Abstract
In this study, the stability of a submicron emulsion to protect an extract obtained from sea grape fruit (Coccoloba uvifera L.) was evaluated. Extract characterization by MS-HPLC revealed the presence of 3 anthocyanins (cyanidin 3-glucoside, malvidin 3-glucoside, and delphinidin 3-glucoside), the content of total phenols was 263.86 ± 1.86 mg gallic acid equivalent/100 g, with an antioxidant capacity determined by ABTS and DPPH of 128.95 ± 1.00 and 26.18 ± 0.60 μg Trolox equivalents/mL, respectively. A submicron emulsion (0.424 μm) by Ultrasound with monomodal distribution, stable over time and low viscosity (1.94 mPa s) classified as a shear-thinning fluid was obtained. The thermogravimetric analysis (TGA) demonstrated the stability of the C. uvifera extract in the emulsion, which is thermostable (212 °C). These emulsions can be added into a beverage as a nutraceutical, dried for later use as pills or incorporated in foods.
Keywords: Anthocyanins, Emulsion, Stability, Thermogravimetric analysis
Introduction
The sea grape fruit (Coccoloba uvifera L.) is a source of compounds with antioxidant properties, comparable to other fruits such as apple, orange, and kiwi (Segura Campos et al., 2015). This plant reaches a height of 8 meters and produces green fruits of approximately 2 cm in diameter, the fruits turn from bright green to purple as they ripen. According to studies, sea grapes have a wide range of compounds of high biological value (CHBV), such as polyphenols (Segura Campos et al., 2015), flavonoids (Bailey et al., 2011), carotenoids (Ramos-Hernández et al., 2018a, b) among others (Shaw et al., 1992). These compounds can reduce the risk of chronic diseases such as cancer, diabetes and cardiovascular disease (Ruiz-Montañez et al., 2017). Interestingly, in sea grape, the presence of anthocyanins (pigments belonging to the group of flavonoids) has been reported. These compounds have an antioxidant capacity that neutralizes the formation of free radicals in the organism that damage cells and tissues (Lima et al., 2017). This damage causes mutations in the DNA, oxidation of glucose molecules, lipid peroxidation, among others, which in turn is related to the development of chronic-degenerative diseases (Halliwell and Whiteman, 2004). The anthocyanins have a wide range of usage in the health area because they prevent the growth of tumors and cancer cells (Zafra-Stone et al., 2007). Additionally, they could decrease the neurodegenerative processes in diseases such as Parkinson’s due to their anti-inflammatory effect (Alinian et al., 2016). The content and structure of these compounds are related to the food matrix; nonetheless, anthocyanin stability has been demonstrated to be affected by various factors such as light, temperature, pH, and presence of oxygen (Patras et al., 2010). One of the alternatives to protect these compounds is the use of emulsions and encapsulation systems. The emulsions are immiscible two-phase systems (an oily and an aqueous) uniformly distributed throughout the other (McClements, 2010). Specifically, submicron emulsions are nano-scale particle dispersions formed with submicron oil droplets (< 500 nm), dispersed within a continuous aqueous phase (Mcclements et al., 2017). Each drop of oil is surrounded by a protective coating of emulsifying (Kale and Deore, 2017; Troncoso et al., 2012). One of the applications of submicron emulsions is the preservation of properties and controlled release of the encapsulated compound, which increases the bioavailability and release in cells and tissues of interest (Santiago et al., 2018). The use of these colloidal systems can increase the concentration of bioactive compounds in specific places of living organisms (Katsouli et al., 2017). These emulsions can be prepared by methods such as high-pressure homogenization, microfluidization, as well as physicochemical methods, which are the so-called “low-energy” methods due to change in temperature or composition (Tadros et al., 2004). There are other methods of high energy dispersion, such as ultrasonic-assisted homogenization, which has been reported as very effective in the formulation of stable emulsions based on the principle of cavitation (Soria and Villamiel, 2010). Moreover, the equipment is affordable, needs little technical support and can be scalable (Peshkovsky et al., 2013). Several studies report the application of ultrasound to stabilize submicron emulsions of curcumin (Abbas et al., 2014), jackfruit extract (Ruiz-Montañez et al., 2019), D-limonene (Jafari et al., 2007) and bay oil (Lima Reis et al., 2018), as well as nanoemulsions of cinnamon oil and D-limonene (Ghosh et al., 2013), demonstrating the potential of this technology in the formulation and stabilization of nano and submicron emulsions. In this sense, the main objective of this study was to obtain a stable submicron emulsion with a protective effect on the encapsulated ethanol extract of sea grape (Coccoloba uvifera L.).
Materials and methods
Preparation of the extract
Sea grape fruits were harvested at Tecolutla, Veracruz, México (22° 28′ N, 17° 09′ S, 93° 36′ E, 98° 39′ W) until maturity for consumption was reached. The samples were frozen at − 80 °C and then lyophilized in FreeZone 4.5 (Labconco, Kansas City, MO, USA.) at − 50 °C and 0.12 Mbar. The seeds were extracted from the sea grapes and then ultrasound-assisted extraction (US) was used to extract anthocyanins. US was performed using a sonicator ultrasonic Branson 1510 (St Louis, MO, USA). Extraction conditions (30 min and 42 kHz) were defined after preliminary studies carried out at 20, 25 and 30 min, frequency of 42, 44, and 46 kHz and controlled temperature (25 ± 1 °C) in ice bath (results not shown). Ethanol–water–citric acid (90:9:1) was used as a solvent. The sample-solvent ratio was 1:10 (g sample/mL solvent).
Determination of total phenolic content
Total phenolic content in the extract was determined using the Folin-Ciocalteu method (Montreau, 1972). 100 mg of extract, 15 mL of water and 1.25 mL of Folin–Ciocalteu reagent were transferred to a tube and allowed to stand in dark for 8 min. Later, 20% (w/v) sodium carbonate solution and deionized water were added to reach a final volume of 25 mL. The tubes were mixed and incubated for two h in the dark. Finally, the absorbance was measured at 765 nm with a microplate reader (Biotek SYNERGY HTX Multi-mode Reader, Winooski, VT, USA). The results were expressed as mg of gallic acid equivalent (GAE) per 100 g of sample.
Evaluation of antioxidant capacity
The antioxidant capacity was calculated by the ATBS (2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) and DPPH (2,2-diphenyl-picrilhidrazilo, D-9132) methods. A microplate reader Biotek SYNERGY HTX (Multi-mode Reader, Winooski, VT, USA) was used for reading the absorbance for both methods.
The ABTS method was performed according to the methodology described by Re et al. (1999). The stock solution of ABTS including 7 mM ABTS in 2.4 mM potassium persulfate solution was kept in dark and incubated at room temperature for 16 h. 20 µL of sea grape extract was mixed with 270 µL of the stock solution ABTS and then the absorbance was measured at 734 nm in a microplate reader. Data were calculated as the percentage of inhibition (% INH) compared to the control sample (solution without extract) according to Eq. 1 and expressed as μg Trolox equivalents (ET) using a relevant calibration curve.
| 1 |
where A absorbance value of the ABTS control solution and B absorbance value of the testing solution.
The antioxidant capacity was determined by the DPPH method using the technique established by Segura Campos et al. (2015). The concentration of the DPPH reagent was 0.1 mM in ethanol. The solution was incubated in dark for 30 min at room temperature. Next, 20 µL of the sample was mixed with 270 µL of DPPH reagent, kept in dark for 30 min and then the absorbance was measured at 517 nm. Percentage inhibition of DPPH radical was calculated according to Eq. 2 and expressed as μg Trolox equivalents (ET), using a calibration curve.
| 2 |
where A absorbance value of the DPPH control solution and B absorbance value of the testing solution.
Anthocyanin identification
Anthocyanin identification was performed by HPLC–MS Agilent Technologies 1200 (Santa Clara, CA, USA) on negative Scan Mode using a Poroshell 120 EC-C18 column of 4.6 × 50 mm internal diameter and 2.7 μm particle size, at 25 °C. 5 μL of the sample was injected and eluted at 0.1 mL/min of flow rate. Acetonitrile (Fermont 99% purity, Mexico) (solvent A) and acidified water (acetic acid) (Jalmek 99.7% purity, Mexico) (solvent B), in a ratio of 90:10 (vA/vB) were both used as the mobile phases. All used solvents were HPLC grade.
Emulsion preparation
Oil-in-water (O/W) emulsion using 5% miglyol as a dispersed phase and 0.5% aqueous solution of sucrose ester (SE) as a continuous phase were prepared, according to Ruiz-Montañez et al. (2017; 2019), who applied the steps for optimization by response surface methodology proposed by Aydar (2018). First, the sucrose ester allowed to hydrate for 16 h and then dispersed phase was added (the extract (0.05%, w/w) was previously incorporated into this phase). Later, the mixture was stirred for 2–4 min and kept in the dark. The mixture was pre-emulsified using an Ultra-turrax (IKA T10, Wilmington, DE, USA) at 10,000 rpm for 10 min. The emulsion was formed employing ultrasound-assisted homogenization (Branson 1510; St Louis, MO, USA) at 42 kHz for 5 min. During the whole process, an ice bath was used to avoid an increase in the temperature of the emulsion.
Determination of particle size distribution
The particle size distribution was performed in a MASTERSIZER 3000 (Malvern Instruments, Malvern, UK) by laser dispersion, equipped with a liquid cell (Hydro 3000). The particle size was characterized by the distribution curves in volume (%) vs particle size, considering the refractive index (1.44) and density (0.940 g/mL) of the dispersed phase (miglyol).
Viscosity
2 mL of sample were analyzed in a TA Instrument rheometer (model Discovery HR-1, New Castle, DE, USA) with concentric cylinder geometry using a shear rate of 74 s−1 at 25 °C.
Stability
The stability was measured through the technique of accelerated aging (Ruiz-Montañez et al., 2015). For this experiment, 10 mL of sample was centrifuged in a HERMLE centrifuge (model Z36K, Wihingen, Germany) at 2350 × g at room temperature for 5 min, observing the presence or absence of phase separation. The emulsion was preserved at room temperature and refrigerated at 4 °C for 3 months to evaluate the stability.
Thermogravimetric analysis
This analysis was performed in a TA Instruments thermobalance (model TGA 550 equipment, New Castle, DE, USA) using 2–5 mg of sample, in a heating range of 25–500 °C, at 5 °C/min rate, under nitrogen atmosphere. The analysis of emulsion and each component were carried out by triplicate (Ramos-Hernández et al., 2018a, b).
Loading efficiency
Loading efficiency was calculated according to Eq. 3, which expresses the relation between the final extract quantified by TGA and the calculated theoretical extract initially added in the emulsion (Layre et al., 2005).
| 3 |
Statistical analysis
The results were analyzed by analysis of variance with the STATISTICA 10 software.
Results and discussion
Determination of total phenols
The content of total phenols in sea grape extract was 263.86 ± 1.86 mg GAE/100 g. Hence, it was classified with an intermediate content (100–500 mg GAE/100 g). This content is comparable with the apple “Gala” (132 mg GAE/100 g), the orange (217 mg GAE/100 g) and the kiwi fruits (273 mg GAE/100 g) (Segura Campos et al., 2015).
The high-frequency waves generated by ultrasound, allow the breaking of the plant cell wall, which increases the interaction between the compounds of interest and the solvent, resulting in a major amount of compounds (Wang et al., 2016).
Antioxidant capacity evaluation
The antioxidant capacity of the sea grape extract obtained by the ABTS and DPPH methods was 128.95 ± 1.00 and 26.18 ± 0.60 μg ET/mL, respectively. These are complementary methods whose mechanism of action is the transfer of electrons, with the difference that the ABTS method determines the activity of compounds of hydrophilic and hydrophobic nature, while DPPH only detects the compounds of polar nature. Higher antioxidant capacity value was obtained by ABTS compared to DPPH. This indicates that the US technique allows the extraction of polar compounds (such as anthocyanins), which can react with free radicals.
On the other hand, we obtained superior results to those reported by Segura Campos et al. (2015) who determined the antioxidant capacity in a macerated extract of sea grapes, reaching values of 2.23 μg ET/mL and 7.19 μg ET/mL by the ABTS and DPPH assays, respectively. The results obtained in this work are possible due to the factors such as fruit maturity index, hours of sun exposure, cultivation conditions, part of the plant material considered, and mainly to the extraction method since the ultrasonication supports (qualitatively and quantitatively) the extraction of high biological value compounds (Both et al., 2013; Ramos-Hernández et al., 2018a, b). Because of the chemical structure of the HBVC, they can react with the reactive oxygen species due to the hydrogen atom (of its hydroxyl group) can be donated to the radical. In turn, the content of total phenols and the antioxidant capacity are closely related, since the extract with the highest phenol content showed greater antioxidant capacity (p < 0.05). The correlation of both indicators was positive for Folin–ABTS and Folin–DPPH, with a value of r = 0.7059, (p < 0.05) and r = 0.9618, (p < 0.05), respectively. Likewise, due to the high correlation between the content of total phenols and the antioxidant capacity for the DPPH method, it can be suggested that phenolic compounds of polar nature play an important role in the antioxidant capacity of this extract. This shows the richness of polar compounds in the extract.
Identification of anthocyanins
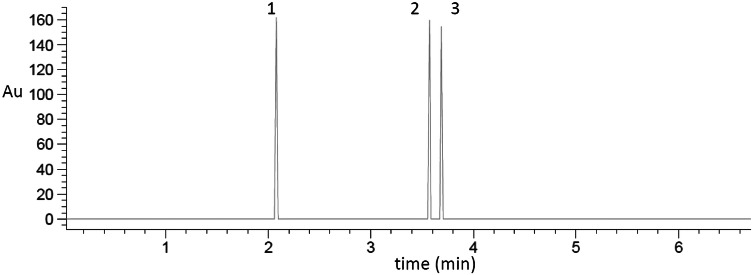
Liquid chromatography coupled to HPLC–MS masses revealed the presence of 3 anthocyanins in the sea grape extract, which coincides with the reported by Bailey et al. (2011). We identified cyanidin-3-glucoside, malvidin-3-glucoside and petunidin-3-glucoside with molecular weights of 449, 493 and 479 g/mol, respectively (Fig. 1). Other anthocyanins of similar molecular weight have been identified in jamaica (Hibiscus sabdariffa) (Sangoluisa et al., 2019) and cranberry (Vaccinium myrtillus) (Castañeda-Ovando et al., 2009). In addition to anthocyanins, some acids such as gallic, ferulic and chlorogenic were also identified in the extract.
Fig. 1.
Identification of anthocyanins in the sea grape extract by HPLC–MS
The particle size distribution of the emulsion
The particle size distribution in the emulsion without and with extract showed a monomodal droplet size distribution with a value of 0.409 μm and 0.424 μm, respectively and a polydispersity index (PDI) of 0.5 (Fig. 2A, B).
Fig. 2.
Emulsion particle size distribution without extract (A) and with extract (B)
This particle size is attributed to the homogenization process by ultrasound and to its principle of cavitation that causes implotting droplets, this process produces a size reduction and allows the formation of submicron emulsions (Silva et al., 2015). This monomodal behavior indicates the stability of the emulsion and coincides with reported studies by other authors who obtained submicron oil-in-water emulsions using ultrasound (Alvarado et al., 2015; Jafari et al., 2007).
Emulsion viscosity
The emulsions without and with extract showed shear-thinning behavior at high cutting speeds and low apparent viscosity, (1.72 mPa s ± 0.05 and 1.94 ± 0.16), for a shear rate of 74 s−1 (Fig. 3A, B). These low viscosity values contribute to the use of these emulsions for possible applications such as packaging for direct use, additives in food processing or pharmaceutical products, and make them suitable for the spray drying process. High viscosity values affect the pumping capacity of the emulsion to the atomizer and therefore, difficult its processing (Gharsallaoui et al., 2007). These results coincide with that reported by Ruiz-Montañez et al. (2017), who determined an apparent viscosity in the range of 1.06 to 2.35 ± 0.06 mPa with shear rates of 74 s−1 for emulsions with jackfruit extract.
Fig. 3.
Emulsion viscosity without extract (A) and with extract (B)
Stability
In this study, the emulsions without and with extract were evaluated by the accelerated aging technique. No phenomena of separation such as coalescence, cremation, flocculation, and sedimentation were observed. This result agrees with that reported by Ruiz-Montañez et al. (2017). One factor that affects emulsion stability is the small size of the obtained drop due to the ultrasonic action since it avoids the coalition of the drop. Another important factor is the emulsifying action of the sucrose ester that allows the interfacial tension between the phases to decrease and therefore, avoids the emulsion separation (Ruiz-Montañez et al., 2017). The emulsion remained stable after 3 months of preservation at room and refrigeration temperatures (4 °C).
Thermogravimetric analysis
The thermogravimetric analysis was carried out on both emulsions (without and with extract), as well as on all the components such as sucrose ester, miglyol, and sea grape extract. In the thermogravimetric analysis, we noticed that the extract lost 50% of its initial mass at 60 °C (which corresponds to the aqueous fraction) in the first stage of decomposition. In the second stage, the extract lost 20% of its mass at 192 °C, corresponding to the mass fraction of the extract (Fig. 4A). The thermogram (Fig. 4B) indicate the miglyol decomposition temperature in the range of 268–304 °C. This thermogram presents a single stage of mass variation since a purified fraction of the oil was analyzed. The sucrose ester lost more than 80% of its mass at a temperature range of 189–233 °C, according to the thermogram (Fig. 4C). Similar behavior was found in the thermograms of the emulsions without and with extract (Fig. 4D). The only difference was observed in the temperature range of 212–282 °C since the curve of the emulsion with the extract (Fig. 4D (Δ)) has a higher slope in comparison to the curve of the emulsion without extract (Fig. 4D (□)), which corresponds to the decomposition of the mass fraction of the extract. The thermograms demonstrated the presence of the compounds that form the emulsions because of the decomposition temperatures coincide with those evaluated for each component.
Fig. 4.
Thermogravimetric analysis of the emulsion and its components. Ethanolic extract (A), miglyol (B), sucrose ester (C), emulsions (D) with extract (Δ) and without extract (□). T thermogram and D derivate from the thermogram
Loading efficiency
The loading efficiency of the extract in the emulsion was 100%, confirming that the whole extract initially loaded in the emulsion remained there after the emulsion was processed. In this regard, the thermogravimetric analysis revealed that after 3 months of storage at room temperature and refrigeration, the loading efficiency of the stored emulsion does not experience significant changes.
We obtained an emulsion containing the ethanolic extract of sea grape with a good content of phenolic compounds (predominantly three anthocyanins) and high antioxidant capacity compared to other fruits, which demonstrates the potential value of this fruit. The emulsion showed a monomodal behavior with submicron drop size, low viscosity, and shear-thinning behavior. Further, it was stable for 90 days at room and refrigeration temperature. The thermogravimetric analysis revealed the different mass variations for each component of the emulsion and the presence of the sea grape extract was confirmed by the calculation of the loading efficiency.
Acknowledgements
The authors thank Tecnológico Nacional de México (Project Code 374(2545).17-P) for their support in conducting the work throughout this project, CYTED thematic network code 319RT0576, and Spanish Ministry of Science, Innovation and Universities (Project Code RTI2018-097249-B-C21). The authors would also like to thank CONACYT (Mexico) for the scholarship Granted Number 638096 to Surelys Ramos Bell.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Surelys Ramos-Bell, Email: dylan150113@gmail.com.
Montserrat Calderón-Santoyo, Email: montserratcalder@gmail.com.
Julio César Barros-Castillo, Email: juliocesar_bc@hotmail.com.
Juan Arturo Ragazzo-Sánchez, Email: jragazzo@ittepic.edu.mx.
References
- Abbas S, Bashari M, Akhtar W, Li WW, Zhang X. Process optimization of ultrasound-assisted curcumin nanoemulsions stabilized by OSA-modified starch. Ultrason. Sonochem. 2014;21:1265–1274. doi: 10.1016/j.ultsonch.2013.12.017. [DOI] [PubMed] [Google Scholar]
- Alinian S, Razmjoo J, Zeinali H. Flavonoids, anthocyanins, phenolics and essential oil produced in cumin (Cuminum cyminum L.) accessions under different irrigation regimes. Ind. Crops Prod. 2016;81:49–55. doi: 10.1016/j.indcrop.2015.11.040. [DOI] [Google Scholar]
- Alvarado HL, Abrego G, Souto EB, Garduño-Ramirez ML, Clares B, García ML, Calpena AC. Nanoemulsions for dermal controlled release of oleanolic and ursolic acids: in vitro, ex vivo and in vivo characterization. Colloids Surfaces B Biointerfaces. 2015;130:40–47. doi: 10.1016/j.colsurfb.2015.03.062. [DOI] [PubMed] [Google Scholar]
- Aydar AY. Utilization of response surface methodology in optimization of extraction of plant materials. In: Silva V, editor. Statistical Approaches with Emphasis on Design of Experiments Applied to Chemical Processes. London: IntechOpen; 2018. pp. 157–159. [Google Scholar]
- Bailey C, Christian KR, Pradhan S, Nair MG, Christian OE. Anti-inflammatory and antioxidant activities of Coccoloba uvifera (Seagrapes) Curr. Top. Phytochemistry. 2011;10:55–60. [Google Scholar]
- Both S, Chemat F, Strube J. Extraction of polyphenols from black tea – Conventional and ultrasound assisted extraction. Ultrason. Sonochem. 2013;21:1030–1034. doi: 10.1016/j.ultsonch.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Castañeda-Ovando A, Pacheco-Hernández ML, Paez-Hernández ME, Rodríguez JA, Galán-Vidal CA. Chemical studies of anthocyanins: a review. Food Chemistry. 2009;113:859–871. doi: 10.1016/j.foodchem.2008.09.001. [DOI] [Google Scholar]
- Gharsallaoui A, Roudaut G, Chambin O, Voilley A, Saurel R. Applications of spray-drying in microencapsulation of food ingredients: an overview. Food Res. Int. 2007;40:1107–1121. doi: 10.1016/j.foodres.2007.07.004. [DOI] [Google Scholar]
- Ghosh V, Saranya S, Mukherjee A, Chandrasekaran N. Cinnamon Oil Nanoemulsion Formulation by Ultrasonic Emulsification: investigation of Its Bactericidal Activity. J. Nanosci. Nanotechnol. 2013;13:114–122. doi: 10.1166/jnn.2013.6701. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br. J. Pharmacol. 2004;142:231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari SM, He Y, Bhandari B. Production of sub-micron emulsions by ultrasound and microfluidization techniques. J. Food Eng. 2007;82:478–788. doi: 10.1016/j.jfoodeng.2007.03.007. [DOI] [Google Scholar]
- Kale SN, Deore SL. Emulsion Micro Emulsion and Nano Emulsion: a Review. Syst. Rev. Pharm. 2017;8:39–47. doi: 10.5530/srp.2017.1.8. [DOI] [Google Scholar]
- Katsouli M, Polychniatou V, Tzia C. Optimization of water in olive oil nano-emulsions composition with bioactive compounds by response surface methodology. LWT - Food Sci. Technol. 2017;89:740–748. doi: 10.1016/j.lwt.2017.11.046. [DOI] [Google Scholar]
- Layre AM, Gref R, Richard J, Requier D, Chacun H, Appel M, Domb AJ, Couvreur P. Nanoencapsulation of a crystalline drug. Int. J. Pharm. 2005;298:323–327. doi: 10.1016/j.ijpharm.2005.02.039. [DOI] [PubMed] [Google Scholar]
- Lima ÁS, Soares CMF, Paltram R, Halbwirth H, Bica K. Extraction and consecutive purification of anthocyanins from grape pomace using ionic liquid solutions. Fluid Phase Equilib. 2017;451:68–78. doi: 10.1016/j.fluid.2017.08.006. [DOI] [Google Scholar]
- Lima Reis PMC, Mezzomo N, Aguiar GPS, Lemus Senna EMT, Hense H, Ferreira SRS. Ultrasound-assisted emulsion of laurel leaves essential oil (Laurus nobilis L.) encapsulated by SFEE. J. Supercrit. Fluids. 2018;147:284–292. doi: 10.1016/j.supflu.2018.11.018. [DOI] [Google Scholar]
- McClements DJ. Emulsion Design to Improve the Delivery of Functional Lipophilic Components. Annu. Rev. Food Sci. Technol. 2010;1:241–269. doi: 10.1146/annurev.food.080708.100722. [DOI] [PubMed] [Google Scholar]
- McClements DJ, Bai L, Chung C. Recent Advances in the Utilization of Natural Emulsifiers to Form and Stabilize Emulsions. Annu. Rev. Food Sci. Technol. 2017;8:205–236. doi: 10.1146/annurev-food-030216-030154. [DOI] [PubMed] [Google Scholar]
- Montreau F. Sur le dosage des composés phénoliques totaux dans les vins par la méthode Folin-Ciocalteu. OENO One. 1972;6:397–404. doi: 10.20870/oeno-one.1972.6.4.2071. [DOI] [Google Scholar]
- Patras A, Brunton NP, O’Donnell C, Tiwari BK. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010;21:3–11. doi: 10.1016/j.tifs.2009.07.004. [DOI] [Google Scholar]
- Peshkovsky AS, Peshkovsky SL, Bystryak S. Scalable high-power ultrasonic technology for the production of translucent nanoemulsions. Chem. Eng. Process. Process Intensif. 2013;69:77–82. doi: 10.1016/j.cep.2013.02.010. [DOI] [Google Scholar]
- Ramos-Hernández J, Ragazzo-Sánchez JA, Calderón-Santoyo M, Ortiz-Basurto RI, Prieto C, Lagaron J. Use of Electrosprayed Agave Fructans as Nanoencapsulating Hydrocolloids for Bioactives. Nanomaterials. 2018;8:868. doi: 10.3390/nano8110868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Hernández JA, Calderón-Santoyo M, Navarro-Ocaña A, Barros-Castillo JC, Ragazzo-Sánchez JA. Use of emerging technologies in the extraction of lupeol, α-amyrin and β-amyrin from sea grape (Coccoloba uvifera L.) J. Food Sci. Technol. 2018;55:2377–2383. doi: 10.1007/s13197-018-3152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Ruiz-Montañez G, Burgos-Hernández A, Calderón-Santoyo M, López-Saiz CM, Velázquez-Contreras CA, Navarro-Ocaña A, Ragazzo-Sánchez JA. Screening antimutagenic and antiproliferative properties of extracts isolated from Jackfruit pulp (Artocarpus heterophyllus Lam) Food Chem. 2015;175:409–416. doi: 10.1016/j.foodchem.2014.11.122. [DOI] [PubMed] [Google Scholar]
- Ruiz-Montañez G, Ragazzo-Sanchez JA, Picart-Palmade L, Calderón-Santoyo M, Chevalier-Lucia D. Optimization of nanoemulsions processed by high-pressure homogenization to protect a bioactive extract of jackfruit (Artocarpus heterophyllus Lam) Innov. Food Sci. Emerg. Technol. 2017;40:35–41. doi: 10.1016/j.ifset.2016.10.020. [DOI] [Google Scholar]
- Ruiz-Montañez G, Calderón-Santoyo M, Chevalier-Lucia D, Picart-Palmade L, Jimenez-Sánchez DE, Ragazzo-Sánchez JA. Ultrasound-assisted microencapsulation of jackfruit extract in eco-friendly powder particles : characterization and antiproliferative activity. J. Dispers. Sci. Technol. 2019;40:1507–1515. doi: 10.1080/01932691.2019.1566923. [DOI] [Google Scholar]
- Sangoluisa M, Santacruz C, Salvador M. Effect of extraction method of anthocyanins from the hibiscus flower (Hibiscus sabdariffa) on the efficiency of dre-sensitized solar cells. Avances en Ciencia e Ingenierías. 2019;11:352–369. [Google Scholar]
- Santiago D, Paese K, Stanisçuaski S, Jablonski A, Hickmann S, De Oliveira A. Industrial Crops & Products Encapsulation efficiency and thermal stability of norbixin microencapsulated by spray-drying using different combinations of wall materials. Ind. Crop. Prod. 2018;111:846–855. doi: 10.1016/j.indcrop.2017.12.001. [DOI] [Google Scholar]
- Segura Campos MR, Ruiz Ruiz J, Chel-Guerrero L, Betancur Ancona D. Coccoloba uvifera (L.) (Polygonaceae) fruit: phytochemical screening and potential antioxidant activity. J. Chem. 2015;1:1–9. doi: 10.1155/2015/534954. [DOI] [Google Scholar]
- Shaw PE, Moshonas MG, Baldwin EA. Volatile constituents of Coccolobo uvifera. Phytochemistry. 1992;31:3495–3497. doi: 10.1016/0031-9422(92)83714-A. [DOI] [Google Scholar]
- Silva EK, Rosa MTM, Meireles MAA. Ultrasound-assisted formation of emulsions stabilized by biopolymers. Curr. Opin. Food Sci. 2015;5:50–59. doi: 10.1016/j.cofs.2015.08.007. [DOI] [Google Scholar]
- Soria AC, Villamiel M. Effect of ultrasound on the technological properties and bioactivity of food: a review. Trends Food Sci. Technol. 2010;21:323–331. doi: 10.1016/j.tifs.2010.04.003. [DOI] [Google Scholar]
- Tadros T, Izquierdo P, Esquena J, Solans C. Formation and stability of nano-emulsions. Adv. Colloid Interface Sci. 2004;108–109:303–318. doi: 10.1016/j.cis.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Troncoso E, Aguilera JM, McClements DJ. Fabrication, characterization and lipase digestibility of food-grade nanoemulsions. Food Hydrocoll. 2012;27:355–363. doi: 10.1016/j.foodhyd.2011.10.014. [DOI] [Google Scholar]
- Wang W, Jung J, Tomasino E, Zhao Y. Optimization of solvent and ultrasound-assisted extraction for different anthocyanin rich fruit and their effects on anthocyanin compositions. LWT - Food Sci. Technol. 2016;72:229–238. doi: 10.1016/j.lwt.2016.04.041. [DOI] [Google Scholar]
- Zafra-Stone S, Yasmin T, Bagchi M, Chatterjee A, Vinson JA, Bagchi D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol. Nutr. Food Res. 2007;51:675–683. doi: 10.1002/mnfr.200700002. [DOI] [PubMed] [Google Scholar]