Abstract
The purpose of this study was to investigate the endogenous cathepsin activity in each subcellular fraction and the effect of this activity on myofibrillar protein and texture during refrigeration and partial freezing storage of northern pike (Esox lucius) fillets. The results showed that fillets stored under the refrigerated condition were more susceptible to oxidation than partial freezing. Endogenous cathepsin activity indicated that partial freezing destroys the integrity of lysosomes more effectively than refrigeration and inhibits the increase in cathepsin B and B + L in lysosomes. The activity of cathepsin B and B + L in lysosomes, mitochondria and myofibrils under the partial freezing conditions was always lower than that under refrigeration. Texture analysis showed that refrigeration had a negative impact on hardness and springiness. In conclusion, the cathepsin activity in each subcellular fraction was effectively inhibited and better textural characteristics were obtained with partial freezing than refrigeration.
Electronic supplementary material
The online version of this article (10.1007/s10068-020-00781-z) contains supplementary material, which is available to authorized users.
Keywords: Northern pike, Texture, Endogenous cathepsin activity, Subcellular fraction, Protein oxidation
Introduction
Northern pike (Esox lucius) is distributed in cold waters in North America and northern Eurasia. As the top predator in inland waters (Samarin et al., 2016), northern pike is popular for its high nutritional value, low fat content and digestibility. However, due to the geographical limitations of northern pike growth conditions, it takes a long time to transport northern pike from the location of capture to market. Therefore, an efficient storage method to maintain fillet freshness is required to maintain the gratifying sensory attributes and extend the shelf life of the northern pike.
Fresh fishes are highly susceptible to softening and deteriorating due to their biological composition. To reduce the impact of food waste by humans, it is especially important to save food for a long time (Kachele et al., 2017). The most important controllable factor affecting the freshness, quality and shelf life of fish is temperature. The traditional preservation methods of aquatic products are refrigeration, freezing, and partial freezing (− 1 °C to − 3 °C). Partial freezing, also known as partial chilling or deep freezing, is performed by freezing food to a temperature 1–2 °C below its freezing point. The shelf life of most partial freezing foods can be extended to 1.5–4 times that of traditional refrigerated foods, and partial freezing results in a lower degree of protein degradation than freezing, resulting in reduced mechanical damage to muscle structure (Kaale and Eikevik, 2014). Therefore, partial freezing is a more attractive food storage option than refrigeration or freezing.
During storage, fish tissue gradually softens and degenerates, resulting in decreased sensory attributes and commercial value. Many factors affect the textural characteristics of aquatic products, including species, age, size, slaughtering methods, and protein distribution. Protein is the main component of muscle tissue, has a supportive structure, and plays an important role in various physiological and biochemical reactions related to fish softening and degradation. Therefore, the degradation of protein directly affects the quality of fish and represents an important indicator of deteriorated fish quality (Sun et al., 2019). In the early postmortem stage of aquatic products, the degradation of myofibrillar proteins and textural softening are mainly attributed to endogenous proteolytic activity. Lysosomal cathepsin B and cathepsin L are thought to have important biological effects on the degradation of intracellular and extracellular proteins during catabolism (Ahmed et al., 2015). Additionally, protein oxidation can alter the intracellular and intercellular structure of myofibrillar proteins by forming carbonyl compounds, oxidizing sulfhydryl groups to disulfide bonds, and changing surface hydrophobicity, leading to the deterioration of aquatic product quality.
Although effects of endogenous enzyme activity on fish meat have been reported (Bahuaud et al., 2008; Li et al., 2017; Yu et al., 2018), these organisms are very complicated, and no attempt has been made to study the effects of changes in cathepsin B and B + L activity in subcellular fractions on the quality of northern pike fillets during normal storage. The present study aimed to investigate the potential relationships among protein oxidation, changes in endogenous cathepsin activity in the subcellular fractions and protein degradation in fillets to compare the differences between refrigeration (4 °C) and partial freezing (− 3 °C) during 7-days and 21-days storage periods, respectively, and to elucidate the mechanism of the quality deterioration of northern pike fillets under low temperature storage conditions.
Materials and methods
Materials
Fresh northern pikes (weight, 883 ± 50 g; length, 33 ± 1 cm; n = 15), were purchased from a local supermarket (Agricultural Market, Shihezi, Xinjiang, China), covered with crushed ice, were transported to Food College of Shihezi University within 20 min, kept alive before being processed. After percussive stunning, the scales, internal organs, head, tail, skin, and spine were removed, the back muscles were washed and cut into fillets (20 × 20 × 10 mm). Estimated freezing point of northern pike was − 1.5 °C (Fig. S1). The freezing temperature is usually 1–2 °C below the freezing point, so − 3 °C is selected as the storage temperature for the partially freezing. And the fillets were randomly selected into polyethylene bags and placed in a refrigerator (BCD-212YMB/C, Rongsheng Electric Co., Ltd, Guangdong, China) with in (4 ± 1) °C and (− 3 ± 0.5) °C freezer until test, respectively (Fig. S2).
7-Amino-4-methylcoumarin (AMC), Z-Arg-Arg-MCA, Z-Phe-Arg-MCA, dimethyl sulfoxide (DMSO), and EDTA were purchased from Sigma-Aldrich (St. Louis, MO, USA). All of the other chemical reagents used in this study were of analytical grade and from Tianjin Fuyu Fine Chemical Co., Ltd. (Tianjin, China).
Preparation of sarcoplasmic and myofibrillar proteins
The extraction of sarcoplasmic and myofibrillar proteins was performed according to the method of Tadpitchayangkoon et al. (2010) and Xiong et al. (2010) with some modifications. The fish fillets (2 g) were homogenized (15,000 rpm) for 10 s with a homogenizer with 30 mL of icy deionized water. The muscle homogenate was centrifuged at 1000× g for 10 min, and the supernatant and sediment were each collected. A total of 30 mL of 0.3% NaCl solution was added to the precipitate, the above procedure was repeated, and the precipitate was collected again. The two centrifuged supernatants were combined as the sarcoplasmic proteins. The precipitate was added to 30 mL of ice-cold 0.6 M NaCl–20 mM Tris-maleic buffer (pH 7.0), homogenized (15,000 rpm) by a homogenizer for 5 s, and extracted at 4 °C for 1 h to sufficiently dissolve the protein. A total of 8 mL of the protein solution was added to 32 mL of ice-cold deionized water (pH 7.0), the supernatant was diluted to precipitate myofibrillar protein, the sample was centrifuged at 10,000 rpm in a refrigerated centrifuge at 4 °C for 5 min, and the supernatant was discarded. The solution was cleared, and the above procedure was repeated twice. The resulting precipitate was dissolved in 0.6 M NaCl (pH 7.0) as myofibrillar proteins. The protein concentration of the sarcoplasmic and myofibrillar samples was determined using Biuret’s method (Gornall et al., 1949).
Measurement of total sulfhydryl and disulfide bond contents
Myofibrillar proteins were suspended and diluted to 2 mg/mL in 0.6 M NaCl (pH 7.0). The total sulfhydryl and disulfide bond contents were detected using a modified version of Benjakul et al. (1997) and Thannhauser et al. (1987). Five hundreds μL of myofibrillar proteins solution was added to 4.5 mL buffer A (contains 8 M urea, 3 mM EDTA, 1% SDS, and 0.2 M Tris–HCl, pH 8.0) and buffer C (buffer A with 0.1 M Na2SO3, pH 8.0) and mixed well for determination of total sulfhydryl and disulfide bond contents respectively. Then, 4 mL of the mixture were added to 0.5 mL buffer B (contains 10 M DTNB and 0.2 M Tris–HCl, pH 8.0) and buffer D (contains 8 M urea, 3 mM EDTA, 1% SDS, 0.1 M Na2SO3, 10 M NTSB, and 0.2 M Tris–HCl, pH 9.5) respectively, and incubated at 40 °C for 25 min, and the absorbance was measured at 412 nm. The control group used 0.6 M NaCl (pH 7.0) instead of the sample. The calculation was based on a molar absorption of 13,600 L/(mol*cm) and is expressed as mol/105 g protein.
where a is the absorbance value, b is the protein concentration (mg/mL), c is the molecular absorption coefficient with a value of 13,600 L/(mol*cm) and d is the dilution factor with a value of 11.25.
Measurement of carbonyl content
The carbonyl content of myofibrillar proteins was determined by the Oliver et al. (1987) method and expressed as nmol/mg protein. A total of 1 mL of 2 mg/mL myofibrillar proteins was placed in a centrifuge tube, and 1 mL of 10 mM 2,4-dinitrophenylhydrazine (DNPH) was added to each tube. The reaction was performed in a dark room for 1 h (vortexed every 10 min), and 20% trichloroacetic acid (TCA) was added. Then, the supernatant was centrifuged at 8000 rpm for 5 min, and the supernatant was discarded. The precipitate was washed 3 times with a mixture of absolute ethanol and ethyl acetate (1:1 v/v), and the excess reagent was removed. Then, 3 mL of 6 M guanidine hydrochloride solution was added to the precipitate and placed in a water bath (37 °C, 15 min), and the precipitate was dissolved. The insoluble matter was removed by centrifugation at 8000 rpm for 5 min. Then, the supernatant was collected, and the absorbance was measured at 370 nm. The carbonyl content was calculated using a molar extinction coefficient of 22,000 L/(mol*cm).
Measurement of surface hydrophobicity
The surface hydrophobicity of the myofibrillar proteins was detected by ultraviolet spectrophotometry (Cary 50, Varian Medical Systems, California, USA) by measuring the absorbance at 595 nm, according to the method of Chelh et al. (2006) with some modifications. A 2 mg/mL myofibrillar protein solution was added to 200 μL of 1 mg/mL bromophenol blue (BPB), homogeneously mixed and stirred at room temperature for 10 min, and centrifuged at 8000 rpm for 15 min. The absorbance of the supernatant was measured at 595 nm and denoted as D595 nm. The absorbance of the control group was measured at 595 nm with 20 mM phosphate buffer (pH 6.0) plus 200 μL of BPB and recorded as D0.
Subcellular fraction separation
The method of subcellular fraction separation was performed according to Lu et al. (2015). Fish samples (0.5 g) were homogenized with 4.5 mL of buffer (100 mM sucrose, 100 mM KCl, 50 mM Tris, 10 mM sodium pyrophosphate, at 1 mM Na2EDTA, pH = 7.2), and the resulting homogenate was magnetically stirred for 30 min. The mixture was centrifuged to separate myofibrillar (1100× g for 10 min), mitochondrial (3000× g for 20 min) and lysosomal (16,000× g for 30 min) fractions, and the remaining supernatant was the sarcoplasma. Each subcellular fraction was resuspended in storage buffer (85 mM CH3COONa, 15 mM CH3COOH, and 1 mM Na2EDTA), and enzyme activity was analyzed within 2 h.
Measurement of cathepsin B and B + L activity
The activities of cathepsin B and B + L were measured according to the Barrett and Kirschke (1981) method with minor modifications. The substrates Z-Arg-Arg-MCA (cathepsin B) and Z-Phe-Arg-MCA (cathepsin B + L) were dissolved in DMSO to prepare 10 mM solutions and placed at − 20 °C. The solutions were thawed before use and diluted with deionized water. The amount of the fluorescent product AMC released was measured by a fluorescence spectrophotometer (970CRT, Shanghai Precision Instrument Co., Ltd, Shanghai, China), and the excitation wavelength (λex) and the emission wavelength (λem) were 380 and 460 nm, respectively. An enzyme unit was defined as the amount of product hydrolyzed and released in 1 min with 1 mmol of AMC substrate at 40 °C.
SDS-PAGE
The SDS-PAGE method was performed as outlined by Laemmli (1970), with minor modifications. A 12% separation gel and 5% stacking gel were used for the 2 mg/mL solutions of myofibrillar and sarcoplasmic proteins. The sampling wells were loaded with 10 μg of samples and 4 μL of markers in the stacking gel. The initial voltage was maintained at 80 V. When the sample entered the separation gel to a constant voltage of 120 V, the sample was electrophoresed to approximately 1 cm above the bottom of the separation gel, and the power was turned off. The gels were removed, fixed for 1 h in Coomassie blue R-250 staining solution, and rinsed with distilled water several times with a destaining solution until the decoloration showed a clear protein band. Then, the gels were imaged with a scanner (ChemiDoc MP, Bio-Rad Laboratories, Inc., California, USA).
Measurement of texture
Texture analysis was performed according to Yu et al. (2018) with slight modifications by using a TA-XT2i texture analyzer (TA. XT Plus, Stable Micro Systems, Surrey, UK). The texture was determined by using the back muscles of the fish and cutting the fish into cubes of 20 × 20 × 10 mm. The texture profile analysis (TPA) was performed using the flat-bottom cylindrical probe P36R. The test conditions were as follows: premeasuring rate, 1.00 mm/s; test rate, 2.00 mm/s; measured rate, 2.00 mm/s; target compression distance, 5.00 mm; stress deformation, 75.0%; maintenance time, 5.00 s; trigger force, 10.0 g; and data acquisition rate, 200 pps.
Statistical analyses
All measurements were repeated three times. One-way ANOVA and differential significance analysis were performed using the SPSS (version 17.0; SPSS Inc., Chicago, IL, USA.) data analysis software. Multiple comparisons were performed using the Duncan test, and significant differences between the groups were identified by t test. (p < 0.05).
Results and discussion
Changes in total thiol group and disulfide bonds
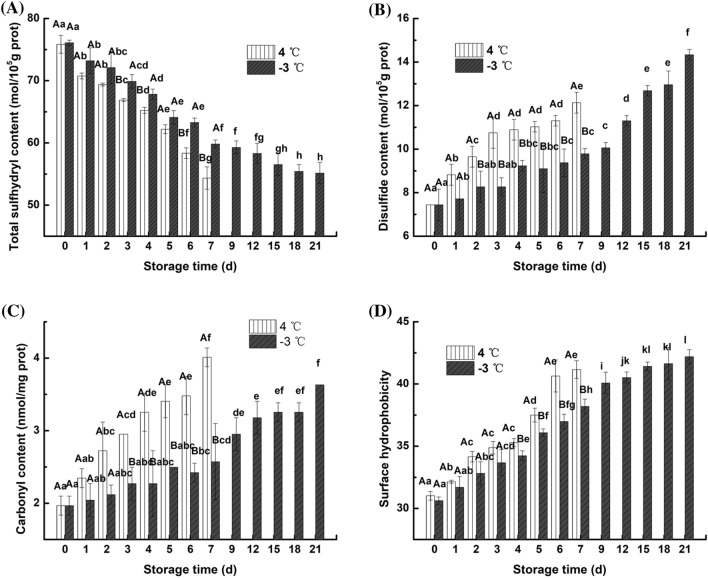
The total thiol group measurement included the active thiol groups on the surface (reactive thiol group) and the thiol groups located inside the protein (Nyaisaba et al., 2019); Urea was added to expose coated sulfhydryl groups to determine the total number of sulfhydryl groups. Figure 1A shows that the total sulfhydryl content of fresh fish was 76.10 mol/105 g protein, and the initial total thiol content was lower than the total thiol content after storage in all samples. After 3 days, the total sulfhydryl content at the two storage temperatures was significantly (p < 0.05) different. The total sulfhydryl content of the samples stored at 4 °C for 7 days and at − 3 °C for 21 days decreased to 71.65% and 72.47% of the initial values, respectively. Kong et al. (2016) also obtained similar results during the frozen storage of common carp (Cyprinus carpio) fillets and believed that the difference in sulfhydryl content of samples kept at different storage temperatures was due to the various oxidative properties of sulfhydryl groups in myofibrillar proteins. The continuous decrease in total sulfhydryl content indicates that the myofibrillar proteins of the fillet was oxidized, and this oxidation may be caused by the aggregation of myosin, which is induced by freezing (Ramirez et al., 2000). This aggregation caused the active sulfhydryl group located outside the myofibrillar proteins to be oxidized to a disulfide bond.
Fig. 1.
Changes in the total sulfhydryl content (A), disulfide content (B), carbonyl content (C) and surface hydrophobicity (D) of myofibrillar proteins from northern pike (Esox lucius) fillets stored at 4 °C and − 3 °C for up to 7 and 21 days, respectively. Effective data were collected within 7 and 21 days for analysis which were the edible periods for the northern pike in 4 °C and − 3 °C storage respectively, same as below. The same capital letter (A–B) indicates no significant difference between the samples stored at 4 °C and − 3 °C for the same length of time (p > 0.05); the same lowercase letter (a-l) indicates no significant difference among samples stored at 4 °C or − 3 °C at different storage times (p > 0.05)
The conversion of sulfhydryl groups to disulfide bonds was one of the earliest protein oxidation-associated phenomena discovered (Deng et al., 2019). As shown in Fig. 1B, the disulfide bond content of myofibrillar proteins showed an increasing trend at 4 °C and − 3 °C with a decrease in total sulfhydryl content. The disulfide bond content of fresh fish meat was 7.44 mol/105 g protein, and the disulfide bond content of the samples stored at 4 °C for 7 days and − 3 °C for 21 days increased by 63.04% and 92.61%, respectively. After 2–7 days of storage, the disulfide bond content at − 3 °C was significantly lower than that at 4 °C. This result indicates that partial freezing storage can protect protein from oxidation better than refrigeration, decreasing denaturation and prolonging the shelf life of the northern pike.
Changes in carbonyl content
The protein oxidation process is complex and produces many oxidation products, and the carbonyl group undergoes the most significant change during muscle protein oxidation (Estévez and Luna, 2017).
The changes in the carbonyl content of the myofibrillar proteins in the fillets are shown in Fig. 1C after storage for 7 days and 21 days under refrigeration and partial freezing conditions. The carbonyl contents of the refrigeration and partial freezing groups were not significantly (p > 0.05) different from 0 to 2 days, and the carbonyl content was significantly increased in the refrigeration group compared with the partial freezing group on days 3–7. In the partial freezing group, the carbonyl content increased by 84.26% after 21 days of storage, while that in the refrigerated group increased by 103.55% after only 7 days of storage. This increased carbonyl content is due to the oxidation of the amino acid side chain by free radical attack. The carbonyl content of the refrigeration storage group was significantly higher than that of the partial freezing group, which may be due to the hydrolysis of protein, the increased number of free amino groups and the induction of oxidative deamination (Sante-Lhoutellier et al., 2007). This assay indicated that refrigeration conditions exacerbate protein oxidation during the same storage time.
Changes in surface hydrophobicity
Surface hydrophobicity is a very sensitive measure of the microstructure of proteins. The surface hydrophobicity of proteins can characterize the conformation of proteins and measure the degree of protein denaturation (Liu et al., 2014).
Figure 1D indicates that the hydrophobicity of the surface of myofibrillar protein at both storage temperatures was significantly increased after storage. Significant differences were observed between samples at different storage temperatures after 4 days of storage. The protein surface hydrophobicity increased by 32.72% and 37.79% after storage for 7 days at 4 °C and 21 days at − 3 °C, respectively. Under refrigeration storage conditions, the surface hydrophobicity of the sample significantly increased over 2 days of storage. Masniyom et al. (2005) also found similar results in a study investigating sea bass fillets stored at 4 °C. Under partial freezing conditions, the surface hydrophobicity of the sample continued to increase. The increased hydrophobicity of proteins may be closely related to the exposure of hydrophobic amino acid residues (Chamba et al., 2014). The disulfide bond (-SS-) and the nondisulfide bond produced by the change in total thiol produce crosslinks, resulting in an increase in the hydrophobic groups within the protein. When the two storage conditions were compared, the surface hydrophobicity of the myofibrillar proteins was always higher in the refrigeration group than in the partial freezing storage group under the same storage length, indicating that partial freezing conditions can better maintain the protein conformation, slow the rate of protein denaturation, and maintain protein integrity. Liu et al. (2014) obtained similar results during the partial freezing storage of carp.
Cathepsin B and B + L activity in subcellular fractions
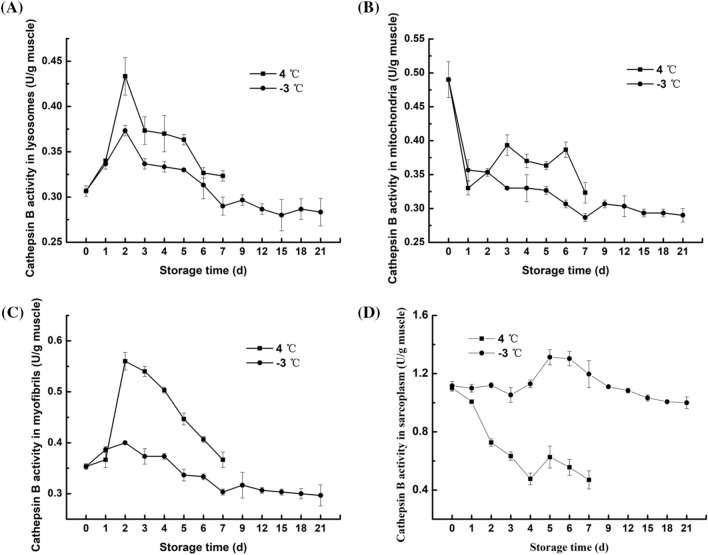
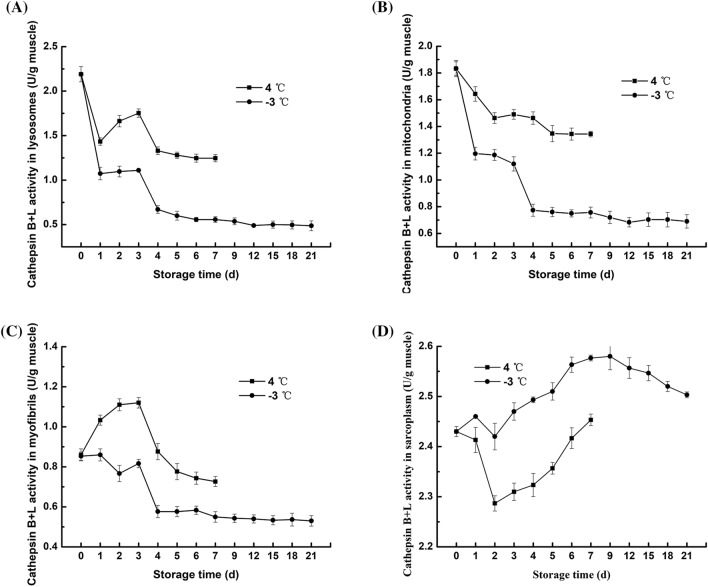
Cathepsin is a type of lysosomal cysteine protease that has a very large impact on the degradation of fish protein after death (Ahmed et al., 2015). As Figs. 2A and 3A shown that during refrigeration and partial freezing storage, the activity of cathepsin B and B + L in the lysosomes was slightly increased in the first 2 days and 1–3 days, respectively, and the enzyme activity decreased gradually in the late storage stage. The increasing trend of cathepsin activity in lysosomes has been demonstrated in other studies, for example, in the first 12 h after the death of the Bighead carp (Lu et al., 2015) and in the Atlantic salmon ice storage for 6 h (Bahuaud et al., 2008); this finding may be due to the fact that cathepsin is transported to lysosomes and proenzyme are gradually activated after death, resulting in an increase in cathepsin activity in lysosomes during storage. At late storage, the gradual decrease in cathepsin activity in lysosomes indicates that the lysosomal membrane is ruptured and cathepsin is released, marking the beginning of fish meat proteolysis. However, compared with refrigeration, partial freezing inhibited the increase in cathepsin and promoted a decrease in cathepsin, which may be due to the fact that partial freezing storage effectively destroys the integrity of lysosomes, reducing the accumulation of cathepsins and accelerating cathepsin release.
Fig. 2.
Changes in the cathepsin B activity of subcellular fractions from northern pike (Esox lucius) fillets stored at 4 °C and − 3 °C for up to 7 and 21 days, respectively. A lysosome; B mitochondria; C myofibrils; and D sarcoplasmic protein
Fig. 3.
Changes in the cathepsin B + L activity of subcellular fractions from northern pike (Esox lucius) fillets stored at 4 °C and − 3 °C for up to 7 and 21 days, respectively. A lysosome; B mitochondria; C myofibrils; and D sarcoplasmic protein
The mitochondrial pathway is the main pathway that controls cell death, and it is characterized by the permeabilization of the outer mitochondrial membrane and the release of several proapoptotic factors. After cathepsins are released into the cytosol, they cleave Bid and degrade antiapoptotic Bcl-2 proteins, inducing the mitochondrial apoptosis pathway (Cesen et al., 2012). During apoptosis, cathepsin B binds to the outer mitochondrial membrane, facilitating the initiation of a series of subsequent apoptotic programs. The changes in the activity of cathepsin B and B + L in mitochondria, ranging from 0.29–0.49 U/g muscle and 0.68–1.84 U/g muscle, respectively. The refrigeration and partial freezing storage are illustrated in Figs. 2B and 3B. On days 1–2, an increase in cathepsin B activity was observed; however, a decrease in cathepsin B activity in the late stage of storage at 4 °C was observed, demonstrating that cathepsin B in lysosomes was transferred to mitochondria beginning on the first day of storage. The activity of cathepsin B + L in mitochondria showed a downward trend at 4 °C and − 3 °C and decreased rapidly at 1–4 days and 3–4 days at 4 °C and − 3 °C, respectively. According to Cleach et al. (2019), the mitochondria of gilthead sea bream maintained significant respiratory activity after 3 days of storage, but from day 4, respiratory activity was significantly decreased due to the rupture of the mitochondrial membrane. This result may be due to mitochondrial inactivation, leading to membrane swelling and rupture and resulting in a rapid decrease in cathepsin B + L activity. At − 3 °C, the activity of cathepsin B and B + L in mitochondria continued to decrease, and the enzyme activity remained lower at − 3 °C than at 4 °C, which demonstrated that partial freezing prolongs mitochondrial activity better than refrigeration and can effectively inhibit the mitochondrial-mediated apoptosis induced by cathepsin.
The activities of cathepsin B and B + L in the myofibrils of fillets during refrigeration and partial freezing storage showed similar trends, varying between 0.30–0.56 U/g muscle and 0.53–1.12 U/g muscle (Figs. 2C and 3C), respectively, which illustrated the effects of cathepsin B on myofibrils are weaker than those of cathepsin L on myofibrils (Ahmed et al., 2015). However, it should be noted that the increase in the activity of cathepsin B and B + L at 4 °C was significantly higher than that at − 3 °C, which may be due to the release of more cathepsin B and B + L from lysosomes into myofibrils at 4 °C that at − 3 °C. In late storage, the activities of both cathepsin enzymes in myofibrils decreased, indicating that the enzyme began to act on certain proteins in myofibrils. During storage, the activities of both cathepsin enzymes in myofibrils were always higher at 4 °C than at − 3 °C, indicating that cathepsins in lysosomes were more easily released to myofibrils at 4 °C than at − 3 °C (Lu et al., 2015).
The changes of cathepsin B and B + L activity in the sarcoplasmic fractions of fillets under refrigeration and partial freezing conditions are shown in Figs. 2D and 3D. For cathepsin B, the enzyme activity at 4 °C decreased from 0 to 4 days, and after a slight increase on the fifth day, it continued to decrease to 42.73% of the initial enzyme activity. The enzyme activity at − 3 °C remained almost unchanged for the first 3 days, increased at 3–5 days, and finally decreased to 89.55% of the initial enzyme activity. For cathepsin B + L, the enzyme activity at 4 °C decreased for the first two days but increased at the late storage stage, while the enzyme activity at − 3 °C showed an overall upward trend in the first 9 days of storage and then decreased. The increase in cathepsin activity in sarcoplasm may be due to the destruction of cells by cryolysis, resulting in the leakage of cathepsins from lysosomes into the sarcoplasm. The decrease in cathepsin activity may be caused by the low temperature during the late storage period. Interestingly, unlike the other three components, the activity of cathepsin B and B + L in the sarcoplasma under partial freezing conditions was always higher than that under refrigeration conditions. These results were in agreement with those of Lu et al. (2015), who reported that partial freezing conditions induce the separation of most myofibrils, resulting in an increase in the sarcoplasmic space between the myofibrils and an increase in the cathepsins released into the sarcoplasm.
Briefly, cathepsin L is released from lysosomes faster than cathepsin B, and cathepsin L acts faster and more efficiently on fillets than cathepsin B, indicating that cathepsin L plays a more important role in the degradation of fillets. Partial freezing can maintain mitochondrial activity and slow the rate of outer membrane destruction, thereby inhibiting the mitochondria-mediated cell apoptosis induced by cathepsins. Cathepsins in myofibrils and sarcoplasma exhibited high enzymatic activity during storage and may adversely affect the degradation of fish texture. In general, partial freezing storage showed much lower activity of cathepsin B and B + L than refrigeration. The large difference in enzyme activity caused by the two storage methods indicates that the partial freezing conditions are more conducive to inhibiting the degradation of fish protein by cathepsins.
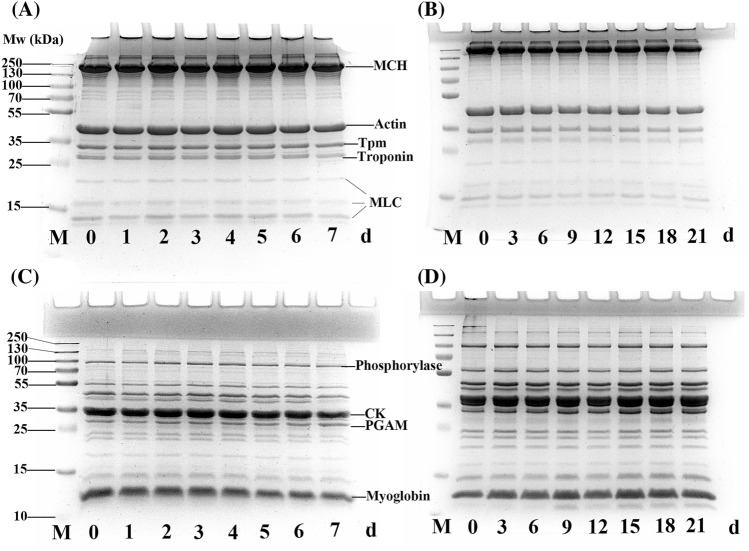
SDS-PAGE
The electrophoretic pattern of the northern pike fillets was monitored by SDS-PAGE (Fig. 4), which showed the molecular weight (Mw; range of 14–250 kDa) of the myofibrillar and sarcoplasmic proteins under the two storage conditions. Figure 4A shows that myosin light chain in the fillets was significantly degraded under the 4 °C storage conditions, and tropomyosin (36 kDa) and troponin (35 kDa) also showed slight degradation, which may have been caused by cathepsins. No degradation of myosin heavy chain (220 kDa) and actin (43 kDa) was observed. In Fig. 4B, no degradation of myosin heavy chain was observed after 21 days of storage at − 3 °C. Other characteristic bands of tropomyosin (36 kDa), troponin (35 kDa) and myosin light chain were slightly lightened. Similar to the results reported by Liu et al. (2013), no new bands were observed under the two storage conditions. Notably, the degradation of myosin light chain under the refrigeration storage condition was more rapid than that of the partial freezing storage condition, which may be due to higher cathepsin B and B + L activity during refrigeration (Yang et al., 2015).
Fig. 4.
SDS-PAGE results of myofibrillar protein and sarcoplasmic protein from northern pike (Esox lucius) stored at 4 °C and − 3 °C for 7 days and 21 days, respectively. A and B show myofibrillar proteins during 4 °C and − 3 °C storage, respectively; C and D show sarcoplasmic protein during 4 °C and − 3 °C storage, respectively. MHC, myosin heavy chain; Tpm, tropomyosin; MLC, myosin light chain; CK, creatine kinase; and PGAM, phosphoglycerate mutas
Sarcoplasmic proteins are soluble proteins found in the sarcoplasm. As shown in Figs. 4C and 4D, there was no significant change in the SDS-PAGE pattern of sarcoplasmic proteins under the 4 °C and − 3 °C storage conditions. However, the protein bands in the refrigeration conditions were slightly reduced in intensity with increasing storage duration, including the bands at 10–25 kDa and 94 kDa. Moreover, in Fig. 4D, near 10 kDa below the Mw of myoglobin, it seems that a new band developed beginning on the 9th day of storage, which indicates that the high enzyme activity in the sarcoplasmic fraction under partial freezing conditions causes the degradation of certain sarcoplasmic proteins. This finding is similar to the severe sarcoplasmic protein degradation observed under partial freezing conditions by Lu et al. (2015).
Texture
Low temperature storage to some extent results in the gradual deterioration of features of aquatic product quality such as textural firmness; thus, the determination and evaluation of the hardness and springiness of fish in low temperature storage is clearly important (Cheng et al., 2014).
The hardness of fish primarily depends on the structure of connective tissue, and the autolytic degradation of proteins and connective tissue caused by endogenous enzymes is a significant factor that decreases hardness (Yu et al., 2018). Table 1 shows that the hardness under the two storage conditions first increases and then continuously decreases. Zhu et al. (2015) observed similar changes in hardness during the ice storage of Songpu mirror carp, mainly due to postmortem stiffness and softening of the fish after slaughtering; moreover, the degradation of protein eventually leads to the softening of muscles. The increase in hardness may be due to disulfide cross-linking (Lu et al., 2017). The decrease in hardness under refrigeration conditions was largely affected by the high cathepsin B and B + L activity in connective tissue (Hassoun and Karoui ,2016) under partial freezing conditions, which may be due to the destruction of the structure of ice crystals formed during storage (Lu et al., 2015). The hardness was significantly higher (p < 0.05) under the partial freezing condition than under the refrigeration condition from days 2–7 of storage (except on the 5th day), which may be attributed to the maintenance of low cathepsin activity and, to a certain extent, the inhibition of the deterioration of the texture of the fish caused by protein oxidation with partial freezing.
Table 1.
The changes in the textural characteristics of northern pike (Esox lucius) fillets during storage at 4 °C and − 3 °C for 7 days and 21 days, respectively
| Storage time (d) | Hardness (g) | Springiness | ||
|---|---|---|---|---|
| 4 °C | − 3 °C | 4 °C | − 3 °C | |
| 0 | 1437.39 ± 133.72Aab | 1374.04 ± 62.35Ab | 0.91 ± 0.02Aa | 0.91 ± 0.02Aa |
| 1 | 1627.34 ± 169.24Aa | 1597.33 ± 182.23Aa | 0.91 ± 0.02Aa | 0.90 ± 0.02Aa |
| 2 | 1255.35 ± 64.22Bb | 1781.78 ± 140.37Aa | 0.90 ± 0.03Aa | 0.90 ± 0.03Aa |
| 3 | 974.37 ± 113.48Bc | 1311.61 ± 29.33Ab | 0.88 ± 0.01Aab | 0.86 ± 0.01Bab |
| 4 | 880.88 ± 150.12Bcd | 1188.09 ± 105.41Ab | 0.85 ± 0.01Bbc | 0.87 ± 0.00Aab |
| 5 | 704.63 ± 133.96Ade | 878.68 ± 127.42Ac | 0.83 ± 0.04Acd | 0.85 ± 0.01Aabc |
| 6 | 514.37 ± 107.70Bef | 866.48 ± 125.36Ac | 0.78 ± 0.01Bde | 0.82 ± 0.02Abcd |
| 7 | 442.91 ± 57.85Bf | 816.02 ± 113.87Acd | 0.75 ± 0.02Ae | 0.79 ± 0.05Acd |
| 9 | 776.30 ± 143.41 cd | 0.77 ± 0.04de | ||
| 12 | 729.08 ± 155.96 cd | 0.76 ± 0.07de | ||
| 15 | 699.19 ± 160.00 cd | 0.71 ± 0.04e | ||
| 18 | 588.13 ± 98.55de | 0.70 ± 0.04e | ||
| 21 | 455.67 ± 76.51e | 0.70 ± 0.06e | ||
Values are given as the mean ± SD (n = 3). Means in the same row with different capital letters (A–B) are significantly different (p < 0.05). Means in the same column with different small letters (a–f) are significantly different (p < 0.05)
Springiness refers to the ability of muscles to undergo deformation by an external force and to return to their original shape after the removal of the deforming force. There was almost no significant difference in the springiness values of fillets stored at the two temperatures. The springiness of fillets did not significantly change during the first 3 days, but it significantly decreased in the late stage. The average daily change rate of springiness under refrigeration and partial freezing conditions was 2.51% and 1.10%, respectively, indicating that refrigeration is more conducive to the reduction in fillet springiness. The decrease in the springiness of the fillets can be due to the denaturation of proteins, the destruction of disulfide bonds and the destruction of cell structure during different storage temperatures (Liu et al., 2014), all of which result in a decrease in the connectivity between fish muscle cells. Compared with refrigeration, partial freezing improved textural characteristics, which markedly prolonged the shelf life.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We acknowledged the financial support from the National Natural Science Foundation of China (Grant No. 31460438) and the National Natural Science Foundation of China (Grant No. 31960460).
Compliance with ethical standards
Conflict of interest
The authors state that they have no conflict of interests associated with this publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hengheng Qiu, Email: qiuheng1994@163.com.
Xin Guo, Email: Guoxin24yjs@163.com.
Xiaorong Deng, Email: dxr20099@163.com.
Xiaobing Guo, Email: gxb_2016@126.com.
Xiaoying Mao, Email: maoxiaoying99@163.com.
Chengjian Xu, Email: helloxj2000@gmail.com.
Jian Zhang, Email: Chj_agr@shzu.edu.cn.
References
- Ahmed Z, Donkor O, Street WA, Vasiljevic T. Calpains- and cathepsins-induced myofibrillar changes in post-mortem fish: impact on structural softening and release of bioactive peptides. Trends Food Sci. Technol. 2015;45:130–146. doi: 10.1016/j.tifs.2015.04.002. [DOI] [Google Scholar]
- Bahuaud D, Morkore T, Langsrud O, Sinnes K, Veiseth E, Ofstad R, Thomassen MS. Effects of -1.5 degrees C Super-chilling on quality of Atlantic salmon (Salmo salar) pre-rigor Fillets: Cathepsin activity, muscle histology, texture and liquid leakage. Food Chem. 2008;111:329–339. doi: 10.1016/j.foodchem.2008.03.075. [DOI] [PubMed] [Google Scholar]
- Barrett AJ, Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80:535–561. doi: 10.1016/S0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- Benjakul S, Seymour TA, Morrissey MT, An HJ. Physicochemical changes in Pacific whiting muscle proteins during iced storage. J. Food Sci. 1997;62:729–733. doi: 10.1111/j.1365-2621.1997.tb15445.x. [DOI] [Google Scholar]
- Cesen MH, Pegan K, Spes A, Turk B. Lysosomal pathways to cell death and their therapeutic applications. Exp. Cell Res. 2012;318:1245–1251. doi: 10.1016/j.yexcr.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Chamba MVM, Hua YF, Katiyo W. Oxidation and structural modification of full-fat and defatted Fflour based soy protein isolates induced by natural and synthetic extraction chemicals. Food Biophys. 2014;9:193–202. doi: 10.1007/s11483-014-9333-8. [DOI] [Google Scholar]
- Chelh I, Gatellier P, Santé-Lhoutellier V. Technical note: A simplified procedure for myofibril hydrophobicity determination. Meat Sci. 2006;74:681–683. doi: 10.1016/j.meatsci.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Cheng JH, Qu JH, Sun DW, Zeng XA. Visible/near-infrared hyperspectral imaging prediction of textural firmness of grass carp (Ctenopharyngodon idella) as affected by frozen storage. Food Res. Int. 2014;56:190–198. doi: 10.1016/j.foodres.2013.12.009. [DOI] [Google Scholar]
- Cleach J, Pasdois P, Marchetti P, Watier D, Duflos G, Goffier E, Lacoste AS, Slomianny C, Grard T, Lencel P. Mitochondrial activity as an indicator of fish freshness. Food Chem. 2019;287:38–45. doi: 10.1016/j.foodchem.2019.02.076. [DOI] [PubMed] [Google Scholar]
- Deng XR, Lei YD, Liu J, Zhang J, Qin JW. Biochemical changes of Coregonus peled myofibrillar proteins isolates as affected by HRGS oxidation system. J. Food Biochem. 2019;43:e12710. doi: 10.1111/jfbc.12710. [DOI] [PubMed] [Google Scholar]
- Estévez M, Luna C. Dietary protein oxidation: A silent threat to human health? Crit. Rev. Food Sci. Nutr. 2017;57:3781–3793. doi: 10.1080/10408398.2016.1165182. [DOI] [PubMed] [Google Scholar]
- Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949;177:751–766. [PubMed] [Google Scholar]
- Hassoun A, Karoui R. Monitoring changes in whiting (Merlangius merlangus) fillets stored under modified atmosphere packaging by front face fluorescence spectroscopy and instrumental techniques. Food Chem. 2016;200:343–353. doi: 10.1016/j.foodchem.2016.01.028. [DOI] [PubMed] [Google Scholar]
- Kaale LD, Eikevik TM. The development of ice crystals in food products during the superchilling process and following storage, a review. Trends Food Sci. Technol. 2014;39:91–103. doi: 10.1016/j.tifs.2014.07.004. [DOI] [Google Scholar]
- Kachele R, Zhang M, Gao ZX, Adhikari B. Effect of vacuum packaging on the shelf-life of silver carp (Hypophthalmichthys molitrix) fillets stored at 4 degrees C. LWT Food Sci. Technol. 2017;80:163–168. doi: 10.1016/j.lwt.2017.02.012. [DOI] [Google Scholar]
- Kong CL, Wang HY, Li DP, Zhang YM, Pan JF, Zhu BW, Luo YK. Quality changes and predictive models of radial basis function neural networks for brined common carp (Cyprinus carpio) fillets during frozen storage. Food Chem. 2016;201:327–333. doi: 10.1016/j.foodchem.2016.01.088. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li Q, Zhang LT, Lu H, Song SJ, Luo YK. Comparison of postmortem changes in ATP-related compounds, protein degradation and endogenous enzyme activity of white muscle and dark muscle from common carp (Cyprinus carpio) stored at 4 degrees C. LWT Food Sci. Technol. 2017;78:317–324. doi: 10.1016/j.lwt.2016.12.035. [DOI] [Google Scholar]
- Liu DS, Liang L, Xia WS, Regenstein JM, Zhou P. Biochemical and physical changes of grass carp (Ctenopharyngodon idella) fillets stored at-3 and 0 degrees C. Food Chem. 2013;140:105–114. doi: 10.1016/j.foodchem.2013.02.034. [DOI] [PubMed] [Google Scholar]
- Liu Q, Chen Q, Kong BH, Han JC, He XY. The influence of superchilling and cryoprotectants on protein oxidation and structural changes in the myofibrillar proteins of common carp (Cyprinus carpio) surimi. LWT Food Sci. Technol. 2014;57:603–611. doi: 10.1016/j.lwt.2014.02.023. [DOI] [Google Scholar]
- Lu H, Liu XC, Zhang YM, Wang H, Luo YK. Effects of chilling and partial freezing on rigor mortis changes of bighead carp (Aristichthys nobilis) fillets: cathepsin activity, protein degradation and microstructure of myofibrils. J. Food Sci. 2015;80:C2725–C2731. doi: 10.1111/1750-3841.13134. [DOI] [PubMed] [Google Scholar]
- Lu H, Zhang LT, Li QZ, Luo YK. Comparison of gel properties and biochemical characteristics of myofibrillar protein from bighead carp (Aristichthys nobilis) affected by frozen storage and a hydroxyl radical-generation oxidizing system. Food Chem. 2017;223:96–103. doi: 10.1016/j.foodchem.2016.11.143. [DOI] [PubMed] [Google Scholar]
- Masniyom P, Benjakul S, Visessanguan W. Combination effect of phosphate and modified atmosphere on quality and shelf-life extension of refrigerated seabass slices. LWT Food Sci. Technol. 2005;38:745–756. doi: 10.1016/j.lwt.2004.09.006. [DOI] [Google Scholar]
- Nyaisaba BM, Liu XX, Zhu SC, Fan XJ, Sun LL, Hatab S, Miao WH, Chen ML, Deng SG. Effect of hydroxyl-radical on the biochemical properties and structure of myofibrillar protein from Alaska pollock (Theragra chalcogramma) LWT Food Sci. Technol. 2019;106:15–21. doi: 10.1016/j.lwt.2019.02.045. [DOI] [Google Scholar]
- Oliver CN, Ahn BW, Moerman EJ, Goldstein S, Stadtman ER. Age-related changes in oxidized proteins. J. Biol. Chem. 1987;262:5488–5491. [PubMed] [Google Scholar]
- Ramirez JA, Martin-Polo MO, Bandman E. Fish myosin aggregation as affected by freezing and initial physical state. J. Food Sci. 2000;65:556–560. doi: 10.1111/j.1365-2621.2000.tb16047.x. [DOI] [Google Scholar]
- Samarin AM, Blecha M, Uzhytchak M, Bytyutskyy D, Zarski D, Flajshans M, Policar T. Post-ovulatory and post-stripping oocyte ageing in northern pike, Esox lucius (Linnaeus, 1758), and its effect on egg viability rates and the occurrence of larval malformations and ploidy anomalies. Aquaculture. 2016;450:431–438. doi: 10.1016/j.aquaculture.2015.08.017. [DOI] [Google Scholar]
- Sante-Lhoutellier V, Aubry L, Gatellier P. Effect of oxidation on in vitro digestibility of skeletal muscle myofibrillar proteins. J. Agric. Food Chem. 2007;55:5343–5348. doi: 10.1021/jf070252k. [DOI] [PubMed] [Google Scholar]
- Sun XY, Guo XB, Ji MY, Wu JL, Zhu WJ, Wang JH, Cheng C, Chen L, Zhang QQ. Preservative effects of fish gelatin coating enriched with CUR/beta CD emulsion on grass carp (Ctenopharyngodon idellus) fillets during storage at 4 degrees C. Food Chem. 2019;272:643–652. doi: 10.1016/j.foodchem.2018.08.040. [DOI] [PubMed] [Google Scholar]
- Tadpitchayangkoon P, Park JW, Mayer SG, Yongsawatdigul J. Structural changes and dynamic rheological properties of sarcoplasmic proteins subjected to pH-shift method. J. Agric. Food Chem. 2010;58:4241–4249. doi: 10.1021/jf903219u. [DOI] [PubMed] [Google Scholar]
- Thannhauser TW, Konishi Y, Scheraga HA. Analysis for disulfide bonds in peptides and proteins. Methods Enzymol. 1987;143:115–119. doi: 10.1016/0076-6879(87)43020-6. [DOI] [PubMed] [Google Scholar]
- Xiong YL, Blanchard SP, Tooru O, Yuanyuan M. Hydroxyl radical and ferryl-generating systems promote gel network formation of myofibrillar protein. J. Food Sci. 2010;75:C215–C221. doi: 10.1111/j.1750-3841.2009.01511.x. [DOI] [PubMed] [Google Scholar]
- Yang F, Rustad T, Xu YS, Jiang QX, Xia WS. Endogenous proteolytic enzymes - A study of their impact on cod (Gadus morhua) muscle proteins and textural properties in a fermented product. Food Chem. 2015;172:551–558. doi: 10.1016/j.foodchem.2014.09.086. [DOI] [PubMed] [Google Scholar]
- Yu DW, Regenstein JM, Zang JH, Xia WS, Xu YS, Jiang QX, Yang F. Inhibitory effects of chitosan-based coatings on endogenous enzyme activities, proteolytic degradation and texture softening of grass carp (Ctenopharyngodon idellus) fillets stored at 4 degrees C. Food Chem. 2018;262:1–6. doi: 10.1016/j.foodchem.2018.04.070. [DOI] [PubMed] [Google Scholar]
- Zhu SC, Zhou ZY, Feng LG, Luo YK. Postmortem changes in physicochemical properties of songpu mirror carp (Cyprinus carpio) during iced storage. Food Biosci. 2015;9:75–79. doi: 10.1016/j.fbio.2014.12.003. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.