Abstract
Purpose
To establish blastocyst freezing criteria for day 7 blastocyst (day 7 BL) for single vitrified-warmed blastocyst transfer (SVBT) by examining the diameter of blastocysts.
Methods
Patients who underwent day 7 BL transfer cycles (1143 cycles, mean age: 38.5 ± 3.5) and randomly selected patients after 1:1 matching who underwent day 6 BL transfer cycles and day 2-single-embryo transfer (SET) cycles were used for analysis. Comparison of the miscarriage (per clinical pregnancy) and live birth rates were made among day 2-SET, day 7 BL, and day 6 BL. These blastocyst groups were stratified into six groups based on blastocyst diameter, namely, 180 μm, 190 μm, 200 μm, 210 μm, over 220 μm, and hatched, for making the freezing criteria.
Results
For each diameter, 180 μm, 190 μm, 200 μm, 210 μm, over 220 μm, and hatched, the live birth rates of day 7 BL after SVBT were 9.0%, 11.9%, 11.5%, 15.6%, 20.0%, and 19.9%, respectively. Compared with the 14.6% live birth rate of the day 2-SET group, the live birth rate of 220 μm day 7 BL was significantly higher (P < 0.05) and was around the same in other diameter groups.
Conclusion
Our study demonstrates that sufficient live birth rates can be obtained after SVBT even from blastocysts on day 7 when blastocysts were vitrified at expanded blastocyst stage of over 180 μm of diameter or at hatched blastocyst stage and were transferred at the optimal time. This is the first study to establish a day 7 blastocyst freezing criteria using blastocyst diameter, which is an objective assessment way.
Keywords: Blastocyst diameter, Blastocyst freezing, Day 7 blastocyst, In vitro fertilization, Single vitrified-warmed blastocyst transfer
Introduction
There are some benefits associated with vitrified-warmed blastocyst (BL) transfer. It makes it possible to select embryos with a high implantation ability by culturing BLs and cryopreserving them once so that the uterine environment is suitable for implantation [1, 2].
Generally, it is rare for a BL to be used for a transfer on its seventh day after fertilization. Studies on day 7 BL usefulness and their clinical outcomes have indicated that pregnancy rates were low compared with those of day 5 and day 6 BLs. One study suggested that day 7 BLs should not be used for in vitro fertilization (IVF) treatment if the BL has not hatched by day 7 [3]. However, several studies suggested that day 7 BL transfer results in pregnancy rates that are lower compared with those of day 6 BLs, and that day 7 BL transfer would be useful for IVF treatment [4, 5]. Furthermore, Du et al. suggested that euploidy rates of day 7 BLs were low [4]. This study suggested that if several day 7 BLs are of good quality, they should be sufficient for implantation and subsequent live birth. Therefore, validation of the clinical application of day 7 BL is important to increase the chance of pregnancy of patients.
To validate the clinical application of day 7 BL, it is necessary to select an embryo that is expected to provide acceptable pregnancy outcomes. Therefore, it is necessary to establish standards that can facilitate decision-making regarding the use of a day 7 BL for BL transfer. Day 2 fresh cleavage single-embryo transfer (day 2-SET) has been one of the strategies of IVF treatment since the first report [6], although it has low live birth rates compared with BL transfer. Therefore, if day 7 BL transfer achieved comparable live birth rates with day 2-SET, it could be acceptable for patients.
Generally, BLs with high viability according to morphology grading, such as the Gardner criteria, are frozen or embryo transfer is performed at the request of the patients [7]. However, BLs at almost day 7 have a low morphological grade according to the Gardner criteria. Thus, the use of morphological grading as freezing standards for BLs may be a difficult decision to make regarding whether to freeze or not. Therefore, it is possible to use embryos obtained more efficiently for IVF treatment by establishing a novel freezing standard that promises a certain level of successful clinical results with day 7 BL.
Grading of some BL characteristics, such as embryo developmental speed [8] and BL diameter [9, 10], is typically performed without using morphology assessments. However, we have reported that using the diameter as a standard for freezing of day 5 and day 6 BLs provides a high BL survival rate after thawing and a high implantation rate after a single vitrified-warmed blastocyst transfer (SVBT) [11]. These parameters could be useful for day 7 BL selection.
In this study, we investigated whether BL diameters influenced live birth as a result of day 7 BL transfer. Subsequently, we evaluated the validity of the freezing standards that use the inner diameter of the day 7 BL by comparing the clinical results of the SVBT of day 7 BLs with inner diameters of 180 μm, 190 μm, 200 μm, 210 μm, over 220 μm, and hatched to the results of SVBT using day 6 BL. We also compared with the results of a day 2 single cleaved embryo transfer (day 2-SET).
Materials and methods
Patients and study design
The present retrospective study was reviewed and approved by the independent Institutional Review Board of Kato Ladies Clinic, Tokyo (IRB approval number: 19-30). Written informed consent was obtained from all the patients who underwent IVF treatment at our center. They were informed that their de-identified data could be used for retrospective analyses. A total of 23,643 patients undergoing 23,643 autologous SVBT cycles (1 patient: 1 cycle) at the center were included between 2006 and 2014. Patients who underwent preimplantation genetic testing for structural rearrangement and those who had intracytoplasmic sperm injection (ICSI) using in vitro matured oocytes were excluded.
In our center, the BL transfer strategy is routinely used for those with tubal factor infertility (tubal obstruction, hydrosalpinx, or a history of extrauterine pregnancy) [12–14] and for those who have experienced failed cycles with single cleavage-stage embryo transfers. This strategy comprises a large number of treatments performed at our center [1].
In study 1, we investigated what factors influence live birth outcomes of day 7 BLs following SVBT using univariable and multivariable logistic regression analyses. The following factors were used for the logistic regression analysis: female age, male age, previous number of egg retrieval cycles, previous number of embryo transfer cycles, insemination methods (conventional IVF or intracytoplasmic sperm injection [ICSI]), etiology of infertility, embryo transfer cycles (natural or hormone replacement), confirmed blastulation times, BL expansion time (blastulation to expanding BL), BL diameter, and BL morphological grading.
In study 2, the validity of the freezing standards that use the inner diameter of day 7 BL was investigated by comparing the live birth rates (LBRs) of SVBT of day 7 BLs with diameters of 180 μm, 190 μm, 200 μm, 210 μm, over 220 μm, and hatched to the results of a day 6 BL transfer and to the results of a day 2-SET using matching (1143 patients on each group, average age: 38.5 ± 3.5 years old). 1:1 matching among day 7 BL, day 6 BL, day 5 BL, and day 2-SET was performed with the cycle of a same-aged patient (in years), treated with the same type of protocol (clomiphene or natural cycle), the same or similar number of treated with egg retrieval, BL morphological grade by Gardner criteria, and BL diameter. If several matching cases were found, random controls using the randomization function in Excel (Microsoft, USA) were selected. No matching patients were found for two patients in day 7 BL. Therefore, they were excluded from study 2. Pregnancy outcomes were ascertained using a written patient questionnaire and/or by the treating obstetrician.
Minimal ovarian stimulation, oocyte retrieval, and fertilization procedures
All patients underwent a clomiphene-based minimal ovarian stimulation protocol or drug-free natural cycle IVF treatment [1, 15]. Ovulation triggering was performed with a gonadotropin-releasing hormone agonist, buserelin (600 μm, Mochida Pharmaceutical Co., Ltd., Fuji Pharma Co., Ltd.), administered in the form of a nasal spray. Oocyte retrieval was performed without any anesthesia, using a fine 21–22-G needle (Kitazato, Japan). Follicular flushing was not used during oocyte retrieval. Conventional insemination was performed approximately 3 h after retrieval, and intracytoplasmic sperm injection (ICSI) was performed 5 h after retrieval. The sperm source was only use ejaculate sperm. Human tubal fluid (HTF; Irvine Scientific, USA) with 10% serum substitute supplement (SSS; Irvine Scientific, USA) was used as the culture medium after insemination.
Embryo culture, BL monitoring, and vitrification
Fertilization assessment was performed 16–20 h after insemination. Normally fertilized zygotes with 2 pronuclei were cultured individually in a drop of 20 or 30 μL of Quinn’s Advantage Protein Plus cleavage medium (SAGE, USA) for 1–3 days. The embryos were transferred to Quinn’s Advantage Protein Plus blastocyst medium (ORIGIO, Denmark) on day 3 and were cultured until days 5–7. All embryos were cultured at 37 °C with 5% O2, 5% CO2, and 90% N2 and 100% humidity in water jacket small multi-gas incubators or dry bench-top incubators (Astec, Japan).
As per the center’s protocol, in order to closely monitor the development of the embryos between days 5 and 7, the embryos were routinely checked twice (morning and evening) daily to fulfill our vitrification criteria. If the embryos still did not reach a BL stage within our vitrification criteria, they were checked again the following day. BL checking was only performed by well-trained senior embryologists and each observation was finished within 1 min. Measurements of the BL’s inner diameter were performed using an inverted microscope (IX-71, Olympus, Japan) and a corresponding imaging software (CellSens, Olympus, Japan or OCTAX EyeWare™, Vitrolife, Sweden). BLs that reach an inner diameter > 160 μm or is a hatching BL by day 5 or 6 were vitrified immediately according to the Cryotop method [11, 16]. If the developing embryo did not fulfill the desired criteria, it was cultured further until day 7. For day 7 BLs, the vitrification criterion was to reach an inner diameter of > 180 μm or hatching. If the embryo did not fulfill the above criteria by day 7, it was discarded.
Post-warming embryo culture and embryo transfer procedure
During the study period, only single-embryo transfers were performed in our center and an exclusive SET policy was strictly observed. Therefore, the cohort was analyzed on a per cycle basis.
Single vitrified-warmed BLs were transferred on day 4.5–5 after ovulation during a spontaneous natural cycle, as mentioned in our previous report [1]. When a patient has blood P4 levels less than 8 ng/mL, SVBT was not carried out. BLs for warming were selected based on BL grading, which was carried out before freezing. After warming, surviving BLs were cultured for 30 min to 2 h until blastocoel re-expansion was confirmed. Only those BLs whose blastocoel size remained the same or increased compared with their pre-vitrification state were transferred. Degenerating BLs were discarded.
Single fresh cleaved ETs were performed on day 2 after oocyte retrieval as described previously [1]. ETs were performed if the embryo met the following criteria: (1) the cell number was three or more and (2) the embryo morphological evaluation based on Veeck’s criteria was grade 1–4. If the embryo did not meet these criteria, ET was not performed.
The embryo transfer procedure was performed under vaginal ultrasonography guidance using a specially designed soft silicone inner catheter (Kitazato, Japan) by placing a single BL suspended in minimal medium volume in the upper part of the uterine cavity. Dydrogesterone (30 mg/day orally; Daiichi-Sankyo, Japan) was routinely administered during the early luteal phase after the transfer procedure. Moreover, intramuscular (125 mg/5 days; Fuji Pharma, Japan) or intravaginal (25 or 50 mg/day; prepared in-house) progesterone was also administered until the 9th week of pregnancy in cases where endogenous progesterone production from the placenta was found to be insufficient (P4 levels of 8–12 ng/mL). During the first trimester, pregnancies were followed weekly with hormone measurements and ultrasound until approximately 9 weeks of ongoing gestation, at which point patients were referred to their treating obstetrician for subsequent care. Neonatal outcomes were ascertained by a written patient questionnaire and/or by the treating obstetrician.
Statistical analysis
A chi-squared test was used to compare categorical variables among groups. Nominal variables were analyzed by the Wilcoxon rank-sum test or the Cochran-Armitage test for trend as appropriate. Univariable and multivariable logistic regression analyses were used to determine factors that influence live birth after SVBT. Only factors with P < 0.1 according to the univariable logistic regression analysis were included in the multivariable logistic regression to calculate the adjusted odds ratios (aORs). JMP software (version 10.0; SAS Institute, Cary, NC) was used for all statistical analyses. Bonferroni correction was applied for each group studied when appropriate. P < 0.05 was considered statistically significant. When Bonferroni correction was applied to statistical analysis, P < 0.0167 was considered statistically significant.
Results
Overall patient characteristics, pregnancy, and neonatal outcomes in SVBT
Table 1 shows all the patient characteristics and clinical outcomes, including the clinical pregnancy (defined as a confirmed gestational sac at 6–7 weeks of pregnancy) rates (CPRs), ongoing pregnancy (defined as a confirmed fetal heartbeat) rates, and LBRs (live birth at ≥ 22 weeks of pregnancy) that were compared among those who underwent day 5 BL SVBT (d5 group), day 6 BL SVBT (d6 group), and day 7 BL SVBT (d7 group). Significantly high age (male and female patients) and previous number of egg retrieval cycles, and significantly low clinical pregnancy rates, ongoing pregnancy rates, and LBRs were observed in patients of the d7 group compared with those of the d6 and d5 groups (P < 0.0167). Table 2 shows the clinical results of the diameter of each BL divided by the day of vitrification. The BL diameter was less than 180 μm on day 5: 6333 cycles and day 6: 2004 cycles; they were excluded from Table 2 for comparison with day 7 BL. For all outcome variables, clinical pregnancy rates and live birth rates were significantly different (P < 0.0167) among groups and decreased progressively on days of late vitrification. Additionally, CPRs and LBRs were increased progressively with increasing diameters of BL.
Table 1.
Patients’ characteristics and clinical outcomes of each blastocyst transfer group before matching
| d5 group | d6 group | d7 group | ||
|---|---|---|---|---|
| N | 12,056 | 10,442 | 1415 | |
| Maternal age | 37.2 ± 4.1a | 38.0 ± 4.1b | 38.5 ± 4.1c | |
| Paternal age | 38.9 ± 5.5a | 39.5 ± 5.6b | 40.1 ± 5.5c | |
| Previous ER | 2.84 ± 0.03a | 3.39 ± 0.04b | 3.78 ± 0.13c | |
| Previous ET | 1.68 ± 0.05a | 1.54 ± 0.02b | 1.68 ± 0.05b | |
| Day of vitrification (%) | 51.0 | 44.2 | 4.8 | |
| Insemination method (ICSI rates, %) | 52.6a | 60.8b | 59.4b | |
| Etiology of infertility (%) | Male factor | 12.4 | 13.8 | 15 |
| Oviduct factor | 19.8 | 19.2 | 18.9 | |
| Others | 0.1 | 0.1 | 0 | |
| Unknown | 65.7 | 64.8 | 62.9 | |
| Mix | 2.1 | 2.1 | 3.2 | |
| Blastocyst morphological grade (%) | Top quality (AA) | 7.4a | 0.6b | 0.0c |
| Good quality (BB, AB, BA) | 35.4a | 8.4b | 0.7c | |
| Poor quality (BC, CB, CC) | 57.1a | 91.0b | 99.3c | |
| Clinical pregnancy (per transfer, %) | 56.9a | 41.7b | 22.0c | |
| Ongoing pregnancy (per transfer, %) | 50.8a | 35.3b | 17.1c | |
| Live birth (per transfer, %) | 44.1a | 29.9b | 14.9c | |
Values are expressed as mean ± SD, mean ± SE, or percentages. ER embryo retrieval, ET embryo transfer, ICSI intracytoplasmic sperm injection, OR oocyte retrieval
Different characters have statistically significant differences across the blastocyst groups (P < 0.05)
A chi-squared test with Bonferroni correction was used to compare categorical variables among groups (P < 0.0167)
Table 2.
Overall clinical outcomes of blastocyst diameters divided by vitrification day among groups
| 180 μm | 190 μm | 200 μm | 210 μm | > 220 μm | Hatched | *P value | ||
|---|---|---|---|---|---|---|---|---|
| CPRs | Day 5 group (%, n) |
57.6 a (1586/2755) |
65.6 a (1001/1526) |
69.8 a (543/778) |
75.5 a (243/322) |
72.9 a (196/269) |
63.0 a (46/73) |
< 0.05 |
| Day 6 group (%, n) |
34.9 b (857/2457) |
42.6 b (849/1994) |
48.3 b (798/1652) |
49.9 b (437/876) |
57.4 b (529/922) |
58.5 b (314/537) |
< 0.05 | |
| Day 7 group (%, n) |
11.8 c (20/169) |
21.6 c (35/162) |
21.6 c (74/343) |
20.8 c (31/149) |
28.8 c (49/170) |
31.2 c (38/122) |
< 0.05 | |
| LBRs | Day 5 group (%, n) |
45.7 a (1258/2755) |
52.0 a (794/1526) |
55.7 a (433/778) |
57.8 a (186/322) |
57.6 a (155/269) |
48.0 a (35/73) |
< 0.05 |
| Day 6 group (%, n) |
24.1 b (593/2457) |
30.8 b (614/1994)) |
34.8 b (574/1652)) |
39.0 b (342/876) |
42.2 b (389/922) |
43.0 b (231/537) |
< 0.05 | |
| Day 7 group (%, n) |
7.7 c (13/169) |
14.2 c (23/162) |
13.4 c (46/343) |
16.1 c (24/149) |
20.0 c (34/170) |
23.0 c (28/122) |
< 0.05 | |
*All clinical outcomes that include clinical pregnancy and live birth rates were significant trend, as demonstrated by the Cochran-Armitage trend test (P < 0.05)
Different characters indicated significant difference in CPRs and LBRs among the day 5 group, day 6 group, and day 7 group on each column (P < 0.0167)
CPRs clinical pregnancy rates, LBRs live birth rates
Table 3 shows the neonatal outcomes of the d5, d6, and d7 groups after SVBT. Maternal age was significantly different within the d5 group and d6 group (P < 0.0167), but not within the d7 group. No significant differences were found for paternal age, gestational age, male rates, congenital malformation rates, birth weight, and cesarean delivery rates.
Table 3.
Comparison of the overall neonatal outcomes among the blastocyst groups
| d5 group | d6 group | d7 group | |
|---|---|---|---|
| Singleton | 5220 | 3080 | 211 |
| Maternal age | 35.9 ± 3.8 a | 36.3 ± 3.7 b | 35.9 ± 4.0 ab |
| Paternal age | 37.8 ± 5.2 | 38.1 ± 5.1 | 38.4 ± 5.3 |
| Gestational age | 38.7 ± 1.9 | 38.7 ± 1.9 | 38.5 ± 1.7 |
| Congenital malformation (%) | 3.7% | 3.7% | 4.8% |
| Male rate (%) | 52.3% | 53.8% | 57.9% |
| Birth weight | 3034 ± 456 | 3063 ± 461 | 3049 ± 454 |
| Caesarean section (%) | 32.1% | 33.7% | 30.7% |
Different characters have statistically significant differences across the blastocyst groups (P < 0.05)
A chi-squared test with Bonferroni correction was used to compare categorical variables among groups (P < 0.0167)
Study 1
The results of the first analysis part are shown in Table 4. The univariable logistic regression analysis indicated P < 0.1 values for female age, male age, previous number of egg retrievals, confirmed blastulation time, expansion time, BL diameter, and BL morphological grade. Therefore, the multivariable logistic regression analysis to calculate aORs included these factors. The multivariable logistic regression analysis revealed that female age (aOR, 0.81; 95% confidence interval [CI], 0.76–0.86; P < 0.05) and BL diameter (aOR, 1.14; 95% CI, 1.06–1.23; P < 0.05) were significantly correlated with live birth after d7 SVBT. Table 5 shows correlation between BL diameter and BL morphological grading on each day of vitrified in BL. In day 5 BL and day 6 BL, as the BL diameter increases, the number of top-quality and good-quality BLs also significant increased (P < 0.05). However, almost all day 7 BLs were poor BLs regardless of their diameter.
Table 4.
Results of univariable and multivariable logistic regression analyses performed to clarify the factors that influence live birth from day 7 blastocysts after single vitrified-warmed blastocyst transfer
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| OR ratio | 95% CI | P value | aOR ratio | 95% CI | P value | |
| Female age | 0.836 | 0.801–0.872 | < .0001 | 0.807 | 0.757–0.857 | < .0001 |
| Male age | 0.936 | 0.906–0.966 | < .0001 | - | - | 0.269 |
| No. of previous ER | 0.919 | 0.864–0.970 | 0.004 | - | - | 0.671 |
| No. of previous ET | - | - | 0.15 | - | - | - |
| Insemination methods (reference: C-IVF) | - | - | 0.694 | - | - | - |
| Etiology of infertility (reference: unknown) | - | - | 0.494 | - | - | - |
| HRT or OVT (reference: OVT) | - | - | 0.514 | - | - | - |
| Confirmed blastulation time | 1.017 | 1.00–1.030 | 0.015 | - | - | 0.19 |
| Expansion time (blastulation to expanding blastocyst) | 0.981 | 0.968–0.994 | 0.006 | 0.949 | 0.896–1.006 | 0.078 |
| Blastocyst diameter | 1.136 | 1.070–1.206 | < .0001 | 1.136 | 1.056–1.222 | 0.001 |
| Blastocyst morphology grading | - | - | 0.147 | |||
| Top quality | - | - | - | - | ||
| Good quality | Reference | - | - | - | - | - |
| Poor quality | 0.166 | 0.039–0.707 | 0.017 | - | - | - |
aOR adjusted odds ratio, CI confidence interval, ER egg retrievals, ET embryo transfer, HRT hormone replacement treatment, OR odds ratio, OVT embryo transfer after ovulation, SVBT single vitrified-warmed blastocyst transfer
Table 5.
Correlation between blastocyst diameters and morphological grading in each vitrified blastocyst
| Inner diameters | 160 | 170 | 180 | 190 | 200 | 210 | 220 | 230 | *P value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Day 5 BL | Top quality (%) | 4.7 | 6.3 | 8.1 | 10.8 | 11.7 | 12.4 | 11.7 | 12.0 | < 0.05 |
| Good quality (%) | 29.0 | 34.4 | 38.1 | 40.0 | 41.2 | 48.8 | 46.8 | 44.4 | < 0.05 | |
| Poor quality (%) | 66.3 | 59.4 | 53.8 | 49.2 | 47.1 | 38.9 | 41.5 | 43.6 | < 0.05 | |
| Day 6 BL | Top quality (%) | 0.2 | 0.2 | 0.4 | 0.4 | 0.7 | 1.5 | 0.7 | 1.6 | < 0.05 |
| Good quality (%) | 4.2 | 4.6 | 5.0 | 7.0 | 11.2 | 12.9 | 15.3 | 17.6 | < 0.05 | |
| Poor quality (%) | 95.6 | 95.2 | 94.6 | 92.6 | 88.1 | 85.5 | 84.0 | 80.8 | < 0.05 | |
| Day 7 BL | Top quality (%) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | NA |
| Good quality (%) | 0.0 | 0.0 | 1.2 | 1.2 | 0 | 0 | 1.0 | 1.8 | N.S. | |
| Poor quality (%) | 100 | 100 | 98.8 | 98.8 | 100 | 100 | 99 | 98.2 | N.S. |
*All clinical outcomes that include clinical pregnancy and live birth rates were significant trend, as demonstrated by the Cochran-Armitage trend test (P < 0.05)
Study 2
Figure 1 shows the clinical results of SVBT using day 7 BL, day 6 BL, and day 2-SET after matching. The clinical pregnancy rates were 23.3%, 44.4%, and 19.2%, respectively, where the clinical pregnancy rate of day 7 BL was significantly lower than that of day 6 BL (P < 0.05) but was around the same as day 2-SET. Furthermore, the live birth rates were 14.5%, 30.3%, and 14.6%, respectively, which meant that the live birth rate of day 7 BL was significantly lower than that of day 6 BL (P < 0.05) but was similar to that of day 2-SET.
Fig. 1.
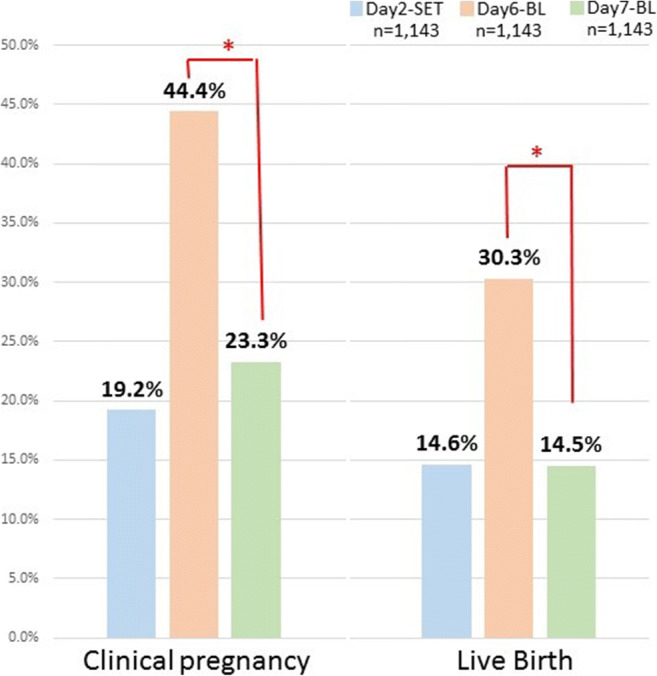
Comparison of clinical pregnancy and live birth rates among blastocyst on day 7 BL transfer, day 6 BL transfer, and day 2 single cleaved embryo transfer (day 2-SET) after matching. An asterisk symbol indicates that there was a significant difference (P < 0.0167)
The survival rates of the day 7 BLs with inner diameters of 180 μm, 190 μm, 200 μm, 210 μm, over 220 μm, and hatched were 97.0%, 95.2%, 92.7%, 94.8%, 91.3%, and 95.9%, respectively, where the survival rates in groups larger than 200 μm and 220 μm were significantly lower (P < 0.05) than the 97.5% in day 6 BL. However, with the survival rates exceeding 90% in all groups (diameters), these rates were acceptable for clinical use.
Figure 2 shows the live birth rate observed with each BL diameter. For each diameter (180 μm, 190 μm, 200 μm, 210 μm, over 220 μm, and hatched), the live birth rates of day 7 BL were 9.0%, 11.9%, 11.5%, 15.6%, 20.0%, and 19.9%, respectively, which meant that compared with the 14.6% in the day 2-SET group, the live birth rates were significantly higher in 220 μm and were similar in other groups (P < 0.05). However, the live birth rates of the day 7 BL group were significantly lower than those of the day 6 BL group among all diameter groups.
Fig. 2.
Comparison of live birth rates between blastocyst on day 7 BL transfer (stratified blastocyst diameter) and day 6 BL transfer (stratified blastocyst diameter), or day 2 single cleaved embryo transfer (day 2-SET). An asterisk symbol indicates a significant difference between the day 7 BL and day 6 BL (P < 0.05). Double asterisk symbols indicate a significant difference between the day 7 BL and day 2-SET group (P < 0.05)
Discussion
Previously, day 7 BLs had no importance in IVF treatment because of their low viability [3]. However, some recent studies of day 7 BL euploidy rates and the usefulness of day 7 BLs for IVF treatment were published as PGT-A is used worldwide [17, 18]. In this study, we were able to obtain a level of live birth results through day 7 BL SVBT that were selected based on freezing conditions where the inner diameter of the BLs should be 180 μm or larger, and we were able to demonstrate the validity of this criterion. This is the first study to establish day 7 BL freezing criteria using BL diameter. In addition, our data supports using day 7 BLs as candidates for PGT-A.
Initially, we showed the overall clinical and neonatal outcomes of SVBT transferred using day 5, day 6, and day 7 BLs. The clinical results of day 7 BLs were significantly lower than those of day 5 and day 6 BLs. The clinical results of day 5 BLs were notably higher than those of day 7 BLs. Therefore, day 5 BLs were not used for matching comparisons. Day 7 BLs had no top-quality BLs and late developing embryos would have low cell proliferation abilities and metabolism. These factors led to day 7 BLs being of poor quality.
Furthermore, in terms of neonatal outcomes, there were no significant differences among groups. Makinen et al. reported that the duration of the embryo culture period is a significant factor that can determine birthweights [19]. However, in this study, the birthweights were not significantly different among the d5, d6, and d7 groups. Furthermore, gestational age, congenital malformation rates, male rates, and cesarean delivery rates were not significantly different among the d5, d6, and d7 groups. However, the number of infants resulting from day 7 BLs was still low. Therefore, further studies of neonatal outcomes from day 7 vitrified BLs are needed.
Study 1 suggests that BL diameter correlated with live birth in day 7 BL transfers, which indicates that large BLs had higher viability than small BLs. BL expansion, which increases the inner diameter, occurs with the flow of water into the blastocoel through the Na+ pump and with an increase in cell number [20, 21]. Water inflow to the blastocoel in the BL stage requires adenosine triphosphate (ATP) [20], which is also needed to increase cell number. Expansion therefore requires active cell metabolism to produce sufficient ATP. Day 7 BLs that result in live birth after transfer require active cell metabolisms, even with late embryo development. A previous study suggested that the embryo metabolism could be a useful biomarker for predicting the genetic integrity in pre-implantation stage embryos [22]. Previous studies and our results suggest that BL diameters can be predicted during euploidy in the BL stage. We also analyzed the correlation between BL diameters and BL morphological grading on each day of vitrification (Table 5). In day 5 BLs and day 6 BLs, as the BL diameter increases, the number of top-quality and good-quality BLs significantly increased. However, almost all day 7 BLs were poor-quality BLs regardless of their diameter. These results suggest that BL diameter could be a good freezing criterion as it reflects BL morphological grading even for day 5 BLs and day 6 BLs.
In study 2, we compared the results of SVBT using day 7 BLs, day 6 BLs, and day 2-SET to examine how effective day 7 BLs are compared with other embryo transfers after matching. As a result, while the clinical pregnancy rates of day 7 BL groups were significantly lower than those of the day 6 BL group, they were not significantly different from those of the day 2-SET group. Furthermore, while the live birth rates in the day 7 BL groups were significantly lower than those in the day 6 BL group, they were not significantly different from those in the day 2-SET group. Based on these results, day 7 BLs led to more acceptable live birth rates compared with the day 2-SET group for IVF treatment, which indicates that day 7 BLs are feasible for clinical application. Accordingly, we compared the clinical results of day 2-SET against the clinical results of each diameter of day 6 and day 7 BLs. As a result, the live birth rates of the 180–210-μm BL diameters and hatched groups were similar to those in the day 2-SET, while those in BLs larger than 220 μm were significantly higher than those in the day 2-SET group. Live birth rates in all the day 7 BL groups were similar to those in the day 2-SET group. From these results, we were able to obtain the clinical outcomes we anticipated by confirming the inner diameter of BLs was over 180 μm in order to meet the freezing standards.
It must be noted that the embryos with slow growth rates may have chromosomal abnormalities [23]. In practice, a report that investigated euploids in an embryo using preimplantation genetic testing for aneuploidy (PGT-A) showed low euploid rates in day 7 BLs [24]. This study suggested that the low LBRs in day 7 BLs were because of chromosome abnormality. Therefore, in future studies, it is necessary to confirm euploidy rates in each day 7 BL diameters for a more accurate day 7 BL freezing criterion and to ultimately increase LBRs.
In this study, BL diameters were analyzed by static observation and BL contractions were not considered for decision of freezing. A recent study suggested that BL contractions would influence clinical outcomes following BL transfer [25]. In future studies, BL contraction should be investigated to correlate BL diameter with pregnancy outcomes.
A limitation of the current study is that we did not evaluate the euploid rates of each BL group. In Japan, PGT-A is not yet allowed for IVF treatment by the Japan Society of Obstetrics and Gynecology. However, this study involved live births. Therefore, the results from this study have the same value as the PGT-A study. Moreover, the day 7 BL criteria that we suggested were based on BL diameters and may be influenced by clinical settings such as culture and environment. Moreover, we used the clomiphene-based minimal ovarian stimulation protocol and the natural cycle IVF treatment for ovarian stimulation. Another limitation is our study’s retrospective nature. Unintentional biases in patient data collection may be possible. We also did not perform endometrium receptivity analysis; in future studies, endometrium receptivity should be analyzed before analysis. Finally, this study did not use morphokinetic data. Time-lapse technology can provide further information about the usefulness of day 7 BLs and should be utilized where possible.
Conclusions
The day 7 BL showed a trend where an increase in size improved clinical results. While day 7 BL is sufficiently applicable for clinical uses of SVBT, patients should be warned that its live birth rate is lower than that of day 6 BL. These results may be a future indicator of whether or not to subject day 7 BL to PGT-A.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study was reviewed and approved by the independent Institutional Review Board of Kato Ladies Clinic, Tokyo (IRB approval number: 19-30).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kato K, Takehara Y, Segawa T, Kawachiya S, Okuno T, Kobayashi T, Bodri D, Kato O. Minimal ovarian stimulation combined with elective single embryo transfer policy: age-specific results of a large, single-center, Japanese cohort. Reprod Biol Endocrinol. 2012;10:35. doi: 10.1186/1477-7827-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roque M, Valle M, Guimaraes F, Sampaio M, Geber S. Freeze-all policy: fresh vs. frozen-thawed embryo transfer. Fertil Steril. 2015;103:1190–1193. doi: 10.1016/j.fertnstert.2015.01.045. [DOI] [PubMed] [Google Scholar]
- 3.Utsunomiya TIH, Nagaki M, Sato J. A prospective, randomized study: day 3 versus hatching blastocyst stage. Hum Reprod. 2004;19:1598–1603. doi: 10.1093/humrep/deh288. [DOI] [PubMed] [Google Scholar]
- 4.Du T, Wang Y, Fan Y, Zhang S, Yan Z, Yu W, et al. Fertility and neonatal outcomes of embryos achieving blastulation on day 7: are they of clinical value? Hum Reprod. 2018; 33:1038–51. [DOI] [PubMed]
- 5.Hammond ER, Cree LM, Morbeck DE. Should extended blastocyst culture include day 7? Hum Reprod. 2018;33:991–997. doi: 10.1093/humrep/dey091. [DOI] [PubMed] [Google Scholar]
- 6.Steptoe P, Edwards R. Birth after the reimplantation of a human embryo. Lancet. 1978;312:366. doi: 10.1016/S0140-6736(78)92957-4. [DOI] [PubMed] [Google Scholar]
- 7.Gardner DKVP, Lane M, Wagley L, Schlenker T, Schoolcraft WB. Culture and transfer of human blastocysts increases implantation rates and reduces the need for multiple embryo transfers. Fertil Steril. 1998;69:84–88. doi: 10.1016/S0015-0282(97)00438-X. [DOI] [PubMed] [Google Scholar]
- 8.Kato K, Ueno S, Yabuuchi A, Uchiyama K, Okuno T, Kobayashi T, Segawa T, Teramoto S. Women’s age and embryo developmental speed accurately predict clinical pregnancy after single vitrified-warmed blastocyst transfer. Reprod BioMed Online. 2014;29:411–416. doi: 10.1016/j.rbmo.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Du QY, Wang EY, Huang Y, Guo XY, Xiong YJ, Yu YP, Yao GD, Shi SL, Sun YP. Blastocoele expansion degree predicts live birth after single blastocyst transfer for fresh and vitrified/warmed single blastocyst transfer cycles. Fertil Steril. 2016;105:910–919. doi: 10.1016/j.fertnstert.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Almagor M, Harir Y, Fieldust S, Or Y, Shoham Z. Ratio between inner cell mass diameter and blastocyst diameter is correlated with successful pregnancy outcomes of single blastocyst transfers. Fertil Steril. 2016;106:1386–1391. doi: 10.1016/j.fertnstert.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Okimura T, Kuwayama M, Segawa T, Takehara Y, Kato K, Kato O. Relations between the timing of transfer, expansion size and implantation ratesin frozen thawed single blastocyst transfer. Fertil Steril. 2009;92:S246. doi: 10.1016/j.fertnstert.2009.07.1619. [DOI] [Google Scholar]
- 12.Akman M, Garcia J, Damewood M, Watts L, Katz E. Hydrosalpinx affects the implantation of previously cryopreserved embryos. Hum Reprod. 1996;11:1013–1014. doi: 10.1093/oxfordjournals.humrep.a019287. [DOI] [PubMed] [Google Scholar]
- 13.Fang C, Huang R, Wei LN, Jia L. Frozen-thawed day 5 blastocyst transfer is associated with a lower risk of ectopic pregnancy than day 3 transfer and fresh transfer. Fertil Steril. 2015;103:655–661. doi: 10.1016/j.fertnstert.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 14.Johnson N, van Voorst S, Sowter MC, Strandell A, Mol BW. Surgical treatment for tubal disease in women due to undergo in vitro fertilisation. Cochrane Database Syst Rev. 2010:CD002125. [DOI] [PMC free article] [PubMed]
- 15.Teramoto SK, O. Minimal ovarian stimulation with clomiphene citrate a large-scale retrospective study. Reprod BioMed Online. 2007;15:134–148. doi: 10.1016/S1472-6483(10)60701-8. [DOI] [PubMed] [Google Scholar]
- 16.Mori C, Yabuuchi A, Ezoe K, Murata N, Takayama Y, Okimura T, Uchiyama K, Takakura K, Abe H, Wada K, Okuno T, Kobayashi T, Kato K. Hydroxypropyl cellulose as an option for supplementation of cryoprotectant solutions for embryo vitrification in human assisted reproductive technologies. Reprod BioMed Online. 2015;30:613–621. doi: 10.1016/j.rbmo.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez-Nieto C, Lee JA, Slifkin R, Sandler B, Copperman AB, Flisser E. What is the reproductive potential of day 7 euploid embryos? Hum Reprod. 2019;34:1697–1706. doi: 10.1093/humrep/dez129. [DOI] [PubMed] [Google Scholar]
- 18.Tiegs AW, Sun L, Patounakis G, Scott RT. Worth the wait? Day 7 blastocysts have lower euploidy rates but similar sustained implantation rates as day 5 and day 6 blastocysts. Hum Reprod. 2019;34:1632–1639. doi: 10.1093/humrep/dez138. [DOI] [PubMed] [Google Scholar]
- 19.Makinen S, Soderstrom-Anttila V, Vainio J, Suikkari AM, Tuuri T. Does long in vitro culture promote large for gestational age babies? Hum Reprod. 2013;28:828–834. doi: 10.1093/humrep/des410. [DOI] [PubMed] [Google Scholar]
- 20.Houghton FDHP, Hawkhead JA, Hall CJ, Leese HJ. Na+, K+, ATPase activity in the human and bovine preimplantation embryo. Dev Biol. 2003;263:360–366. doi: 10.1016/j.ydbio.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Montag MKB, Holmes P, Ven H. Significance of the number of embryonic cells and the state of the zona pellucida for hatching of mouse blastocysts in vitro versus in vivo. Biol Reprod. 2000;62:1738–1744. doi: 10.1095/biolreprod62.6.1738. [DOI] [PubMed] [Google Scholar]
- 22.D’Souza F, Pudakalakatti SM, Uppangala S, Honguntikar S, Salian SR, Kalthur G, Pasricha R, Appajigowda D, Atreya HS, Adiga SK. Unraveling the association between genetic integrity and metabolic activity in pre-implantation stage embryos. Sci Rep. 2016;6:37291. doi: 10.1038/srep37291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sunkara SK, Siozos A, Bolton VN, Khalaf Y, Braude PR, El-Toukhy T. The influence of delayed blastocyst formation on the outcome of frozen-thawed blastocyst transfer: a systematic review and meta-analysis. Hum Reprod. 2010;25:1906–1915. doi: 10.1093/humrep/deq143. [DOI] [PubMed] [Google Scholar]
- 24.Su Y, Li JJ, Wang C, Haddad G, Wang WH. Aneuploidy analysis in day 7 human blastocysts produced by in vitro fertilization. Reprod Biol Endocrinol. 2016;14:20. doi: 10.1186/s12958-016-0157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcos J, Perez-Albala S, Mifsud A, Molla M, Landeras J, Meseguer M. Collapse of blastocysts is strongly related to lower implantation success: a time-lapse study. Hum Reprod. 20152015; 30: 2501–8. [DOI] [PubMed]



