Abstract
This study aimed to evaluate three standard enrichment broth preparations for the recovery of healthy and chlorine-injured E. coli O157:H7 cells in kimchi. The growth of healthy and chlorine-injured cells in kimchi was observed in three different broths for 24 h. Results showed that the three broths were equally effective for the growth of healthy cells, although the broth described by the International Organization for Standardization (ISO) showed better performance in terms of maximum growth rate when compared to the other two broths described by the Korea Food Code (KFC) and the Food and Drug Administration (FDA). In the case of chlorine-injured cells, similar growth patterns were observed in KFC and ISO broths, whereas inhibition or no growth was found in FDA broth. Thus, this study suggests that KFC and ISO broths were more suitable than FDA broth for the enrichment of E. coli O157:H7 cells in kimchi.
Keywords: Escherichia coli O157:H7, Kimchi, Injured cell, Enrichment broth, Recovery
Introduction
Kimchi is a traditional Korean food fermented by lactic acid bacteria and is one of popular side dish in a Korean meal because of its taste and health benefits (Park et al., 2014). The total amount of kimchi production has reached about 450,000 tons in 2017 and its exports have constantly increased from 24,742 tons in 2015 to 28,188 tons in 2019 (KATI, 2020), showing that Korean kimchi has been exported to 68 countries including Japan, United States, Hong Kong and Australia (KATI, 2020). The most popular type of kimchi is ‘Baechu-kimchi’ which is generally made with cabbage (Brassica rapa L. subsp. pekinensis) as the main raw material. For the production of commercial kimchi, cabbage is first cut and brined, followed by quick rinsing with water and draining. The drained cabbage is mixed with seasonings, including chili powder, onions, scallions, garlic, and jeotgal. After fermentation with the help of a starter culture or endogenous lactic acid bacteria, commercial kimchi is packaged and placed in the market for sale (Patra et al., 2016).
Since cabbage and other ingredients, such as onions and scallions, are cultivated on soil, a possibility of contamination with soil-borne pathogenic bacteria might exist (Lee et al., 2018). In addition, there is no heating step involved during kimchi processing and thus the contaminated pathogens could survive and multiply during transportation and storage as well, which could lead to a foodborne outbreak. In 2012, there was an outbreak by consumption of kimchi contaminated with Escherichia coli O169, which caused 1642 cases at seven schools (Cho et al., 2014). Another two incidents associated with kimchi occurred by E. coli O6 contamination in 2013 and 2014 at school cafeterias with a total of 1184 cases reported (Shin et al., 2016). Although kimchi as a fermented food might not be suitable for growth of pathogenic E. coli, a recent study demonstrated that they could survive in kimchi during the fermentation process which might be affected by temperature and time of fermentation (Choi et al., 2018).
One of the proactive approaches to prevent the outbreak of pathogenic E. coli in kimchi is to establish an accurate and effective detection method. Current standard detection protocols for enterohemorrhagic E. coli consist of enrichment and PCR analysis for rapid screening purposes. In the enrichment broth, a modified tryptone soya broth (mTSB) containing the antibiotic novobiocin at different concentrations is used in both the Korea Food Code (KFC) (MFDS, 2018) and the International Organization for Standardization (ISO) (ISO, 2012) protocols, whereas the United States Food and Drug Administration’s Bacteriological Analytical Manual (FDA BAM) recommends the use of modified buffered peptone water (BPW) broth containing three antibiotics (acriflavin–cefsulodin–vancomycin; ACV) (FDA, 2018). The addition of antibiotics in these enrichment broths might increase the selectivity for the detection of pathogenic E. coli since the antibiotics effectively inhibit the growth of background microbiota, providing a better environment for pathogenic E. coli (Hara-Kudo et al., 2000).
The growth of pathogenic E. coli in kimchi might be hindered by high levels of lactic acid bacteria and other endogenous antimicrobials present in certain ingredients, such as chili powder and garlic, during the enrichment process. Zheng et al. (2015) demonstrated that a failure in the recovery of Salmonella Typhimurium on mung bean sprouts in lactose broth during the enrichment process might be due to high levels of indigenous lactic acid bacteria which resulted in a decreased pH. Moreover, pathogenic E. coli might be sub-lethally injured while raw cabbage and other ingredients are washed with chlorinated water for reduction of microbial load (MFDS, 2014). As a result, injured cells may not properly grow in the enrichment broth supplemented with antibiotics. Thus, the selection of appropriate enrichment broth is crucial for the detection of pathogenic E. coli in kimchi, minimizing false negative results.
Although there were some reports regarding the optimization of detection protocol for enterohemorrhagic E. coli (Lee et al., 2016; Weagant and Bound, 2001), no study has been conducted to determine the efficacy of enrichment broth on the growth and the recovery of pathogenic E. coli in kimchi. Therefore, the objective of this study was to compare three standard enrichment broths described in KFC, ISO, and FDA protocols to find the most suitable enrichment method for both healthy and chlorine-injured cells in kimchi.
Materials and methods
Bacterial cultures and preparation of healthy cells
Escherichia coli O157:H7 ATCC 35150 (American Type Culture Collection, Manassas, VA, USA) was used in this study as a model strain. Frozen culture of this strain was activated in 10 mL of tryptone soya broth (TSB, Oxoid, Basingstoke, Hampshire, UK) for 24 h at 37 °C with two consecutive transfers. One mL of the working culture at the stationary phase was centrifuged at 3500×g for 10 min at 4 °C and washed twice with 1 mL of sterilized phosphate-buffered saline (PBS, Biosesang, Seongnam-si, Korea). The cells in the resultant pellet were resuspended in 1 mL of PBS buffer for inoculation which were designated as healthy cells.
Preparation of chlorine-injured cells
Sodium hypochlorite solution (Yuanrox, Yuhanclorox, Seoul, Korea) was diluted with sterilized distilled water and adjusted to a concentration of 1.1 μg/mL. The concentration of free chlorine in chlorinated water was measured using RQflex® 10 Reflectoquant® (Merck, Darmsradt, Germany) according to manufacturer’s instructions. To prepare chlorine-injured E. coli O157:H7 cells, 100 µL of culture suspended in PBS buffer was transferred to 9.9 mL of the prepared chlorinated water and treated for 30 s at room temperature by gentle mixing for uniform sanitizing. After chlorine treatment, cells were immediately transferred to D/E Neutralizing broth (BD Difco™, Becton, Dickinson and Company, Sparks, MD, USA) and serially diluted with 0.1% peptone water (PW). The diluent was spread plated on tryptone soya agar (TSA; Oxoid) and TSA containing 2% NaCl (TSAN), respectively, followed by incubation at 37 °C for 24–48 h. Sublethal cellular injury of E. coli O157:H7 cells were determined by comparing the number of colonies grown on TSA as non-selective agar and TSAN as selective agar. The concentration of NaCl used in this experiment was selected as the maximum concentration of NaCl which did not inhibit the growth of healthy E. coli O157:H7 cells through a preliminary study (data not shown) (Ghate et al., 2013). Thus, the healthy cells could grow on both TSA and TSAN, whereas chlorine-injured cells could only grow on TSA. Based on this preliminary experiment, the percentage of injury was calculated by the following equation (Ghate et al., 2013):
Inoculation on kimchi
Commercial cabbage kimchi products (CJ, Eumseong-gun, Korea) with similar shelf lives were purchased from a local supermarket. The kimchi was cut with a sterilized scissor and weighed to 25 g in a biosafety cabinet. One mL of healthy and chlorine-injured cells as prepared above were spot inoculated on the surface of 25 g of kimchi. The inoculum levels were about 103 and 101 CFU/25 g for healthy cells, and 101 and 100 CFU/25 g for chlorine-injured cells. The inoculated kimchi was placed in a sterile stomacher bag and stored at 8 °C overnight which simulates storage conditions of a supermarket.
Growth of healthy and chlorine-injured E. coli O157:H7 cells in kimchi by three enrichment protocols
For KFC and ISO protocols, 225 mL of modified tryptone soya broth (mTSB; Oxoid) supplemented with 8 mg/L and 20 mg/L of novobiocin (Oxoid), respectively, were transferred into a stomacher bag containing 25 g of inoculated kimchi, and stomached for 2 min, followed by incubation at 37 °C and 41.5 °C, respectively, for 24 h. In the case of FDA protocol (FDA, 2018), 225 mL of modified buffered peptone water (mBPW; HiMedia Laboratories Pvt. Ltd., Mumbai, India) was transferred into 25 g of inoculated kimchi and stomached for 2 min. The cells in mBPW were first enriched for 5 h at 37 °C and then an acriflavin–cefsulodin–vancomycin supplement (ACV; HiMedia Laboratories Pvt. Ltd.) was added into the enrichment broth, followed by incubation at 42 °C for an additional 19 h. The growth of both healthy and chlorine-injured cells was monitored by sampling at selected time intervals during the period of incubation. The number of viable cells (log CFU/mL) was plotted against time and the growth curves were generated by fitting the data to the equation of Baranyi and Roberts (Baranyi and Roberts, 1994) using DMFit 3.5 (www.combase.cc/index.php/en/8-category-en-gb/21-tools). Four growth parameters, namely, lag-phase duration (LPD), maximum growth rate (MGR), maximum population density (MPD), and doubling time (DT) were obtained from the growth modelling. The growth of healthy cells at low level of inoculum and chlorine-injured cells was not fitted to the model since the initial population was too low to be counted.
Enumeration
For enumeration, 0.1 mL of enrichment broth was serially diluted with 0.1% PW and spread plated on Cefixime-Tellurite Sorbitol MacConkey agar (CT-SMAC, Oxoid) for E. coli O157:H7 and MRS agar (BD Difco™) for lactic acid bacteria (LAB). The plates were incubated at 37 °C for 24–48 h and the typical colonies on each agar medium were manually counted. The detection limit of spread plating was 101 CFU/mL.
Monitoring of pH changes of enrichment broth
A 25 g of kimchi inoculated with healthy E. coli O157:H7 cells was incubated in each enrichment broth. The pH of each enrichment broth was measured at specific incubation times of 0, 4, 8, 12 and 24 h using a pH meter (HI 2211 pH/ORP Meter, Hanna® Instruments, Woonsocket, RI, USA).
Statistical analysis
Mean values were obtained from independent triplicate trials with duplicate samples (n = 6). Significant differences in the mean values were statistically analyzed by analysis of variance (ANOVA) test using IBM SPSS Statistical Software (IBM Corporation, Armonk, NY, USA). The differences in the values were judged to be statistically significant at P < 0.05.
Results and discussion
The growth of healthy E. coli O157:H7 in kimchi at the 103 CFU/25 g level was monitored for 24 h in KFC, ISO or FDA enrichment broths, and their growth curves were fitted to the Baranyi model (Fig. 1A). A similar growth pattern was observed in all enrichment broths, although the number of cells in FDA broth were about 0.5–1.0 log lower than those in KFC and ISO broths. To compare the growth kinetics of E. coli O157:H7 cells in these enrichment broths, LPD, MGR, DT and MPD were calculated from the growth model (Table 1). The cells in KFC broth had shorter LPD than those in FDA broth, whereas there were no significant (P ≥ 0.05) differences in these values between KFC and ISO broths. MPD values in KFC and ISO broths were significantly higher (P < 0.05) than that in FDA broth. Unlike LPD and MPD values, ISO broth showed the highest MGR and the lowest DT values among the three enrichment broths. At the inoculum level of 101 CFU/25 g, no growth was observed in all three broths for the first 5 h but the cell populations significantly increased to 3.4, 3.0 and 5.2 CFU/mL at 8 h, and 6.9, 6.9 and 9.3 log CFU/mL at 12 h in KFC, FDA and ISO broths, respectively, revealing that ISO broth promoted the growth of cells for the first 12 h (Fig. 1B). However, there were no significant differences in the number of cells at the end of enrichment period.
Fig. 1.
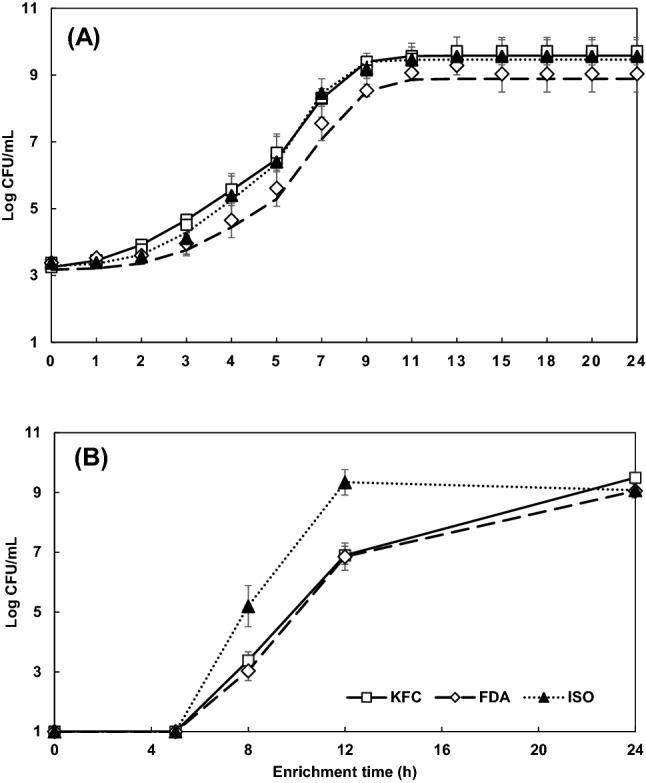
Fitted growth curves of healthy Escherichia coli O157:H7 inoculated on kimchi at the level of 103 CFU/25 g (A) and growth at the level of 101 CFU/25 g (B) in Korea Food Code (KFC), International Organization for Standardization (ISO), and Food and Drug Administration (FDA) enrichment broths for 24 h
Table 1.
Growth parameters§ of Escherichia coli O157:H7 on kimchi in Korean Food Code (KFC), International Organization for Standardization (ISO), and Food and Drug Administration (FDA) enrichment broths for 24 h
| Enrichment methods | LPD (h) | MGR (1/h) | MPD (Log CFU/mL) | DT (h) |
|---|---|---|---|---|
| KFC | 2.09 ± 0.40b | 1.15 ± 0.16b | 9.71 ± 0.45a | 0.62 ± 0.11a |
| FDA | 2.91 ± 0.51a | 1.12 ± 0.18b | 8.59 ± 0.77b | 0.63 ± 0.10a |
| ISO | 2.80 ± 0.89ab | 1.49 ± 0.29a | 9.56 ± 0.55ab | 0.48 ± 0.08b |
LPD lag-phase duration, MGR maximum growth rate, MPD maximum population density, DT doubling time, KFC Korea Food Code, FDA Food and Drug Administration, ISO International Organization for Standardization
§Different letters within the same column indicate significant (P < 0.05) differences for the respective growth parameters
According to the guidelines for the reduction of coliforms in cabbage kimchi (MFDS, 2014), raw cabbage and other raw ingredients, such as garlic and onions, are recommended to be treated with 100 ppm sodium hypochlorite solution during the process of commercial kimchi. Since this washing with chlorinated water could sub-lethally injure pathogenic E. coli, the efficacy of three enrichment protocols in recovering chlorine-injured E. coli O157:H7 cells was evaluated. For this study, E. coli O157:H7 cells were treated with 1.1 ppm of free chlorine for 30 s to achieve 75-80% of injury. The chorine-injured cells were inoculated on kimchi at the levels of 101 and 100 CFU/25 g, and incubated in KFC, FDA and ISO enrichment broths for 24 h, respectively (Fig. 2). At the 101 CFU/25 g inoculum level, the populations of chlorine-injured cells in KFC and ISO broths increased to 8.3–8.6 log CFU/mL after 12 h and remained at the same levels until the end of incubation period (Fig. 2A). The growth of cells in FDA broth was similar to those in KFC and ISO broths during the first 4 h of enrichment but cell populations dramatically decreased to undetectable level at 8 h and increased to 4.2 log CFU/mL at the end of enrichment period. The injured cells at the inoculum level of 100 CFU/25 g in KFC and ISO broths grew completely, reaching 7.9–8.5 log CFU/mL after 12 h, whereas the cells in FDA broth were totally undetectable during the 24 h enrichment period (Fig. 2B), exhibiting that both KFC and ISO broths might be more suitable for the repair and growth of chlorine-injured cells than FDA broth.
Fig. 2.
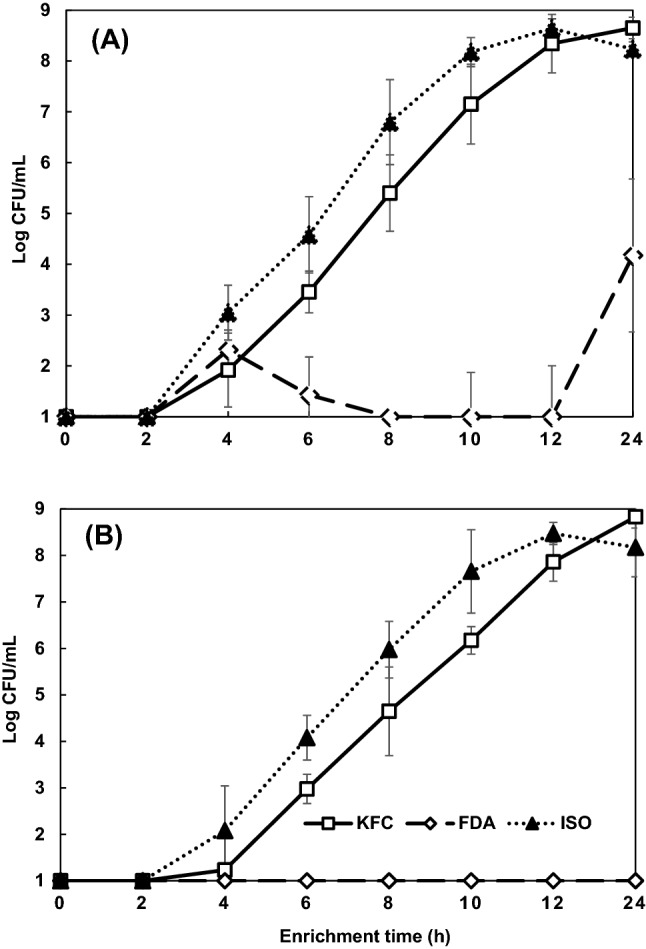
Recovery of chlorine-injured Escherichia coli O157:H7 in Korea Food Code (KFC), Food and Drug Administration (FDA) and International Organization for Standardization (ISO) enrichment broths with the inoculum levels at 101 (A) and 100 CFU/25 g (B) of kimchi
To identify the cause of inhibition of chlorine-injured E. coli O157:H7 cell growth in FDA enrichment broth, changes in pH values and the growth of lactic acid bacteria (LAB) in kimchi were monitored during the incubation in the three enrichment broths for 24 h (Fig. 3). The initial pH values of three broths containing the inoculated kimchi were approximately 7.3–7.4, exhibiting no significant (P ≥ 0.05) differences in pH values (Fig. 3A). As expected, pH values significantly decreased over a 24-h incubation and the final pH values were 5.7, 6.5 and 5.3 for KFC, FDA and ISO broths at the end of enrichment, respectively. These results show that pH value of FDA broth remained significantly (P < 0.05) higher than those of KFC and ISO broths after a 12-h incubation period. For LAB, the initial counts of kimchi in the enrichment broths were approximately 7.5–8.3 log CFU/mL (Fig. 3B). The populations of LAB in FDA broth were not significantly (P ≥ 0.05) altered, whereas those in KFC and ISO broths were augmented by 1.3–2.1 log CFU/mL over a 24-h incubation period. Although there were significant differences in the counts of LAB among the three enrichment broths during the incubation time, similar counts of LAB were observed with the range of 8.8–9.9 log CFU/mL after 24 h enrichment. The pH decreases in the three enrichment broths during the incubation period might be due to the production of organic acids, such as, lactic and acetic acids by LAB (Zheng et al., 2015). However, the extent of pH decrease in FDA broth formulated with BPW was smaller than those in KFC and ISO broths based on TSB even though there were no differences in the final populations of LBA among the three broths. This is because BPW consists of a phosphate buffer system that helps to maintain a constant pH by removing hydrogen ions dissociated from organic acids.
Fig. 3.
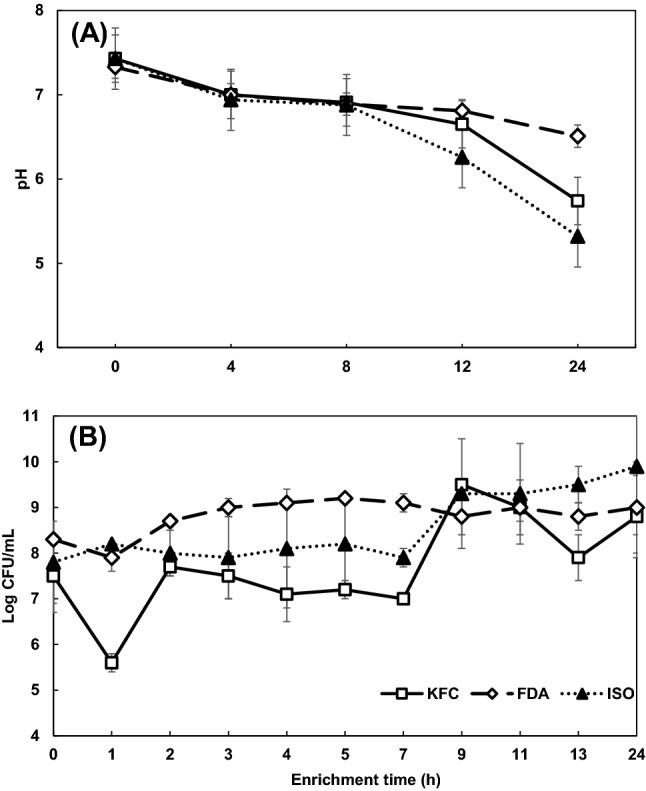
Changes in pH values (A) and counts of lactic acid bacteria (B) of kimchi in Korea Food Code (KFC), Food and Drug Administration (FDA) and International Organization for Standardization (ISO) enrichment broths during the enrichment period of 24 h
It is known that the effective enrichment broth for the detection of pathogenic bacteria in foods could not only allow the growth of target pathogen with the inhibition of background microbiota that compete for nutrients but also provide an environment for the repair of injured target pathogen to avoid false negative results at the end of detection (Restaino et al., 2001). Considering these two criteria, the effectiveness of three standard enrichment protocols in recovering healthy and chlorine-injured cells in kimchi were compared. For healthy cells which had not been exposed to any stress conditions before the inoculum, the efficiencies of all three enrichment protocols in cell growth were equivalent during the incubation period, regardless of inoculum levels, although the growth parameters and the cell growth pattern at low inoculum levels revealed that ISO broth was better than KFC and FDA broths. Unlike healthy cells, poor or no growth of chlorine-injured cells was observed in FDA broth depending on the inoculum level. These results indicate that the enrichment conditions used in the FDA protocol might not be suitable for chlorine-injured E. coli O157:H7 cells in kimchi when low number of the injured cells would be present in the product.
There are several factors including the growth of LAB, the pH of the broth and antibiotic supplements in the enrichment broth affecting the recovery of chlorine-injured cells in kimchi during the enrichment period. Among these factors, changes in pH values and the increased LAB counts might not be the most dominant factors influencing the poor performance of the FDA broth for chlorine-injured cells since the pH values of the FDA broth were maintained almost neutral and the level of LAB in the broth was also similar to those in KFC and ISO broths during the incubation period. Thus, the three antibiotics (ACV) supplemented in FDA broth could be a possible factor which hinders the recovery of injured cells. A previous study conducted by Stephens and Joynson (1998) also showed that fewer acid/salt-stressed E. coli O157:H7 cells were recovered in BPW containing VCC (vancomycin–cefsulodin–cefixime) antibiotics compared with non-selective medium. These results indicate that the levels of antibiotics in FDA broth could provide an unfavorable condition to chlorine-injured cells contaminated in kimchi during the enrichment period.
Unlike the present results, Weagant and Bound (2001) reported that E. coli O157:H7 cells inoculated on alfalfa sprout samples at the level of 0.12–0.42 CFU/g were detected on CT-SMAC agar after enrichment in mBPW broth supplemented with ACV for 24 h, whereas no colonies were found when the samples were enriched in mTSB containing novobiocin. Moreover, physiological states of target cells might influence the effectiveness of enrichment broth on the resuscitation of stressed or injured cells. For example, Jasson et al. (2009) reported that freeze-injured cells inoculated on the food samples such as fermented sausage and raw minced beef were detected by PCR after 6 h of enrichment in BPW broth containing vancomycin but not in mTSB broth containing novobiocin. Thus, these previous studies revealed that not only the levels and types of antibiotics but also the degree of injury might have profound effects on the recovery of stressed or injured cells in the enrichment broths.
In conclusion, the present results showed that there were no significant differences in the final populations of healthy E. coli O157:H7 cells in the three enrichment broths at the end of incubation period, although the cell growth in ISO broth was better with the highest MGR and the lowest DT than those in KFC and FDA broths during the period of enrichment. The growth of chlorine-injured cells in KFC and ISO broths were observed, whereas the cell growth in FDA broth were inhibited, probably due to the presence of three antibiotics. Thus, these results suggest that both KFC and ISO enrichment protocols might be suitable for the growth and the recovery of healthy and chlorine-injured E. coli O157:H7 cells in kimchi to avoid false negative results on the selective agar medium or during further PCR analysis. More research is needed to validate these standard enrichment protocols for kimchi with different pathogenic E. coli strains and serotypes as well as stress conditions.
Acknowledgements
No specific grant was used to carry out this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Baranyi J, Roberts TA. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994;23:277–294. doi: 10.1016/0168-1605(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Cho SH, Kim J, Oh KH, Hu JK, Seo J, Oh SS, Hur MJ, Choi YH, Youn SK, Chung GT, Choe YJ. Outbreak of enterotoxigenic Escherichia coli O169 enteritis in schoolchildren associated with consumption of kimchi, Republic of Korea, 2012. Epidemiol. Infect. 2014;142:616–623. doi: 10.1017/S0950268813001477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Lee S, Kim HJ, Lee H, Kim S, Lee J, Ha J, Oh H, Choi KH, Yoon Y. Pathogenic Escherichia coli and Salmonella can survive in kimchi during fermentation. J. Food Prot. 2018;81:942–946. doi: 10.4315/0362-028X.JFP-17-459. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration (FDA). BAM: Diarrheagenic Escherichia coli. Available from: https://www.fda.gov/food/laboratory-methods-food/bam-diarrheagenic-escherichia-coli. Accessed Feb. 20, 2020 (2018)
- Ghate VS, Ng KS, Zhou W, Yang H, Khoo GH, Yoon WB, Yuk HG. Antibacterial effect of light emitting diodes of visible wavelengths on selected foodborne pathogens at different illumination temperatures. Int. J. Food Microbiol. 2013;166:399–406. doi: 10.1016/j.ijfoodmicro.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Hara-Kudo Y, Ikedo M, Kodaka H, Nakagawa H, Goto K, Masuda T, Konuma H, Kojuma T, Kumagai S. Selective enrichment with a resuscitation step for isolation of freeze-injured Escherichia coli O157:H7 from foods. Appl. Environ. Microbiol. 2000;66:2866–2872. doi: 10.1128/AEM.66.7.2866-2872.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Organization for Standardization (ISO). Microbiology of food and animal feed—Real-time polymerase chain reaction (PCR)-based method for the detection of food-borne pathogens—Horizontal method for the detection of Shiga toxinproducing Escherichia coli (STEC) and the determination of O157, O111, O26, O103 and O145 serogroups. ISO/TS 13136 (2012)
- Jasson V, Rajkovic A, Baert L, Debevere J, Uyttendaele M. Comparison of enrichment conditions for rapid detection of low numbers of sublethally injured Escherichia coli O157 in food. J. Food Prot. 2009;72:1862–1868. doi: 10.4315/0362-028X-72.9.1862. [DOI] [PubMed] [Google Scholar]
- Korea Agricultural Trade Information (KATI). Available from: https://www.kati.net/product/basisInfo.do?lcdCode=MD161. Accessed Feb. 21, 2020.
- Lee DY, Kim H, Seo DW, Cho YS. Evaluation of enrichment broth and selective media for the detection of non-O157 enterohemorrhagic Escherichia coli. Korean J. Food Sci. Technol. 2016;48:323–328. doi: 10.9721/KJFST.2016.48.4.323. [DOI] [Google Scholar]
- Lee J, Ha J, Lee H, Lee JY, Hwang Y, Lee HM, Kim SH, Kim S. Analysis of microbiological contamination in kimchi and its ingredients. J. Food Hyg. Saf. 2018;33:94–101. doi: 10.13103/JFHS.2018.33.2.94. [DOI] [Google Scholar]
- Ministry of Food and Drug Safety (MFDS). Guideline for the reduction of coliforms in cabbage kimchi. Available from: https://www.mfds.go.kr/brd/m_227/view.do?seq=17405&srchFr=&srchTo=&srchWord=%EB%B0%B0%EC%B6%94%EA%B9%80%EC%B9%98&srchTp=0&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&Data_stts_gubun=C9999&page=1. Accessed Feb. 27, 2020 (2014)
- Ministry of Food and Drug Safety (MFDS). Available from: https://www.foodsafetykorea.go.kr/portal/safefoodlife/food/foodRvlv/foodRvlv.do. Accessed Feb. 21, 2020 (2018).
- Park KY, Jeong JK, Lee YE, Daily JW., 3rd Health benefits of kimchi (Korean fermented vegetables) as a probiotic food. J. Med. Food. 2014;17:6–20. doi: 10.1089/jmf.2013.3083. [DOI] [PubMed] [Google Scholar]
- Patra JK, Das G, Paramithiotis S, Shin HS. Kimchi and other widely consumed traditional fermented foods of Korea: a review. Front. Microbiol. 2016;7:1493. doi: 10.3389/fmicb.2016.01493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restaino L, Frampton EW, Spitz H. Repair and growth of heat- and freeze-injured Escherichia coli O157:H7 in selective enrichment broths. Food Microbiol. 2001;18:617–629. doi: 10.1006/fmic.2001.0427. [DOI] [Google Scholar]
- Shin J, Yoon KB, Jeon DY, Oh SS, Oh KH, Chung GT, Kim SW, Cho SH. Consecutive outbreaks of enterotoxigenic Escherichia coli O6 in schools in south Korea caused by contamination of fermented vegetable kimchi. Foodborne Pathog. Dis. 2016;13:535–543. doi: 10.1089/fpd.2016.2147. [DOI] [PubMed] [Google Scholar]
- Stephens PJ, Joynson JA. Direct inoculation into media containing bile salts and antibiotics is unsuitable for the detection of acid/salt stressed Escherichia coli O157:H7. Lett. Appl. Microbiol. 1998;27:147–151. doi: 10.1046/j.1472-765X.1998.00415.x. [DOI] [PubMed] [Google Scholar]
- Weagant SD, Bound AJ. Evaluation of techniques for enrichment and isolation of Escherichia coli O157:H7 from artificially contaminated sprouts. Int. J. Food Microbiol. 2001;71:87–92. doi: 10.1016/S0168-1605(01)00558-X. [DOI] [PubMed] [Google Scholar]
- Zheng Q, Miks-Krajnik M, D’Souza C, Yang Y, Heo DJ, Kim SK, Lee SC, Yuk HG. Growth of healthy and sanitizer-injured Salmonella cells on mung bean sprouts in different commercial enrichment broths. Food Microbiol. 2015;52:159–168. doi: 10.1016/j.fm.2015.07.013. [DOI] [PubMed] [Google Scholar]


