Abstract
Rosemary extract (RE) has significant antioxidant and antibacterial properties; however, the application of RE to areas with an aqueous solution is limited due to its poor solubility. There is a need for research focused on finding a method to improve water solubility for incorporating RE into aqueous systems, such as food and cosmetic. Therefore, in this study, the micellar solubilization of RE is conducted using four types of surfactants (Tween 20, polyglyceryl-10-laurate, polyglyceryl-10-myristate, and polyglyceryl-10-monooleate) to increase the water solubility of RE and the effects of various surfactant types and concentration on solubility were investigated. Antibacterial activities of the mixture solutions containing RE and surfactants were also examined. The water solubility of RE significantly improved when surfactants were added into the RE solution and especially in polyglyceryl-10-monooleate, with the longest tail, was the most effective for increasing solubility. In terms of the antibacterial effect on Bacillus subtilis, it was observed that a relatively lower concentration of surfactants was effective. The results of this study provide useful information for the development of a new RE-loaded delivery system for food and cosmetic application.
Keywords: Rosemary extract, Micelle, Surfactant, Solubility, Antibacterial activity
Introduction
Processed food products require the use of preservatives to ensure longer shelf-life and to inhibit natural aging and discoloration in the food product. Recently, there has been a growing interest in replacing artificial preservatives with plant-derived alternatives because consumers are looking for safe food products with natural ingredients (Aschemann-Witzel et al., 2019; Savoia, 2012). Research on the use of plant extracts as natural antibacterial compounds has increased. Plants are known for their defensive mechanisms against fungal and bacterial attacks due to their phenolic compounds. Phenolic compounds that are widespread in plant and plant-derived foods include flavonoids, tannins, lignans, coumarins, curcuminoids, and quinones. These compounds can be used as natural food preservatives due to their antioxidant and antibacterial activity (Ambriz-Pérez et al., 2016).
Among plant extracts that are used as replacements for synthetic antioxidant and antibacterial agents, many studies have reported the potential benefits of using rosemary extract (RE). Rosemary (Rosmarinus officinalis L.) is a natural spice with good antioxidant activity and is widely used for medicinal purposes, beauty care, and spices (Yoon et al., 2011). RE is commonly used in the food industry to extend the shelf life of several products, and research regarding antibacterial activity has also increased in recent times (Aguilar et al., 2008; Lalas and Dourtoglou, 2003; Robbins and Sewalt, 2005). The major bioactive components of RE are carnosic acid (CaA), carnosol, and rosmarinic acid (Pérez-Fons et al., 2009).
Although there are various beneficial activities of RE, its application to industry is limited due to its poor water solubility, similar to other hydrophobic natural components. RE is primarily insoluble in the aqueous phase because of its highly hydrophobic structure (Zhang et al., 2012). Therefore, the use of various techniques, such as encapsulation, solid dispersion, microemulsion, and micellization, have been attempted to overcome the problem of enhancing its water solubility, which would promote the practical use of RE (Kumar and Singh, 2016). Micellization using surfactants is a method that has been widely used in the field of pharmacy to increase the water solubility and stability of insoluble active compounds. This method is based on the characteristics of surfactants that can form micelle. In recent studies, different types of synthetic and natural amphiphilic compounds are used for the encapsulation of hydrophobic drugs, such as single surfactant or polymer micelles, binary surfactant/cosurfactant, or surfactant/polymer systems (Zhang et al., 2012). An important property of surfactants is the formation of micelles. Once surfactant concentration reaches a critical micelle concentration (CMC), it starts to self-assemble into small particles to reduce the contact area. In micelles, non-polar tails of surfactants are placed within the hydrophobic core, whereas their polar heads are placed in outside. Due to the structure of surfactant micelle, non-polar (hydrophobic) molecules are solubilized within the hydrophobic interiors of surfactants and consequently, they can be dispersed into an aqueous phase.
Therefore, in this study, the degree of increase in water solubility of RE due to various types of surfactants and the accompanied antibacterial activity were measured. As surfactants, tween 20 (TW20), polyglyceryl-10-laurate (PG-10-LR), polyglyceryl-10-myristate (PG-10-MS), and polyglyceryl-10-monooleate (PG-10-MO) were selected. TW20 is one of the most universal surfactants used in the food, pharmaceutical and cosmetic industries. It is nonionic surfactant formed by the ethoxylation of sorbitan before the addition of laurate (Gelardi et al., 2016). PG-10-LR is a surfactant commonly used for cosmetics and has a TW20-like tail (laurate, C12), but its head consists of 10 glycerol, so it is specialized in the production of oil in water (O/W) emulsions (Matsumoto et al., 2013). As with PG-10-LR, the remaining two kinds of surfactants (PG-10-MS, PG-10-MO) have the same head composed of 10 glycerols. However, the structure of the tail part is different. It is found that PG-10-MS and PG-10-MO are respectively composed of myristate (C14) and monooleate (C18), so form a tail of different lengths. Consequently, based on the structural association between each of the selected surfactants, their characteristics (effects on RE) could be expressed.
Materials and methods
Materials
Commercial RE (containing 61.07% of CaA through the HPLC) and 4 types of surfactants—tween 20 (TW20), polyglyceryl-10-laurate (PG-10-LR), polyglyceryl-10-myristate (PG-10-MS), and polyglyceryl-10-monooleate (PG-10-MO) were supplied by Dyne Soze (Yongin, Korea). A strain of Bacillus subtilis subsp. spizizenii ATCC 6633 was used in antibacterial test. Paper disc was obtained from Advantec (Ehime, JAPAN, 8 mm diameter). All other chemicals were of analytical grade and were purchased from Duksan Pure Chemicals (Ansan, Korea).
Preparation of surfactant solution containing RE (Micellization)
The surfactant solutions were prepared by dissolving adequate amounts of surfactants into 5 mM phosphate buffer (pH 7) to vary the surfactant concentrations (0.1, 0.5, 1, 2, and 4 wt %) and a sufficient concentration of confirmed antibacterial activity in RE powder (1000 ppm) was then put into each surfactant solution. These solutions were ultrasound-treated (Power sonic 410, Hwashin Tech., Gwangju, Korea) for 30 min (400 W) and then dissolved for 3 h. Each sample solution was filtered through a 0.45 μm membrane filter (Sartorius, Goettingen, Germany) to remove the insoluble RE components.
Solubility of RE in surfactant solutions
RE was first dissolved in 95% ethanol and then filtered through a 0.45 μm membrane filter (Sartorius, Goettingen, Germany). The absorption spectrum of 95% ethanol containing dissolved RE was measured using the UV–vis spectrophotometer (JP/UV-1650PC, Shimadzu Co., Kyoto, Japan) within 200–600 nm. A particularly high peak appeared at 285 nm and a standard curve was achieved by measuring the optical density (O.D.) of 0–0.02% RE in 95% ethanol at this wavelength. In order to measure the O.D. value of only rosemary in this wavelength, the O.D. value of ethanol is subtracted as blank.
The RE content in surfactant solutions was determined based on the standard curve. Using the formula obtained from the standard curve, the amount of RE dissolved in the supernatant was calculated and compared with the first amount.
ζ-potential measurement
The ζ-potential measurements of RE-surfactant complex were performed using the zetasizer (Nano Series ZS, Malvern Instruments, Malvern, UK) through dynamic light scattering (DLS). The ζ-potential was determined by measuring the direction and velocity that the RE-surfactant complex moved in the applied electric field. The sample was diluted in a suitable concentration (0.1%–1%, v/v) to prevent the particle interactions from affecting the results of the experiment. Each individual ζ-potential was determined from the average of 12 readings per sample by nanosizer.
Measurement of antibacterial effect
The paper disc method was conducted to determine the antibacterial effects of RE and each RE-surfactant complex samples. First, the agar medium (20 ml) was poured into the petri dish and hardened, thus forming the bottom agar. Second, the new agar medium (top agar, 10 ml) was inoculated with B.subtilis and then poured on the bottom agar. Third, after the two layers of agar medium were prepared, the paper discs were placed on top and 50 μl of the RE-surfactant samples were dropped onto the discs. Petri dishes were incubated at 37 °C for 24 h (overnight).
Statistical analysis
Data presented were the mean ± standard deviation. A statistical analysis was performed using SPSS (IBM Corp., USA). A one-way ANOVA test, followed by Duncan’s multiple range test, were conducted to identify the statistical significances (P < 0.05) between the samples. All experiments were performed in triplicate.
Results and discussion
Solubilization of RE in surfactant micelles
The solubilization of RE in surfactant solutions was first examined by observing changes in the transparency of RE-TW20 solutions, wherein RE was added into TW20 solutions in different concentrations. Appearance of the RE-TW20 solution by the surfactant concentration is shown in Fig. 1.
Fig. 1.

The appearance of the mixture of rosemary extract (1000 ppm) and tween 20 solutions (①: 0%, ②: 0.1%, ③: 0.5%, ④: 1%, ⑤: 2%, ⑥: 4%)
When RE was dissolved in an aqueous solution (5 mM phosphate buffer, pH 7) without TW20, the rosemary did not dissolve well and the solution became cloudy. However, when the TW20 concentration in the solution reached 0.5 wt %, the RE-TW20 solution became transparent and the transparency increased as the concentration of TW20 increased. The raised transparency indicates a decrease in light scattering by RE molecules, this means that the RE size has become sufficiently small and the light has passed through without scattering (Bohren and Huffman, 2008). Thus, the observation of transparency indicated that the solubilization process occurred around 0.5% TW20. Also, the presence of surfactants was thought to affect the stability as well as the solubility of the rosemary extract. The rosemary extract in 5 mM phospate buffers, tween 20, and polyglycerl-10-monoolate solutions showed light green color in all sample. However, after two weeks of storage at room temperature, observations showed that only the phosphate buffer sample turned red. Based on this, it is believed that the presence of surfactants has maintained rosemary’s stability.
Above the CMC, surfactant molecules form micelles with hydrophobic interior and hydrophilic surfaces in water (Baeurle and Kroener, 2004). The CMC of TW20 is known to be 0.06 mM (Mahmood and Al-Koofee, 2013); 0.06 mM is close to around 0.1%. Therefore, TW20 is present in the form of micelle above 0.5% and the hydrophobic interior of micelles provided a suitable condition for RE, thereby allowing the RE molecules to be incorporated into micelles (Stoyanova et al., 2016). It was determined that being inserted into micelles formed by TW20 prevented RE molecules from being aggregated. The size of micelles containing RE molecules was smaller than the wavelength of visible light; therefore, it became transparent and optically invisible (Silva et al., 2013). In contrast, solutions with surfactant concentrations of 0% and 0.1% were still turbid. This is because the micelle structure was starting to form at these concentrations. Therefore, it was determined that several large clustered rosemary particles remained in the solution.
The absorption spectrum of RE (dissolved in 95% ethanol) measured in the range of 200–600 nm showed a particularly high peak at 285 nm (Fig. 2). This was consistent with the absorption wavelength of CaA, which is commonly known as the standard substance of RE (Pérez-Fons et al., 2009; Zhang et al., 2012). As the concentration of the added TW20 increased, the optical density at 285 nm also increased, thereby indicating that the amount of CaA dissolved in TW20 solutions increased. This result supported the experimental result that incorporating RE into the micelle of TW20 led to improved water solubility.
Fig. 2.
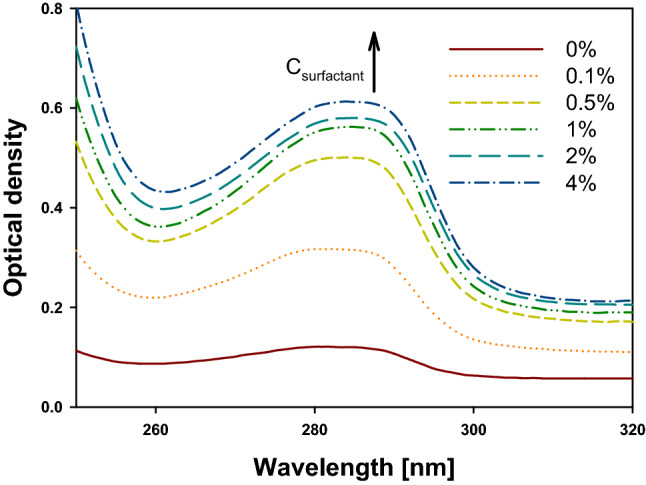
UV–vis spectra of rosemary extract saturated solutions in the presence of different Tween 20 concentrations
Influence of surfactant type and concentration
To examine the effect of various types and concentrations of surfactants on RE solubility, RE was dissolved into four types of surfactant solutions (TW20, PG-10-LR, PG-10-MS, and PG-10-MO), with varying concentrations (0%, 0.1%, 0.5%, 1.0%, 2.0%, and 4.0%). Changes in RE solubility were analyzed based on the types and concentrations of surfactants (Fig. 3).
Fig. 3.

Effects of the type and concentration of surfactants on the solubility of rosemary extract (TW20: tween20, PG-10-LR: polyglyceryl-10-laurate, PG-10-MS: polyglyceryl-10-myristate, PG-10-MO: polyglyceryl-10-monooleate)
In the absence of surfactant, the amount of RE was dissolved only 18.8% in the aqueous solvent, while considering the amount of CaA. However, in the presence of surfactants, the solubility of the RE in 5 mM phosphate buffer increased with surfactant concentrations. In addition to 0.1% surfactants, RE solubility increased up to 39.2% on average regardless of the type of surfactant used and it was 2.09 times higher than that of the buffer solution without surfactants. The RE solubility increased rapidly until the addition of 0.5% surfactant and then steadily increased with the increasing surfactant concentration beyond the CMC. As a result, an increase of 3.68 times in solubility was achieved when up to 4% surfactants were added regardless of the surfactant type.
Many studies have shown that surfactants increase the solubility of hydrophobic material in aqueous solvents (Mirgorodskaya et al., 2017; Stoyanova et al., 2016; Tehrani-Bagha and Holmberg, 2013). Holmberg et al. (2002) have shown that surfactant micelles tremendously affect the solubility of hydrophobic materials and have indicated that the beginning point of solubilization was the CMC of the surfactant. Notably, the effectiveness of the solubilization of surfactants is determined based on numerous factors, such as the structure of surfactants, solubilizate molecules, the morphology of micelle, the presence of additives and pH, and the ionic strength of medium (Adamczak, 2013). Studies have also reported that the interaction of solubilizate with micelles depended on whether the solubilizate is charge, polarized, or simple hydrophobic; therefore, the effectiveness of solubilization would differ (Mirgorodskaya et al., 2017; Rangel-Yagui et al., 2005; Vinarov et al., 2018).
The polyglycerol esters of fatty acids (PG-10-LR, PG-10-MS, and PG-10-MO) used in this experiment, were synthetic surfactant derived from various vegetable oils such as coconut, palm, and olive oils. These substances have the same head group composed of 10 glycerols and different length of carbon chain tail. The carbon number of PG-10-LR, PG-10-MS, and PG-10-MO tails are 12, 14, and 18, respectively. TW20 and PG-10-LR have a same carbon chain length of 12; however, they have different head groups (Head part of TW20 is composed of polyoxyethylene sorbitan) (Gelardi et al., 2016). The solubility was the highest in the PG-10-MO solution, which had the longest chain length (C18), followed by PG-10-MS > PG-10-LR = TW20. As the carbon number increased, the solubility of CaA increased, thereby suggesting that surfactant tail length can affect the solubility of hydrophobic materials. Adamczak (2013) noted that an increase of surfactant hydrophobic tail length enhances the solubilization capacity of hydrophobic compounds. If the tail length is longer with the same head part, the size of the core will increase during the micelle formation. Therefore, more hydrophobic molecules can enter the core. Vinarov et al. (2018) also investigated progesterone solubilization by using various surfactants with different hydrophilic head group charge and variable hydrophobic chain length. They reported that the increase of hydrophobic chain length led to increased progesterone solubilization, regardless of the type and charge of the hydrophilic head. Consistent with past results, there was no difference in RE solubility for PG-10-LR and TW20 with the same carbon chain length and different head groups.
However, our study was conducted using particular surfactants that are commonly used in the food and cosmetic industry, only non-ionic surfactants were used and the specific differences caused by head groups could not be identified. Therefore, future studies may analyze the effects of surfactant properties, such as surfactant charge and hydrophilic-liphophilic properties.
ζ-potential of RE-surfactant mixture
The ζ-potential is used to measure the surface charge of nanoparticles and provide general information about surface charge character. Insight regarding interactions between materials can be obtained by measuring the ζ-potential value (Smith et al., 2017).
In this section, the ζ-potential values of the RE-surfactant mixture solutions were evaluated. When the RE was added into the buffer in the absence of surfactants, the zeta-potential value were − 40.1 to − 48.5 mV (Fig. 4). One of the reasons for the RE solution to be able to maintain the turbid solution in the absence of surfactants and without precipitation, may be this electrical repulsive force. The ζ-potential values of ± 40– ± 50 mV are known as the proper range for good colloidal stability (Lu and Gao, 2010). On the other hand, the surfactant-only solutions without RE showed negative charges (− 5.8 to − 17.1 mV) (Fig. 4), even though this study used non-ionic surfactants. This may be caused by the anionic impurities present in the surfactants and the values ranging from − 10 to + 10 mV are generally considered neutral (McClements, 2015).
Fig. 4.
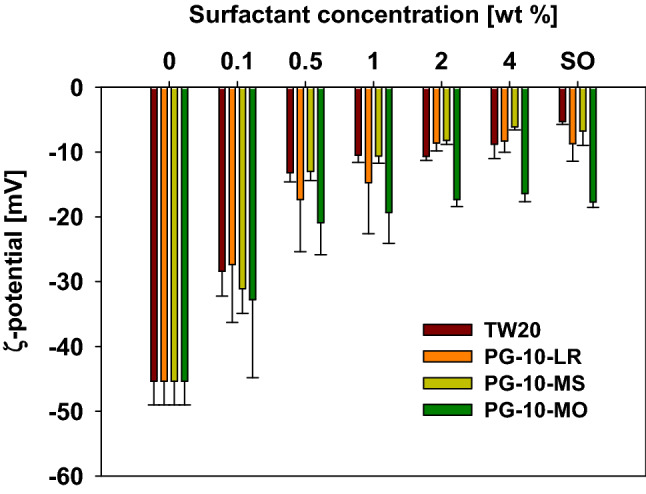
ζ-potential values of rosemary extract-surfactant mixture solutions (TW20: tween20, PG-10-LR: polyglyceryl-10-laurate, PG-10-MS: polyglyceryl-10-myristate, PG-10-MO: polyglyceryl-10-monooleate, SO: surfactant only)
As the concentration of the surfactants increased, the ζ-potential of the RE-surfactant solutions increased significantly until 0.5% was added. Above 0.5%, the increase in value was comparatively subtle and reached a similar value to those of only-surfactant solutions. This result was consistent with that of changes in appearance and solubility of RE-surfactant mixture solutions. The addition of surfactant above CMC effectively screened the negative charges of RE molecules, thereby suggesting that the RE molecules completely entered the core of those micelles.
Antibacterial effect
Antibacterial test was conducted using dissolved RE molecules, which have been incorporated into each surfactant micelle. The degree of antibacterial activity was analyzed by measuring the diameter of the inhibition zone formed around the paper disc. As the amount of surfactants increased, that of dissolved RE molecules also increased, as shown in Fig. 3; therefore, it had been expected that antibacterial activity would also increase. However, the results differed from the expectations.
Figure 5 shows the change in the size of the inhibition zone according to the concentration of surfactant in the RE-surfactant solution. Initially, as the concentration of surfactants increased, so did the inhibition zone. The early increase in antibacterial activity is seen as the result of large amounts of RE molecules being dissolved in samples due to the rise in RE solubility caused by the addition of surfactants. However, if the amount of surfactant increases to a certain level or more, the area of the inhibition zone decreases despite the more dissolved RE. Antibacterial activity started to decrease at lower concentrations in the order of PG-10-MO, PG-10-MS, PG-10-LR ≈ TW20. As shown in Fig. 3, the solubilization of RE using PG-10-MO was significantly advanced compared to other surfactants; however, antibacterial activity began to decrease when concentrations reached 0.1%. These results may be related to the structure of surfactants. Each surfactant has a different tail length that forms different micelle. Therefore, PG-10-MO has a significantly longer tail length than PG-10-MS, PG-10-LR, or TW20, which would manufacture an increasingly hydrophobic environment and thicker micelle barrier, thus allowing dissolved RE molecules to exist inside the micelle. Different physical and chemical properties of micelles can be affected, based on the tail length of the surfactant.
Fig. 5.
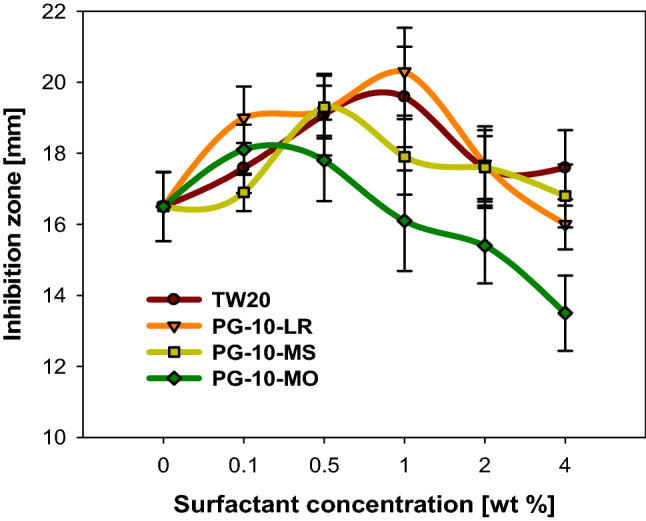
Effect of surfactant type on the inhibition zone (TW20: tween20, PG-10-LR: polyglyceryl-10-laurate, PG-10-MS: polyglyceryl-10-myristate, PG-10-MO: polyglyceryl-10-monooleate)
Chaiyasit et al. (2000) discovered that the surfactant hydrophobic tail group size can alter lipid oxidation in oil-in-water (O/W) emulsions. They manufactured O/W emulsion with two different surfactants: polyoxyethylene 10 lauryl ether and polyoxyethylene 10 stearyl ether. They have structurally different hydrophobic tails and similar hydrophilic heads. Notably, the surfactant with the longer tail (polyoxyethylene 10 stearyl ether) produced improved antioxidant effects. This is due to the thick barrier provided by the larger hydrophobic tails, which made it difficult for free-radical to come into contact with the fatty acids in the lipid. Materials in micelle were more strongly packed by intermolecular interaction (e.g., van der Waals interactions). Similar to antioxidation, the antibacterial activity is also activated by direct contact. Research has yet to address how phenolic compounds represent antibacterial activity against microbial agents. However, these compounds are believed to physically interact with the bacterial membrane and cause the disruption of proteins that play important roles in the entire bacteria (Bazaka et al., 2015). Urzúa et al. (2008) noted that the antibacterial activity of natural extracts was due to the diterpenoid structure they had and hydrogen-bond-donor (HBD) group strategically positioned in the molecule was an important requirement for activity. HBD group is known to be associated with physical interactions and lysis of cell membranes (Urzúa et al., 2008). These disruptions then conduct the fatal phenomena in cell growth, such as membrane expansion, inhibition of respiration, and abnormal ion exchange. Therefore, the physical combination of bacteria and antibacterial agent is considered an important factor in antibacterial activity. Therefore, if RE molecules are inserted in the micelles of a relatively large surfactant, with big hydrophilic head and long hydrophobic tail, the contact of RE and bacteria walls would be interrupted. In fact, after measuring the size of micelles composed of RE-surfactant solution, the average particle size of a micelles made of TW20, PG-10-LR, PG-10-MS, PG-10-MO was shown in order 61.47, 47.89, 58.37, 360.5 d.nm, respectively. There was not much difference between samples of TW20, PG-10-LR, PG-10-MS, but PG-10-MO showed relatively large particle sizes. This was consistent with our assumption that the early decay of antibacterial activity in PG-10-MO sample was due to the thick micelle layer. Therefore, it is possible to explain the antibacterial effects based on the structures of surfactants.
Figure 6 showed the changes in concentration-contrasting antibacterial activities of RE due to the concentration of surfactant (regardless of type). This result indicated that the presence of surfactants negatively affected antibacterial activity, even though more RE molecules could be dissolved in the solution with surfactants. When antibacterial trend lines at each concentration were drawn, the slope values were 0.048, 0.031, 0.027, 0.023, 0.019, and 0.015 in the order of 0%, 0.1%, 0.5%, 1%, 2%, and 4%, respectively. These values indicated that when the same amount of RE molecules were present in the aqueous phase, the solution containing less surfactant showed improved antibacterial activity. If the values of the antibacterial activity slope were plotted into a graph, it would almost match the shape of the upturned solubility graph. However, rosemary does not dissolve thoroughly in non- or low-surfactant concentrations; therefore, they showed relatively low antibacterial activity. Thus, the result suggested an optimum concentration (0.5–1%) determination was required to satisfy both the water solubility and antibacterial activity.
Fig. 6.
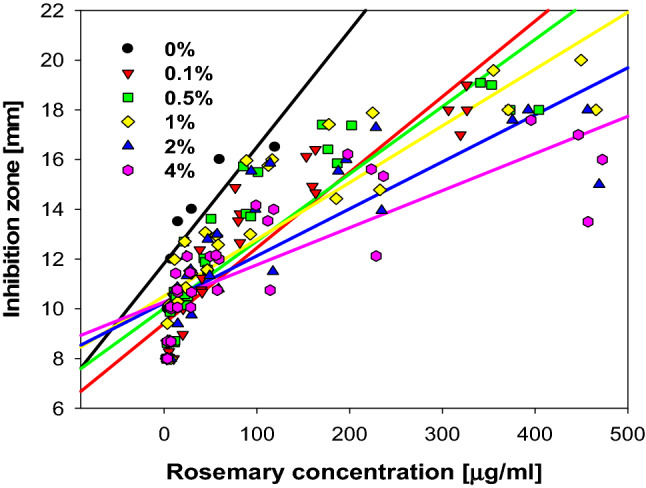
Changes in concentration-contrasting antibacterial activities of rosemary due to the concentration of surfactant (regardless of type)
In this study, four types of surfactants (tween 20, polyglyceryl-10-laurate, polyglyceryl-10-myristate and polyglyceryl-10-monooleate) were used to overcome the disadvantages of RE, which is difficult to dissolve in water and has many restrictions on industrial use. The micellar solubilization of RE was conducted by adding RE to solutions prepared by dissolving surfactants of various types and concentrations. The RE solubility was the highest in the PG-10-MO solution, which had the longest chain length (C18), followed by PG-10-MS > PG-10-LR = TW20. Therefore, it was suggested that surfactant tail length can affect the solubility of hydrophobic materials. Through the ζ-potential measurement, the addition of surfactant above CMC screened the negative charges of RE molecules (± 40 ~ ± 50 mV), thereby suggesting that the RE molecules completely entered into the core of those micelles. The addition of surfactant raised the amount of dissolved RE molecules in the solution, resulting in increased antibacterial activity. However, the presence of surfactants above a certain concentration (1%) reduced its effect, because surfactants prevented the RE molecules from sticking to the outer membrane of the microorganism and engaging in antibacterial activity. Based on these results, when micellizing rosemary, it was suggested that the optimum surfactant concentration (0.5% ~ 1%) should be determined to satisfy both the water solubility and antibacterial activity.
Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF) grant funded by Korea government (MSIT) (No. 2019R1A2C1010708).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shinjae Park, Email: psj0037@snu.ac.kr.
Saehun Mun, Email: saehun@snu.ac.kr.
Yong-Ro Kim, Email: yongro@snu.ac.kr.
References
- Adamczak M. Surfactants, polyelectrolytes and nanoparticles as building blocks for nanocarriers. PhD thesis, AGH University of Science and Technology, Krakow, Poland (2013)
- Aguilar F, Autrup H, Barlow S, Castle L, Crebelli R, Engel K, Gontard N, Gott D, Grilli S. Use of rosemary extracts as a food additive-Scientific opinion of the panel on food additives, flavourings, processing aids and materials in contact with food. Eur. Food Safe. Auth. 2008;6(6):1–29. [Google Scholar]
- Ambriz-Pérez DL, Leyva-López N, Gutierrez-Grijalva EP, Heredia JB. Phenolic compounds: Natural alternative in inflammation treatment. A Review. Cogent Food Agric. 2016;2(1):1131412. [Google Scholar]
- Aschemann-Witzel J, Varela P, Peschel AO. Consumers’ categorization of food ingredients: Do consumers perceive them as ‘clean label’ producers expect? An exploration with projective mapping. Food Qual. Prefer. 2019;71:117–128. doi: 10.1016/j.foodqual.2018.06.003. [DOI] [Google Scholar]
- Baeurle SA, Kroener J. Modeling effective interactions of micellar aggregates of ionic surfactants with the Gauss-core potential. J. Math. Chem. 2004;36:409–421. doi: 10.1023/B:JOMC.0000044526.22457.bb. [DOI] [Google Scholar]
- Bazaka K, Jacob M, Chrzanowski W, Ostrikov K. Anti-bacterial surfaces: natural agents, mechanisms of action, and plasma surface modification. Int. J. Food Microbiol. 2015;5:48739–48759. [Google Scholar]
- Bohren CF, Huffman DR. Absorption and scattering of light by small particles. Hoboken: Wiley; 2008. pp. 130–157. [Google Scholar]
- Chaiyasit W, Silvestre M, McClements DJ, Decker EA. Ability of surfactant hydrophobic tail group size to alter lipid oxidation in oil-in-water emulsions. J. Agr. Food Chem. 2000;48:3077–3080. doi: 10.1021/jf000323e. [DOI] [PubMed] [Google Scholar]
- Gelardi G, Mantellato S, Marchon D, Palacios M, Eberhardt AB, Flatt RJ. 9 - Chemistry of chemical admixtures. Woodhead Publishing, pp. 149-218 (2016)
- Holmberg K, JoÈnsson B, Kronberg B, Lindman B. Surfactant and Polymers in Aqueous Solution. Hoboken, NJ, USA: Wiley-Blackwell; 2002. pp. 39–66. [Google Scholar]
- Kumar S, Singh P. Various techniques for solubility enhancement: an overview. Pharm. Innov. J. 2016;5:23–28. doi: 10.7897/2277-4572.0516. [DOI] [Google Scholar]
- Lalas S, Dourtoglou V. Use of rosemary extract in preventing oxidation during deep-fat frying of potato chips. J. Am. Oil. Chem. Soc. 2003;80:579–583. doi: 10.1007/s11746-003-0741-x. [DOI] [Google Scholar]
- Lu GW, Gao P. Emulsions and microemulsions for topical and transdermal drug delivery. Amsterdam: Elsevier; 2010. pp. 59–94. [Google Scholar]
- Mahmood ME, Al-Koofee DA. Effect of temperature changes on critical micelle concentration for tween series surfactant. Global J. Sci. Front. Res. 2013;13:1–8. [Google Scholar]
- Matsumoto K, Igarashi Y, Shirai D, Hayashi K. Investigation of the influence of surfactant on the degree of supercooling (coexisting system of solid–liquid and gas–liquid interfaces) Int. J. Refrig. 2013;36(4):1302–1309. doi: 10.1016/j.ijrefrig.2013.02.007. [DOI] [Google Scholar]
- McClements DJ. Food emulsions: principles, practices, and techniques. Boca Raton: CRC Press; 2015. [Google Scholar]
- Mirgorodskaya AB, Mamedov VA, Zakharova LY, Valeeva FG, Mamedova VL, Galimullina VR, Kushnasarova RA, Sinyashin OG. Surfactant solutions for enhancing solubility of new arylquinolin-2-ones. J. Mol. Liq. 2017;242:732–738. doi: 10.1016/j.molliq.2017.07.055. [DOI] [Google Scholar]
- Pérez-Fons L, GarzÓn MaT, Micol V. Relationship between the antioxidant capacity and effect of rosemary (Rosmarinus officinalis L.) polyphenols on membrane phospholipid order. J. Agr. Food Chem. 58 (1): 161-171 (2009) [DOI] [PubMed]
- Rangel-Yagui CO, Hsu HWL, Pessoa-Jr A, Tavares LC. Micellar solubilization of ibuprofen: influence of surfactant head groups on the extent of solubilization. Rev. Bras. Cienc. Farm. 2005;41:237–246. doi: 10.1590/S1516-93322005000200012. [DOI] [Google Scholar]
- Robbins K, Sewalt V. Extending freshness with rosemary extract. Food Tech. 2005;16:8. [Google Scholar]
- Savoia D. Plant-derived antimicrobial compounds: alternatives to antibiotics. Future Microbiol. 2012;7:979–990. doi: 10.2217/fmb.12.68. [DOI] [PubMed] [Google Scholar]
- Silva DGd, Sarruf FD, Oliveira LCDd, Arêas EPG, Kaneko TM, Consiglieri VO, Velasco MVR, Baby AR. Influence of particle size on appearance and in vitro efficacy of sunscreens. Braz. J. Pharm. Sci. 49: 251-261 (2013)
- Smith MC, Crist RM, Clogston JD, McNeil SE. Zeta potential: a case study of cationic, anionic, and neutral liposomes. Anal. Bioanal. Chem. 2017;409:5779–5787. doi: 10.1007/s00216-017-0527-z. [DOI] [PubMed] [Google Scholar]
- Stoyanova K, Vinarov Z, Tcholakova S. Improving Ibuprofen solubility by surfactant-facilitated self-assembly into mixed micelles. J. Drug Deliv. Sci. Tec. 2016;36:208–215. doi: 10.1016/j.jddst.2016.10.011. [DOI] [Google Scholar]
- Tehrani-Bagha AR, Holmberg K. Solubilization of hydrophobic dyes in surfactant solutions. Mater. 2013;6:580–608. doi: 10.3390/ma6020580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urzúa A, Rezende MC, Mascayano C, Vásquez L. A structure-activity study of antibacterial diterpenoids. Molecules. 2008;13:882–891. doi: 10.3390/molecules13040822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinarov Z, Dobreva P, Tcholakova S. Effect of surfactant molecular structure on progesterone solubilization. J. Drug Deliv. Sci. Tec. 2018;43:44–49. doi: 10.1016/j.jddst.2017.09.014. [DOI] [Google Scholar]
- Yoon S, Kim J, Jo B, Kim J, Lee S, Ahn B, Cho Y. Isolation and identification of antimicrobial compounds against Helicobacter pylori from Rosemary (Rosmarinus officinalis L.) extracts. J. Appl. Biol. Chem. 2011;54(3):159–165. doi: 10.3839/jabc.2011.027. [DOI] [Google Scholar]
- Zhang Y, Smuts JP, Dodbiba E, Rangarajan R, Lang JC, Armstrong DW. Degradation study of carnosic acid, carnosol, rosmarinic acid, and rosemary extract (Rosmarinus officinalis L.) assessed using HPLC. J. Agr. Food Chem. 2012;60:9305–9314. doi: 10.1021/jf302179c. [DOI] [PubMed] [Google Scholar]


