Abstract
Purpose
To study the effector mechanism against pathogens of polymorphonuclear neutrophils (PMN) and macrophages, called ETosis, involving the release of extracellular traps (ETs) in patients with acute epididymitis. To assess the different ET phenotypes present in semen samples and to identify correlations between ETosis and clinical parameters.
Materials and methods
Samples from patients diagnosed with acute epididymitis were examined and compared with samples from uninfected controls. Biochemical analyses of seminal fluid included determination of peroxidase, α-glucosidase, fructose, and elastase levels. ETosis in semen was determined through presence of citrullinated histones, global histones, and extracellular DNA. Different ETosis phenotypes such as spread ETs, aggregated ETs, and diffuse ETs were identified by co-localisation of extruded DNA with myeloperoxidase and global histones. Anti-CD15+ and anti-CD68+ antibodies were used to identify different cell lines.
Results
Revealed a high number of ETs compared with the control group. The mean number of CD15+PMN and CD68+ macrophages was higher in the acute epididymitis group. ETosis increase in ejaculates correlated with clinical parameters such as enhancement of elastase concentrations and diminution of fructose in the semen.
Conclusions
This work shows for the first time the presence of ETs and their components in semen from patients with acute epididymitis. The presence of infections is an important factor for induction of ETs in semen. Furthermore, the presence of ETosis in ejaculates is suggestive of developing infectious processes and might possibly have a diagnostic value.
Keywords: Infertility, Leucocytospermia, Microbial infections, Extracellular traps
Introduction
Infertility, with a prevalence of 48.5 million couples worldwide, is attributable to male factors in 50% of cases; 15% of these are caused by bacterial infections of the male reproductive tract [1]. Although infection/inflammation is usually asymptomatic during the andrological workup of patients presenting with subfertility/infertility, they frequently could harbour opportunistic infections that commonly are different to classical STDs [2]. In addition to asymptomatic inflammation/infection of the urogenital tract, acute symptomatic bacterial infections (prostatitis, epididymitis) are important. In this context, acute bacterial epididymitis resulting from bacterial ascension through the urogenital tract is the most common infection; it might be caused by sexually transmitted diseases (e.g. Chlamydia trachomatis) as well as typical uropathogens (e.g. Escherichia coli) in males of reproductive age [3].
We know that not all male accessory gland infections cause leucocytosis; however, many of them have been associated with a massive presence of leucocytes in the semen [4]. Infiltration of leucocytes into the seminal fluid has been associated with altered sperm function, reduced sperm motility, impaired acrosome reaction, and reduced capacity to fuse with the oocyte [5–7]. Presence of leucocytes in semen has been described as one of the main causes for the loss of sperm quality; reactive oxygen species (ROS) are the principal molecules involved in causing this damage [8]. Although the classical effector mechanisms of leucocytes, i.e. phagocytosis, degranulation, and ROS production, have been described previously in great detail [9–11], release of extracellular traps (ETs) from polymorphonuclear leucocytes (PMN) may also be involved and might explain reductions in sperm quality and progressive motility, and even fertility problems as demonstrated elsewhere [12]. Among leucocytes, neutrophils play a key role in early host innate immune response; they are the most abundant cells in the blood and form the first line of defence [13]. Monocytes and macrophages carry out the same functions in cases of chronic urogenital infections [14]. The most recently described effector mechanism of these cells is NETosis/METosis, characterised by citrullination of nuclear histones by the enzyme peptidylarginine deiminase 4 (PAD4). In this post-translational modification, arginine residues are changed into citrullin and highly condensed chromatin changes into its decondensed form; the process concludes with the release of neutrophil extracellular traps (NETs) and macrophage/monocyte-derived extracellular traps (METs), known generically as extracellular traps (ETs) or ETosis process (mechanism of PMN that releases ETs) [15–17]. ETs are sticky, fibrous extracellular DNA structures decorated with histones (H1, H2A/H2B, H3, H4) and other granule components such as neutrophil elastase (NE), myeloperoxidase (MPO), permeability-increasing protein, cathepsin G, lactoferrin, metalloproteinase-9, pentraxin, and LL-37. ETs are highly effective antimicrobial structures, capable of capturing and eliminating foreign agents such as bacteria, fungi, viruses, and large-sized parasites [15, 18–20]. To date, different in vitro, ex vivo, and in vivo studies in the reproduction of domestic animals have been published, showing signalling pathways in detail and highlighting the biological importance of ETosis against sperm and pathogens [21]. However, little is known to date about their role in human reproduction, and much less about their pivotal role in the immunopathogenesis of infectious diseases of the male urogenital tract [21, 22].
The purpose of the present work was to determine the presence of ETosis in semen samples from men diagnosed with epididymitis ex vivo, and to link ETosis with immunopathology derived from patients suffering bacterial epididymitis, associating ETosis levels with semen parameters in these patients.
Materials and methods
Reagents
All reagents were obtained from Sigma-Aldrich unless otherwise stated.
Study population
Patients were recruited between 2015 and 2017 from an ongoing prospective study at the Urology Clinic of the Justus Liebig University Giessen, Germany. The inclusion criterion was acute epididymitis, defined as onset within the last 2 weeks, enlarged epididymis on palpation typically associated with pain, epididymal hyperaemia on scrotal ultrasound, urine analysis showing infection of the genitourinary tract, and ultrasound for final confirmation [3]. Semen samples were collected after diagnosis and 2–7 days of sexual abstinence to assess the seminal inflammation in the acute phase (n = 4). Healthy fertile men (median: 44 years, range: 33–54) requesting vasectomy were recruited from December 2016 to May 2017 as controls (n = 4).
Microbiological analysis
All patients with sexual activity within the last 12 months received PCR screening for sexual transmission disease; it was based on 5-mL first-void urine. For bacterial culture, 10-μL urine specimens were inoculated on MacConkey 5% sheep-blood and Sabouraud agar plates (Oxoid, Wesel, Germany), but no cultures were performed from semen. The cultured bacteria were identified by the MALDI-TOF technology [3].
Collection and preparation of semen samples
Semen analysis was carried out following the WHO 2010 recommendations [23]. To develop the ET evaluations, semen samples were immediately smeared on a sterile glass slide, using 15 μL of each sample, and were dried at room temperature. After drying, the specimens were stored at room temperature (RT) until immunostaining was performed for the detection of extracellular DNA decorated with classical components of ET structures.
Spermiogram and biochemical analysis of semen
The semen was analysed within 1 h after sample collection; in addition to the standard parameters (see Table 1), the peroxidase-positive leucocytes were determined using the protocol described by Politch et al. [23]. Briefly, 0.0375% of H2O2 was added to 4 mL of a solution of benzidine (0.0125% w/v of benzidine, Sigma Chemical Co., St. Louis, MO, USA, in 50% ethanol). A total of 20 μL of ejaculates was mixed with 20 μL of the recently prepared benzidine-H2O2 solution. After 5 min of incubation, 160 μL of sterile PBS was added.
Table 1.
Semen characteristics of epididymitis patients and controls
| Patients | Medical diagnosis | Etiology | Volume (mL) | pH | Appearance | Liquefaction | Sperm concentration (106/mL) | Progressive motility (%) | Leucocytes (1 × 106) | Normal morphology (%) | Eosin test (%) | Peroxidase cells (1 × 106/mL−1) | Elastase (ng/mL) | Glucosidase (mIU/mL) | Fructose (ng/mL) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EPI-1 | Epididymitis | Ureaplasma urealyticum | 2.5 | 7.6 | Ordinary | Normal | 110.5 | 64 | 11.9 | 12 | 84 | 13.4 | 1967 | 13.9 | 7.5 |
| EPI-2 | Epididymitis | Chlamydia trachomatis | 1.2 | 7.6 | Clear | Liquid | 25.4 | 45 | 8.7 | 6 | 77 | 1.0 | 747 | 11.9 | 3.8 |
| EPI-3 | Epididymitis | E. coli | 1.5 | 8 | Ordinary | Normal | 15.8 | 68 | 15 | 8 | 89 | 9.9 | 1941 | 11.8 | 6.4 |
| EPI-4 | Epididymitis | E. coli | 1.4 | 9 | Clear | Normal | 8 | 25 | 16.1 | 0 | 70 | 1.7 | 869 | 6.9 | 2.3 |
| C1 | - | - | 4.7 | 7.2 | Ordinary | Normal | 62.4 | 60 | < 1 | 7 | 73 | 0.2 | 81 | 21.6 | 13.9 |
| C2 | - | - | 2.7 | 7.6 | Ordinary | Normal | 258.5 | 63 | < 1 | 13 | 90 | 0.0 | 18 | 21.2 | 10.7 |
| C3 | - | - | 1.6 | 7.4 | Ordinary | Normal | 97 | 50 | < 1 | 7 | 86 | 0.3 | 97 | 35.1 | 22.2 |
| C4 | - | - | 7.8 | 8.7 | Ordinary | Normal | 70 | 69 | < 1 | 9 | 91 | 0.0 | 35 | 10.4 | 17.4 |
The peroxidase-positive cells (stained brown) were counted in a haemocytometer using a phase contrast microscope (Olympus). The number of peroxidase-positive cells was expressed in per millilitre of ejaculate [24].
The levels of α-glucosidase were also determined by the hydrolysis reaction of 4-nitrophenyl α-d-glucopyranoside in 4-nitrophenol and α-d-glucopyranoside, identifying the 4-nitrophenol by spectrophotometry. The results are presented in milli-international units per millilitre. The acid isoenzyme was inhibited by addition of sodium dodecyl sulphate 1% at pH 6.8, thereby ensuring that only the neutral isoform of α-glucosidase was measured [25]. Fructose was identified using 0.1% of resorption in ethanol. The intensity of the resulting bright red colour was recorded at 490 nm against a blank which did not contain fructose, using a Specol-211® spectrophotometer (Thermo Scientific, Germany) [26]; the results were expressed in nanogrammes per millilitre. The granulocyte-elastase levels of the seminal plasma were obtained by centrifuging seminal fluids at 300×g for 10 min at RT. The concentration of neutrophil-elastase released into the seminal plasma and bonded to the a1-protease inhibitor [27] was determined by a commercial ELISA method and expressed in nanogrammes per millilitre (Milenia Biotec, Bad Nauheim, Germany).
Identification of extracellular DNA release decorated with global histones and citrunillated H4Cit3 in human semen samples ex vivo
Semen samples were blocked with BSA 20% and 0.005% saponin in sterile PBS for 30 min at RT. Then, two washes were performed with 500 μL of sterile PBS for each sample. Subsequently, the following primary antibodies were added: anti-histones (anti-mouse monoclonal, clone H11-4, MAB3422, Merck, Millipore) and anti-citrunillated H4Cit3 (anti-rabbit polyclonal, 07-596, Merck Millipore). After incubation (1 h at RT), the samples were washed twice with 500 μL of PBS, and the secondary conjugated antibodies were then added (Alexa Fluor 488 goat anti-mouse IgG [ref. A11001, Invitrogen] for detection of global histones [H1, H2A/H2B, H3, H4]; Alexa Fluor 405 goat anti-rabbit IgG [ref. A31556, Invitrogen] for detection of H4Cit3). After incubation for 1 h at RT in the dark, the specimens were washed three times with 500 μL of PBS, and the DNA was stained with Sytox Orange® (ref. 18495, Invitrogen) for 20 min in the dark. Finally, the samples were washed twice with 500 μL of PBS and mounted face down on a glass coverslip (Nunc) to which one drop of anti-fading mounting medium (Fluoromount G®, ref. 495802, Invitrogen) was added. The WHO in its manual evaluates the presence of leucocytes in semen expressing it in per millilitre of ejaculate; therefore, it is independent of the volume; the same criteria were used to evaluate ET formation in this work [23]. The samples were viewed using an Olympus IX81® inverted fluorescence microscope equipped with a XM10® digital camera (Olympus).
To identify global histones (i.e. H1, H2A/H2B, H3, H4) and citrunillated histone H4 (H4Cit3), these proteins were viewed simultaneously by immunofluorescence microscopy (IFM) analysis. Twenty-five power vision field pictures were taken per seminal smear at × 40 magnification. The presence of ETs showing co-localisation of DNA with global histones and H4Cit3 was assessed individually using the ImageJ® analysis program. Results were expressed as the mean number of ET-positive events for global histones (green fluorescence) and the mean number of cells positive for H4Cit3 (blue fluorescence).
Assessment of different phenotypes of ETs
To reveal the different ET phenotypes, such as spread ETs (sprETs), diffuse ETs (diffETs), and aggregated ETs (aggETs), double immunofluorescence staining was performed following the steps as described above. The following antibodies were used: to mark human MPO, the monoclonal anti-MPO antibody (ref. 11073 anti-rabbit, Biorbyt orb) was used, and thereafter incubated in the presence of the second antibody conjugated with Alexa Fluor 405 (anti-rabbit ref. A31556, Invitrogen). At the same time, the anti-global histone antibody was used (ref. MAB 3422 clone H 11-4 anti-mouse, Invitrogen), conjugated with Alexa Fluor 488 anti-mouse antibody (Molecular Probes). To view the leucocyte nuclei and the DNA released into the extracellular space, Sytox Orange® (dilution 1:2000) was used in PBS 1×. The samples were analysed using an Olympus IX81® inverted fluorescence microscope equipped with an Olympus XM10® digital camera. Visualisation of sprETs, diffETs, and aggETs was analysed microscopically using four images selected at random for each group and morphologically classified according to Muñoz-Caro et al. [19, 28]: (i) diff ETs composed of network of decondensed extracellular chromatin, spotted with compact, globular antimicrobial proteins of 15–20 μm diameter; (ii) sprETs, which consist of structures in the form of soft, elongated bands of decondensed chromatin with antimicrobial proteins composed of fine fibres of 15–17 μm diameter; and (iii) very high density, stable released NETs forming aggregates and defined as aggETs [29]. In each sample, all these phenotypes were counted and compared with the uninfected control group. The data obtained were expressed in mean numbers of sprETs, aggETs, and diffETs in the epididymitis group and the uninfected control group.
Identification of seminal macrophages and neutrophils
To identify macrophage and neutrophil populations, seminal smears from each cell suspension were prepared and are described in the “Collection and preparation of semen samples” section. Mouse anti-human monoclonal antibody CD68-FITC (RRID: AB 795842; Thermo Fisher Scientific) was used to identify macrophages, and mouse anti-human monoclonal antibody (ref. 11-0159-42; Thermo Fisher Scientific) to identify neutrophils according to Schulz et al. [21]. After incubation (1 h, RT) in darkness, samples were washed three times with sterile PBS and mounted face down on glass coverslips (Nunc) and one drop of anti-fading mounting buffer (Fluoromount G®, ref. 495802, Invitrogen) was added. In total, 200 cells were viewed per power vision field with × 40 magnification using an inverted IX81® inverted fluorescence microscope (Olympus) equipped with an XM10® digital camera (Olympus). The results were presented as the mean number of CD15+ cells and the mean number of CD68+ cells.
Statistical analysis
As the data analysed did not present normal distribution, the Mann-Whitney-Wilcoxon test was applied to compare the epididymitis group with the control group. Differences were considered significant at P value < 0.05. The statistical analyses were performed using the GraphPad Prism® program. The data were expressed as box plot with median and interquartile range. The Spearman correlation test was applied between the following variables: level of elastase and peroxidase-positive cells; level of fructose and peroxidase-positive cells; levels of fructose and elastase; level of ETs and sperm concentration; level of ETs and peroxidase-positive cells; level of ETs and fructose; level of ETs and elastase.
Results
Spermiogram findings and biochemical semen analysis
The principal date of the spermiogram and biochemical analysis of seminal fluids are shown in Table 1 which summarises the macroscopic and microscopic findings of the semen samples and identification of the microorganisms founded in urine. All the samples diagnosed with acute epididymitis derived from microbial infections (i.e. Ureaplasma urealyticum, Chlamydia trachomatis, and E. coli). All the samples with infection presented leucocytospermia (leucocyte counts > 1 million).
Epididymitis patients present different phenotypes of neutrophil- and macrophage-derived ETs
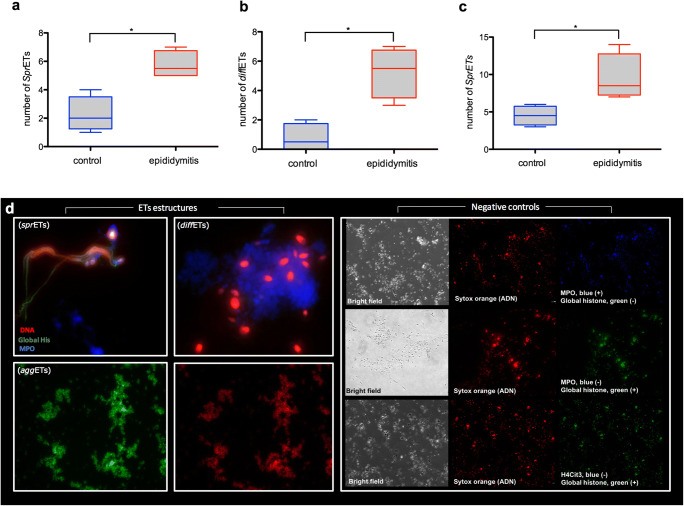
Staining with Sytox Orange® confirmed the classical nature of DNA-enriched ET-like structures extruded from CD15+-stained neutrophils and CD68+-positive macrophages. Furthermore, co-location studies revealed presence of MPO and global histones (i.e. H1, H2A/H2B, H3, and H4) simultaneously in these ET structures (Fig. 1a–c), induced most probably by the different microorganisms found in the ejaculates obtained from epididymitis patients (Fig. 1d). Quantitative analyses of diverse ET phenotypes resulted in larger mean numbers of sprETs (5.75; SEM = 0.478), aggETs (9.5; SEM = 1.555), and diffETs (5.25; SEM = 0.8539) in semen samples of patients with epididymitis when compared with those in semen samples from uninfected donors, where the mean numbers were as follows: 2.25 (SEM = 0.629), 4.5 (SEM = 0.6455), and 0.75 (SEM = 0.478), respectively (p < 0.05).
Fig. 1.
Microscope epifluorescence images showing the different phenotypes of ET structures (sprETs, diffETs, aggETs) in semen samples from epididymitis patients: DNA (red stain, Sytox Orange); MPO (blue stain, Alexa Fluor 405); global histones (green stain, Alexa Fluor 488) and negative controls of immunofluorescences. Graphs a, b, and c are box plot with median and interquartile range differences of quantification of the different phenotypes of ETs represented in the images comparing semen samples from epididymitis patients and control group. *P < 0.05
Presence of extracellular DNA, global histones, and H4Cit3 in semen samples from epididymitis patients
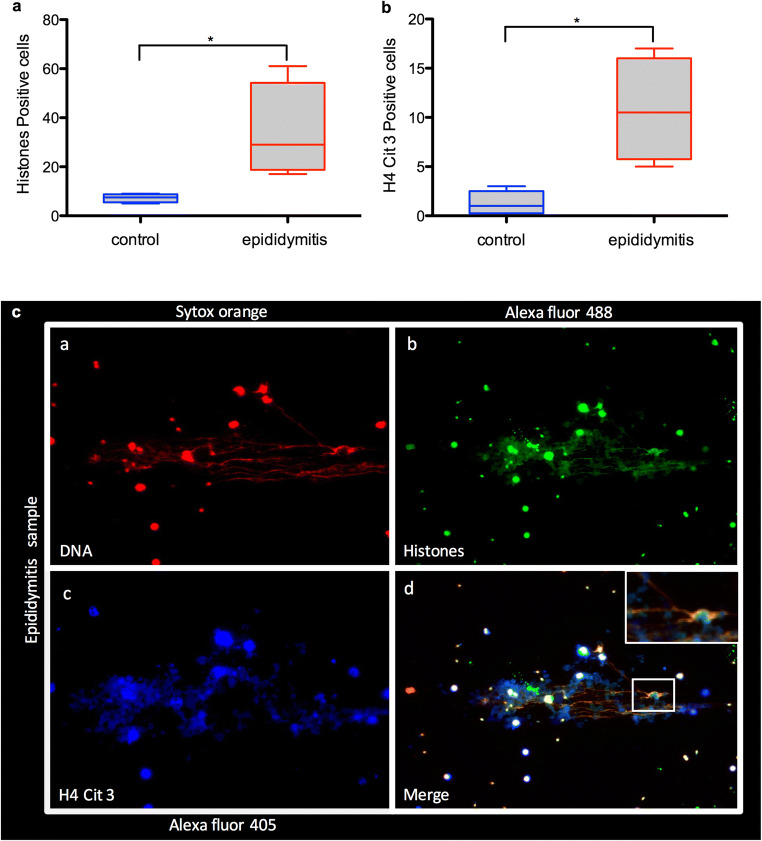
The samples from epididymitis patients showed that the presence of microorganism infections such as U. urealyticum, C. trachomatis, and E. coli significantly increased the formation of ETs in neutrophils and macrophages present in the ejaculates (P < 0.05). Quantification of cells positive for classical ET-derived proteins, such as global histones (epididymitis group SEM = 9.652; control group SEM = 0.853) and H4Cit3 (epididymitis group SEM = 2.658; control group SEM = 0.629), showed a larger number of these proteins in the epididymitis group than in the control group (P < 0.05) (Fig. 2a, b). Likewise, the images of the semen samples where epididymitis had been diagnosed showed a greater presence of extracellular DNA released by neutrophils and macrophages than the control group (Fig. 2c).
Fig. 2.
Microscope epifluorescence images showing semen from patients with epididymitis, (a) marking DNA with Sytox Orange (red); (b) Alexa Fluor 488 marking global histones (green); (c) Alexa Fluor 405 marking H4Cit3 (blue); and (d) a merge of channels. Magnification × 20. Graphs a and b show box plot with median and interquartile range differences of cells stained positive to global histones and citrullinated histones (H4Cit3), respectively. *P < 0.05
Leucocyte subpopulations in semen samples of epididymitis patients
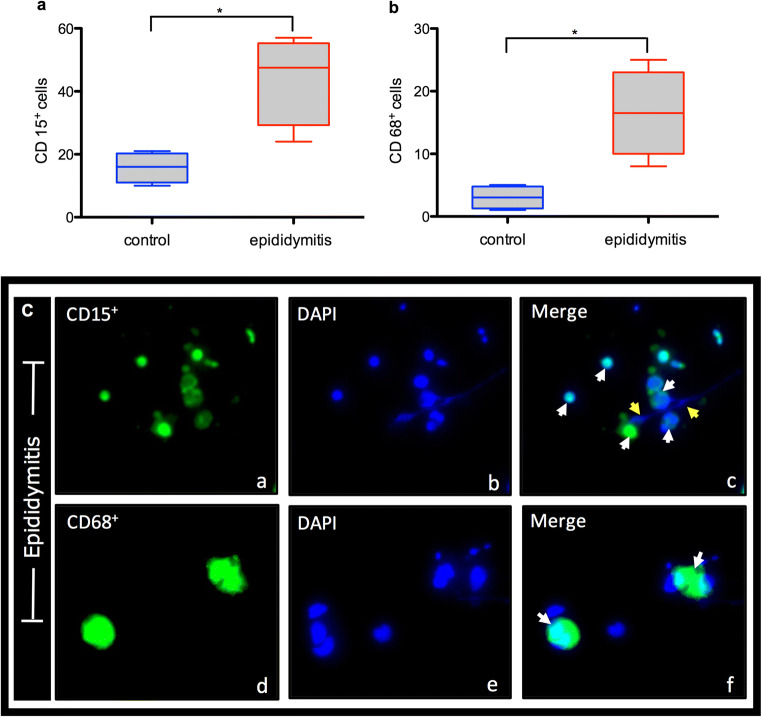
The number of leucocyte subpopulations positive for CD15 ([CD15+], neutrophils) and for CD68 ([CD68+], macrophages) was assessed in semen samples of epididymitis patients and compared with that of the control group (Fig. 3). The results showed a mean of 44 (SEM = 7.106) CD15+ cells in the study group. This number was significantly higher (P < 0.05) than the mean observed in the control group, where 15.75 (SEM = 2.394) CD15+ cells were counted (Fig. 3a–c). The number of CD68+ was also observed to be higher (16.5; SEM = 3.476) (P < 0.05) in the epididymitis group, whilst the mean cell count of CD68+-positive cells in the control group was 3 (SEM = 0.9129) (Fig. 3b, c).
Fig. 3.
Microscope epifluorescence images showing the mean numbers of CD15+ and CD68+ cells. White arrows indicate neutrophils (a–c) and macrophages (d–f). Yellow arrows mark ETs. Blue dye staining DNA with DAPI, green dye staining global histones with Alexa Fluor 488. Graphs a and b show the number of CD15+ and CD68+ cells in the semen samples of the epididymitis and control groups. Data are shown as mean ± SD, *P < 0.05
Correlation of semen parameters and levels of ET release
Correlation analyses (Table 2) showed a strong correlation (P = 0.0027, r = 0.7785) between the levels of elastase and the percentage of peroxidase-positive cells. The fructose levels presented a moderate correlation with the percentage of peroxidase-positive cells (P = 0.132, r = 0.5988). There was a strong correlation between the levels of elastase and fructose (P = 0.27, r = 0.7857). Comparison of data between the levels of ETs detected in the samples and the sperm concentration showed a strong negative correlation (P = 0.057, r = − 0.7109). Comparison of data between the ET levels and the percentage of peroxidase-positive cells showed a weak correlation (P = 0.0481, r = 0.2927). Comparison of the same data with fructose levels showed a strong negative correlation (P = 0.01, r = − 0.7857), and with elastase levels, they showed a very strong correlation (P = 0.001, r = 0.9398).
Table 2.
Correlation between clinical and biological parameters of semen
| Spearman’s correlation (r) | P value | |
|---|---|---|
| Elastase/peroxidase-positive cells | 0.7785 | 0.0279* |
| Fructose/peroxidase-positive cells | − 0.5988 | 0.1323 |
| Fructose/elastase | − 0.7857 | 0.0278* |
| ETs/sperm concentration | − 0.7109 | 0.0576 |
| ETs/peroxidase-positive cells | 0.5927 | 0.0481* |
| ETs/fructose | − 0.7952 | 0.0218* |
| ETs/elastase | 0.9398 | 0.0011** |
Spearman’s correlation data between the different semen analyses carried out on epididymitis patients and healthy donors (n = 8). Single and double asterisks represent a significant difference between the correlated data with P value < 0.05 and < 0.001, respectively
Discussion
Sperm-mediated ET formation by seminal neutrophils and macrophages in vitro and ex vivo was recently reported to occur in different mammal species including humans [12, 21, 22, 30, 31]. Here we investigated for the first time the possible interactions of human neutrophils/macrophages in semen samples obtained from acute epididymitis patients, analysing the molecular and morphological characteristics of seminal ETs ex vivo.
New evidence indicates adverse effects of neutrophils and/or macrophages on sperm function after exposure to extruded ETs [12, 21, 22]. Although it is known that seminal fluid contains macrophages and neutrophils as well as spermatozoa [32]. These professional phagocytes recruited into the focus of inflammation empty their granule contents, releasing peptides/proteins such as elastase, MMP9, pentraxin, MPO, lactoferrin, cathepsin, and peroxidase described previously during degranulation process [33]. Today, it is well-known that ETs have a high content of these enzymes, which are released into the extracellular space together with other antimicrobial proteins, DNA, and histones (e.g. H2A) originating mostly from the nucleus [15]. This translates into increased extracellular elastase in the seminal plasma, which can also be explained by the presence of ETosis—most probably triggered by microorganisms in the epididymis as shown in this study.
Seminal ETs ex vivo can be inferred in semen from epididymitis patients, since induction of ETs has previously been reported for E. coli, U. urealyticum, and C. trachomatis in vitro, with different activation times and reaction intensities [34, 35]. Interestingly, patients with epididymitis and leucocytospermia presented all three morphological phenotypes of ETs (diffETs, aggETs, and sprETs) [36], with high presence of aggETs entrapping vast amounts of spermatozoa as has been previously described in humans in vitro and ex vivo [12]. These different ET phenotypes were confirmed by co-localisation of DNA-enriched extracellular structures decorated with global histones, MPO, and H4Cit3 [15, 16]. Additionally, phenotypes of ETs are associated with different leucocyte populations depending on the source cell by which they are released. So far, neutrophils can cast all three characteristic morphologies, whilst macrophages/monocytes mainly release diffETs [22]. These morphological differences are often related with the type of stimulus, causing the formation of different ETs [21, 22].
Interestingly, recent studies dealing with infertility in male patients have related extrusion of aggETs with leucocytospermia and bacteriospermia, which would stimulate both migration and a marked response of these urogenital-derived leucocytes [37]. Conversely, presence of all three types of ETs have also been described in human seminal fluid with no microbial infection; these are most probably associated with other conditions—such as chronic aseptic inflammation or immunological disorders—which would result in the stimulation of seminal neutrophils and macrophages [12, 21].
The findings of the present study showed that extruded ETs can be found in samples from uninfected healthy donors, indicating that base physiological concentrations may be present in normozoospermic donors without producing any alteration in their semen parameters; or that the triggered ETs are under control or digested/removed adequately by DNases also present in human seminal plasma [21]. Furthermore, it is known that the spermatozoon itself constitutes a biological stimulus for the formation of ETs [21]. It is still unclear whether sperm motility or size would be the triggering mechanisms for leucocyte-derived ETosis; this was not clarified by the current study in contrast with other reports [38, 39].
In patients with epididymitis, inflammation of the seminal vesicle glands has been seen in a high percentage of cases (92.3%) [40]; this significantly affects gland function, reducing the secretion of different factors such as ascorbic acid, prostaglandins, and fructose, which—among other functions—act to reduce or prevent sperm agglutination [41, 42]. We found low levels of fructose in the semen samples from patients with epididymitis, correlating with high levels of elastase and ETs, leading us to suggest that spermatozoa might lose their defence mechanisms in presence of microbial infection. The diminution of fructose levels, combined with other factors, may be one of the factors that make spermatozoa more likely to form aggregations and agglutinations, principally due to the presence of ETs, as we have seen in this study.
In conclusion, our data confirm previous observations and show for the first time the presence of ETs and their different structures in ejaculates ex vivo from men with acute epididymitis. ETosis might correlate with other parameters, leading us to expect that when clinical results show high levels of elastase and low levels of fructose, the semen samples will also present high contents of ETs. However, further studies will be necessary to determine the implications of the presence of ETs, and their diagnostic value for infections in the men urogenital tract.
Authors’ contributions
FZ, IC, MS, and PU performed the quantification of ETs and image analysis. AT, RS, and CH cooperated in research design, data analysis, and manuscript review. AP, HS, and FW cooperated in manuscript review. AP cared for the patients and controls and provided the clinical data. All the authors checked and accepted the final manuscript.
Funding information
This work was funded by the Institute of Parasitology, Justus Liebig University Giessen, Germany. FZ was a recipient of a post-doctoral scholarship financed by the Universidad de La Frontera, Chile.
Data availability
The data and material can be requested to the corresponding author.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval
This study was approved by the Ethics Committees of the Universidad de La Frontera and University of Giessen (Ref. N°100/7-Clinical trials: DRKS00003325). All work has been carried out in compliance with the Helsinki Declaration.
Consent to participate
Patients and controls were enrolled after signing consent.
Consent for publication
Not applicable for that section.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update. 2015;21(4):411–426. doi: 10.1093/humupd/dmv016. [DOI] [PubMed] [Google Scholar]
- 2.Pilatz A, Kilb J, Kaplan H, et al. High prevalence of urogenital infection/inflammation in patients with azoospermia does not impede surgical sperm retrieval. Andrologia. 2019;51(10):e13401. doi: 10.1111/and.13401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pilatz A, Hossain H, Kaiser R, Mankertz A, Schüttler CG, Domann E, Schuppe HC, Chakraborty T, Weidner W, Wagenlehner F. Acute epididymitis revisited: impact of molecular diagnostics on etiology and contemporary guideline recommendations. Eur Urol. 2015;68(3):428–435. doi: 10.1016/j.eururo.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez R, Villegas J, Pena P, Miska W, Schill WB. Determination of peroxidase positive cells in semen: is it a secure parameter for the diagnosis of silent genital infections? Rev Med Chil. 2003;131(6):613–616. [PubMed] [Google Scholar]
- 5.Aitken RJ. A free radical theory of male infertility. Reprod Fertil Dev. 1994;6(1):19–23. doi: 10.1071/RD9940019. [DOI] [PubMed] [Google Scholar]
- 6.Plante M, de Lamirande E, Gagnon C. Reactive oxygen species released by activated neutrophils, but not by deficient spermatozoa, are sufficient to affect normal sperm motility. Fertil Steril. 1994;62(2):387–393. doi: 10.1016/S0015-0282(16)56895-2. [DOI] [PubMed] [Google Scholar]
- 7.Baker HW, Brindle J, Irvine DS, Aitken RJ. Protective effect of antioxidants on the impairment of sperm motility by activated polymorphonuclear leukocytes. Fertil Steril. 1996;65(2):411–419. doi: 10.1016/S0015-0282(16)58109-6. [DOI] [PubMed] [Google Scholar]
- 8.Kumar N, Singh AK. Reactive oxygen species in seminal plasma as a cause of male infertility. J Gynecol Obstet Hum Reprod. 2018;47:565–572. doi: 10.1016/j.jogoh.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Easterhoff D, Ontiveros F, Brooks LR, Kim Y, Ross B, Silva JN, Olsen JS, Feng C, Hardy DJ, Dunman PM, Dewhurst S. Semen-derived enhancer of viral infection (SEVI) binds bacteria, enhances bacterial phagocytosis by macrophages, and can protect against vaginal infection by a sexually transmitted bacterial pathogen. Antimicrob Agents Chemother. 2013;57(6):2443–2450. doi: 10.1128/AAC.02464-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurzawa R. Modulation of peritoneal macrophage function: effect of selected drugs on their activity and sperm phagocytosis. Ann Acad Med Stetin. 1997;43:79–97. [PubMed] [Google Scholar]
- 11.Marey MA, Liu J, Kowsar R, Haneda S, Matsui M, Sasaki M, Shimizu T, Hayakawa H, Wijayagunawardane MPB, Hussein FM, Miyamoto A. Bovine oviduct epithelial cells downregulate phagocytosis of sperm by neutrophils: prostaglandin E2 as a major physiological regulator. Reproduction. 2014;147(2):211–219. doi: 10.1530/REP-13-0375. [DOI] [PubMed] [Google Scholar]
- 12.Zambrano F, Carrau T, Gartner U, et al. Leukocytes coincubated with human sperm trigger classic neutrophil extracellular traps formation, reducing sperm motility. Fertil Steril. 2016;106(5):1053–1060. doi: 10.1016/j.fertnstert.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Uhl B, Vadlau Y, Zuchtriegel G, Nekolla K, Sharaf K, Gaertner F, Massberg S, Krombach F, Reichel CA. Aged neutrophils contribute to the first line of defense in the acute inflammatory response. Blood. 2016;128(19):2327–2337. doi: 10.1182/blood-2016-05-718999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castanheira FVS, Kubes P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood. 2019;133(20):2178–2185. doi: 10.1182/blood-2018-11-844530. [DOI] [PubMed] [Google Scholar]
- 15.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 16.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176(2):231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Granger V, Faille D, Marani V, Noël B, Gallais Y, Szely N, Flament H, Pallardy M, Chollet-Martin S, Chaisemartin L. Human blood monocytes are able to form extracellular traps. J Leukoc Biol. 2017;102(3):775–781. doi: 10.1189/jlb.3MA0916-411R. [DOI] [PubMed] [Google Scholar]
- 18.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191(3):677–691. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munoz-Caro T, Rubio RM, Silva LM, et al. Leucocyte-derived extracellular trap formation significantly contributes to Haemonchus contortus larval entrapment. Parasit Vectors. 2015;8:607. doi: 10.1186/s13071-015-1219-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munoz-Caro T, Conejeros I, Zhou E, et al. Dirofilaria immitis microfilariae and third-stage larvae induce canine NETosis resulting in different types of neutrophil extracellular traps. Front Immunol. 2018;9:968. doi: 10.3389/fimmu.2018.00968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schulz M, Zambrano F, Schuppe HC, et al. Determination of leucocyte extracellular traps (ETs) in seminal fluid (ex vivo) in infertile patients-a pilot study. Andrologia. 2019;51(9). 10.1111/and.13356. [DOI] [PubMed]
- 22.Schulz M, Zambrano F, Schuppe HC, et al. Monocyte-derived extracellular trap (MET) formation induces aggregation and affects motility of human spermatozoa in vitro. Syst Biol Reprod Med. 2019;65(5):357–66. 10.1080/19396368.2019.1624873. [DOI] [PubMed]
- 23.World Health Organization . WHO laboratory manual for the examination and processing of human semen. Geneva: WHO Press; 2010. [Google Scholar]
- 24.Wolff H, Panhans A, Zebhauser M, Meurer M. Comparison of three methods to detect white blood cells in semen: leukocyte esterase dipstick test, granulocyte elastase enzymeimmunoassay, and peroxidase cytochemistry. Fertil Steril. 1992;58(6):1260–1262. doi: 10.1016/S0015-0282(16)55584-8. [DOI] [PubMed] [Google Scholar]
- 25.Cooper TG, Yeung CH, Nashan D, Jockenhovel F, Nieschlag E. Improvement in the assessment of human epididymal function by the use of inhibitors in the assay of alpha-glucosidase in seminal plasma. Int J Androl. 1990;13(4):297–305. doi: 10.1111/j.1365-2605.1990.tb01035.x. [DOI] [PubMed] [Google Scholar]
- 26.Gonzales GF, Villena A. True corrected seminal fructose level: a better marker of the function of seminal vesicles in infertile men. Int J Androl. 2001;24(5):255–260. doi: 10.1046/j.1365-2605.2001.00306.x. [DOI] [PubMed] [Google Scholar]
- 27.Neumann S, Gunzer G, Hennrich N, Lang H. “PMN-elastase assay”: enzyme immunoassay for human polymorphonuclear elastase complexed with alpha 1-proteinase inhibitor. J Clin Chem Clin Biochem. 1984;22(10):693–697. [PubMed] [Google Scholar]
- 28.Lange MK, Penagos-Tabares F, Munoz-Caro T, et al. Gastropod-derived haemocyte extracellular traps entrap metastrongyloid larval stages of Angiostrongylus vasorum, Aelurostrongylus abstrusus and Troglostrongylus brevior. Parasit Vectors. 2017;10(1):50. doi: 10.1186/s13071-016-1961-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knopf J, Leppkes M, Schett G, Herrmann M, Munoz LE. Aggregated NETs sequester and detoxify extracellular histones. Front Immunol. 2019;10:2176. doi: 10.3389/fimmu.2019.02176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alghamdi AS, Foster DN. Seminal DNase frees spermatozoa entangled in neutrophil extracellular traps. Biol Reprod. 2005;73(6):1174–1181. doi: 10.1095/biolreprod.105.045666. [DOI] [PubMed] [Google Scholar]
- 31.Alghamdi AS, Lovaas BJ, Bird SL, Lamb GC, Rendahl AK, Taube PC, Foster DN. Species-specific interaction of seminal plasma on sperm-neutrophil binding. Anim Reprod Sci. 2009;114(4):331–344. doi: 10.1016/j.anireprosci.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 32.Eggert-Kruse W, Zimmermann K, Geissler W, Ehrmann A, Boit R, Strowitzki T. Clinical relevance of polymorphonuclear (PMN-) elastase determination in semen and serum during infertility investigation. Int J Androl. 2009;32(4):317–329. doi: 10.1111/j.1365-2605.2007.00852.x. [DOI] [PubMed] [Google Scholar]
- 33.Parzy E, Bouchaud V, Massot P, Voisin P, Koonjoo N, Moncelet D, Franconi JM, Thiaudière E, Mellet P. Overhauser-enhanced MRI of elastase activity from in vitro human neutrophil degranulation. PLoS One. 2013;8(2):e57946. doi: 10.1371/journal.pone.0057946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajeeve K, Das S, Prusty BK, Rudel T. Chlamydia trachomatis paralyses neutrophils to evade the host innate immune response. Nat Microbiol. 2018;3(7):824–835. doi: 10.1038/s41564-018-0182-y. [DOI] [PubMed] [Google Scholar]
- 35.Yu Y, Kwon K, Pieper R. Detection of neutrophil extracellular traps in urine. Methods Mol Biol. 2019;2021:241–257. doi: 10.1007/978-1-4939-9601-8_21. [DOI] [PubMed] [Google Scholar]
- 36.Munoz-Caro T, Mena Huertas SJ, Conejeros I, et al. Eimeria bovis-triggered neutrophil extracellular trap formation is CD11b-, ERK 1/2-, p38 MAP kinase- and SOCE-dependent. Vet Res. 2015;46:23. doi: 10.1186/s13567-015-0155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hahn S, Giaglis S, Hoesli I, Hasler P. Neutrophil NETs in reproduction: from infertility to preeclampsia and the possibility of fetal loss. Front Immunol. 2012;3:362. doi: 10.3389/fimmu.2012.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haidl G, Allam JP, Schuppe HC. Chronic epididymitis: impact on semen parameters and therapeutic options. Andrologia. 2008;40(2):92–96. doi: 10.1111/j.1439-0272.2007.00819.x. [DOI] [PubMed] [Google Scholar]
- 39.Xin S, Hao Y, Zhi-Peng M, Nanhe L, Bin C. Chronic epididymitis and leptin and their associations with semen characteristics in men with infertility. Am J Reprod Immunol. 2019;82(1):e13126. doi: 10.1111/aji.13126. [DOI] [PubMed] [Google Scholar]
- 40.Furuya R, Takahashi S, Furuya S, Kunishima Y, Takeyama K, Tsukamoto T. Is seminal vesiculitis a discrete disease entity? Clinical and microbiological study of seminal vesiculitis in patients with acute epididymitis. J Urol. 2004;171(4):1550–1553. doi: 10.1097/01.ju.0000116288.59223.e9. [DOI] [PubMed] [Google Scholar]
- 41.Okamura N, Tajima Y, Ishikawa H, Yoshii S, Koiso K, Sugita Y. Lowered levels of bicarbonate in seminal plasma cause the poor sperm motility in human infertile patients. Fertil Steril. 1986;45(2):265–272. doi: 10.1016/S0015-0282(16)49166-1. [DOI] [PubMed] [Google Scholar]
- 42.Marconi M, Pilatz A, Wagenlehner F, Diemer T, Weidner W. Impact of infection on the secretory capacity of the male accessory glands. Int Braz J Urol. 2009;35(3):299–308. doi: 10.1590/S1677-55382009000300006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and material can be requested to the corresponding author.