Abstract
The muscle stem cells of domestic animals are of interest to researchers in the food and biotechnology industries for the production of cultured meat. For producing cultured meat, it is crucial for muscle stem cells to be efficiently isolated and stably maintained in vitro on a large scale. In the present study, we aimed to optimize the method for the enrichment of pig muscle stem cells using a magnetic-activated cell sorting (MACS) system. Pig muscle stem cells were collected from the biceps femoris muscles of 14 d-old pigs of three breeds [Landrace×Yorkshire×Duroc (LYD), Berkshire, and Korean native pigs] and cultured in skeletal muscle growth medium-2 (SkGM-2) supplemented with epidermal growth factor (EGF), dexamethasone, and a p38 inhibitor (SB203580). Approximately 30% of total cultured cells were nonmyogenic cells in the absence of purification in our system, as determined by immunostaining for cluster of differentiation 56 (CD56) and CD29, which are known markers of muscle stem cells. Interestingly, following MACS isolation using the CD29 antibody, the proportion of CD56+/CD29+ muscle stem cells was significantly increased (91.5±2.40%), and the proportion of CD56 single-positive nonmyogenic cells was dramatically decreased. Furthermore, we verified that this method worked well for purifying muscle stem cells in the three pig breeds. Accordingly, we found that CD29 is a valuable candidate among the various marker genes for the isolation of pig muscle stem cells and developed a simple sorting method based on a single antibody to this protein.
Keywords: pig, muscle stem cells, purification, magnetic-activated cell sorting (MACS), cluster of differentiation 29 (CD29)
Introduction
Muscle stem cells reside beneath the basal lamina of myofibers and are responsible for the regeneration of muscle tissues (Motohashi et al., 2012). The muscle stem cells have been used for studies on muscle physiology and regeneration and recently have been considered as an important candidate for the production of cultured meat in domestic animals (Post, 2012). Because the in vivo niche of muscle stem cells is composed of various type of tissues and cells, such as muscle fibers, connective tissues, and stromal cells, as well as various stem cell populations, the isolation of muscle stem cells from muscle tissues requires sequential steps (Liu et al., 2015). First, the dissected muscle tissues from the animals are dissociated into single cells using proteinases such as trypsin, pronase, and collagenase. The digested tissues are filtered with a cell strainer to separate the dissociated single cells from tissue debris and myofibers. The resulting cell population contains various types of cells such as somatic cells, blood cells, stromal cells, and muscle stem cells. Therefore, various sorting approaches have been developed to obtain highly purified muscle stem cells based on their physical, biological, and molecular features.
Density gradient centrifugation and preplating are widely used methods for sorting muscle stem cells because no special devices are required. The density gradient centrifugation separates cells based on their density. Because the muscle stem cells and other somatic cells have different densities, the stem cells can be isolated from the mixed populations via centrifugation using a solution with a density gradient made of dense substrates (Bischoff, 1997). Because muscle stem cells and fibroblasts prefer laminin and collagen as an adherent niche, respectively (Kühl et al., 1986), the preplating technique divides the cell populations using this difference in adhering ability onto the culture plate or the substrates. At 40–60 min after seeding on the collagen-coated culture plate, the stem cell population can be obtained by harvesting the supernatant, since most of the fibroblasts and epithelial cells remain attached to the culture plate (Rando and Blau, 1994; Richler and Yaffe, 1970). However, density gradient centrifugation and the preplating technique reportedly show wide variations and low fidelities (Ding et al., 2017). Advances in molecular biology allow us to analyze and separate the cells based on their molecular features. Fluorescence-activated cell sorting (FACS) and magnetic-activated cell sorting (MACS) systems isolate the muscle stem cells using fluorescence and magnetic microbead-conjugated antibodies against the marker genes of the stem cells, respectively (Blanco-Bose et al., 2001; Liu et al., 2015). FACS and MACS are considered to be more precise methods for isolating muscle stem cells compared to the aforementioned approaches (Ding et al., 2017).
Every cell in the body has its markers that it exclusively expresses compared to other cells, and FACS and MACS analyze and sort the cells through a recognition of markers using antibodies. To date, various markers, including cluster of differentiation 29 (CD29; integrin β1), CD34, CD56 (neural cell adhesion molecule, NCAM), C-X-C chemokine receptor 4 (CXCR4), vascular cell adhesion molecule (VCAM), integrin α7, and SM/C-2.6, have been used for the sorting of muscle stem cells (Liu et al., 2015). Both antibody-based methods have shown a consistently high efficiency for isolating muscle stem cells. While FACS allows us to conduct a more precise analysis using flow cytometry, MACS especially is relatively less harmful to the cells during a sorting procedure and is more suitable for scale-up. For producing cultured meat, it is crucial for muscle stem cells to be efficiently isolated and stably maintained in vitro at a large scale. In a previous study, we optimized the in vitro culture conditions to maintain the stemness of pig muscle stem cells for an expanded period (Choi et al., 2020). For the purification of pig muscle stem cells, the density gradient centrifugation and preplating techniques have been widely used in pig studies. However, only a few protocols using FACS and MACS for pig muscle stem cells have been reported (Ding et al., 2017). Accordingly, in the present study, we aimed to develop a scalable method for the enrichment of pig muscle stem cells using the MACS system.
Materials and Methods
Animal care
The care and experimental use of pigs were approved by the Institutional Animal Care and Use Committee (IACUC) at Seoul National University (approval no. SNU-180612-2). The experiments were conducted according to the standard protocol of the Institute of Laboratory Animal Resources at Seoul National University.
Isolation and culture of pig muscle stem cells
Pig muscle stem cells were isolated from the biceps femoris muscle of 14 d-old crossbred (Landrace×Yorkshire×Duroc, LYD), Berkshire, and Korean native pigs, which were euthanized through CO2 inhalation and exsanguination. The biceps femoris muscles were collected and washed with Dulbecco’s phosphate-buffered saline (DPBS; Welgene, Gyeongsan, Korea) containing 2×antibiotic-antimycotic (AA; Gibco, Gaithersburg, MD, USA), after which the excessive connective tissues and blood vessels were removed. The 30 g of collected tissues was minced by a meat grinder and digested with 0.8 mg/mL Pronase (Sigma-Aldrich, St. Louis, MO, USA) for 40 min at 37°C with vortexing every 10 min. The resultant mixture was harvested by centrifugation at 1,200×g for 15 min and resuspended in minimum essential medium (MEM) containing 10% fetal bovine serum (FBS, Gibco). For separation of the undigested tissues from the digested cells containing the muscle stem cell population, the digested muscle tissues were centrifuged at 300×g for 5 min and the supernatant was collected. The supernatant was filtered by a 100 μm cell strainer and harvested by centrifugation at 1,200×g for 15 min. The resulting cells were cryopreserved in 10 vials with MEM containing 10% FBS and 10% dimethyl sulfoxide (DMSO) until subsequent use.
The cryopreserved muscle stem cells were thawed and cultured on Matrigel-coated dishes (MatrixTM; SPL Life Science, Pocheon, Korea) in basic GM consisting of the Skeletal Muscle Cell Growth Medium-2 BulletKitTM (SkGM-2, Lonza, Basel, Switzerland) supplemented with 20 μM SB203580 (Cayman Chemical, Ann Arbor, MI, USA) as described previously (Choi et al., 2020). The pig muscle stem cells were subcultured every 3 d. When the cells reached approximately 90% confluency, the cultured cells were dissociated using TrypLE™ Express (Gibco). These dissociated cells were transferred onto new Matrigel-coated culture dishes at a 1:10 split ratio. The medium was changed every 24 h, and the cells were cultured under humidified conditions in an atmosphere containing 5% CO2 at 37°C.
Myogenic differentiation of pig muscle stem cells
At three days after the subculture (four days in the case of the Korean native pig cells), the muscle stem cells at 90% confluency were used for myogenic differentiation. The cells were cultured in a differentiation media consisting of MEM containing 2% (v/v) horse serum (Biowest, Nuaillé, France), 1×GlutaMAX, 1×AA, and 0.1 mM β-mercaptoethanol (all purchased from Gibco) for 2 d without media changes (3 d in the case of the Korean native pig cells). After myofiber formation from the muscle stem cells was evident, the cells were fixed with 4% paraformaldehyde for further analysis.
Purification of pig muscle stem cells by magnetic-activated cell sorting (MACS)
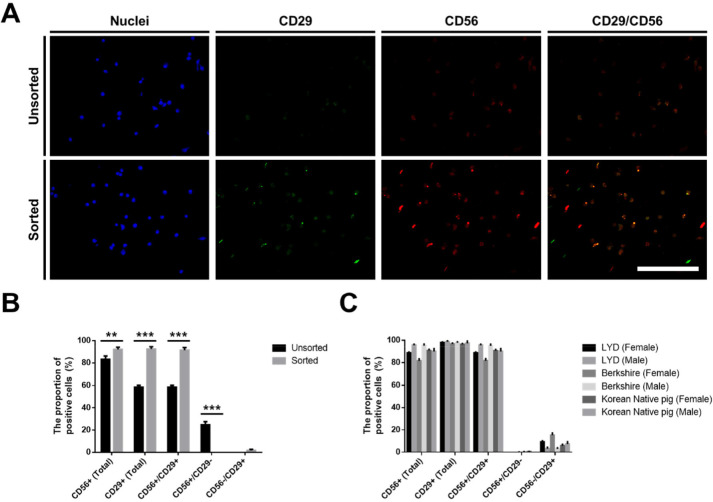
The CD29-positive pig muscle stem cells were sorted using a MACS Cell Separation System (Miltenyi Biotec, Bergisch Gladbach, Germany) to remove the nonmyogenic cells during the in vitro culture. When the cells reached approximately 90% confluency, the cultured cells were dissociated using TrypLE™ Express (Gibco). The dissociated pig stem cells were reacted with an anti-CD29 antibody (1:100; MAB17783, R&D Systems, Minneapolis, MN, USA) and anti-mouse IgG microbeads (1:5; Miltenyi Biotec). The CD29-positive cells were sorted on an MS column (capacity, 1×107 magnetically labeled cells; Miltenyi Biotec) according to the manufacturer’s instructions. The identification of the sorted CD29-positive cells as pig muscle stem cells was verified by immunostaining with CD29 and CD56 antibodies as described below (Fig. 1A).
Fig. 1. Purification of pig muscle stem cells by magnetic-activated cell sorting (MACS) using a CD29 antibody.
Muscle stem cells isolated from the biceps femoris muscle of 14 d-old LYD pigs were sorted by MACS using a CD29 antibody. (A) The expression pattern of CD29 and CD56 in pig muscle stem cells as determined by immunostaining. Blue, green, and red fluorescence represent nuclei, CD29, and CD56, respectively. (B) The proportion of CD29- and CD56-positive cells in pig muscle stem cells as measured by immunostaining. (C) The proportion of CD29- and CD56-positive cells following MACS sorting was measured by immunostaining in cells from the various breeds (LYD, Berkshire, and Korean native pigs). Data are represented as mean±SEM. The significance of the differences was determined between the unsorted and sorted groups. * p<0.05, ** p<0.01, and *** p<0.001. Scale bar=200 μm. LYD, Landrace×Yorkshire×Duroc; CD, cluster of differentiation.
Immunocytochemical staining
For immunocytochemical staining, the cell samples were preincubated for 10 min at 4°C and fixed in 4% (w/v) paraformaldehyde for 30 min. After washing twice with DPBS (Welgene), the samples were treated for 15 min with 0.2% (v/v) Triton X-100 (Sigma-Aldrich) and blocked for 1 h with 10% (v/v) goat serum in DPBS to prevent nonspecific binding. Serum-treated cells were incubated overnight at 4°C with primary antibodies against the following: CD29 (1:200; MAB17783, R&D Systems), CD56 (1:200; 710388, Thermo Fisher Scientific, Waltham, MA, USA), and myosin heavy chain (1:200; 05-716, Sigma-Aldrich). After incubation with the primary antibody, the cells were treated overnight at 4°C with the appropriate Alexa Fluor-conjugated secondary antibodies. The nuclei were stained with Hoechst 33342 (Molecular Probes, Eugene, OR, USA). Images of the stained cells were captured using an inverted fluorescence microscope (Eclipse TE2000-U, Nikon, Konan, Japan).
Statistical analysis
The data obtained in this study are presented as the mean±standard error of the mean (SEM) and were analyzed using Prism 6 software (GraphPad Software, San Diego, CA, USA). The significance of the differences was determined by two-way analyses of variance followed by Fisher’s least significant difference test. Differences were considered significant at p<0.05 (* p<0.05, ** p<0.01, and *** p<0.001 in the figure).
Results and Discussion
The purification process of pig muscle stem cells from other type of cells is required for studies on myogenesis and cultured meat production. Without purification, the muscle stem cells show a low myogenic potential, as shown by the myotube fusion rate during in vitro culture (Doumit and Merkel, 1992; Jeong et al., 2013). For these reasons, sorting methods have been applied for the enrichment of pig muscle stem cells in various studies. Density gradient centrifugation and preplating technique have been widely used in pig studies. The muscle stem cells, with a greater than 90% purity after sorting by density gradient centrifugation, expressed muscle stem cell marker genes such as NCAM (also known as CD56) and desmin, as determined by immunostaining (Mau et al., 2008; Mesires and Doumit, 2002; Perruchot et al., 2012). In addition, the purity of the myogenic cells was increased following preplating (Redshaw and Loughna, 2012; Redshaw et al., 2010). However, these methods reportedly have a wide variation and low fidelity for muscle stem cells (Ding et al., 2017). To solve these problems, sorting systems using cell surface markers, such as FACS and MACS, have been developed. In a pig study, Ding and colleagues attempted to separate the CD56+CD29+CD31–CD45– cell population from the dissociated muscle tissues using FACS, and the 94% of the sorted cells expressed PAX7, as determined by immunostaining (Ding et al., 2017). However, no MACS protocols for pig muscle stem cells have been reported.
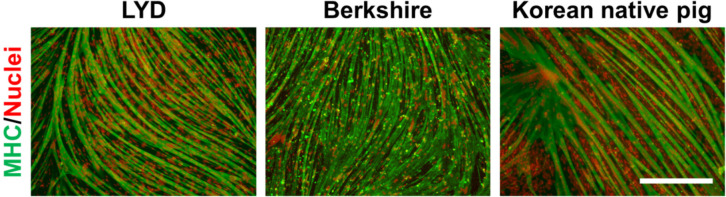
Previously, we optimized the in vitro culture conditions for maintaining the stemness of pig muscle stem cells (Choi et al., 2020). Unfortunately, approximately 30% of the nonmyogenic cells in the total cultured cells was observed without the purification process in our system, as determined by immunostaining for CD56 and CD29 (Fig. 1A and B), which are known as the marker genes in pig muscle stem cells, as well as in those of other mammals (Ding et al., 2017). Moreover, the unsorted cells showed low myogenic potential after differentiation induction (data not shown), which indicates that the development of the purification method is required along with the optimization of the culture conditions in pig muscle stem cells. In the present study, we attempted to find a surface marker for the enrichment of the cultured pig muscle stem cells using a MACS system. First, we conducted an experiment using muscle stem cells isolated from 14 d-old LYD pigs to find an optimized protocol. MACS is a cell sorting technique by which the cells tagged with magnetic microbead-conjugated antibodies against the cell surface protein can be isolated. The marker gene should be highly expressed in muscle stem cells exclusively for use in MACS. Immunostaining revealed that CD29 was exclusively expressed in the pig muscle stem cells while CD56 was expressed in most cells (Fig. 1A and B). Furthermore, CD56 single-positive cells represented approximately 25% of the unsorted cell population. For these reasons, we selected CD29 as a candidate for the purification of pig muscle stem cells using MACS. Interestingly, after MACS isolation using the CD29 antibody, the proportion of CD56+/CD29+ muscle stem cells was significantly increased (91.5±2.40%), and the proportion of CD56 single-positive nonmyogenic cells was dramatically decreased (Fig. 1B). Furthermore, we verified that this method can be applied to purify muscle stem cells from other breeds, including Berkshire and Korean native pigs (Fig. 1C). Finally, the cells sorted by the CD29 antibody represented those with the high differentiation potential, as determined by immunostaining of the myosin heavy chain (Fig. 2).
Fig. 2. The myogenic potential of pig muscle stem cells sorted by magnetic-activated cell sorting (MACS) using a CD29 antibody.
The myogenic ability of pig muscle stem cells purified by MACS was examined and defined using immunostaining of the myosin heavy chain (MHC) in cells from various breeds (Landrace×Yorkshire×Duroc (LYD), Berkshire, and Korean native pigs). Red and green fluorescence represent nuclei and MHC, respectively. Scale bar=400 μm. CD, cluster of differentiation.
Integrin is a transmembrane receptor, which plays an important role in adhesion onto extracellular matrix (ECM) proteins, thereby activating the intracellular signaling involved in cell proliferation and the development of various tissues (Guilak et al., 2009). In particular, CD29, also known as integrin β1 (ITGB1), recognizes laminin and participates in the myogenesis, adhesion and migration of myoblasts via heterodimerization with integrin α7 (Crawley et al., 1997; Schwander et al., 2003). In combination with FGF2, CD29 synergistically upregulates the mitogen-activated protein kinases (MAPK)/extracellular signal-regulated kinases (ERK) signaling pathway, thereby enhancing muscle regeneration in aged mice (Rozo et al., 2016). In addition, the genetic ablation of CD29 in muscle stem cells induces failure in the maintenance of the quiescent state and reduces the regenerative ability by the decline of proliferation (Rozo et al., 2016). Reportedly, CD29 is coexpressed in over 95% of mouse and human satellite cells with Pax7 (Bosnakovski et al., 2008; Xu et al., 2015) and is highly expressed in pig muscle stem cells as well (Ding et al., 2017), which indicates that CD29 is a crucial candidate for the purification of pig muscle stem cells. Our preliminary study showed that CD56 is also one of the important marker genes for muscle stem cells, but sorting using the CD56 antibody was not efficient for the enrichment of muscle stem cells, as in a previous study (Park et al., 2006). Although negative selections using CD90 (Thy1; fibroblast marker), CD31 (PECAM-1; endothelial cell marker), and CD45 (PTPRC; hematopoietic cell marker) were also examined, they were not suitable for the purification of pig muscle stem cells (data not shown). Accordingly, we found that CD29 is a valuable candidate among the various marker genes for the isolation of pig muscle stem cells and developed a simple sorting method based on a single antibody to this protein. In addition, this method could be applied for the removal of cells losing their myogenic potential during in vitro culturing. Finally, this method could be applied for the enrichment of pig muscle stem cells during their in vitro culture and isolation process and for the production of cultured meat because of its ease of scale up.
Acknowledgements
This work was supported by the BK21 Plus Program, the National Research Foundation of Korea (NRF) grant funded by the Korean government (NRF-2019R1C1C1004514), the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry, and Fisheries (IPET) through the Development of High Value-Added Food Technology Program funded by the Ministry of Agriculture, Food, and Rural Affairs (MAFRA; 118042-03).
Conflicts of Interest
The authors declare no potential conflicts of interest.
Author Contributions
Conceptualization: Choi KH, Kim M, Yoon JW, Jo C, Lee CK. Formal analysis: Choi KH, Kim M, Yoon JW. Methodology: Choi KH, Kim M, Yoon JW. Validation: Jeong J, Ryu M. Writing - original draft: Choi KH, Kim M. Writing - review & editing: Choi KH, Kim M, Yoon JW, Jeong J, Ryu M, Jo C, Lee CK.
Ethics Approval
The care and experimental use of pigs were approved by the Institutional Animal Care and Use Committee (IACUC) at Seoul National University (approval no. SNU-180612-2). The experiments were conducted according to the standard protocol of the Institute of Laboratory Animal Resources at Seoul National University.
References
- Bischoff R. Chemotaxis of skeletal muscle satellite cells. Dev Dyn. 1997;208:505–515. doi: 10.1002/(SICI)1097-0177(199704)208:4<505::AID-AJA6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Blanco-Bose WE, Yao CC, Kramer RH, Blau HM. Purification of mouse primary myoblasts based on α7 integrin expression. Exp Cell Res. 2001;265:212–220. doi: 10.1006/excr.2001.5191. [DOI] [PubMed] [Google Scholar]
- Bosnakovski D, Xu Z, Li W, Thet S, Cleaver O, Perlingeiro RCR, Kyba M. Prospective isolation of skeletal muscle stem cells with a pax7 reporter. Stem Cells. 2008;26:3194–3204. doi: 10.1634/stemcells.2007-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KH, Yoon JW, Kim M, Jeong J, Ryu M, Park S, Jo C, Lee CK. Optimization of culture conditions for maintaining pig muscle stem cells in vitro. Food Sci Anim Resour. 2020;40:659–667. doi: 10.5851/kosfa.2020.e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley S, Farrell EM, Wang W, Gu M, Huang HY, Huynh V, Hodges BL, Cooper DNW, Kaufman SJ. The α7β1 integrin mediates adhesion and migration of skeletal myoblasts on laminin. Exp Cell Res. 1997;235:274–286. doi: 10.1006/excr.1997.3671. [DOI] [PubMed] [Google Scholar]
- Ding S, Wang F, Liu Y, Li S, Zhou G, Hu P. Characterization and isolation of highly purified porcine satellite cells. Cell Death Discov. 2017;3:17003. doi: 10.1038/cddiscovery.2017.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doumit ME, Merkel RA. Conditions for isolation and culture of porcine myogenic satellite cells. Tissue Cell. 1992;24:253–262. doi: 10.1016/0040-8166(92)90098-R. [DOI] [PubMed] [Google Scholar]
- Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5:17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JY, Kim JM, Rajesh RV, Suresh S, Jang GW, Lee KT, Kim TH, Park M, Jeong HJ, Kim KW, Cho YM, Lee HJ. Comparison of gene expression levels of porcine satellite cells from postnatal muscle tissue during differentiation. Reprod Dev Biol. 2013;37:219–224. doi: 10.12749/RDB.2013.37.4.219. [DOI] [Google Scholar]
- Kühl U, Öcalan M, Timpl R, Von Der Mark K. Role of laminin and fibronectin in selecting myogenic versus fibrogenic cells from skeletal muscle cells in vitro. Dev Biol. 1986;117:628–635. doi: 10.1016/0012-1606(86)90331-3. [DOI] [PubMed] [Google Scholar]
- Liu L, Cheung TH, Charville GW, Rando TA. Isolation of skeletal muscle stem cells by fluorescence-activated cell sorting. Nat Protoc. 2015;10:1612–1624. doi: 10.1038/nprot.2015.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mau M, Oksbjerg N, Rehfeldt C. Establishment and conditions for growth and differentiation of a myoblast cell line derived from the semimembranosus muscle of newborn piglets. In Vitro Cell Dev Biol Anim. 2008;44:1–5. doi: 10.1007/s11626-007-9069-6. [DOI] [PubMed] [Google Scholar]
- Mesires NT, Doumit ME. Satellite cell proliferation and differentiation during postnatal growth of porcine skeletal muscle. Am J Physiol Cell Physiol. 2002;282:C899–906. doi: 10.1152/ajpcell.00341.2001. [DOI] [PubMed] [Google Scholar]
- Motohashi N, Alexander MS, Kunkel LM. Skeletal muscle regeneration and muscle progenitor cells. J Phys Fit Sports Med. 2012;1:151–154. doi: 10.7600/jpfsm.1.151. [DOI] [Google Scholar]
- Park YG, Moon JH, Kim J. A comparative study of magnetic-activated cell sorting, cytotoxicity and preplating for the purification of human myoblasts. Yonsei Med J. 2006;47:179–183. doi: 10.3349/ymj.2006.47.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perruchot MH, Ecolan P, Sorensen IL, Oksbjerg N, Lefaucheur L. In vitro characterization of proliferation and differentiation of pig satellite cells. Differentiation. 2012;84:322–329. doi: 10.1016/j.diff.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Post MJ. Cultured meat from stem cells: Challenges and prospects. Meat Sci. 2012;92:297–301. doi: 10.1016/j.meatsci.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Rando TA, Blau HM. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol. 1994;125:1275–1287. doi: 10.1083/jcb.125.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redshaw Z, Loughna PT. Oxygen concentration modulates the differentiation of muscle stem cells toward myogenic and adipogenic fates. Differentiation. 2012;84:193–202. doi: 10.1016/j.diff.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Redshaw Z, McOrist S, Loughna P. Muscle origin of porcine satellite cells affects in vitro differentiation potential. Cell Biochem Funct. 2010;28:403–411. doi: 10.1002/cbf.1670. [DOI] [PubMed] [Google Scholar]
- Richler C, Yaffe D. The in vitro cultivation and differentiation capacities of myogenic cell lines. Dev Biol. 1970;23:1–22. doi: 10.1016/S0012-1606(70)80004-5. [DOI] [PubMed] [Google Scholar]
- Rozo M, Li L, Fan CM. Targeting β1-integrin signaling enhances regeneration in aged and dystrophic muscle in mice. Nat Med. 2016;22:889–896. doi: 10.1038/nm.4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwander M, Leu M, Stumm M, Dorchies OM, Ruegg UT, Schittny J, Müller U. β1 integrins regulate myoblast fusion and sarcomere assembly. Dev Cell. 2003;4:673–685. doi: 10.1016/S1534-5807(03)00118-7. [DOI] [PubMed] [Google Scholar]
- Xu X, Wilschut KJ, Kouklis G, Tian H, Hesse R, Garland C, Sbitany H, Hansen S, Seth R, Knott PD, Hoffman WY, Pomerantz JH. Human satellite cell transplantation and regeneration from diverse skeletal muscles. Stem Cell Reports. 2015;5:419–434. doi: 10.1016/j.stemcr.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]