoplasmic organelles, leading to the dysfunction of intracellular enzymes and preventing the fusion of viral envelope protein with the endosomal membrane [64].
Interferons (IFNs) impart immunomodulatory action against viral infections and the IFN system is crucial for the innate immune response to an acute viral infection. Interferon-gamma (IFN-γ) degrades RNA and inhibits protein synthesis and RNA mismatching [65]. Studies suggested that CQ affected the recognition of viral antigen by plasmacytoid dendritic cells via suppressing toll-like receptor (TLR) signaling [66] and postponing cell-mediated adaptive immune responses [65]. Moreover, CQ inhibits the production of chemokines, cytokines, nitric oxide (NO), reactive oxygen species (ROS), and other mediators that contribute to the severity of viral infections. It also inhibits cytokine storm and reduces the proinflammatory response as well as the activation of macrophages 2, 61, 62, 63, 64.
Limitations of chloroquine as an antiviral therapy
The analysis of data from clinical trials or animal experiments suggests that long-term treatment with CQ is pernicious 65, 67 and reduces its potential therapeutic effect over time [68]. Sufficient and stable concentrations of CQ are required for maximal therapeutic effect. First, it is difficult to increase and maintain the pH of acidic organelles at neutral levels. Either the steady-state plasma concentration of CQ must be maintained at least at 3.125 μM/l or the whole-blood concentration should be maintained at 16 μM/l [68]. Second, the narrow therapeutic indexes and poor penetration to specific tissues are controversial [67]. Third, acute stage, severity of infections, and dose regimens are also vital considerations for therapeutic effect.
To overcome these limitations, feasible solutions, such as combination therapies and sustained-release dosing, have been developed. For example, nanoparticle drug delivery systems (NDDSs) improve drug distribution, drug release rate, and tissue targeting [69]. Improved pharmacokinetics seen in in vitro results, such as bioavailability and steady-state concentration, might also confer in vivo efficacy [70].
Application of chloroquine and hydroxychloroquine for COVID-19
A new pneumonia caused by a novel coronavirus emerged in China in December 2019. The WHO named the disease ‘Coronavirus Disease 2019 (COVID-19)’. The Coronavirus Study Group (CSG) of the International Committee denominated the virus as ‘Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2)’. COVID-19 has since spread to numerous countries, causing a global pandemic.
As of July 12, 2020, WHO announced that >12 550 000 cases of COVID-19 have been confirmed worldwide which included 561 617 deaths [71]. COVID-19 spreads through droplets, respiratory secretions, direct contacts, and fecal swabs. The patients show symptoms such as fever, fatigue, cough, sputum production, diarrhea, and vomiting, and a few severely ill patients rapidly develop acute respiratory distress syndrome, multiple organ failure, and even die [72]. Unfortunately besides remdesivir (RDV), no drugs or vaccines has been formally approved for the treatment of COVID-19 so far.
The invasive routes of SARS-CoV-2
The Spike (S) glycoprotein of SARS-CoV-2 and SARS-CoV shares 76% amino acid sequence identity [73]. This virion of coronavirus can attach to the angiotensin-converting enzyme 2 (ACE2) receptor on the surface of human cells, mediating viral entry. Hoffmann et al. provided evidence that SARS-CoV-2 depended on the ACE2 receptor to enter host cells, similar to SARS-CoV [74]. Blocking the activation of angiotensin receptor 1 (AT1R) and upregulating the expression of ACE2 can increase angiotensin 1–7, which could protect the lung from injury. Researchers speculated that AT1R antagonists, such as losartan, might offer protection from severe symptoms among patients with SARS-CoV-2 [75].
In addition, viral entry can be blocked by an inhibitor of the cellular transmembrane protease serine 2 (TMPRSS2), and it is closely related to the binding and priming of S glycoprotein. Studies found that TMPRSS2 inhibitors, such as camostat mesylate, blocked SARS-CoV-2 infection of lung cells [74]. Wang et al. verified that CD147 was a novel receptor for SARS-CoV-2 invasion and anti-CD147 antibody, such as meplazumab, could competitively inhibit the binding of S glycoproteins and CD147, preventing virus replication [73]. However, the efficacy of these products needs further exploration.
Treating COVID-19 with chloroquine and hydroxychloroquine
Research into safe, effective, and low-toxicity anti-SARS-CoV-2 agents is urgently needed. Wang et al. evaluated the antiviral effect of seven antiviral drugs in vitro, and demonstrated the preliminary efficacy of RDV [half-maximal effective concentration (EC50) = 0.77 μM] and CQ (EC50 = 1.13 μM) in inhibiting SARS-CoV-2 [76]. HCQ was also reported to be potentially effective in inhibiting SARS-CoV-2 infection in vitro [77].
Results from >100 patients in China showed that CQ was superior to the control group in alleviating the exacerbation of pneumonia, improving pulmonary images, increasing the negative conversion of the virus, and reducing the disease course [78]. Gautreta et al. indicated that HCQ combined with azithromycin caused viral load reduction or disappearance among 36 patients in an open-label nonrandomized clinical trial [79]. Nevertheless, some scientists cast doubt on the efficacy of HCQ because of the study limitations, including small sample size and short-term follow-up [80].
Recently, a 2019-nCoVr model was designed to help develop therapies and vaccines against SARS-CoV-2. Mefloquine hydrochloride, a quinoline derivative, presented potential therapeutic effects in this model and a clinical trial (NCT04347031) was initiated to study its off-label use for the treatment of COVID-19 [81].
In a multinational registry analysis, 96 032 patients with COVID-19 were assessed, of whom 14 888 received CQ or HCQ with a macrolide treatment. Results showed that all four regimens increased the risk of de novo ventricular arrhythmias and in-hospital mortality, but the study was retracted on June 5, 2020 [82]. Based on the existing scientific data with no benefit on mortality or in speeding recovery, the US Food and Drug Administration (FDA) revoked the emergency use authorization for CQ and HCQ on June 15, 2020. WHO discontinued the Solidarity Trial of HCQ and lopinavir/ritonavir (LPV/RTV) arms on July 4, 2020, because these interim trial results indicated that both they produced little or no reduction in mortality of patients hospitalized with COVID-19 when compared with standard care. There is an urgent need for additional high-quality randomized clinical trials to demonstrate the efficacy of CQ and HCQ in treating COVID-19.
Several clinical trials are underway using CQ and HCQ for the treatment of COVID-19. As of July 12, 2020, there are more than 2500 ongoing clinical trials on ClinicalTrials.gov investigating potential therapeutic options for the prevention and/or treatment of COVID-19, including 80 clinical trials with CQ interventions and 239 with HCQ interventions.
Prudent use of chloroquine and hydroxychloroquine
CQ and HCQ have become the focus of the global scientific community because of promising results in some in vitro studies or clinical trials. However, indiscriminate promotion and widespread deployment of CQ in Africa for COVID-19 have led to extensive shortages and increased market prices, which hindered the treatment of patients with malaria [83]. Although the safety of CQ and HCQ has been well established in the treatment of malaria or auto-immune disease, patients with COVID-19 might be more susceptible to adverse effects due to their older age and complications such as diabetes, obesity, and cardiovascular diseases, as well as prevalent polypharmacy [84].
According to the investigation of cardiac adverse drug reactions by the network of French pharmacovigilance centers, there are several unknown consequences with off-label use of CQ, HCQ, LPV/RTV, and azithromycin in COVID-19. As a result, 120 reports of cardiac adverse drug reactions had been notified in a month, most of which were associated with HCQ alone (86%) or combined with azithromycin (60%) [85]. CQ and HCQ might cause QTc prolongation and sodium-channel inhibition, leading to ventricular arrhythmias and cardiovascular collapse, and the symptoms described early are exacerbated when combined with other QTc prolonging agents, such as azithromycin [86]. Another clinical trial demonstrated that patients with COVID-19 treated with CQ showed a gradually increasing QTc interval [87]. Thus, an initial cardiac evaluation is needed for patients before administering CQ or HCQ against SARS-CoV-2 in the clinic.
A recommended dose of CQ for adults (>50 kg) is 1000 mg/day for 7 days as well as for adults (<50 kg) 1000 mg/day for first 2 days and 500 mg/day for 5 days. The common dosage regimen of HCQ in clinical trials is 400 mg for 5 days or 600 mg for 10 days [88]. These dose regimens are notably longer than the therapeutic schedule for malaria or RA. Both CQ and HCQ have a large volume of distribution and a long elimination half-life, resulting in a tendency to accumulate in metabolic organs and tissues at a higher level compared with the plasma concentration [89]. Therefore, pharmacological changes and dose-related adverse effects must be closely monitored and long term follow-up is of great necessity.
Concluding remarks
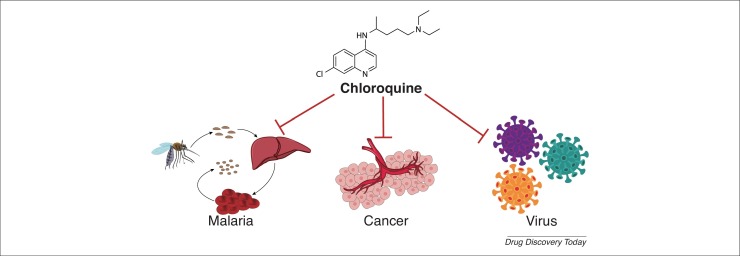
In summary, as a marketed old drug with known pharmacokinetics, the safety of CQ in a range of populations is controllable and certifiable. In addition to its antimalarial, anticancer, and antiviral effects summarized here (Fig. 4 ), CQ also exhibits promising therapeutic potential in autoimmune diseases, metabolic disorders, cardiovascular diseases, and neurodegenerative diseases, with mechanisms of toxin inhibition, modification of melanin synthesis, and anti-inflammation. CQ and its derivatives increase the pH in lysosomes because of the existence of an alkaline quinoline ring, leading to the accumulation of free hematin, which kills parasites. The adjuvant anticancer action of CQ is closely related to its autophagic inhibition and tumor vascular normalization. Its lysosomotropic and immunomodulatory properties allow CQ to suppress viral infection and replication. Further studies are needed on the exact mechanisms of action of CQ. Although several antiviral agents, including CQ and HCQ, have been evaluated for the treatment of COVID-19, the small sample size of clinical evidence and cardiotoxic adverse effects hinder their demonstratable efficacy. Thus, additional studies are urgently needed to define the roles and mechanisms of CQ and related drugs for managing COVID-19.
Figure 4.
The roles of chloroquine (CQ) in malaria, cancer, and viral diseases.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (U1903126, 81773888 and 81872901) and the Guangdong Basic and Applied Basic Research Foundation (2020A1515010005 and 2020A1515010605).
References
- 1.Thomé R., et al. Chloroquine: modes of action of an undervalued drug. Immunol. Lett. 2013;153(1):50–57. doi: 10.1016/j.imlet.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Al-Bari M.A.A. Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases. Pharmacol. Res. Perspect. 2017;5(1):e00293. doi: 10.1002/prp2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y., et al. The utility of chloroquine in cancer therapy. Curr. Med. Res. Opin. 2015;31(5):1009–1013. doi: 10.1185/03007995.2015.1025731. [DOI] [PubMed] [Google Scholar]
- 4.Aguiar A.C.C., et al. Chloroquine analogs as antimalarial candidates with potent in vitro and in vivo activity. Int. J. Parasitol. Drugs Drug Resist. 2018;8(3):459–464. doi: 10.1016/j.ijpddr.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Titus E.O. Recent developments in the understanding of the pharmacokinetics and mechanism of action of chloroquine. Ther. Drug Monit. 1989;11(4):369–379. [PubMed] [Google Scholar]
- 6.E.P, et al. Antimalarial 4-aminoquinolines: mode of action and pharmacokinetics. Fundam. Clin. Pharmacol. 1994;8(1):1–17. doi: 10.1111/j.1472-8206.1994.tb00774.x. [DOI] [PubMed] [Google Scholar]
- 7.Verbaanderd C., et al. Repurposing Drugs in Oncology (ReDO)-chloroquine and hydroxychloroquine as anti-cancer agents. E-cancer Med. Sci. 2017;11:781. doi: 10.3332/ecancer.2017.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Law I., et al. Transfer of chloroquine and desethylchloroquine across the placenta and into milk in Melanesian mothers. Br. J. Clin. Pharmacol. 2008;65(5):674–679. doi: 10.1111/j.1365-2125.2008.03111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blignaut M., et al. Revisiting the cardiotoxic effect of chloroquine. Cardiovasc. Drugs Ther. 2019;33(1):1–11. doi: 10.1007/s10557-018-06847-9. [DOI] [PubMed] [Google Scholar]
- 10.Knox J.M., et al. The chloroquine mystery: including antimalarial agents in general. Arch. Dermatol. 1966;94(2):205–214. [PubMed] [Google Scholar]
- 11.Mushtaque M., et al. Reemergence of chloroquine (CQ) analogs as multi-targeting antimalarial agents: a review. Eur. J. Med. Chem. 2015;90:280–295. doi: 10.1016/j.ejmech.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 12.WHO . WHO; 2018. World Malaria Report 2018. [Google Scholar]
- 13.Verma R., et al. Malaria vaccine can prevent millions of deaths in the world. Hum. Vaccin. Immunother. 2013;9(6):1268–1271. doi: 10.4161/hv.23950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Commons R.J., et al. The effect of chloroquine dose and primaquine on Plasmodium vivax recurrence: a WorldWide Antimalarial Resistance Network systematic review and individual patient pooled meta-analysis. Lancet Infect. Dis. 2018;18(9):1025–1034. doi: 10.1016/S1473-3099(18)30348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu Y., et al. Quinoline hybrids and their antiplasmodial and antimalarial activities. Eur. J. Med. Chem. 2017;139:22–47. doi: 10.1016/j.ejmech.2017.07.061. [DOI] [PubMed] [Google Scholar]
- 16.Pelfrene E., et al. Artemisinin-based combination therapy in the treatment of uncomplicated malaria: review of recent regulatory experience at the European Medicines Agency. Int. Health. 2015;7(4):239–246. doi: 10.1093/inthealth/ihv017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Popovici J., et al. Therapeutic and transmission-blocking efficacy of dihydroartemisinin/piperaquine and chloroquine against Plasmodium vivax malaria, Cambodia. Emerg. Infect. Dis. 2018;24(8):1516–1519. doi: 10.3201/eid2408.170768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raobela O., et al. Efficacy of artesunate–amodiaquine in the treatment of falciparum uncomplicated malaria in Madagascar. Malar. J. 2018;17(1):284. doi: 10.1186/s12936-018-2440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurth F., et al. Pyronaridine-artesunate combination therapy for the treatment of malaria. Curr. Opin. Investig. Drugs. 2011;24(6):564–569. doi: 10.1097/QCO.0b013e32834cabdb. [DOI] [PubMed] [Google Scholar]
- 20.Ayalew M.B. Therapeutic efficacy of artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in Ethiopia: a systematic review and meta-analysis. Infect. Dis. Poverty. 2017;6(1):157. doi: 10.1186/s40249-017-0372-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milner D.A., Jr. Malaria pathogenesis. CSH Perspect. Med. 2018;8(1):a025569. doi: 10.1101/cshperspect.a025569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skrzypek R., et al. The ‘pushmi-pullyu’ of resistance to chloroquine in malaria. Essays Biochem. 2017;61(1):167–175. doi: 10.1042/EBC20160060. [DOI] [PubMed] [Google Scholar]
- 23.Kaur K., et al. Quinolines and structurally related heterocycles as antimalarials. Eur. J. Med. Chem. 2010;45(8):3245–3264. doi: 10.1016/j.ejmech.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Lee A.H., et al. DNA repair mechanisms and their biological roles in the malaria parasite Plasmodium falciparum. Microbiol. Mol. Biol. Rev. 2014;78(3):469–486. doi: 10.1128/MMBR.00059-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slater A.F.G. Chloroquine: mechanism of drug action and resistance in plasmodium falciparum. Pharmacol. Ther. 1993;57(2):203–235. doi: 10.1016/0163-7258(93)90056-j. [DOI] [PubMed] [Google Scholar]
- 26.Wellems T.E., et al. Chloroquine-resistant malaria. J. Infect. Dis. 2001;184(6):770–776. doi: 10.1086/322858. [DOI] [PubMed] [Google Scholar]
- 27.Huang W., et al. Exosomes with low miR-34c-3p expression promote invasion and migration of non-small cell lung cancer by upregulating integrin α2β1. Signal. Transduct. Target Ther. 2020;5(1):39. doi: 10.1038/s41392-020-0133-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishida J., et al. Repurposing of approved cardiovascular drugs. J. Transl. Med. 2016;14:269. doi: 10.1186/s12967-016-1031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.García-Serradilla M., et al. Drug repurposing for new, efficient, broad spectrum antivirals. Virus Res. 2019;264:22–31. doi: 10.1016/j.virusres.2019.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burikhanov R., et al. Chloroquine-inducible Par-4 secretion is essential for tumor cell apoptosis and inhibition of metastasis. Cell Rep. 2017;18(2):508–519. doi: 10.1016/j.celrep.2016.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnaout A., et al. A randomized, double-blind, window of opportunity trial evaluating the effects of chloroquine in breast cancer patients. Breast Cancer Res. Treat. 2019;178(2):327–335. doi: 10.1007/s10549-019-05381-y. [DOI] [PubMed] [Google Scholar]
- 32.Gallagher L.E., et al. Lysosomotropism depends on glucose: a chloroquine resistance mechanism. Cell Death Dis. 2017;8(8):e3014. doi: 10.1038/cddis.2017.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu R., et al. The clinical value of using chloroquine or hydroxychloroquine as autophagy inhibitors in the treatment of cancers: a systematic review and meta-analysis. Medicine (Baltimore) 2018;97(46):e12912. doi: 10.1097/MD.0000000000012912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panagiotaki K.N., et al. A triphenylphosphonium-functionalized mitochondriotropic nanocarrier for efficient co-delivery of doxorubicin and chloroquine and enhanced antineoplastic activity. Pharmaceuticals (Basel) 2017;10(4):91. doi: 10.3390/ph10040091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun J., et al. Co-delivery nanoparticles of doxorubicin and chloroquine for improving the anti-cancer effect in vitro. Nanotechnology. 2019;30(1361–6528):085101. doi: 10.1088/1361-6528/aaf51b. [DOI] [PubMed] [Google Scholar]
- 36.Bishop E., et al. Autophagy modulation: a prudent approach in cancer treatment? Cancer Chemother. Pharmacol. 2018;82(6):913–922. doi: 10.1007/s00280-018-3669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levy J.M.M., et al. Targeting autophagy in cancer. Nat. Rev. Cancer. 2017;17(9):528–542. doi: 10.1038/nrc.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawabata T., et al. Autophagosome biogenesis and human health. Cell Discov. 2020;6:33. doi: 10.1038/s41421-020-0166-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu Y., et al. The role of autophagy in colitis-associated colorectal cancer. Signal Transduct. Target Ther. 2018;3:31. doi: 10.1038/s41392-018-0031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kimura T., et al. Chloroquine in cancer therapy: a double-edged sword of autophagy. Cancer Res. 2013;73(1):3. doi: 10.1158/0008-5472.CAN-12-2464. [DOI] [PubMed] [Google Scholar]
- 41.Ou C., et al. Chloroquine promotes gefitinib-induced apoptosis by inhibiting protective autophagy in cutaneous squamous cell carcinoma. Mol. Med. Rep. 2019;20(6):4855–4866. doi: 10.3892/mmr.2019.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo Z., et al. Anti-cancer effects of CQBTO, a chloroquine, and benzo(e)triazine oxide conjugate. Chem. Biol. Drug Des. 2019;93(5):874–882. doi: 10.1111/cbdd.13477. [DOI] [PubMed] [Google Scholar]
- 43.Mandhair H.K., et al. Molecular modulation of autophagy: new venture to target resistant cancer stem cells. World J. Stem Cells. 2020;12(5):303–322. doi: 10.4252/wjsc.v12.i5.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Viry E., et al. Autophagy: an adaptive metabolic response to stress shaping the antitumor immunity. Biochem. Pharmacol. 2014;92(1):31–42. doi: 10.1016/j.bcp.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 45.Varisli L., et al. Dissecting pharmacological effects of chloroquine in cancer treatment: interference with inflammatory signaling pathways. Immunology. 2020;159(3):257–278. doi: 10.1111/imm.13160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moballegh Nasery M., et al. Curcumin delivery mediated by bio-based nanoparticles: a review. Molecules. 2020;25(3):689. doi: 10.3390/molecules25030689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prasannan R., et al. Key cell signaling pathways modulated by zerumbone: role in the prevention and treatment of cancer. Biochem. Pharmacol. 2012;84(10):1268–1276. doi: 10.1016/j.bcp.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 48.Siveen K.S., et al. Thymoquinone overcomes chemoresistance and enhances the anticancer effects of bortezomib through abrogation of NF-κB regulated gene products in multiple myeloma xenograft mouse model. Oncotarget. 2014;5(3):634–648. doi: 10.18632/oncotarget.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rajendran P., et al. Honokiol inhibits signal transducer and activator of transcription-3 signaling, proliferation, and survival of hepatocellular carcinoma cells via the protein tyrosine phosphatase SHP-1. J. Cell. Physiol. 2012;227(5):2184–2195. doi: 10.1002/jcp.22954. [DOI] [PubMed] [Google Scholar]
- 50.Chen Q., et al. The independence of and associations among apoptosis, autophagy, and necrosis. Signal Transduct. Target Ther. 2018;3:18. doi: 10.1038/s41392-018-0018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cortegiani A., et al. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J. Crit. Care. 2020;57:279–283. doi: 10.1016/j.jcrc.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amaravadi R.K., et al. Principles and current strategies for targeting autophagy for cancer treatment. Clin. Cancer Res. 2011;17(4):654. doi: 10.1158/1078-0432.CCR-10-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pascolo S. Time to use a dose of chloroquine as an adjuvant to anti-cancer chemotherapies. Eur. J. Pharmacol. 2016;771:139–144. doi: 10.1016/j.ejphar.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 54.Eng C.H., et al. Macroautophagy is dispensable for growth of KRAS mutant tumors and chloroquine efficacy. Proc. Natl. Acad. Sci. U. S. A. 2016;113(1):182–187. doi: 10.1073/pnas.1515617113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Viallard C., et al. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis. 2017;20(4):409–426. doi: 10.1007/s10456-017-9562-9. [DOI] [PubMed] [Google Scholar]
- 56.Li Q., et al. Chloroquine inhibits tumor growth and angiogenesis in malignant pleural effusion. Tumour Biol. 2016;37(12):16249–16258. doi: 10.1007/s13277-016-5441-z. [DOI] [PubMed] [Google Scholar]
- 57.Maes H., et al. Chloroquine anticancer activity is mediated by autophagy-independent effects on the tumor vasculature. Mol. Cell Oncol. 2016;3(1):e970097. doi: 10.4161/23723548.2014.970097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maes H., et al. Tumor vessel normalization by chloroquine independent of autophagy. Cancer Cell. 2014;26(2):190–206. doi: 10.1016/j.ccr.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 59.Kumberger P., et al. Multiscale modeling of virus replication and spread. FEBS Lett. 2016;590(13):1972–1986. doi: 10.1002/1873-3468.12095. [DOI] [PubMed] [Google Scholar]
- 60.Yao X., et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020;71:732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Savarino A., et al. Effects of chloroquine on viral infections: an old drug against today's diseases. Lancet Infect. Dis. 2003;3(11):722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang S., et al. Chloroquine inhibits endosomal viral RNA release and autophagy-dependent viral replication and effectively prevents maternal to fetal transmission of Zika virus. Antiviral Res. 2019;169:104547. doi: 10.1016/j.antiviral.2019.104547. [DOI] [PubMed] [Google Scholar]
- 63.Li C., et al. Saikosaponin D suppresses enterovirus A71 infection by inhibiting autophagy. Signal. Transduct. Target Ther. 2019;4(1):4. doi: 10.1038/s41392-019-0037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shiryaev S.A., et al. Repurposing of the anti-malaria drug chloroquine for Zika Virus treatment and prophylaxis. Sci. Rep. 2017;7(1):15771. doi: 10.1038/s41598-017-15467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roques P., et al. Paradoxical effect of chloroquine treatment in enhancing Chikungunya virus infection. Viruses. 2018;10(5):268. doi: 10.3390/v10050268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Diebold S.S., et al. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303(5663):1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 67.Dowall S.D., et al. Chloroquine inhibited Ebola virus replication in vitro but failed to protect against infection and disease in the in vivo guinea pig model. J. Gen. Virol. 2015;96(12):3484–3492. doi: 10.1099/jgv.0.000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Akpovwa H. Chloroquine could be used for the treatment of filoviral infections and other viral infections that emerge or emerged from viruses requiring an acidic pH for infectivity. Cell Biochem. Funct. 2016;34(4):191–196. doi: 10.1002/cbf.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lima L.T., et al. Improving encapsulation of hydrophilic chloroquine diphosphate into biodegradable nanoparticles: a promising approach against Herpes Virus simplex-1 infection. Pharmaceutics. 2018;10(4):255. doi: 10.3390/pharmaceutics10040255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chauhan A., et al. The enigma of the clandestine association between chloroquine and HIV-1 infection. HIV Med. 2015;16(10):585–590. doi: 10.1111/hiv.12295. [DOI] [PubMed] [Google Scholar]
- 71.WHO . WHO; 2020. Coronavirus disease 2019 (COVID-19) Situation Report. [Google Scholar]
- 72.Guo Y., et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Mil. Med. Res. 2020;7(1):11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang K., et al. 2020. SARS-CoV-2 invades host cells via a novel route: CD147-Spike protein BioRxiv. (published March 14, 2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hoffmann M., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev. Res. 2020;81:537–540. doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang M., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu J., et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gao J., et al. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 79.Gautret P., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;56:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mégarbane B. Chloroquine and hydroxychloroquine to treat COVID-19: between hope and caution. Clin. Toxicol. (Phila). 2020 doi: 10.1080/15563650.2020.1748194. (published online April 2, 2020) [DOI] [PubMed] [Google Scholar]
- 81.Fan H.H., et al. Repurposing of clinically approved drugs for treatment of Coronavirus Disease 2019 in a 2019-novel coronavirus-related coronavirus model. Chin. Med. J. (Engl.) 2020;133(9):1051–1056. doi: 10.1097/CM9.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mehra M.R., et al. RETRACTED: hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020 doi: 10.1016/S0140-6736(20)31180-6. Published online May 22, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 83.Abena P.M., et al. Chloroquine and hydroxychloroquine for the prevention or treatment of Novel Coronavirus Disease (COVID-19) in Africa: caution for inappropriate off-label use in healthcare settings. Am. J. Trop. Med. Hyg. 2020;102(6):1184–1188. doi: 10.4269/ajtmh.20-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gevers S., et al. Safety considerations for chloroquine and hydroxychloroquine in the treatment of COVID-19. Clin. Microbiol. Infect. 2020;26:1276–1277. doi: 10.1016/j.cmi.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gérard A., et al. ‘Off-label’ use of hydroxychloroquine, azithromycin, lopinavir-ritonavir and chloroquine in COVID-19: a survey of cardiac adverse drug reactions by the French Network of Pharmacovigilance Centers. Therapie. 2020;75:371–379. doi: 10.1016/j.therap.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gupta N., et al. Chloroquine in COVID-19: the evidence. Monaldi Arch. Chest Dis. 2020;90:1. doi: 10.4081/monaldi.2020.1290. [DOI] [PubMed] [Google Scholar]
- 87.Sinkeler F.S., et al. The risk of QTc-interval prolongation in COVID-19 patients treated with chloroquine. Neth. Heart J. 2020;28:418–423. doi: 10.1007/s12471-020-01462-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pastick K.A., et al. Review: hydroxychloroquine and chloroquine for treatment of SARS-CoV-2 (COVID-19) Open Forum Infect. Dis. 2020;7(4) doi: 10.1093/ofid/ofaa130. ofaa130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wong Y.K., et al. Caution and clarity required in the use of chloroquine for COVID-19. Lancet Rheumatol. 2020;2(5):e255. doi: 10.1016/S2665-9913(20)30093-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu S., et al. Chloroquine exerts antitumor effects on NB4 acute promyelocytic leukemia cells and functions synergistically with arsenic trioxide. Oncol. Lett. 2018;15(2):2024–2030. doi: 10.3892/ol.2017.7488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gao L., et al. Histone deacetylase inhibitor trichostatin A and autophagy inhibitor chloroquine synergistically exert anti-tumor activity in H-ras transformed breast epithelial cells. Mol. Med. Rep. 2018;17(3):4345–4350. doi: 10.3892/mmr.2018.8446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ou C., et al. Chloroquine promotes gefitinib-induced apoptosis by inhibiting protective autophagy in cutaneous squamous cell carcinoma. Mol. Med. Rep. 2019;20(6):4855–4866. doi: 10.3892/mmr.2019.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ke X., et al. Heterogeneous responses of gastric cancer cell lines to Tenovin-6 and synergistic effect with chloroquine. Cancers. 2020;12(2):365. doi: 10.3390/cancers12020365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim M., et al. Combination therapy with a PI3K/mTOR dual inhibitor and chloroquine enhances synergistic apoptotic cell death in Epstein-Barr Virus-infected gastric cancer cells. Mol. Cells. 2019;42(6):448–459. doi: 10.14348/molcells.2019.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Helmy S.A., et al. Chloroquine upregulates TRAIL/TRAILR2 expression and potentiates doxorubicin anti-tumor activity in thioacetamide-induced hepatocellular carcinoma model. Chem. Biol. Interact. 2018;279:84–94. doi: 10.1016/j.cbi.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 96.Ryabaya O.O., et al. Autophagy inhibitors chloroquine and LY294002 enhance temozolomide cytotoxicity on cutaneous melanoma cell lines in vitro. Anticancer Drugs. 2017;28(3):307–315. doi: 10.1097/CAD.0000000000000463. [DOI] [PubMed] [Google Scholar]
- 97.Shani A.P., et al. Combining chloroquine with RAD001 inhibits tumor growth in a NEN mouse model. Endocr. Relat. Cancer. 2018;25(6):677–686. doi: 10.1530/ERC-18-0121. [DOI] [PubMed] [Google Scholar]
- 98.Aga T., et al. Inhibition of autophagy by chloroquine makes chemotherapy in nasopharyngeal carcinoma more efficient. Auris Nasus Larynx. 2019;46(3):443–450. doi: 10.1016/j.anl.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 99.Datta S., et al. Autophagy inhibition with chloroquine reverts paclitaxel resistance and attenuates metastatic potential in human nonsmall lung adenocarcinoma A549 cells via ROS mediated modulation of β-catenin pathway. Apoptosis. 2019;24(5):414–433. doi: 10.1007/s10495-019-01526-y. [DOI] [PubMed] [Google Scholar]
- 100.Yin P., et al. ABT-737, a Bcl-2 selective inhibitor, and chloroquine synergistically kill renal cancer cells. Oncol. Res. 2016;24(1):65–72. doi: 10.3727/096504016X14587366983838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Grimaldi A., et al. Antagonistic effects of chloroquine on autophagy occurrence potentiate the anticancer effects of everolimus on renal cancer cells. Cancer Biol. Ther. 2015;16(4):567–579. doi: 10.1080/15384047.2015.1018494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fleisher B., et al. Chloroquine sensitizes MDA-MB-231 cells to osimertinib through autophagy-apoptosis crosstalk pathway. Breast Cancer (Dove Med Press) 2019;11:231–241. doi: 10.2147/BCTT.S211030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lin S., et al. Pituitary tumor suppression by combination of cabergoline and chloroquine. J. Clin. Endocrinol. Metab. 2017;102(10):3692–3703. doi: 10.1210/jc.2017-00627. [DOI] [PubMed] [Google Scholar]
- 104.Chou H., et al. Combination therapy of chloroquine and C2-ceramide enhances cytotoxicity in lung cancer H460 and H1299 cells. Cancers. 2019;11(3):370. doi: 10.3390/cancers11030370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lv X., et al. Honokiol exhibits enhanced antitumor effects with chloroquine by inducing cell death and inhibiting autophagy in human non-small cell lung cancer cells. Oncol. Rep. 2015;34(3):1289–1300. doi: 10.3892/or.2015.4091. [DOI] [PubMed] [Google Scholar]
- 106.Tang M., et al. Chloroquine enhances gefitinib cytotoxicity in gefitinib-resistant nonsmall cell lung cancer cells. PLoS ONE. 2015;10(3):e0119135. doi: 10.1371/journal.pone.0119135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhu J., et al. Low concentration of chloroquine enhanced efficacy of cisplatin in the treatment of human ovarian cancer dependent on autophagy. Am. J. Transl. Res. 2017;9(9):4046–4058. [PMC free article] [PubMed] [Google Scholar]
- 108.Wang H., et al. Gambogic acid induces autophagy and combines synergistically with chloroquine to suppress pancreatic cancer by increasing the accumulation of reactive oxygen species. Cancer Cell Int. 2019;19:7. doi: 10.1186/s12935-018-0705-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hu J., et al. ROS-mediated activation and mitochondrial translocation of CaMKII contributes to Drp1-dependent mitochondrial fission and apoptosis in triple-negative breast cancer cells by isorhamnetin and chloroquine. J. Exp. Clin. Cancer Res. 2019;38(1):225. doi: 10.1186/s13046-019-1201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]